Mixture Design and Doehlert Matrix for Optimization of Energized Dispersive Guided Extraction (EDGE) of Theobromine and Caffeine from Cocoa Bean Shells
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material and Chemicals
2.2. EDGEs
2.3. Optimization Strategy
2.4. Liquid Chromatographic Analysis
2.5. Validation Procedures
2.6. Analytical Greenness Metric Tool
2.7. Determination of Total Phenolic Content (TPC)
2.8. Determination of Antioxidant Activity
2.8.1. DPPH Radical Scavenging Analysis
2.8.2. ABTS Radical Scavenging Analysis
3. Results
3.1. Optimization of the Extractor Solution
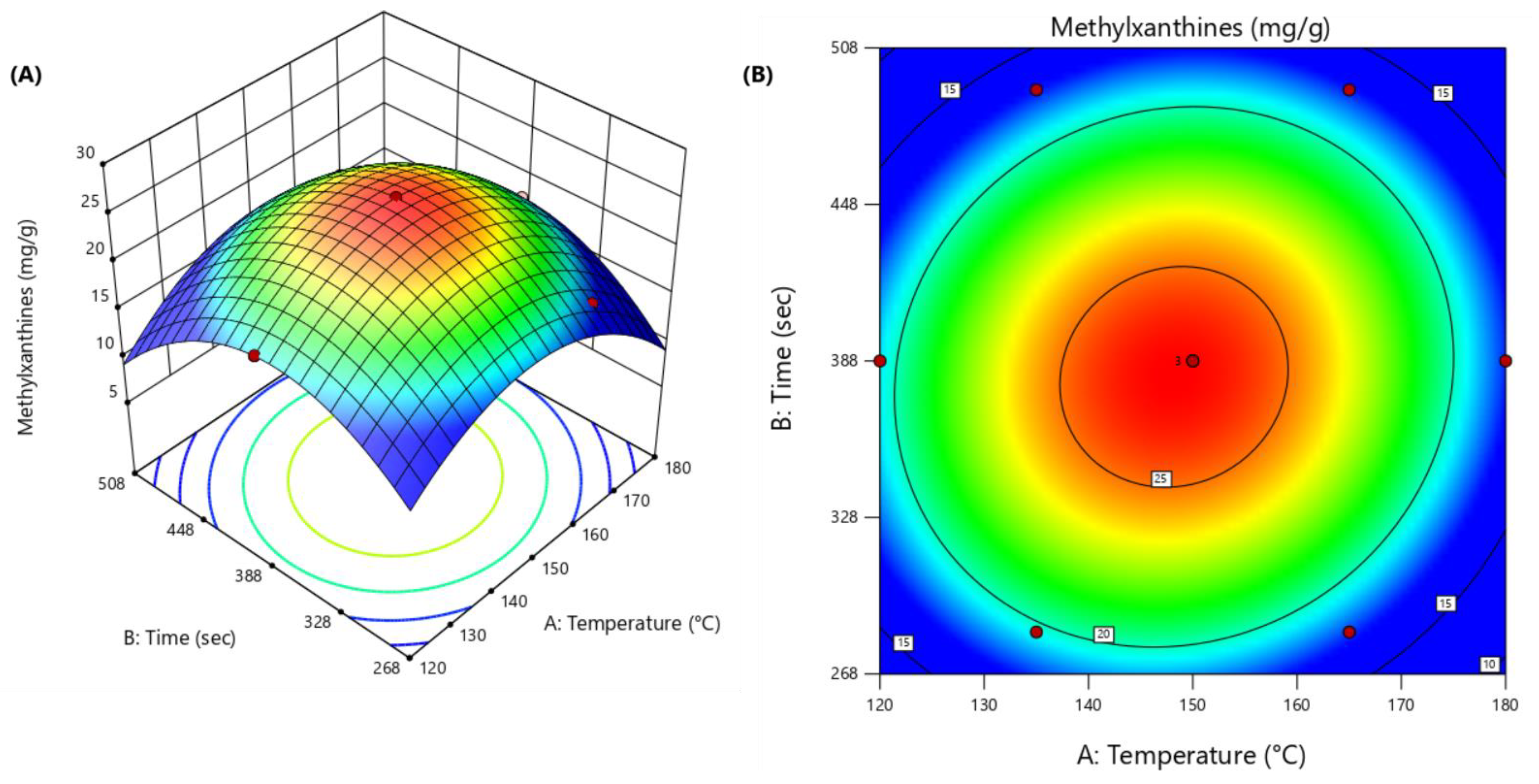
3.2. Optimization of EDGE Conditions
3.3. Effect of EDGE Cycles on Methylxanthines, Phenolic Content, and Antioxidant Activity
3.4. Analytical Performance
3.5. Comparison with Other Extraction Methods
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ANOVA | Analysis of variance |
| BB | Box–Behnken |
| CBS | Cocoa bean shell |
| CCD | Central Composite Design |
| DD | Doehlert Design |
| DM | Dry matter |
| EDGE | Energized Dispersive Guided Extraction |
| LOD | Limit of detection |
| LOQ | Limit of quantification |
| MAE | Microwave-assisted extraction |
| MD | Mixture design |
| TE | Trolox Equivalent |
| TPC | Total phenolic content |
References
- Quarterly Bulletin of Cocoa Statistics. Available online: https://www.icco.org/wp-content/uploads/Grindings_QBCS-L-No.-1.pdf (accessed on 17 March 2024).
- Nascimento, L.L.; Pereira, M.S.; de Almeida, L.S.; da Silveira Ferreira, L.; de Moura Pita, B.L.; de Souza, C.O.; Ribeiro, C.D.F.; Fricks, A.T. Innovation in Cocoa Fermentation: Evidence from Patent Documents and Scientific Articles. Fermentation 2024, 10, 251. [Google Scholar] [CrossRef]
- Sánchez, M.; Laca, A.; Laca, A.; Díaz, M. Cocoa Bean Shell: A By-Product with High Potential for Nutritional and Biotechnological Applications. Antioxidants 2023, 12, 1028. [Google Scholar] [CrossRef] [PubMed]
- Grillo, G.; Boffa, L.; Binello, A.; Mantegna, S.; Cravotto, G.; Chemat, F.; Dizhbite, T.; Lauberte, L.; Telysheva, G. Cocoa Bean Shell Waste Valorisation; Extraction from Lab to Pilot-Scale Cavitational Reactors. Food Res. Int. 2019, 115, 200–208. [Google Scholar] [CrossRef] [PubMed]
- Pagliari, S.; Domínguez-Rodríguez, G.; Cifuentes, A.; Ibáñez, E.; Labra, M.; Campone, L. Pressurized Liquid Extraction of Glucosinolates from Camelina sativa (L.) Crantz by-Products: Process Optimization and Biological Activities of Green Extract. Food Chem. X 2024, 22, 101324. [Google Scholar] [CrossRef] [PubMed]
- Parry, D.; Iqbal, S.; Harrap, I.; Oeppen, R.S.; Brennan, P.A. Caffeine: Benefits and Drawbacks for Technical Performance. Br. J. Oral Maxillofac. Surg. 2023, 61, 198–201. [Google Scholar] [CrossRef]
- Zhang, M.; Zhang, H.; Jia, L.; Zhang, Y.; Qin, R.; Xu, S.; Mei, Y. Health Benefits and Mechanisms of Theobromine. J. Funct. Foods 2024, 115, 106126. [Google Scholar] [CrossRef]
- Dos Santos, P.N.A.; Conrado, N.M.; Neubauer, T.M.; dos Santos, A.L.; Krause, L.C.; Caramão, E.B. Optimization of Energized Dispersive Guided Extraction (EDGE) of Antioxidants from Eugenia uniflora L. (Pitanga) Leaves Using Response Surface Methodology. Microchem. J. 2023, 187, 108411. [Google Scholar] [CrossRef]
- Drača, N.; Aladić, K.; Banožić, M.; Šubarić, D.; Jokić, S.; Nemet, I. Chamomile Waste: A Comprehensive Insight on Phytochemical and Safety Profile, Extraction Techniques and Potential Application. Biocatal. Agric. Biotechnol. 2025, 63, 103468. [Google Scholar] [CrossRef]
- Conrado, N.M.; dos Santos, N.C.T.; dos Santos, A.L.; Farrapeira, R.O.; Andrade, Y.B.; dos Santos, P.N.A.; Krause, L.C.; Freitas, L.d.S.; Caramão, E.B.; Polidoro, A.S. Innovative Açaí Seed Pre-Treatment: Energized Dispersive Guided Extraction for Small-Scale Fixed-Bed Reactor Fast-Pyrolysis. Waste Biomass Valorization 2024, 15, 1. [Google Scholar] [CrossRef]
- Santos, P.N.A.; Andrade, Y.B.; Granja, H.S.; Freitas, L.S.; Polidoro, A.S.; Caramão, E.B. Energized Dispersive Guided Extraction (EDGE): A Promising Tool for Analytical Chemistry Applications. Anal. Methods 2025, 17, 871–881. [Google Scholar] [CrossRef]
- López-Lorente, Á.I.; Pena-Pereira, F.; Pedersen-Bjergaard, S.; Zuin, V.G.; Ozkan, S.A.; Psillakis, E. The Ten Principles of Green Sample Preparation. TrAC Trends Anal. Chem. 2022, 148, 116530. [Google Scholar] [CrossRef]
- Wojnowski, W.; Tobiszewski, M.; Pena-Pereira, F.; Psillakis, E. AGREEprep—Analytical Greenness Metric for Sample Preparation. TrAC Trends Anal. Chem. 2022, 149, 116553. [Google Scholar] [CrossRef]
- Tasfiyati, A.N.; Antika, L.D.; Dewi, R.T.; Septama, A.W.; Sabarudin, A.; Ernawati, T. An Experimental Design Approach for the Optimization of Scopoletin Extraction from Morinda citrifolia L. Using Accelerated Solvent Extraction. Talanta 2022, 238, 123010. [Google Scholar] [CrossRef] [PubMed]
- Souza, M.R.R.; Santos, E.; Moraes, A.S.; Ribeiro, I.C.S.; Pinto, K.B.; Caramão, E.B.; Bjerk, T.R.; Krause, L.C. Green Extraction of Commiphora leptophloeos Mart.—J. B. Gillett Aiming to Increase the Content of Hinokinin, an “Emerging Bioactive”. Sustain. Chem. Pharm. 2023, 33, 101128. [Google Scholar] [CrossRef]
- Katiyar, P.; Gupta, K.; Yadav, S. Selective Extraction of Anti-Fungal Components from Carrot Seeds: Design of Experiments and Artificial Neural Network Modeling. J. Supercrit. Fluids 2024, 205, 106122. [Google Scholar] [CrossRef]
- Bezerra, M.A.; Lemos, V.A.; Novaes, C.G.; de Jesus, R.M.; Filho, H.R.S.; Araújo, S.A.; Alves, J.P.S. Application of Mixture Design in Analytical Chemistry. Microchem. J. 2020, 152, 104336. [Google Scholar] [CrossRef]
- Cerqueira, U.M.F.M.; Bezerra, M.A.; Ferreira, S.L.C.; de Jesus Araújo, R.; da Silva, B.N.; Novaes, C.G. Doehlert Design in the Optimization of Procedures Aiming Food Analysis—A Review. Food Chem. 2021, 364, 130429. [Google Scholar] [CrossRef]
- Khalid, W.; Koraqi, H.; Benmebarek, I.E.; Moreno, A.; Alsulami, T.; Mugabi, R.; Nayik, G.A. Optimization of UAE-NADES Green Extraction of Bioactive Compounds from Chickpea (Cicer arietinum L.) Sprouts Using Simplex Lattice Mixture Design Methodology. Ultrason. Sonochem. 2025, 112, 107186. [Google Scholar] [CrossRef]
- Design Expert; Design Expert Inc.: Minneapolis, MN, USA, 2005.
- IUPAC, R. Analytical Chemistry Division, Commission on Analytical Nomenclature. Pure Appl. Chem. 2000, 72, 1851–2082. [Google Scholar]
- Pena-Pereira, F.; Tobiszewski, M.; Wojnowski, W.; Psillakis, E. A Tutorial on AGREEprep an Analytical Greenness Metric for Sample Preparation. Adv. Sample Prep. 2022, 3, 100025. [Google Scholar] [CrossRef]
- Singleton, V.L.; Rossi, J.A., Jr. Colorimetry of Total Phenolics with Phosphomolybdic-Phosphotungstic Acid Reagents. Am. J. Enol. Vitic. 1965, 16, 144–158. [Google Scholar] [CrossRef]
- Kupina, S.; Fields, C.; Roman, M.C.; Brunelle, S.L. Determination of Total Phenolic Content Using the Folin-C Assay: Single-Laboratory Validation, First Action 2017.13. J. AOAC Int. 2019, 102, 320–321. [Google Scholar] [CrossRef]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a Free Radical Method to Evaluate Antioxidant Activity. LWT—Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Mellinas, A.C.; Jiménez, A.; Garrigós, M.C. Optimization of Microwave-Assisted Extraction of Cocoa Bean Shell Waste and Evaluation of Its Antioxidant, Physicochemical and Functional Properties. LWT 2020, 127, 109361. [Google Scholar] [CrossRef]
- Barreto, P.K.C.; da Silva, A.L.S.; de Jesus, R.M.; Coutinho, J.P.; Lôbo, I.P.; Santos, H.M.; de Jesus, R.M. Use of Design of Experiments to Optimize the Chromatographic Separation and Ultrasound-Assisted Extraction for Simultaneous Determination of Methylxanthines and Flavonoids in Guarana. Food Anal. Methods 2024, 17, 930–944. [Google Scholar] [CrossRef]
- Santana, Á.L.; Macedo, G.A. Effects of Hydroalcoholic and Enzyme-Assisted Extraction Processes on the Recovery of Catechins and Methylxanthines from Crude and Waste Seeds of Guarana (Paullinia cupana). Food Chem. 2019, 281, 222–230. [Google Scholar] [CrossRef]
- Machado, K.N.; de Freitas, A.A.; Cunha, L.H.; Faraco, A.A.G.; de Pádua, R.M.; Braga, F.C.; Vianna-Soares, C.D.; Castilho, R.O. A Rapid Simultaneous Determination of Methylxanthines and Proanthocyanidins in Brazilian Guaraná (Paullinia cupana Kunth.). Food Chem. 2018, 239, 180–188. [Google Scholar] [CrossRef]
- Llerena, W.; Samaniego, I.; Vallejo, C.; Arreaga, A.; Zhunio, B.; Coronel, Z.; Quiroz, J.; Angós, I.; Carrillo, W. Profile of Bioactive Components of Cocoa (Theobroma cacao L.) By-Products from Ecuador and Evaluation of Their Antioxidant Activity. Foods 2023, 12, 2583. [Google Scholar] [CrossRef]
- Benítez-Correa, E.; Bastías-Montes, J.M.; Acuña-Nelson, S.; Muñoz-Fariña, O. Effect of Choline Chloride-Based Deep Eutectic Solvents on Polyphenols Extraction from Cocoa (Theobroma cacao L.) Bean Shells and Antioxidant Activity of Extracts. Curr. Res. Food Sci. 2023, 7, 100614. [Google Scholar] [CrossRef]
- Shahidi, F.; Zhong, Y. Measurement of Antioxidant Activity. J. Funct. Foods 2015, 18, 757–781. [Google Scholar] [CrossRef]
- Ramos-Escudero, F.; Rojas-García, A.; Cádiz-Gurrea, M.d.l.L.; Segura-Carretero, A. High Potential Extracts from Cocoa Byproducts through Sonotrode Optimal Extraction and a Comprehensive Characterization. Ultrason. Sonochem. 2024, 106, 106887. [Google Scholar] [CrossRef] [PubMed]
- Sánchez, M.; Bernal, T.; Laca, A.; Laca, A.; Díaz, M. Hydrothermal Hydrolysis of Cocoa Bean Shell to Obtain Bioactive Compounds. Processes 2024, 12, 956. [Google Scholar] [CrossRef]
- Da Silva, R.F.; Carneiro, C.N.; de Sousa, C.B.d.C.; Gomez, F.J.V.; Espino, M.; Boiteux, J.; de los Á. Fernández, M.; Silva, M.F.; de S. Dias, F. Sustainable Extraction Bioactive Compounds Procedures in Medicinal Plants Based on the Principles of Green Analytical Chemistry: A Review. Microchem. J. 2022, 175, 107184. [Google Scholar] [CrossRef]
- Pagliari, S.; Celano, R.; Rastrelli, L.; Sacco, E.; Arlati, F.; Labra, M.; Campone, L. Extraction of Methylxanthines by Pressurized Hot Water Extraction from Cocoa Shell By-Product as Natural Source of Functional Ingredient. LWT 2022, 170, 114115. [Google Scholar] [CrossRef]
- Román-Hidalgo, C.; López-Pérez, G.; Villar-Navarro, M.; Martín-Valero, M.J. Green Electromembrane Extraction Procedure Based on Biodegradable Chitosan Films for Determination of Polyphenolic Compounds in Food Samples: Greenness Assessment of the Sample Preparation Approach. Talanta 2023, 253, 124034. [Google Scholar] [CrossRef]
- Pavlović, N.; Jokić, S.; Jakovljević, M.; Blažić, M.; Molnar, M. Green Extraction Methods for Active Compounds from Food Waste—Cocoa Bean Shell. Foods 2020, 9, 140. [Google Scholar] [CrossRef]
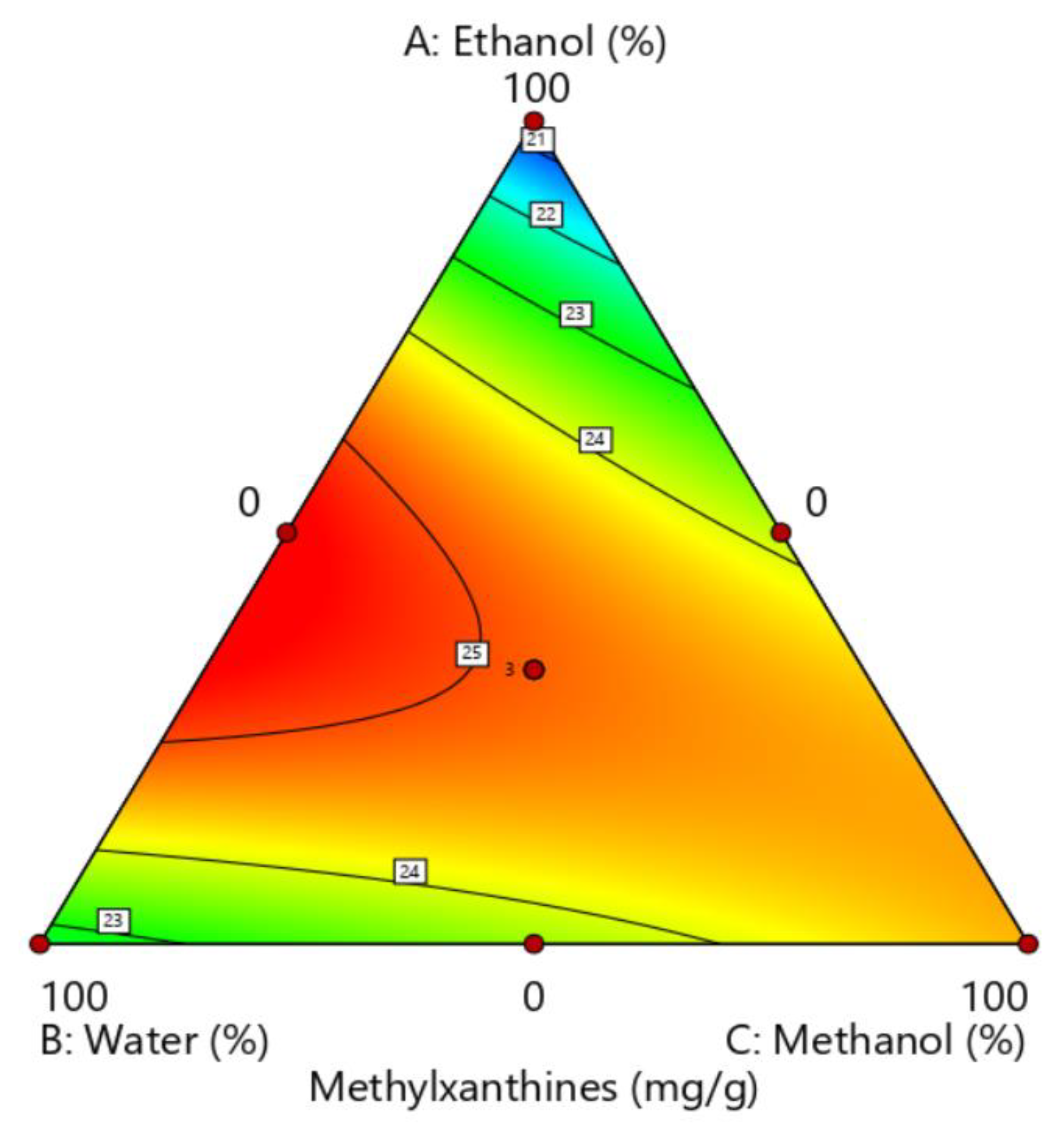
| # | Ethanol (%) | Water (%) | Methanol (%) | Methylxanthines (mg g−1 DM) |
|---|---|---|---|---|
| 1 | 50 | 50 | 0 | 25.3 |
| 2 | 50 | 0 | 50 | 23.7 |
| 3 | 0 | 0 | 100 | 24.5 |
| 4 | 0 | 100 | 0 | 22.7 |
| 5 | 100 | 0 | 0 | 20.6 |
| 6 | 33.33 | 33.33 | 33.33 | 25.1 |
| 7 | 0 | 50 | 50 | 23.6 |
| # | Temperature (°C) | Time (s) | Methylxanthines (mg g−1 DM) |
|---|---|---|---|
| 1 (C) | 0 (150) | 0 (388) | 25.9 |
| 2 (C) | 0 (150) | 0 (388) | 28.9 |
| 3 (C) | 0 (150) | 0 (388) | 25.8 |
| 4 | 1 (180) | 0 (388) | 17.4 |
| 5 | 0.5 (165) | 0.86 (491.92) | 17.4 |
| 6 | −1 (120) | 0 (388) | 19.3 |
| 7 | −0.5 (135) | −0.86 (284.08) | 19.4 |
| 8 | 0.5 (165) | −0.86 (284.08) | 17.8 |
| 9 | −0.5 (135) | 0.86 (491.92) | 17.2 |
| Cycle | Theobromine (mg g−1 DM) | Caffeine (mg g−1 DM) | TPC (mg GAE g−1 DM) | ABTS (mg TE g−1 DM) | DPPH (mg TE g−1 DM) |
|---|---|---|---|---|---|
| 1 | 20.14 ± 0.21 a | 3.53 ± 0.04 a | 80.10 ± 2.89 a | 227.73 ± 4.23 a | 104.45 ± 1.99 a |
| 2 | 1.78 ± 0.02 b | 0.26 ± 0.01 b | 10.63 ± 0.53 b | 9.84 ± 0.95 b | 6.61 ± 0.54 b |
| 3 | 0.29 ± 0.01 c | 0.04 ± 0.002 c | 1.88 ± 0.04 c | 6.77 ± 0.03 b | 4.74 ± 0.56 c |
| Compound | Regression Equation a | R2 b | LOD (mg L−1) c | LOQ (mg L−1) d | Precision e | Recovery f (%) (n = 3) | |
|---|---|---|---|---|---|---|---|
| Intraday (n = 7) | Interday (n = 7) | ||||||
| Theobromine | y = 18,972x + 31,443 | 0.9991 | 1.3 | 3.9 | 0.55 | 0.23 | 105 |
| Caffeine | y = 29,406x − 4342 | 0.9996 | 1.1 | 3.4 | 0.65 | 0.67 | 102 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lordêlo Nascimento, L.; Alves dos Santos, P.N.; Granja, H.S.; da Silveira Ferreira, L.; Ferreira Lima, J.V.; de Moura Pita, B.L.; dos Santos Polidoro, A.; dos Santos Freitas, L.; Caramão, E.B.; de Souza Dias, F.; et al. Mixture Design and Doehlert Matrix for Optimization of Energized Dispersive Guided Extraction (EDGE) of Theobromine and Caffeine from Cocoa Bean Shells. Foods 2025, 14, 740. https://doi.org/10.3390/foods14050740
Lordêlo Nascimento L, Alves dos Santos PN, Granja HS, da Silveira Ferreira L, Ferreira Lima JV, de Moura Pita BL, dos Santos Polidoro A, dos Santos Freitas L, Caramão EB, de Souza Dias F, et al. Mixture Design and Doehlert Matrix for Optimization of Energized Dispersive Guided Extraction (EDGE) of Theobromine and Caffeine from Cocoa Bean Shells. Foods. 2025; 14(5):740. https://doi.org/10.3390/foods14050740
Chicago/Turabian StyleLordêlo Nascimento, Luciana, Paulo Natan Alves dos Santos, Honnara Santos Granja, Larissa da Silveira Ferreira, João Victor Ferreira Lima, Bruna Louise de Moura Pita, Allan dos Santos Polidoro, Lisiane dos Santos Freitas, Elina Bastos Caramão, Fabio de Souza Dias, and et al. 2025. "Mixture Design and Doehlert Matrix for Optimization of Energized Dispersive Guided Extraction (EDGE) of Theobromine and Caffeine from Cocoa Bean Shells" Foods 14, no. 5: 740. https://doi.org/10.3390/foods14050740
APA StyleLordêlo Nascimento, L., Alves dos Santos, P. N., Granja, H. S., da Silveira Ferreira, L., Ferreira Lima, J. V., de Moura Pita, B. L., dos Santos Polidoro, A., dos Santos Freitas, L., Caramão, E. B., de Souza Dias, F., & Fricks, A. T. (2025). Mixture Design and Doehlert Matrix for Optimization of Energized Dispersive Guided Extraction (EDGE) of Theobromine and Caffeine from Cocoa Bean Shells. Foods, 14(5), 740. https://doi.org/10.3390/foods14050740






