Near-Infrared Spectroscopy Detection of Off-Flavor Compounds in Tench (Tinca tinca) After Depuration in Clean Water
Abstract
1. Introduction
2. Materials and Methods
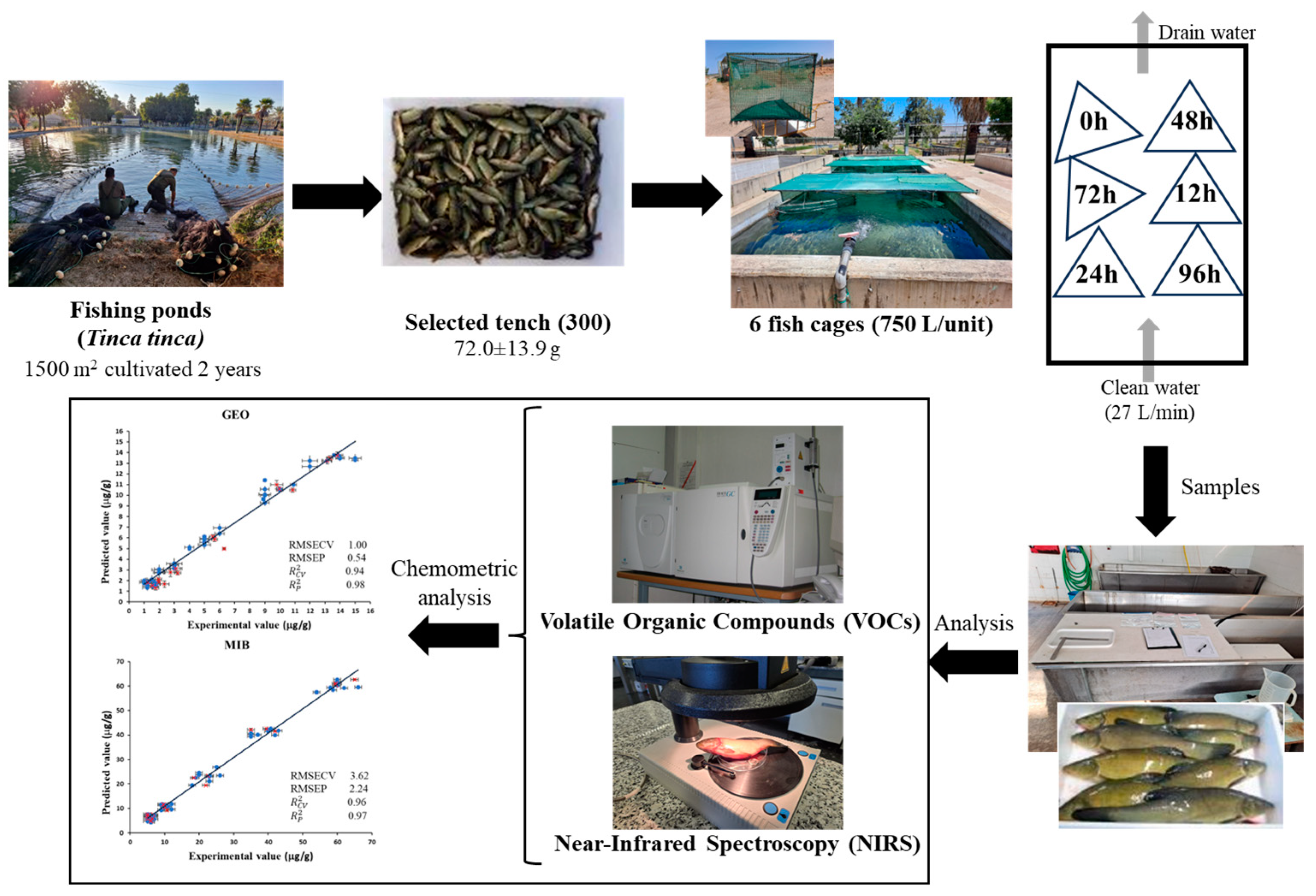
2.1. Samples
2.2. Analysis of VOCs in Tench
2.3. Near-Infrared Spectroscopy Analysis
2.4. Multivariate Data Analysis
3. Results and Discussion
3.1. Effect of Water Depuration on Volatile Organic Compounds
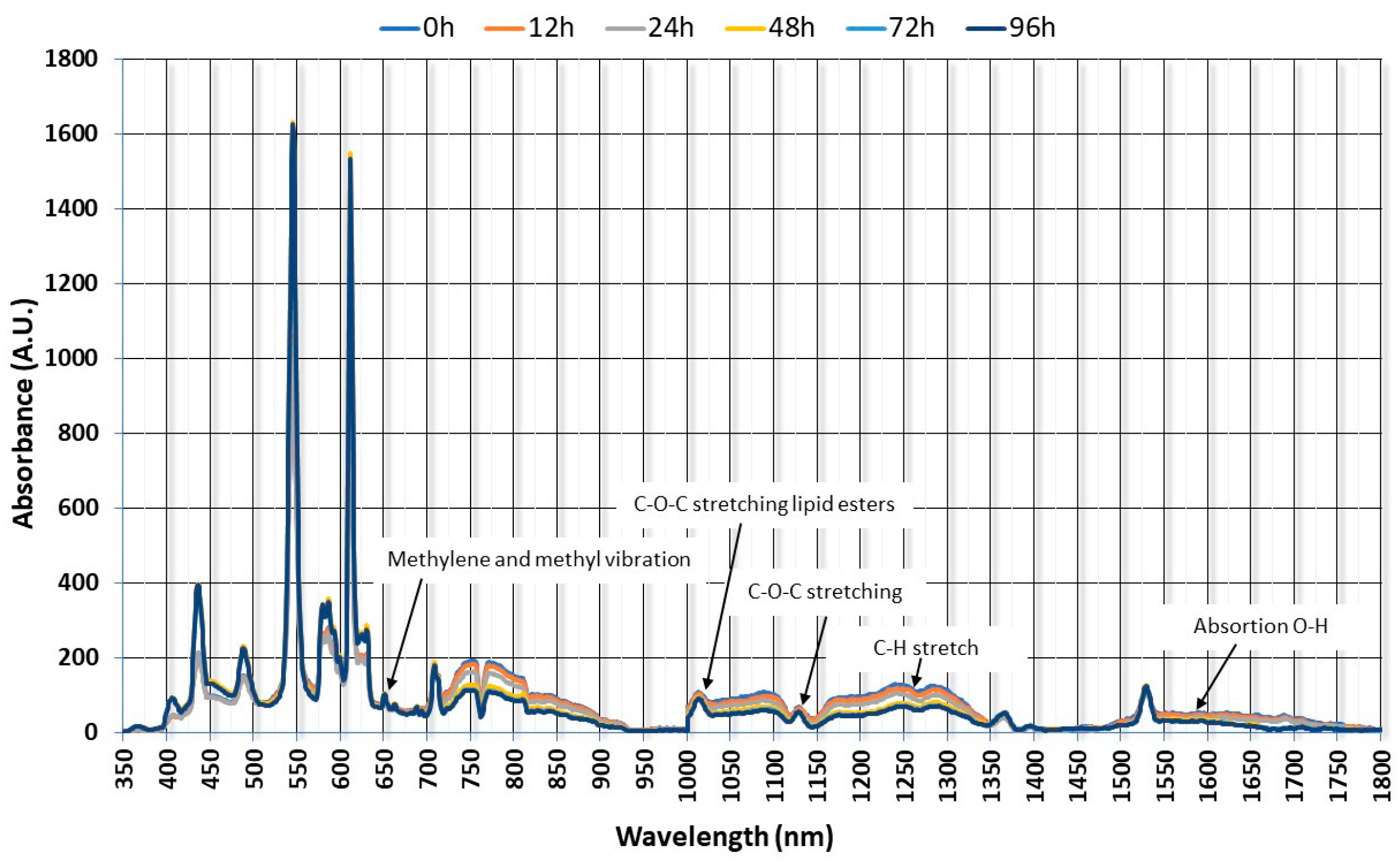
3.2. Evaluation of NIRS Spectra Data
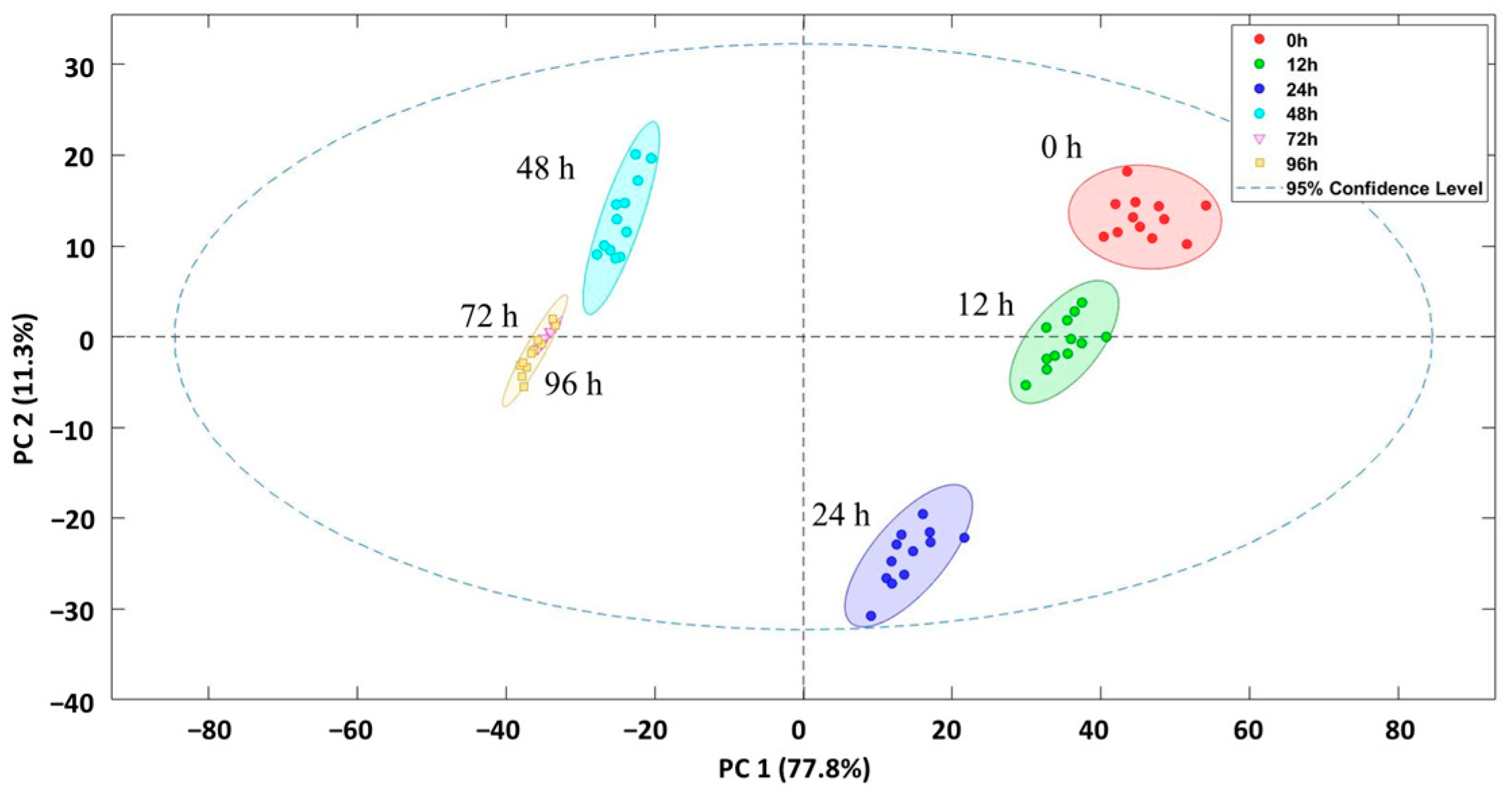
3.3. NIRS Discrimination Samples After the Application of Different Clean Water Depuration Processes
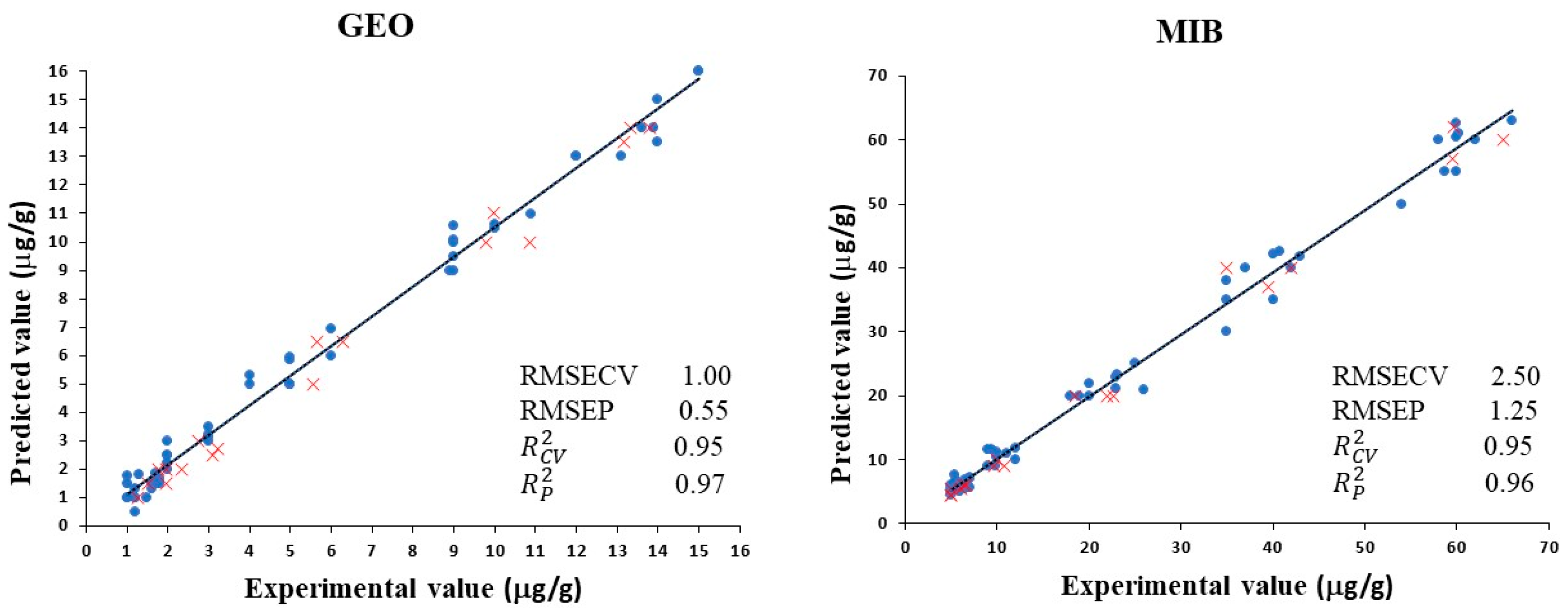
3.4. Prediction of Dimethyl-8-Hydronaphtalen and 2-Methylisoborneol Using NIRS Technology
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- FAO. El Estado Mundial de la Pesca y la Acuicultura 2022. Hacia la Transformación Azul; FAO: Rome, Italy, 2022. [Google Scholar]
- Tucker, C.S. Off-flavor problems in aquaculture. Rev. Fish. Sci. 2000, 8, 45–88. [Google Scholar] [CrossRef]
- Moretto, J.A.; Freitas, P.N.; Souza, J.P.; Oliveira, T.M.; Brites, I.; Pinto, E. Off-flavors in aquacultured fish: Origins and implications for consumers. Fishes 2022, 7, 34. [Google Scholar] [CrossRef]
- Kottelat, M.; Freyhof, J. Handbook of European Freshwater Fishes; Publications Kottelat: Cornol, Switzerland; Freyhof: Berlin, Germany, 2007; p. 646. [Google Scholar]
- Lajbner, Z.; Linhart, O.; Kotlík, P. Human-aided dispersal has altered but not erased the phylogeography of the tench: Human-aided dispersal in fish phylogeography. Evol. Appl. 2011, 4, 545–561. [Google Scholar] [CrossRef] [PubMed]
- Avlijaš, S.; Ricciardi, A.; Mandrak, N.E. Eurasian tench (Tinca tinca): The next Great Lakes invader. Can. J. Fish. Aquat. Sci. 2018, 75, 169–179. [Google Scholar] [CrossRef]
- Fernández, I.; Larrán, A.M.; de Paz, P.; Riesco, M.F. The direct effects of climate change on tench (Tinca tinca) sperm quality under a real heatwave event scenario. Animals 2024, 14, 778. [Google Scholar] [CrossRef]
- Asociación Empresarial de Acuicultura de España (APROMAR). Report on Aquaculture in Spain 2024; APROMAR: Madrid, Spain, 2024; Available online: https://apromar.es/wp-content/uploads/2024/12/Aquaculture-in-Spain-2024_layout-editing_v2.pdf (accessed on 1 June 2024).
- Pula, H.J.; Trenzado, C.E.; García-Mesa, S.; Fallola, C.; Sanz, A. Effects of different culture systems on growth, immune status, and other physiological parameters of tench (Tinca tinca). Aquaculture 2018, 485, 101–110. [Google Scholar] [CrossRef]
- Rendón, P.M.; Gallardo, J.M.; Ceballos, E.G.; Regadera, J.J.P.; García, J.C.E. Determination of substrate preferences of Tench, Tinca tinca (L.), under controlled experimental conditions. J. Appl. Ichthyol. 2003, 19, 138–141. [Google Scholar] [CrossRef]
- Freeman, K.S. Harmful algal blooms: Musty warnings of toxicity. Environ. Health Perspect. 2010, 118, A473. [Google Scholar] [CrossRef]
- Lukassen, M.B.; de Jonge, N.; Bjerregaard, S.M.; Podduturi, R.; Jørgensen, N.O.; Petersen, M.A.; Gianmarco, S.D.; da Silva, R.J.; Nielsen, J.L. Microbial production of the off-flavor geosmin in tilapia production in Brazilian water reservoirs: Importance of bacteria in the intestine and other fish-associated environments. Front. Microbiol. 2019, 10, 2447. [Google Scholar] [CrossRef]
- Li, Z.; Hobson, P.; An, W.; Burch, M.D.; House, J.; Yang, M. Earthy odor compounds production and loss in three cyanobacterial cultures. Water Res. 2012, 46, 5165–5173. [Google Scholar] [CrossRef]
- Olsen, B.K.; Chislock, M.F.; Wilson, A.E. Eutrophication mediates a common off-flavor compound, 2-methylisoborneol, in a drinking water reservoir. Water Res. 2016, 92, 228–234. [Google Scholar] [CrossRef] [PubMed]
- Podduturi, R.; Petersen, M.A.; Vestergaard, M.; Jørgensen, N.O. Geosmin fluctuations and potential hotspots for elevated levels in recirculated aquaculture system (RAS): A case study from pikeperch (Stizostedion lucioperca) production in Denmark. Aquaculture 2020, 514, 734501. [Google Scholar] [CrossRef]
- Bonpunt, E. The off-flavors management in the production of farmed sturgeon. In The Siberian Sturgeon (Acipenser baerii, Brandt, 1869) Volume 2—Farming; Springer: Cham, Switzerland, 2018; pp. 175–180. [Google Scholar]
- Adebo, O.A.; Oyeyinka, S.A.; Adebiyi, J.A.; Feng, X.; Wilkin, J.D.; Kewuyemi, Y.O.; Abrahams, A.M.; Tugizimana, F. Application of gas chromatography–mass spectrometry (GC-MS)-based metabolomics for the study of fermented cereal and legume foods: A review. Int. J. Food Sci. Technol. 2021, 56, 1514–1534. [Google Scholar] [CrossRef]
- Zhou, J.; Wu, X.; Chen, Z.; You, J.; Xiong, S. Evaluation of freshness in freshwater fish based on near infrared reflectance spectroscopy and chemometrics. LWT 2019, 106, 145–150. [Google Scholar] [CrossRef]
- Warm, K.; Martens, H.; Nielsen, J.; Martens, M. Sensory quality criteria for five fish species predicted from near-infrared (NIR) reflectance measurement. J. Food Qual. 2001, 24, 389–403. [Google Scholar] [CrossRef]
- Ritthiruangdej, P.; Suwonsichon, T. Relationships between NIR spectra and sensory attributes of Thai commercial fish sauces. Anal. Sci. 2007, 23, 809–814. [Google Scholar] [CrossRef]
- Sánchez, R.; Martín-Tornero, E.; Lozano, J.; Fernández, A.; Arroyo, P.; Meléndez, F.; Martín-Vertedor, D. Electronic nose application for the discrimination of sterilization treatments applied to Californian-style black olive varieties. J. Sci. Food Agric. 2022, 102, 2232–2241. [Google Scholar] [CrossRef]
- de Carvalho, D.S.; Dionísio, A.P.; dos Santos, R.; Boguzs, S., Jr.; Godoy, H.T.; Pastore, G.M. Production of 1-octen-3-ol by Neurospora species isolated from beiju in different culture medium. Procedia Food Sci. 2011, 1, 1694–1699. [Google Scholar] [CrossRef]
- Maggi, F.; Papa, F.; Cristalli, G.; Sagratini, G.; Vittori, S. Characterisation of the mushroom-like flavour of Melittis melissophyllum L. subsp. melissophyllum by headspace solid-phase microextraction (HS-SPME) coupled with gas chromatography (GC–FID) and gas chromatography–mass spectrometry (GC–MS). Food Chem. 2010, 123, 983–992. [Google Scholar] [CrossRef]
- Lu, Y.; Tang, F.; Wang, Y.; Zhao, J.; Zeng, X.; Luo, Q.; Wang, L. Biodegradation of dimethyl phthalate, diethyl phthalate and di-n-butyl phthalate by Rhodococcus sp. L4 isolated from activated sludge. J. Hazard. Mater. 2009, 168, 938–943. [Google Scholar] [CrossRef]
- Xiao, Z.; Liu, L.; Niu, Y.; Zhang, J.; Wang, D.; Zhou, C. Mushroom alcohol (1-octen-3-ol) and other 7 aroma compounds selected from Chinese dry-cured hams can enhance saltiness perception. Meat Sci. 2024, 208, 109398. [Google Scholar] [CrossRef] [PubMed]
- Iglesias, J.; Medina, I. Solid-phase microextraction method for the determination of volatile compounds associated to oxidation of fish muscle. J. Chromatogr. A 2008, 1192, 9–16. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Yuan, L.; Liu, H.; Liu, H.; Zhou, Y.; Li, M.; Gao, R. Analysis of the changes of volatile flavor compounds in a traditional Chinese shrimp paste during fermentation based on electronic nose, SPME-GC-MS and HS-GC-IMS. Food Sci. Hum. Wellness 2023, 12, 173–182. [Google Scholar] [CrossRef]
- Zhang, Q.; Liu, C.; Sun, Z.; Hu, X.; Shen, Q.; Wu, J. Authentication of edible vegetable oils adulterated with used frying oil by Fourier Transform Infrared Spectroscopy. Food Chem. 2012, 132, 1607–1613. [Google Scholar] [CrossRef]
- Socrates, G. Infrared and Raman Characteristic Group Frequencies: Tables and Charts; John Wiley & Sons: Hoboken, NJ, USA, 2004; 98p. [Google Scholar]
- Aenugu, H.P.R.; Kumar, D.S.; Srisudharson, N.P.; Ghosh, S.; Banji, D. Near infrared spectroscopy—An overview. Int. J. ChemTech Res. 2011, 3, 825–836. [Google Scholar]
- Panagou, E.Z.; Papadopoulou, O.; Carstensen, J.M.; Nychas, G.-J.E. Potential of multispectral imaging technology for rapid and non-destructive determination of the microbiological quality of beef filets during aerobic storage. Int. J. Food Microbiol. 2014, 174, 1–11. [Google Scholar] [CrossRef]
- Workman, J., Jr.; Weyer, L. Practical Guide to Interpretive Near-Infrared Spectroscopy; CRC Press: Boca Raton, FL, USA, 2007; 344p. [Google Scholar]
- Khodabux, K.; L’Omelette, M.S.S.; Jhaumeer-Laulloo, S.; Ramasami, P.; Rondeau, P. Chemical and near-infrared determination of moisture, fat and protein in tuna fishes. Food Chem. 2007, 102, 669–675. [Google Scholar] [CrossRef]
- Osborne, B.G. Near infrared spectroscopy in food analysis. In Encyclopedia of Analytical Chemistry: Applications, Theory and Instrumentation; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2006; pp. 1–14. [Google Scholar]
- Agyekum, A.A.; Hutsanedzie, F.Y.H.; Annavaram, V.; Mintah, B.K.; Asare, E.K.; Wang, B. FT-NIR coupled chemometric methods rapid prediction of K-value in fish. Vib. Spectrosc. 2020, 108, 103044. [Google Scholar] [CrossRef]
- Grassi, S.; Casiraghri, E.; Alamprese, C. Handheld NIR device: A non-targeted approach to assess authenticity of fish fillets and patties. Food Chem. 2018, 243, 382–388. [Google Scholar] [CrossRef]
- Currò, S.; Savini, F.; Fasoleto, L.; Indio, V.; Tomasello, F.; Rampazzo, G.; Zironi, E.; Pagliuca, G.; Gazzotti, T.; Prandini, L.; et al. Application of near-infrared spectroscopy as at line method for the evaluation of histamine in tuna fish (Thunnus albacares). Food Control 2025, 167, 110778. [Google Scholar] [CrossRef]
- Cheng, J.H.; Sun, D.W. Partial least squares regression (PLSR) applied to NIR and HSI spectral data modeling to predict chemical properties of fish muscle. Food Eng. Rev. 2017, 9, 36–49. [Google Scholar] [CrossRef]
- Khoshnoudi-Nia, S.; Moosavi-Nasab, M. Prediction of various freshness indicators in fish fillets by one multispectral imaging system. Sci. Rep. 2019, 9, 14704. [Google Scholar] [CrossRef] [PubMed]
- Sanhueza, M.I.; Montes, C.S.; Sanhueza, I.; Montoya-Gallardo, N.I.; Escalona, F.; Luarte, D.; Escribano, R.; Torres, S.; Godoy, S.E.; Amigo, J.M.; et al. VIS-NIR hyperspectral imaging and multivariate analysis for direct characterization of pelagic fish species. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2024, 328, 125451. [Google Scholar] [CrossRef] [PubMed]
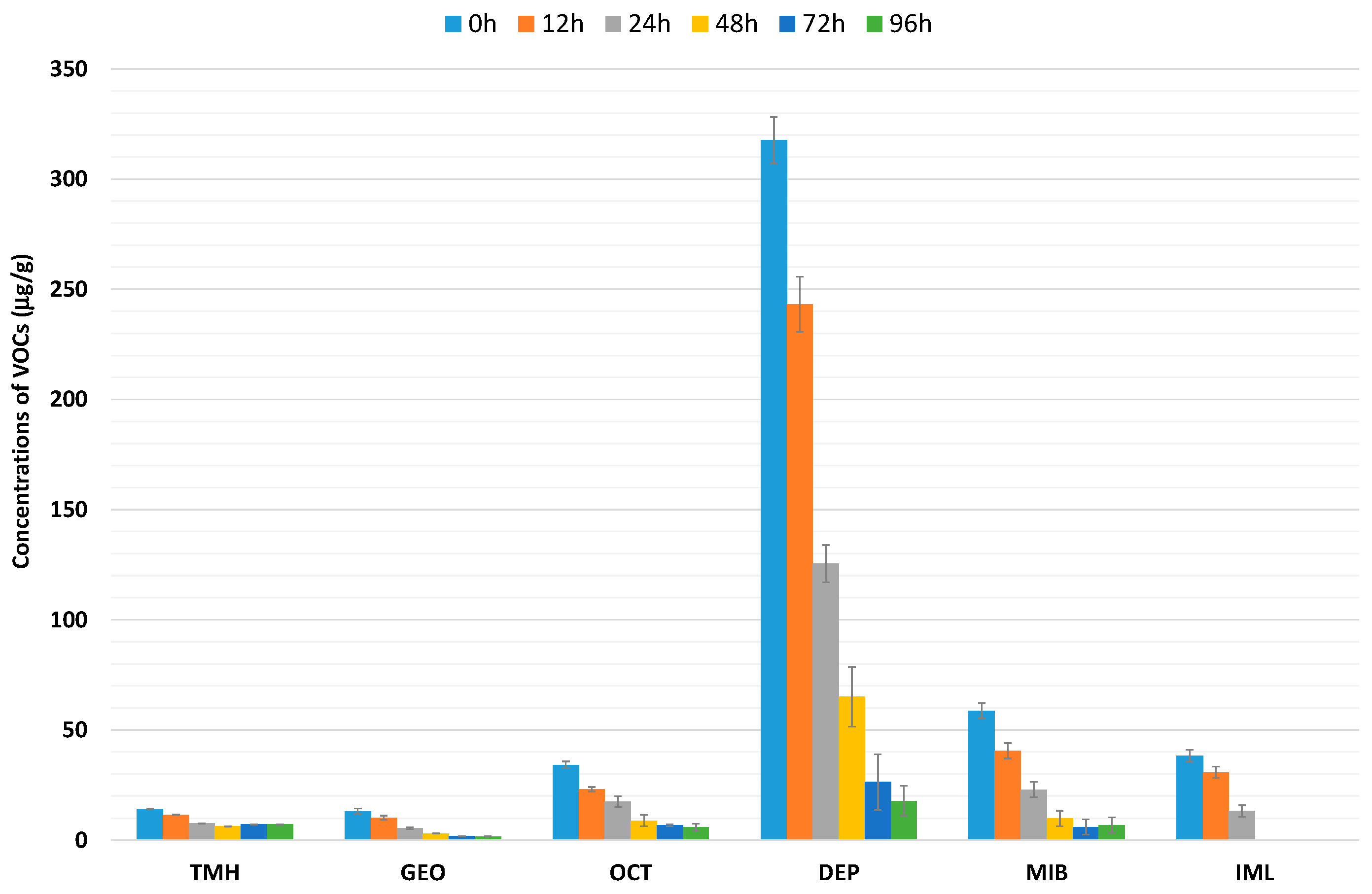
| 0 h | 12 h | 24 h | 48 h | 72 h | 96 h | |
|---|---|---|---|---|---|---|
| Hydrocarbons | 10.7 ± 1.3 | 12.5 ± 1.1 | 12.1 ± 1.0 | 16.7 ± 1.2 | 15.4 ± 1.5 | 15.8 ± 1.6 |
| Alcohols | 8.5 ± 1.4 | 7.8 ± 1.1 | 12.7 ± 0.3 | 17.9 ± 1.3 | 18.8 ± 1.6 | 21.4 ± 2.2 |
| Acid derivates | 59.1 ± 2.1 | 58.7 ± 1.1 | 54.6 ± 3.2 | 48.5 ± 2.6 | 47.7 ± 3.8 | 45.1 ± 5.4 |
| Terpenes | 12.5 ± 1.0 | 11.1 ± 1.3 | 11.5 ± 1.3 | 9.9 ± 1.1 | 9.0 ± 1.2 | 9.5 ± 2.1 |
| Ketones | 6.9 ± 2.1 | 7.7 ± 1.0 | 6.8 ± 1.5 | 2.1 ± 0.3 | 4.8 ± 0.6 | 4.1 ± 0.6 |
| Phenols | 2.3 ± 0.6 | 2.2 ± 0.5 | 2.1 ± 0.5 | 4.7 ± 1.2 | 4.1 ± 1.1 | 3.8 ± 0.6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martín-Vertedor, D.; Ramírez-López, J.C.; Aleman, R.S.; Martín-Tornero, E.; Montero-Fernández, I. Near-Infrared Spectroscopy Detection of Off-Flavor Compounds in Tench (Tinca tinca) After Depuration in Clean Water. Foods 2025, 14, 739. https://doi.org/10.3390/foods14050739
Martín-Vertedor D, Ramírez-López JC, Aleman RS, Martín-Tornero E, Montero-Fernández I. Near-Infrared Spectroscopy Detection of Off-Flavor Compounds in Tench (Tinca tinca) After Depuration in Clean Water. Foods. 2025; 14(5):739. https://doi.org/10.3390/foods14050739
Chicago/Turabian StyleMartín-Vertedor, Daniel, Juan Carlos Ramírez-López, Ricardo S. Aleman, Elisabet Martín-Tornero, and Ismael Montero-Fernández. 2025. "Near-Infrared Spectroscopy Detection of Off-Flavor Compounds in Tench (Tinca tinca) After Depuration in Clean Water" Foods 14, no. 5: 739. https://doi.org/10.3390/foods14050739
APA StyleMartín-Vertedor, D., Ramírez-López, J. C., Aleman, R. S., Martín-Tornero, E., & Montero-Fernández, I. (2025). Near-Infrared Spectroscopy Detection of Off-Flavor Compounds in Tench (Tinca tinca) After Depuration in Clean Water. Foods, 14(5), 739. https://doi.org/10.3390/foods14050739







