Effects of Lemongrass Essential Oil on Key Aromas of Pickled Radish During Storage Using HS–GC–IMS and in Silico Approaches
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Chemicals
2.3. Headspace–Gas Chromatography–Ion Mobility Spectrometry (HS–GC–IMS) Analysis
2.3.1. HS Conditions
2.3.2. GC–IMS Detection
2.3.3. Qualitative and Quantitative of Aroma Volatile Compounds
2.4. ROAV Calculated
2.5. MD Analysis
2.6. Statistical Analysis
3. Results and Discussion
3.1. Analysis of Qualitative and Quantitative of Aroma Volatile Compounds
3.2. Fingerprint Spectra Analysis of Aromas
3.3. Multivariate Statistical Analysis of Aromas
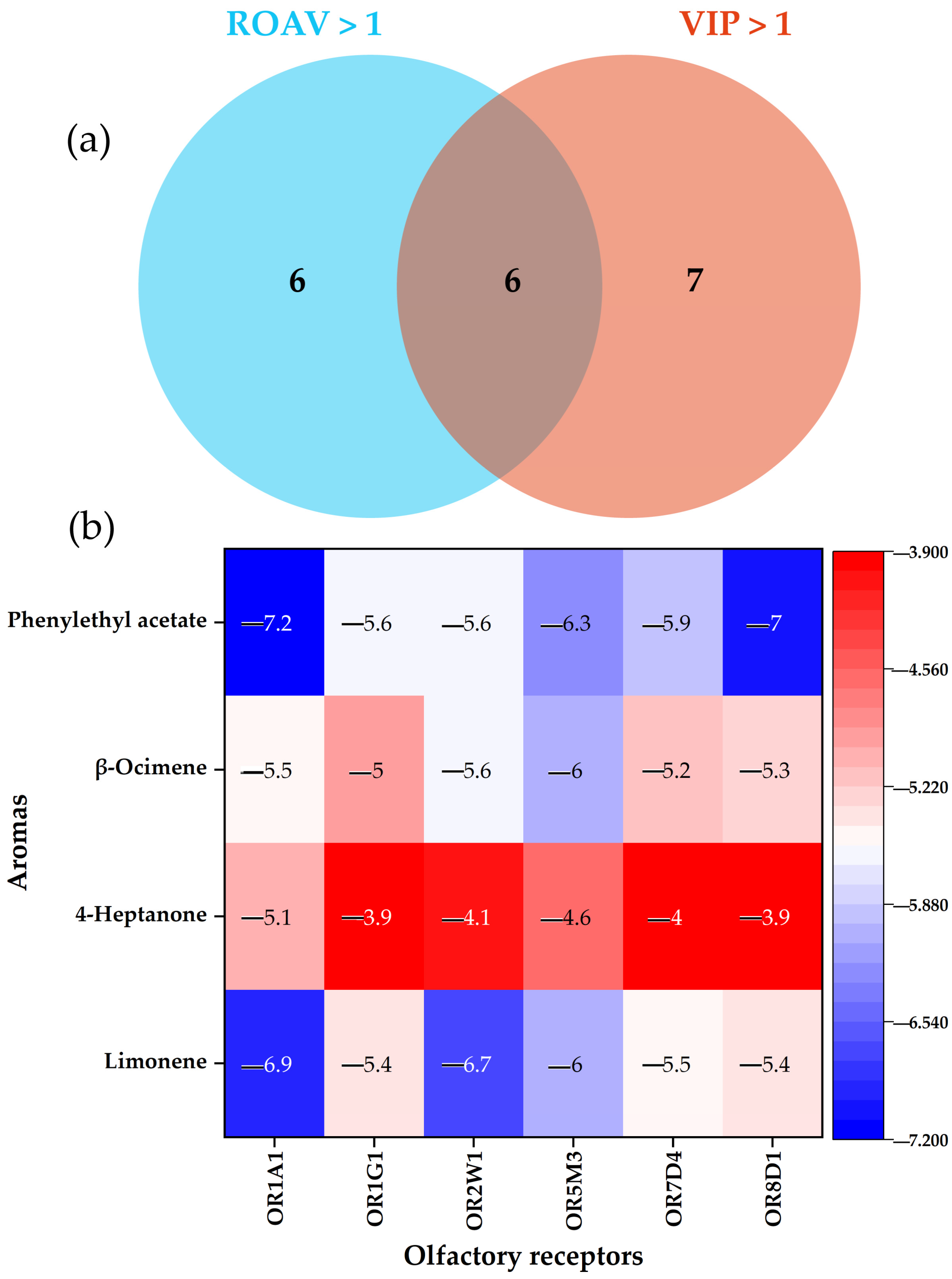
3.4. Key Aromas Screening
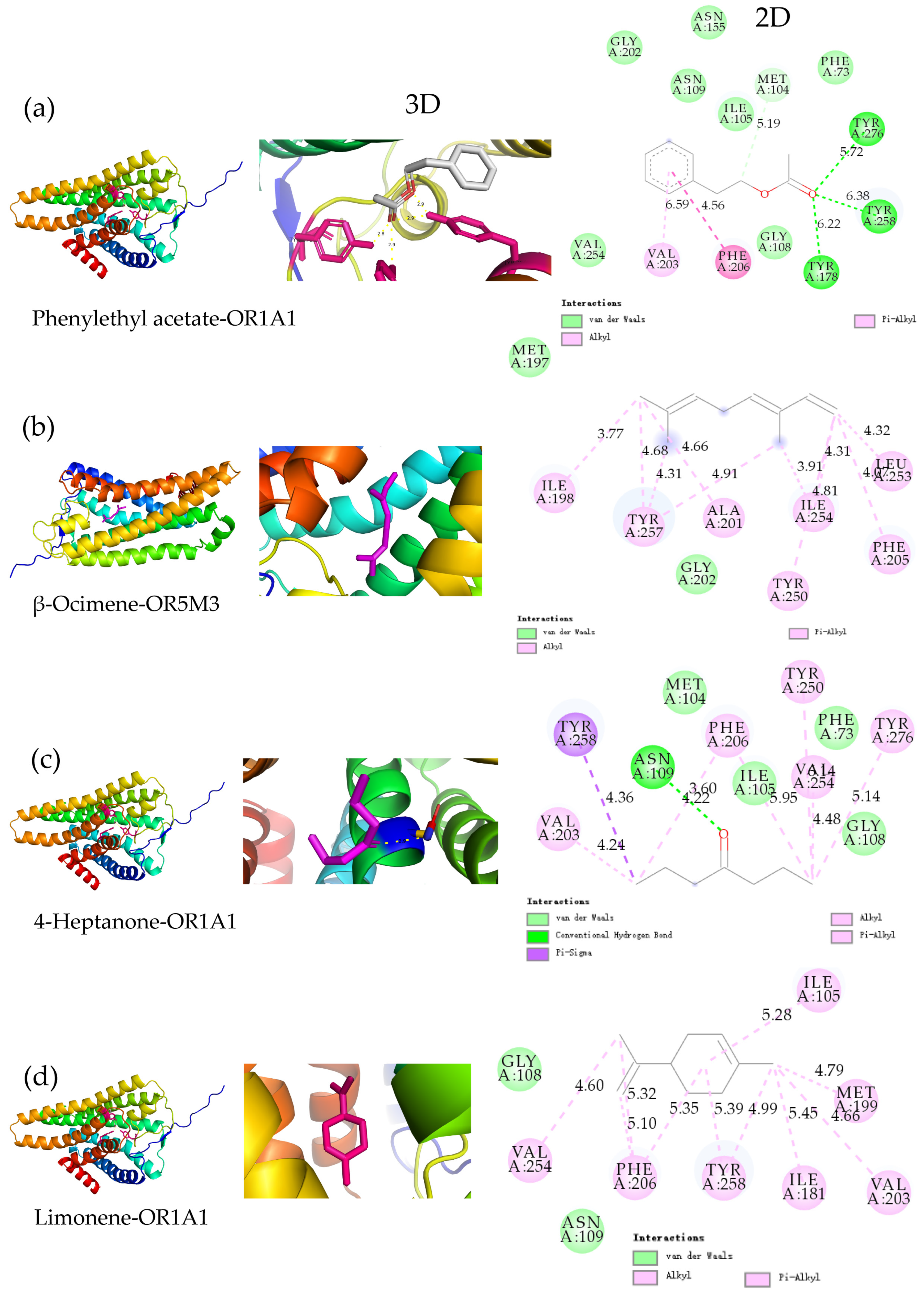
3.5. Analysis of MD Results
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Xing, Y.; Yi, R.; Yue, T.; Bi, X.; Wu, L.; Pan, H.; Liu, X.; Che, Z. Effect of dense phase carbon dioxide treatment on the flavor, texture, and quality changes in new-paocai. Food Res. Int. 2023, 165, 112431. [Google Scholar] [CrossRef]
- Liu, W.; Zhang, L.; Karrar, E.; Wu, D.; Chen, C.; Zhang, Z.; Li, J. A cooperative combination of non-targeted metabolomics and electronic tongue evaluation reveals the dynamic changes in metabolites and sensory quality of radish during pickling. Food Chem. 2024, 446, 138886. [Google Scholar] [CrossRef] [PubMed]
- Kumakura, K.; Kato, R.; Kobayashi, T.; Sekiguchi, A.; Kimura, N.; Takahashi, H.; Takahashi, A.; Matsuoka, H. Nutritional content and health benefits of sun-dried and salt-aged radish (takuan-zuke). Food Chem. 2017, 231, 33–41. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Zhang, L.; Wu, D.; Liu, J.; Li, G.; Zhang, Z.; Li, J. Three dietary phenols from pickled radish improve uric acid metabolism disorder in hyperuricemia mice associated with the altered gut microbiota composition. Food Biosci. 2024, 61, 104802. [Google Scholar] [CrossRef]
- Liu, X.; Wu, D.; Liu, J.; Li, G.; Zhang, Z.; Chen, C.; Zhang, L.; Li, J. Characterization of xanthine oxidase inhibitory activities of phenols from pickled radish with molecular simulation. Food Chem. X 2022, 14, 100343. [Google Scholar] [CrossRef]
- Xiong, S.; Xu, X.; Zhang, L.; Du, T.; Huang, T.; Huang, J.; Ren, H.; Xiong, T.; Xie, M. Integrated metatranscriptomics and metabolomics reveal microbial succession and flavor formation mechanisms during the spontaneous fermentation of Laotan Suancai. Food Res. Int. 2024, 177, 113865. [Google Scholar] [CrossRef]
- Zhao, C.; Liu, X.; Tian, H.; Li, Z. Integrated characterization of arabica coffee husk tea using flavoromics, targeted screening, and in silico approaches. Food Chem. X 2024, 23, 101556. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Lian, Y.; Yin, S.; Suo, H.; Zeng, F.; Wang, H.; Song, J.; Zhang, Y. Inhibition of Lactobacillus fermentum SHY10 on the white membrane production of soaked pickled radish. Food Sci. Nutr. 2022, 10, 2236–2244. [Google Scholar] [CrossRef]
- Rao, Y.; Qian, Y.; Tao, Y.; She, X.; Li, Y.; Che, Z.; Li, H.; Liu, L. Influence of oxygen exposure on fermentation process and sensory qualities of Sichuan pickle (paocai). RSC Adv. 2019, 9, 38520–38530. [Google Scholar] [CrossRef] [PubMed]
- Dong, A.; Malo, A.; Leong, M.; Ho, V.T.T.; Turner, M.S. Control of Listeria monocytogenes on ready-to-eat ham and fresh cut iceberg lettuce using a nisin containing Lactococcus lactis fermentate. Food Control 2021, 119, 107420. [Google Scholar] [CrossRef]
- Singh, S.; Kurmi, A.; Singh, V.; Singh, M.K.; Mishra, S.; Shankar, U.; Savita, A.; Gupta, H.; Yadav, N.P.; Sakia, D.; et al. Cymbopogon distans: A source of essential oil with potential antibacterial, antifungal, and mosquito-repelling properties. Food Biosci. 2024, 61, 104931. [Google Scholar] [CrossRef]
- Ashaq, B.; Rasool, K.; Habib, S.; Bashir, I.; Nisar, N.; Mustafa, S.; Ayaz, Q.; Nayik, G.A.; Uddin, J.; Ramniwas, S.; et al. Insights into chemistry, extraction and industrial application of lemon grass essential oil -A review of recent advances. Food Chem. X 2024, 22, 101521. [Google Scholar] [CrossRef] [PubMed]
- Zulfa, Z.C.C.T.; Rukayadi, Y. In vitro antimicrobial activity of Cymbopogon citratus (lemongrass) extracts against selected foodborne pathogens. Int. Food Res. J. 2016, 3, 1262–1267. [Google Scholar]
- Saeed, K.; Pasha, I.; Jahangir Chughtai, M.F.; Ali, Z.; Bukhari, H.; Zuhair, M. Application of essential oils in food industry: Challenges and innovation. J. Essent. Oil Res. 2022, 34, 97–110. [Google Scholar] [CrossRef]
- Zhang, X.; Li, Y.; Zhao, Y.; Guan, H.; Jin, C.; Gong, H.; Sun, X.; Wang, P.; Li, H.; Liu, W. Effect of Levilactobacillus brevis as a starter on the flavor quality of radish paocai. Food Res. Int. 2023, 168, 112780. [Google Scholar] [CrossRef]
- Chen, Q.; Yang, X.; Hong, P.; Liu, M.; Li, Z.; Zhou, C.; Zhong, S.; Liu, S. GC-MS, GC-IMS, and E-nose analysis of volatile aroma compounds in wet-marinated fermented golden pomfret prepared using different cooking methods. Foods 2024, 13, 390. [Google Scholar] [CrossRef]
- Pu, D.; Meng, R.; Qiao, K.; Cao, B.; Shi, Y.; Wang, Y.; Zhang, Y. Electronic tongue, proton-transfer-reaction mass spectrometry, spectral analysis, and molecular docking characterization for determining the effect of α-amylase on flavor perception. Food Res. Int. 2024, 181, 114078. [Google Scholar] [CrossRef] [PubMed]
- Sun, P.; Lin, S.; Li, X.; Li, D. Different stages of flavor variations among canned Antarctic krill (Euphausia superba): Based on GC-IMS and PLS-DA. Food Chem. 2024, 459, 140465. [Google Scholar] [CrossRef] [PubMed]
- Guan, H.; Yang, C.; Tian, Y.; Feng, C.; Gai, S.; Liu, D.; Diao, X. Changes in stability and volatile flavor compounds of self-emulsifying chicken soup formed during the stewing process. LWT-Food Sci. Technol. 2023, 175, 114520. [Google Scholar] [CrossRef]
- Piasecki, B.; Biernasiuk, A.; Skiba, A.; Skalicka-Woźniak, K.; Ludwiczuk, A. Composition, anti-MRSA activity and toxicity of essential oils from Cymbopogon Species. Molecules 2021, 26, 7542. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Q.; Zheng, Z.; Wu, Y.; Zhang, X.; Jia, Z.; Zhong, K.; Gao, H. Unraveling the core bacterial community responsible for quality and flavor improvement of the radish paocai during spontaneous fermentation. Food Biosci. 2023, 55, 102956. [Google Scholar] [CrossRef]
- Choi, Y.; Yong, S.; Lee, M.J.; Park, S.J.; Yun, Y.; Park, S.; Lee, M. Changes in volatile and non-volatile compounds of model kimchi through fermentation by lactic acid bacteria. LWT-Food Sci. Technol. 2019, 105, 118–126. [Google Scholar] [CrossRef]
- Hong, S.P.; Lee, E.J.; Kim, Y.H.; Ahn, D.U. Effect of fermentation temperature on the volatile composition of kimchi. J. Food Sci. 2016, 81, 13517. [Google Scholar] [CrossRef]
- Guo, W.; Cheng, M.; Dong, X.; Liu, C.; Miao, Y.; Du, P.; Chu, H.; Li, C.; Liu, L. Analysis of flavor substances changes during fermentation of Chinese spicy cabbage based on GC-IMS and PCA. Food Res. Int. 2024, 192, 114751. [Google Scholar] [CrossRef]
- Xing, Y.; Qiu, Y.; Yue, T.; Yi, R.; Xu, Q.; Rao, Y.; Huang, J.; Pan, H. Correlation between microbial communities and volatile flavor compounds in the fermentation of new pickle fermentation broth. Food Biosci. 2023, 56, 103167. [Google Scholar] [CrossRef]
- Yun, L.; Mao, B.; Cui, S.; Tang, X.; Zhang, H.; Zhao, J.; Chen, W. Gas chromatography-mass spectrometry-based metabolomics analysis of metabolites in commercial and inoculated pickles. J. Sci. Food Agric. 2021, 101, 1436–1446. [Google Scholar] [CrossRef] [PubMed]
- Faheem, F.; Liu, Z.W.; Rabail, R.; Haq, I.; Gul, M.; Bryła, M.; Roszko, M.; Kieliszek, M.; Din, A.; Aadil, R.M. Uncovering the industrial potentials of lemongrass essential oil as a food preservative: A review. Antioxidants 2022, 11, 720. [Google Scholar] [CrossRef]
- Xie, J.; Wang, L.; Deng, Y.; Yuan, H.; Zhu, J.; Jiang, Y.; Yang, Y. Characterization of the key odorants in floral aroma green tea based on GC-E-Nose, GC-IMS, GC-MS and aroma recombination and investigation of the dynamic changes and aroma formation during processing. Food Chem. 2023, 427, 136641. [Google Scholar] [CrossRef] [PubMed]
- Meng, Y.; Wang, X.; Li, Y.; Chen, J.; Chen, X. Microbial interactions and dynamic changes of volatile flavor compounds during the fermentation of traditional kombucha. Food Chem. 2024, 430, 137060. [Google Scholar] [CrossRef]
- Wang, D.; Chen, G.; Tang, Y.; Ming, J.; Huang, R.; Li, J.; Ye, M.; Fan, Z.; Yin, L.; Zhang, Q.; et al. Effect of non-core microbes on the key odorants of paocai. LWT-Food Sci. Technol. 2022, 172, 114211. [Google Scholar] [CrossRef]
- Zhang, Y.; Kang, S.; Yan, H.; Xie, D.; Chen, Q.; Lv, H.; Lin, Z.; Zhu, Y. Insights into characteristic volatiles in Wuyi rock teas with different cultivars by chemometrics and gas chromatography olfactometry/mass spectrometry. Foods 2022, 11, 4109. [Google Scholar] [CrossRef] [PubMed]
- Park, D.H.; Kim, M.; Park, S.J.; Lee, M.; Choi, Y.; Choi, M.; Park, S.H. Effect of long-term supercooling preservation using a stepwise algorithm on the freshness of kimchi cabbage. Food Control 2024, 164, 110565. [Google Scholar] [CrossRef]
- Wang, J.; Gao, Y.; Feng, Z.; Deng, S.; Chen, J.; Wang, F.; Li, Y.; Zhang, Y.; Yin, J.; Zeng, L.; et al. Chemometrics and sensomics-assisted identification of key odorants responsible for retort odor in shelf-stored green tea infusion: A case study of Biluochun. Food Res. Int. 2024, 195, 114953. [Google Scholar] [CrossRef] [PubMed]
- van Gemert, L.J. Odour Thresholds; Oliemans Punter & Partners BV: Zeist, The Netherlands, 2011; p. 486. [Google Scholar]
- Zhu, L.; Song, X.; Li, X.; Geng, X.; Zheng, F.; Li, H.; Sun, J.; Huang, M.; Sun, B. Interactions between kafirin and pickle-like odorants in soy sauce flavor Baijiu: Aroma profile change and binding mechanism. Food Chem. 2023, 400, 133854. [Google Scholar] [CrossRef] [PubMed]
- Tong, W.; Li, Y.; Huang, Z.; Wang, S.; Chen, G.; Liu, X.; Yang, Y.; Zhao, L. Mechanism of molecular interaction between pyrazine flavor substances and Lysozyme: Based on spectroscopy and molecular docking studies. J. Mol. Liq. 2023, 389, 122876. [Google Scholar] [CrossRef]
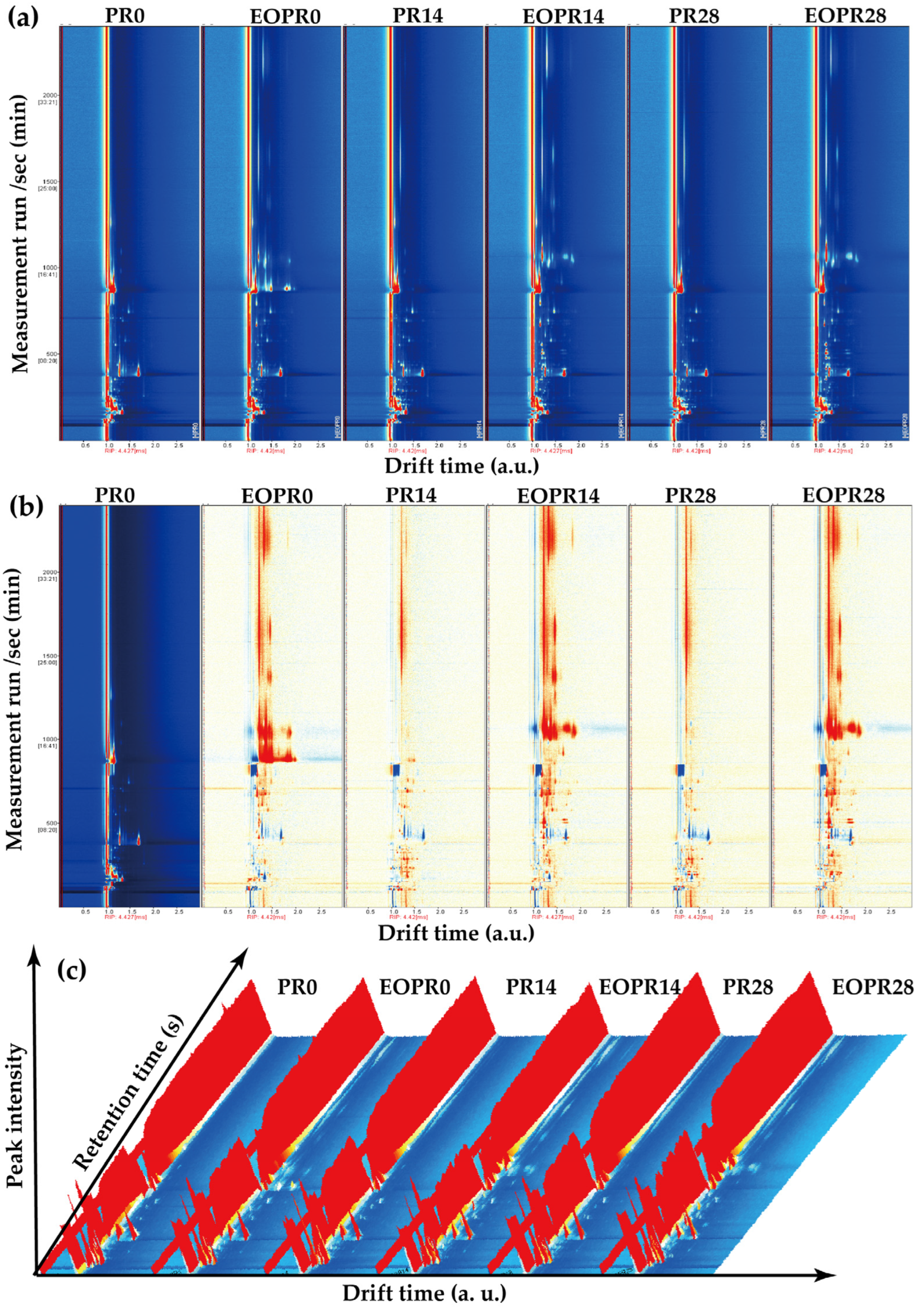
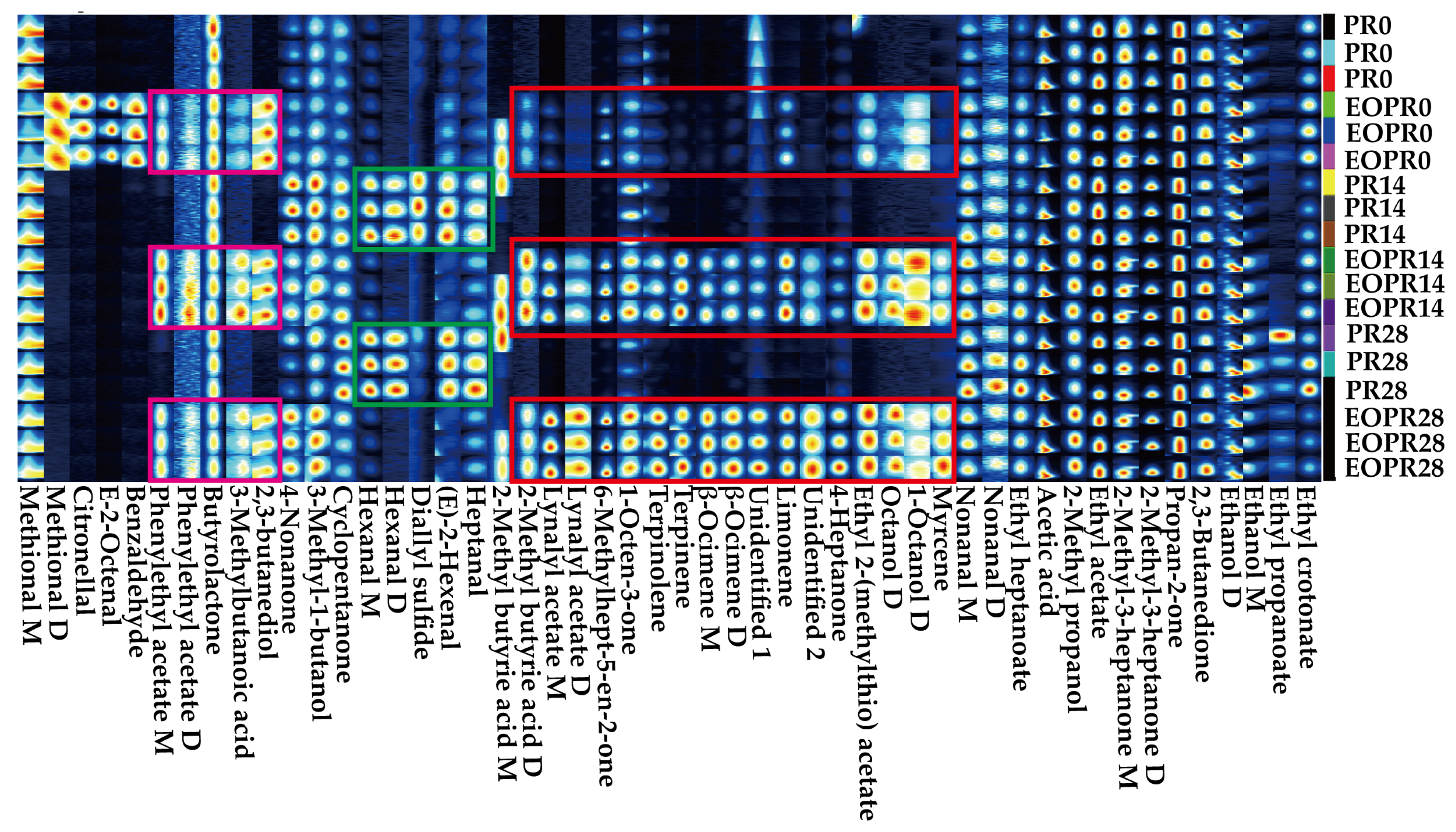

| No. | Aroma Volatile Compounds | CAS | 0 Days | 14 Days | 28 Days | |||
|---|---|---|---|---|---|---|---|---|
| PR0 | EOPR0 | PR14 | EOPR14 | PR28 | EOPR28 | |||
| Ketones | ||||||||
| 1 | Propan-2-one | C103457 | 14.44 ± 0.15 a | 13.03 ± 0.46 b | 14.39 ± 0.38 a | 11.42 ± 0.41 c | 15.10 ± 0.63 a | 11.25 ± 0.13 c |
| 2 | 6-Methylhept-5-en-2-one | C110930 | 0.21 ± 0.02 d | 1.19 ± 0.08 c | 0.20 ± 0.01 d | 1.66 ± 0.13 b | 0.24 ± 0.02 d | 1.83 ± 0.05 a |
| 3 | 4-Heptanone | C123193 | 0.10 ± 0.00 c | 0.12 ± 0.01 c | 0.12 ± 0.01 c | 0.21 ± 0.02 b | 0.14 ± 0.02 c | 0.40 ± 0.05 a |
| 4 | 4-Nonanone | C4485090 | 0.29 ± 0.01 cd | 0.27 ± 0.01 d | 0.58 ± 0.03 a | 0.34 ± 0.03 c | 0.26 ± 0.06 d | 0.42 ± 0.02 b |
| 5 | 1-Octen-3-one | C4312996 | 0.17 ± 0.01 c | 0.28 ± 0.01 b | 0.32 ± 0.01 b | 0.40 ± 0.03 a | 0.19 ± 0.02 c | 0.43 ± 0.02 a |
| 6 | 2-Methyl-3-heptanone D | C13019200 | 12.08 ± 0.21 a | 9.88 ± 1.11 bc | 11.31 ± 0.45 a | 9.15 ± 0.39 cd | 10.92 ± 0.82 ab | 8.22 ± 0.48 d |
| 7 | 2-Methyl-3-heptanone M | C13019200 | 6.66 ± 0.02 a | 5.62 ± 0.59 b | 5.23 ± 0.17 bc | 4.35 ± 0.32 de | 4.83 ± 0.21 cd | 4.01 ± 0.22 e |
| 8 | Cyclopentanone | C120923 | 0.27 ± 0.03 b | 0.23 ± 0.02 cd | 0.36 ± 0.02 a | 0.25 ± 0.02 bc | 0.35 ± 0.01 a | 0.21 ± 0.03 d |
| 9 | 2,3-Butanedione | C431038 | 0.96 ± 0.03 a | 0.94 ± 0.06 a | 0.79 ± 0.08 b | 0.81 ± 0.05 b | 0.83 ± 0.10 b | 0.66 ± 0.04 c |
| Acids | ||||||||
| 10 | 2-Methyl butanoic acid D | C116530 | 0.39 ± 0.06 d | 0.86 ± 0.04 c | 0.31 ± 0.02 d | 1.41 ± 0.14 a | 0.36 ± 0.03 d | 1.11 ± 0.05 b |
| 11 | 2-Methylbutyric acid | C116530 | 0.76 ± 0.15 a | 3.06 ± 2.15 a | 2.31 ± 2.41 a | 3.04 ± 2.06 a | 2.97 ± 2.65 a | 2.62 ± 1.29 a |
| 12 | 3-Methylbutanoic acid | C503742 | 0.22 ± 0.03 c | 0.44 ± 0.01 b | 0.21 ± 0.03 c | 0.57 ± 0.04 a | 0.22 ± 0.03 c | 0.47 ± 0.02 b |
| 13 | Acetic acid | C64197 | 25.88 ± 0.38 ab | 16.04 ± 0.07 d | 24.92 ± 0.79 b | 20.75 ± 0.46 c | 26.88 ± 1.72 a | 21.64 ± 0.16 c |
| Esters | ||||||||
| 14 | Butyrolactone | C96480 | 1.31 ± 0.07 a | 1.14 ± 0.03 c | 1.22 ± 0.03 b | 0.91 ± 0.04 d | 1.18 ± 0.04 bc | 0.84 ± 0.00 d |
| 15 | Lynalyl acetate M | C115957 | 0.42 ± 0.13 d | 2.87 ± 0.10 c | 0.44 ± 0.05 d | 7.45 ± 0.16 b | 0.66 ± 0.08 d | 9.47 ± 0.25 a |
| 16 | Lynalyl acetate D | C115957 | 0.14 ± 0.02 c | 0.19 ± 0.01 c | 0.16 ± 0.04 c | 0.58 ± 0.04 b | 0.19 ± 0.03 c | 0.86 ± 0.05 a |
| 17 | Ethyl heptanoate | C106309 | 0.3 ± 0.03 c | 0.46 ± 0.01 b | 0.38 ± 0.04 bc | 0.45 ± 0.04 b | 0.63 ± 0.15 a | 0.41 ± 0.02 bc |
| 18 | Ethyl crotonate | C623701 | 0.29 ± 0.02 ab | 0.37 ± 0.01 a | 0.20 ± 0.04 bc | 0.25 ± 0.03 bc | 0.39 ± 0.13 a | 0.15 ± 0.01 c |
| 19 | Phenylethyl acetate M | C111875 | 0.53 ± 0.07 c | 3.34 ± 0.24 b | 0.56 ± 0.09 c | 3.85 ± 0.17 a | 0.71 ± 0.25 c | 2.97 ± 0.12 b |
| 20 | Phenylethyl acetate D | C13877913 | 0.31 ± 0.04 c | 0.42 ± 0.02 ab | 0.34 ± 0.06 c | 0.44 ± 0.01 a | 0.36 ± 0.03 bc | 0.35 ± 0.02 c |
| 21 | Ethyl acetate | C141786 | 10.59 ± 0.13 b | 7.01 ± 0.37 de | 11.84 ± 0.31 a | 6.61 ± 0.24 e | 7.47 ± 0.78 cd | 8.01 ± 0.16 c |
| 22 | ethyl 2-(Methylthio)acetate | C4455134 | 0.12 ± 0.13 c | 0.44 ± 0.03 b | 0.05 ± 0.01 c | 0.57 ± 0.02 a | 0.05 ± 0.01 c | 0.62 ± 0.02 a |
| 23 | Ethyl propanoate | C105373 | 0.02 ± 0.00 b | 0.03 ± 0.00 b | 0.02 ± 0.00 b | 0.01 ± 0.00 b | 0.07 ± 0.03 a | 0.03 ± 0.00 b |
| Alcohols | ||||||||
| 24 | 2,3-Butanediol | C513859 | 0.14 ± 0.01 d | 1.59 ± 0.06 a | 0.17 ± 0.02 d | 1.17 ± 0.03 b | 0.19 ± 0.06 d | 1.05 ± 0.05 c |
| 25 | 1-Octanol D | C111875 | 0.05 ± 0.01 d | 0.22 ± 0.01 b | 0.07 ± 0.01 d | 0.27 ± 0.05 a | 0.07 ± 0.01 d | 0.18 ± 0.01 c |
| 26 | 3-Methyl-1-butanol | C123513 | 0.21 ± 0.01 bc | 0.19 ± 0.02 c | 0.31 ± 0.01 a | 0.23 ± 0.02 b | 0.24 ± 0.02 b | 0.27 ± 0.02 a |
| 27 | Octanol D | C13877913 | 0.13 ± 0.01 c | 0.38 ± 0.02 b | 0.13 ± 0.00 c | 0.57 ± 0.07 a | 0.14 ± 0.03 c | 0.51 ± 0.04 a |
| 28 | 2-Methylpropanol | C78831 | 0.20 ± 0.01 a | 0.22 ± 0.01 a | 0.24 ± 0.00 a | 0.20 ± 0.00 a | 0.20 ± 0.00 a | 0.21 ± 0.01 a |
| 29 | Ethanol | C64175 | 13.10 ± 0.38 a | 13.09 ± 0.37 a | 12.10 ± 0.47 b | 11.85 ± 0.53 b | 13.38 ± 0.49 a | 10.74 ± 0.17 c |
| 30 | Ethanol M | C64175 | 0.36 ± 0.01 cd | 0.59 ± 0.01 b | 0.27 ± 0.02 d | 0.48 ± 0.03 bc | 0.84 ± 0.20 a | 0.39 ± 0.01 cd |
| Aldehydes | ||||||||
| 31 | Benzaldehyde | C100527 | 0.23 ± 0.03 b | 2.93 ± 0.12 a | 0.22 ± 0.02 b | 0.20 ± 0.02 b | 0.21 ± 0.02 b | 0.16 ± 0.01 b |
| 32 | Methional M | C3268493 | 7.28 ± 0.88 a | 4.88 ± 0.13 b | 7.25 ± 0.09 a | 5.45 ± 0.26 b | 6.7 ± 0.23 a | 4.95 ± 0.12 b |
| 33 | Methional D | C3268493 | 0.09 ± 0.01 b | 0.90 ± 0.03 a | 0.10 ± 0.02 b | 0.06 ± 0.01 c | 0.09 ± 0.01 bc | 0.06 ± 0.00 c |
| 34 | Nonanal M | C124196 | 0.57 ± 0.03 d | 0.75 ± 0.02 bc | 0.84 ± 0.08 ab | 0.70 ± 0.06 cd | 0.94 ± 0.14 a | 0.63 ± 0.03 cd |
| 35 | Nonanal D | C124196 | 0.06 ± 0.01 c | 0.07 ± 0.01 b | 0.08 ± 0.01 ab | 0.07 ± 0.01 bc | 0.09 ± 0.01 a | 0.06 ± 0.00 c |
| 36 | E-2-Octenal | C2548870 | 0.21 ± 0.02 b | 3.37 ± 0.34 a | 0.24 ± 0.04 b | 0.16 ± 0.01 b | 0.21 ± 0.02 b | 0.16 ± 0.00 b |
| 37 | Citronellal | C106230 | 0.09 ± 0.01 b | 1.34 ± 0.17 a | 0.12 ± 0.01 b | 0.07 ± 0.01 b | 0.11 ± 0.02 b | 0.07 ± 0.00 b |
| 38 | (E)-2-Hexenal | C6728263 | 0.06 ± 0.01b c | 0.08± 0.00 b | 0.19 ± 0.03 a | 0.04 ± 0.00 d | 0.20 ± 0.02 a | 0.05 ± 0.00 cd |
| 39 | Hexanal M | C66251 | 0.16 ± 0.01 b | 0.15 ± 0.01 b | 0.56 ± 0.06 a | 0.15 ± 0.01 b | 0.62 ± 0.07 a | 0.12 ± 0.02 b |
| 40 | Hexanal D | C66251 | 0.01 ± 0.00 b | 0.01 ± 0.00 b | 0.06 ± 0.01 a | 0.01 ± 0.00 b | 0.07 ± 0.01 a | 0.01 ± 0.00 b |
| 41 | Heptanal | C111717 | 0.07 ± 0.01 b | 0.08± 0.01 b | 0.17 ± 0.01 a | 0.09 ± 0.00 b | 0.19 ± 0.03 a | 0.08 ± 0.00 b |
| Alkenes | ||||||||
| 42 | Terpinene | C103457 | 0.04 ± 0.00 c | 0.10 ± 0.02 c | 0.05 ± 0.00 c | 0.63 ± 0.05 b | 0.07 ± 0.01 c | 0.70 ± 0.06 a |
| 43 | Limonene | C138863 | 0.07 ± 0.00 d | 0.36 ± 0.06 c | 0.07 ± 0.01 d | 0.60 ± 0.04 a | 0.09 ± 0.01 d | 0.54 ± 0.03 b |
| 44 | β-Ocimene M | C586629 | 0.06 ± 0.01 c | 0.09 ± 0.01 c | 0.05 ± 0.00 c | 0.66 ± 0.05 b | 0.07 ± 0.01 c | 0.93 ± 0.05 a |
| 45 | β-Ocimene D | C99854 | 0.05 ± 0.01 c | 0.07 ± 0.00 c | 0.05 ± 0.00 c | 0.26 ± 0.01 b | 0.06 ± 0.01 c | 0.40 ± 0.03 a |
| 46 | Myrcene | C123353 | 0.02 ± 0.00 b | 0.03 ± 0.00 b | 0.03 ± 0.00 b | 0.08 ± 0.01 a | 0.02 ± 0.00 b | 0.10 ± 0.01 ab |
| 47 | Terpinolene | C67641 | 0.24 ± 0.01 c | 0.23 ± 0.03 c | 0.22 ± 0.02 c | 0.5 ± 0.04 b | 0.14 ± 0.02 d | 0.64 ± 0.01 a |
| Ethers | ||||||||
| 48 | Diallyl sulfide | C592881 | 0.05 ± 0.00 c | 0.03 ± 0.00 d | 0.16 ± 0.01 a | 0.03 ± 0.00 d | 0.07 ± 0.01 b | 0.03 ± 0.00 d |
| No. | Aroma Volatile Compounds | T (µg/kg) [34] | Odorant Description [34] | ROAV | |||||
|---|---|---|---|---|---|---|---|---|---|
| PR0 | EOPR0 | PR14 | EOPR14 | PR28 | EOPR28 | ||||
| 1 | Acetic acid | 1.82 | sour, spicy | 2.74 | 1.70 | 2.64 | 2.20 | 2.85 | 2.29 |
| 2 | Benzaldehyde | 0.35 | bitter almond, cherry, nutty | 0.13 | 1.62 | 0.12 | 0.11 | 0.12 | 0.09 |
| 3 | Nonanal M | 0.0011 | rose, citrus, strong oily | 100.00 | 131.58 | 147.37 | 122.81 | 164.91 | 110.53 |
| 4 | Nonanal D | 0.0011 | rose, citrus, strong oily | 10.53 | 12.28 | 14.04 | 12.28 | 15.79 | 10.53 |
| 5 | Limonene | 0.058 | lemon, sweet, orange, pine oil | 0.23 | 1.20 | 0.23 | 2.00 | 0.30 | 1.80 |
| 6 | 4-Heptanone | 0.0082 | Fruity | 2.35 | 2.82 | 2.82 | 4.94 | 3.29 | 9.41 |
| 7 | Phenylethyl acetate M | 0.25 | citrus, sweet, rose, honey, fruity | 0.41 | 2.58 | 0.43 | 2.98 | 0.55 | 2.30 |
| 8 | Citronellal | 0.0052 | lemon, lemongrass, rose | 3.34 | 49.73 | 4.45 | 2.60 | 4.08 | 2.60 |
| 9 | 4-Nonanone | 0.0082 | green | 6.82 | 6.35 | 13.65 | 8.00 | 6.12 | 9.88 |
| 10 | β-Ocimene M | 0.034 | fresh woody, sweet, citrus | 0.34 | 0.51 | 0.28 | 3.75 | 0.40 | 5.28 |
| 11 | β-Ocimene D | 0.034 | fresh woody, sweet, citrus | 0.28 | 0.40 | 0.28 | 1.48 | 0.34 | 2.27 |
| 12 | 2,3-Butanedione | 0.059 | butter, popcorn, sweet taste, sour rice | 3.14 | 3.07 | 2.58 | 2.65 | 2.71 | 2.16 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Z.; Gao, Z.; Li, C.; Wu, Y.; Xia, Y.; Ni, L.; Yan, J.; Hu, Y.; Wang, D.; Niu, Z.; et al. Effects of Lemongrass Essential Oil on Key Aromas of Pickled Radish During Storage Using HS–GC–IMS and in Silico Approaches. Foods 2025, 14, 727. https://doi.org/10.3390/foods14050727
Li Z, Gao Z, Li C, Wu Y, Xia Y, Ni L, Yan J, Hu Y, Wang D, Niu Z, et al. Effects of Lemongrass Essential Oil on Key Aromas of Pickled Radish During Storage Using HS–GC–IMS and in Silico Approaches. Foods. 2025; 14(5):727. https://doi.org/10.3390/foods14050727
Chicago/Turabian StyleLi, Zelin, Ziqi Gao, Chao Li, Yanghuan Wu, Yiqiu Xia, Linyu Ni, Jing Yan, Yifan Hu, Dongyu Wang, Zhirui Niu, and et al. 2025. "Effects of Lemongrass Essential Oil on Key Aromas of Pickled Radish During Storage Using HS–GC–IMS and in Silico Approaches" Foods 14, no. 5: 727. https://doi.org/10.3390/foods14050727
APA StyleLi, Z., Gao, Z., Li, C., Wu, Y., Xia, Y., Ni, L., Yan, J., Hu, Y., Wang, D., Niu, Z., Cao, C., Tian, H., & Liu, X. (2025). Effects of Lemongrass Essential Oil on Key Aromas of Pickled Radish During Storage Using HS–GC–IMS and in Silico Approaches. Foods, 14(5), 727. https://doi.org/10.3390/foods14050727






