1H NMR Spectroscopy Primitivo Red Wine Screening After Grape Pomace Repassage for Possible Toxin Contamination Removal
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Wine Sampling
- Controls, namely, untreated OTA-free Primitivo di Gioia red wine: three samples.
- Repassage over Primitivo di Gioia fresh pomace: two different biological samples.
- Repassage over Primitivo di Gioia stabilized pomace, in duplicate: three different biological samples.
- Repassage over Aglianico fresh pomace: three different biological samples.
- Enological activated carbon treatment: a total of 5 L of Primitivo stored in a steel tank containing 1.5 g of enological activated carbon, which was stirred for few seconds and left in static conditions for 24 h: three biological samples.
2.3. Repassage of Red Wines over Grape Pomace from Different Varieties
2.4. NMR Measurements
2.5. Multivariate Data Analysis
3. Results
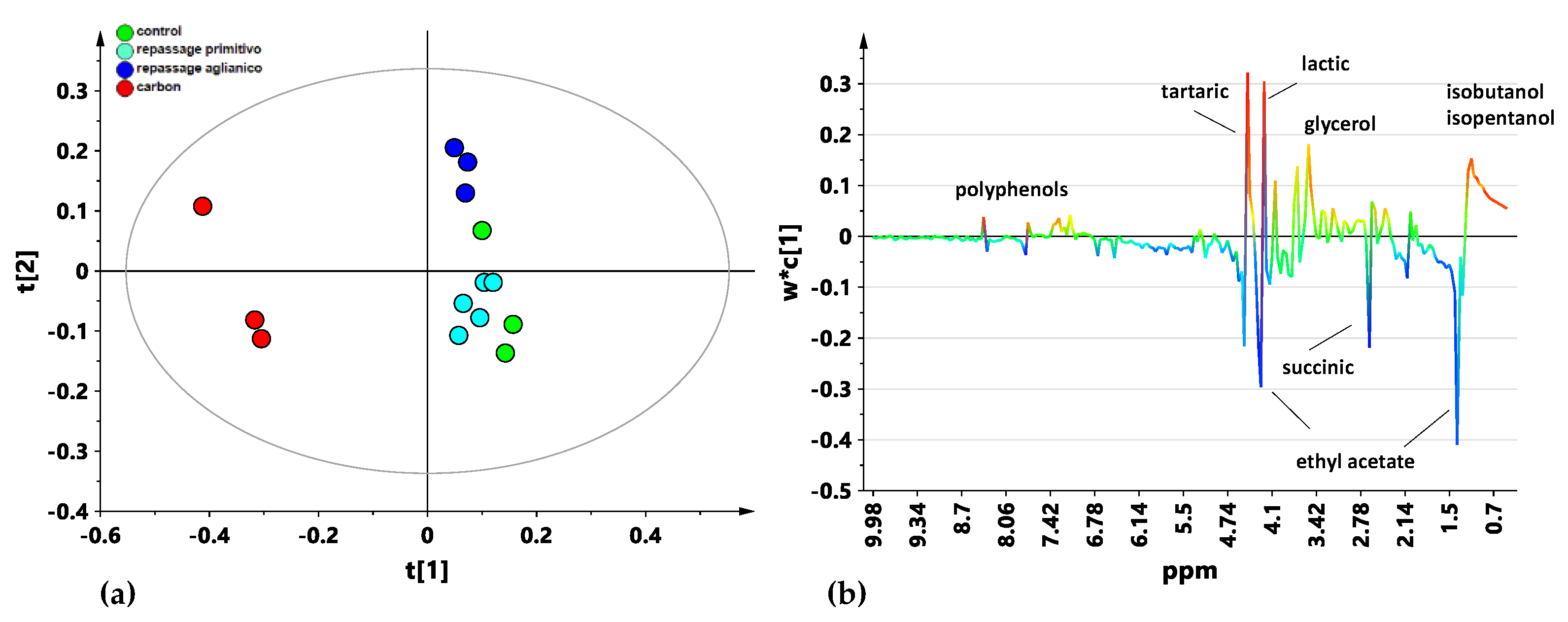
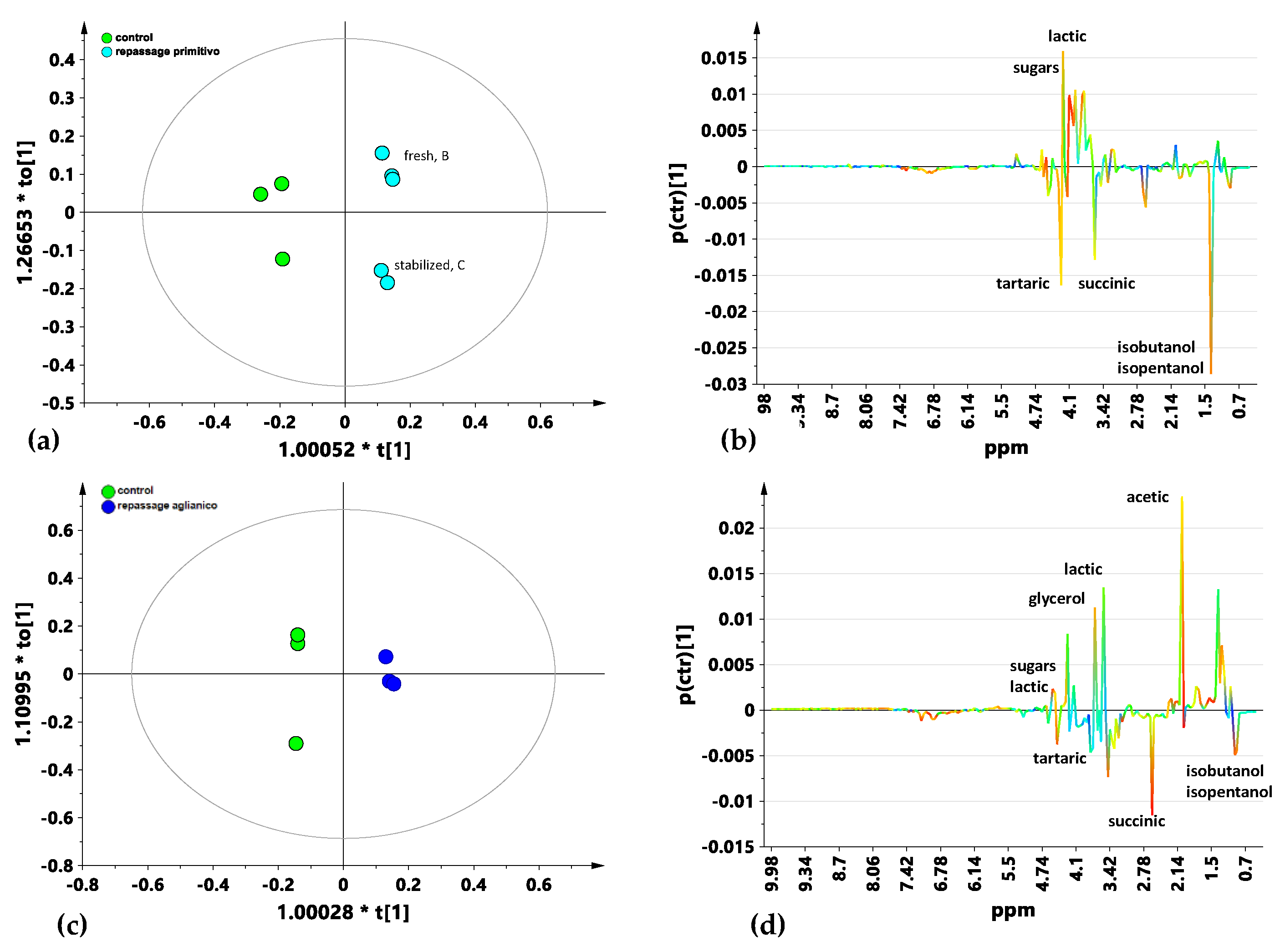
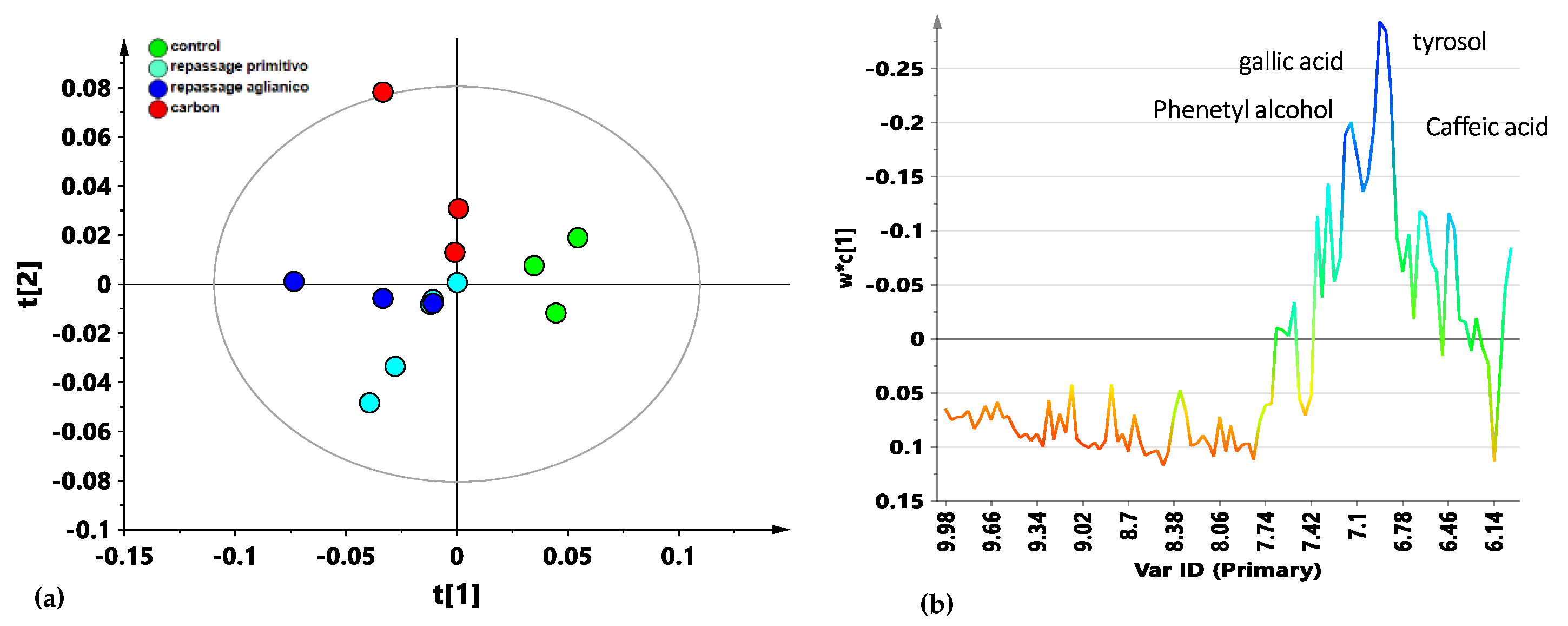
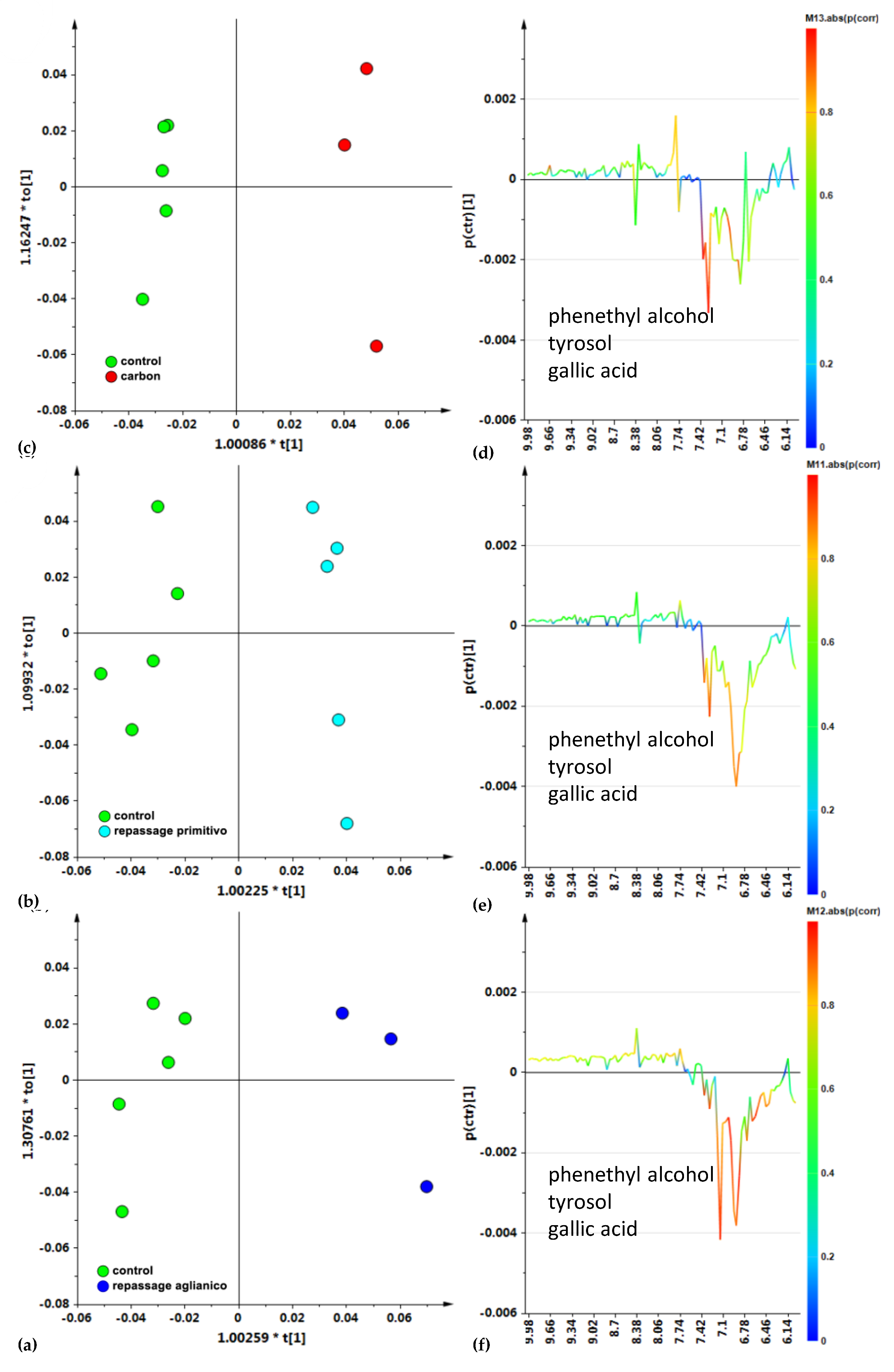
3.1. 1H NMR Spectroscopy
3.2. Multivariate Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cosme, F.; Ribeiro, M.; Filipe-Ribeiro, L.; Nunes, M.F. Review of Mycotoxins in Grapes and Grape Products. In Grapes and Grape Products—Chemistry and Technology; IntechOpen: Rijeka, Croatia, 2024. [Google Scholar]
- Pfohl-Leszkowicz, A.; Manderville, R.A. An Update on Direct Genotoxicity as a Molecular Mechanism of Ochratoxin a Carcinogenicity. Chem. Res. Toxicol. 2012, 25, 252–262. [Google Scholar] [CrossRef] [PubMed]
- IARC Working Group on the Evaluation of Carcinogenic Risks to Humans, International Agency for Research on Cancer, and World Health Organization. Some Naturally Occurring Substances: Food Items and Constituents, Heterocyclic Aromatic Amines and Mycotoxins; International Agency for Research on Cancer, Ed.; IARC monographs on the evaluation of the carcinogenic risk of chemicals to humans; IARC: Lyon, France, 1993; ISBN 978-92-832-1256-0. [Google Scholar]
- Gil-Serna, J.; Vázquez, C.; González-Jaén, M.; Patiño, B. Wine Contamination with Ochratoxins: A Review. Beverages 2018, 4, 6. [Google Scholar] [CrossRef]
- Liu, J.; Wang, Y.; Cui, J.; Xing, L.; Shen, H.; Wu, S.; Lian, H.; Wang, J.; Yan, X.; Zhang, X. Ochratoxin A Induces Oxidative DNA Damage and G1 Phase Arrest in Human Peripheral Blood Mononuclear Cells in Vitro. Toxicol. Lett. 2012, 211, 164–171. [Google Scholar] [CrossRef] [PubMed]
- La Placa, L.; Tsitsigiannis, D.; Camardo Leggieri, M.; Battilani, P. From Grapes to Wine: Impact of the Vinification Process on Ochratoxin A Contamination. Foods 2023, 12, 260. [Google Scholar] [CrossRef]
- Opinion of the Scientific Panel on Contaminants in the Food Chain [CONTAM] Related to Ochratoxin A in Food|EFSA. Available online: https://www.efsa.europa.eu/en/efsajournal/pub/365 (accessed on 5 July 2024).
- Commission Regulation (EU) 2023/915 of 25 April 2023 on Maximum Levels for Certain Contaminants in Food and Repealing Regulation (EC) No 1881/2006 (Text with EEA Relevance)Text with EEA Relevance; European Union: Brussels, Belgium, 2024.
- Ding, L.; Han, M.; Wang, X.; Guo, Y. Ochratoxin A: Overview of Prevention, Removal, and Detoxification Methods. Toxins 2023, 15, 565. [Google Scholar] [CrossRef]
- Wang, L.; Wang, Q.; Wang, S.; Cai, R.; Yuan, Y.; Yue, T.; Wang, Z. Bio-Control on the Contamination of Ochratoxin A in Food: Current Research and Future Prospects. Curr. Res. Food Sci. 2022, 5, 1539–1549. [Google Scholar] [CrossRef]
- Petruzzi, L.; Sinigaglia, M.; Corbo, M.R.; Campaniello, D.; Speranza, B.; Bevilacqua, A. Decontamination of Ochratoxin A by Yeasts: Possible Approaches and Factors Leading to Toxin Removal in Wine. Appl. Microbiol. Biotechnol. 2014, 98, 6555–6567. [Google Scholar] [CrossRef]
- Behfar, M.; Heshmati, A.; Mehri, F.; Khaneghah, A.M. Removal of Ochratoxin A from Grape Juice by Clarification: A Response Surface Methodology Study. Foods 2022, 11, 1432. [Google Scholar] [CrossRef]
- Solfrizzo, M.; Avantaggiato, G.; Panzarini, G.; Visconti, A. Removal of Ochratoxin A from Contaminated Red Wines by Repassage over Grape Pomaces. J. Agric. Food Chem. 2010, 58, 317–323. [Google Scholar] [CrossRef]
- Visconti, A.; Perrone, G.; Cozzi, G.; Solfrizzo, M. Managing Ochratoxin A Risk in the Grape-Wine Food Chain. Food Addit. Contam. Part A 2008, 25, 193–202. [Google Scholar] [CrossRef]
- Filipe-Ribeiro, L.; Milheiro, J.; Matos, C.C.; Cosme, F.; Nunes, F.M. Reduction of 4-ethylphenol and 4-ethylguaiacol in Red Wine by Activated Carbons with Different Physicochemical Characteristics: Impact on Wine Quality. Food Chem. 2017, 229, 242–251. [Google Scholar] [CrossRef]
- Solfrizzo, M.; Perrone, G.; Piemontese, L.; Zivoli, R.; Ferrara, M.; Gambacorta, L.; Magistà, D.; Fracchiolla, R.; Leo, R. Process and Plant for the Treatment of Musts and Wines; Consiglio Nazionale delle Ricerche e Industrie Fracchiolla SpA: Bari, Italy, 2016. [Google Scholar]
- Guerrero, R.F.; Smith, P.; Bindon, K.A. Application of Insoluble Fibers in the Fining of Wine Phenolics. J. Agric. Food Chem. 2013, 61, 4424–4432. [Google Scholar] [CrossRef]
- Jiménez-Martínez, M.D.; Gómez-Plaza, E.; Molero, N.; Bautista-Ortín, A.B. Fining of Red Wines with Pomace Cell Wall Material: Effect on Wine Phenolic Composition. Food Bioprocess Technol. 2017, 10, 1531–1539. [Google Scholar] [CrossRef]
- Jiménez-Martínez, M.D.; Gil-Muñoz, R.; Gómez-Plaza, E.; Bautista-Ortín, A.B. Performance of Purified Grape Pomace as a Fining Agent to Reduce the Levels of Some Contaminants from Wine. Food Addit. Contam. Part A 2018, 35, 1061–1070. [Google Scholar] [CrossRef] [PubMed]
- Niculescu, V.-C.; Ionete, R.-E. An Overview on Management and Valorisation of Winery Wastes. Appl. Sci. 2023, 13, 5063. [Google Scholar] [CrossRef]
- Calò, F.; Girelli, C.R.; Wang, S.C.; Fanizzi, F.P. Geographical Origin Assessment of Extra Virgin Olive Oil via NMR and MS Combined with Chemometrics as Analytical Approaches. Foods 2022, 11, 113. [Google Scholar] [CrossRef] [PubMed]
- Colì, C.S.; Girelli, C.R.; Cesari, G.; Hussain, M.; Fanizzi, F.P. Biodynamic, Organic and Integrated Agriculture Effects on cv. Italia Table Grapes Juice, over a 3-Year Period Experiment: An 1H NMR Spectroscopy-Based Metabolomics Study. Chem. Biol. Technol. Agric. 2024, 11, 35. [Google Scholar] [CrossRef]
- Cevallos-Cevallos, J.M.; Reyes-De-Corcuera, J.I.; Etxeberria, E.; Danyluk, M.D.; Rodrick, G.E. Metabolomic Analysis in Food Science: A Review. Trends Food Sci. Technol. 2009, 20, 557–566. [Google Scholar] [CrossRef]
- Spraul, M.; Schütz, B.; Humpfer, E.; Mörtter, M.; Schäfer, H.; Koswig, S.; Rinke, P. Mixture analysis by NMR as Applied to Fruit Juice Quality Control. Magn. Reson. Chem. 2009, 47, S130–S137. [Google Scholar] [CrossRef]
- Girelli, C.R.; Papadia, P.; Pagano, F.; Miglietta, P.P.; Fanizzi, F.P.; Cardinale, M.; Rustioni, L. Metabolomic NMR Analysis and Organoleptic Perceptions of Pomegranate Wines: Influence of Cultivar and Yeast on the Product Characteristics. Heliyon 2023, 9, e16774. [Google Scholar] [CrossRef]
- Mao, I.L.; Costa, G.D.; Richard, T. 1H-NMR Metabolomics for Wine Screening and Analysis. OENO One 2023, 57, 15–31. [Google Scholar] [CrossRef]
- Mascellani, A.; Hoca, G.; Babisz, M.; Krska, P.; Kloucek, P.; Havlik, J. 1H NMR Chemometric Models for Classification of Czech Wine Type and Variety. Food Chem. 2021, 339, 127852. [Google Scholar] [CrossRef] [PubMed]
- Bambina, P.; Spinella, A.; Lo Papa, G.; Chillura Martino, D.F.; Lo Meo, P.; Cinquanta, L.; Conte, P. 1H-NMR Spectroscopy Coupled with Chemometrics to Classify Wines According to Different Grape Varieties and Different Terroirs. Agriculture 2024, 14, 749. [Google Scholar] [CrossRef]
- Serio, F.; Girelli, C.R.; Acito, M.; Imbriani, G.; Sabella, E.; Moretti, M.; Fanizzi, F.P.; Valacchi, G. Preliminary Characterization of “Salice Salentino” PDO Wines from Salento (South Italy) Negroamaro Grapes: NMR-Based Metabolomic and Biotoxicological Analyses. Foods 2024, 13, 3554. [Google Scholar] [CrossRef] [PubMed]
- Gerginova, D.; Simova, S. Chemical Profiling of Wines Produced in Bulgaria and Distinction from International Grape Varieties. ACS Omega 2023, 8, 18702–18713. [Google Scholar] [CrossRef]
- Viskić, M.; Bandić, L.M.; Korenika, A.-M.J.; Jeromel, A. NMR in the Service of Wine Differentiation. Foods 2021, 10, 120. [Google Scholar] [CrossRef]
- Solovyev, P.A.; Fauhl-Hassek, C.; Riedl, J.; Esslinger, S.; Bontempo, L.; Camin, F. NMR Spectroscopy in Wine Authentication: An Official Control Perspective. Compr. Rev. Food Sci. Food Saf. 2021, 20, 2040–2062. [Google Scholar] [CrossRef]
- Pittari, E.; Moio, L.; Piombino, P. Screening of the Volatile Composition and Olfactory Properties of Aglianico and Primitivo, Two Southern Italian Red Wines. Appl. Sci. 2023, 13, 2165. [Google Scholar] [CrossRef]
- International Code of Oenological Practices|OIV. Available online: https://www.oiv.int/standards/international-code-of-oenological-practices (accessed on 9 July 2024).
- Singleton, V.L.; Rossi, J.A. Colorimetry of Total Phenolics with Phosphomolybdic-Phosphotungstic Acid Reagents. Am. J. Enol. Vitic. 1965, 16, 144–158. [Google Scholar] [CrossRef]
- Amargianitaki, M.; Spyros, A. NMR-Based Metabolomics in Wine Quality Control and Authentication. Chem. Biol. Technol. Agric. 2017, 4, 9. [Google Scholar] [CrossRef]
- Losciale, P.; Conti, L.; Seripierri, S.; Alba, V.; Mazzone, F.; Rustioni, L.; di Leo, G.; Tarricone, F.; Tarricone, L. Effect of Different Deficit Irrigation Regimes on Vine Performance, Grape Composition and Wine Quality of the “Primitivo” Variety Under Mediterranean Conditions. Irrig. Sci. 2024, 42, 877–890. [Google Scholar] [CrossRef]
- Godelmann, R.; Fang, F.; Humpfer, E.; Schütz, B.; Bansbach, M.; Schäfer, H.; Spraul, M. Targeted and Nontargeted Wine Analysis by 1H NMR Spectroscopy Combined with Multivariate Statistical Analysis. Differentiation of Important Parameters: Grape Variety, Geographical Origin, Year of Vintage. J. Agric. Food Chem. 2013, 61, 5610–5619. [Google Scholar] [CrossRef] [PubMed]
- Monakhova, Y.B.; Schäfer, H.; Humpfer, E.; Spraul, M.; Kuballa, T.; Lachenmeier, D.W. Application of Automated Eightfold Suppression of Water and Ethanol Signals in 1H NMR to Provide Sensitivity for Analyzing Alcoholic Beverages. Magn. Reson. Chem. MRC 2011, 49, 734–739. [Google Scholar] [CrossRef] [PubMed]
- López-Rituerto, E.; Savorani, F.; Avenoza, A.; Busto, J.H.; Peregrina, J.M.; Engelsen, S.B. Investigations of La Rioja Terroir for Wine Production Using 1H NMR Metabolomics. J. Agric. Food Chem. 2012, 60, 3452–3461. [Google Scholar] [CrossRef]
- Mulder, F.A.A.; Tenori, L.; Licari, C.; Luchinat, C. Practical Considerations for Rapid and Quantitative NMR-Based Metabolomics. J. Magn. Reson. 2023, 352, 107462. [Google Scholar] [CrossRef]
- Vignoli, A.; Ghini, V.; Meoni, G.; Licari, C.; Takis, P.G.; Tenori, L.; Turano, P.; Luchinat, C. High-Throughput Metabolomics by 1D NMR. Angew. Chem. Int. Ed. 2019, 58, 968–994. [Google Scholar] [CrossRef]
- van den Berg, R.A.; Hoefsloot, H.C.; Westerhuis, J.A.; Smilde, A.K.; van der Werf, M.J. Centering, Scaling, and Transformations: Improving the Biological Information Content of Metabolomics Data. BMC Genom. 2006, 7, 142. [Google Scholar] [CrossRef]
- Trygg, J.; Wold, S. Orthogonal projections to latent structures (O-PLS). J. Chemom. 2002, 16, 119–128. [Google Scholar] [CrossRef]
- Eriksson, L.; Byrne, T.; Johansson, E.; Trygg, J.; Vikström, C. Multi-and Megavariate Data Analysis Basic Principles and Applications; Umetrics Academy: Umeå, Sweden, 2013; Volume 1, ISBN 91-973730-5-2. [Google Scholar]
- Wheelock, Å.M.; Wheelock, C.E. Trials and Tribulations of ‘Omics Data Analysis: Assessing Quality of SIMCA-Based Multivariate Models Using Examples from Pulmonary Medicine. Mol. Biosyst. 2013, 9, 2589. [Google Scholar] [CrossRef]
- Triba, M.N.; Le Moyec, L.; Amathieu, R.; Goossens, C.; Bouchemal, N.; Nahon, P.; Rutledge, D.N.; Savarin, P. PLS/OPLS Models in Metabolomics: The Impact of Permutation of Dataset Rows on the K-Fold Cross-Validation Quality Parameters. Mol. Biosyst. 2015, 11, 13–19. [Google Scholar] [CrossRef]
- Girelli, C.R.; Del Coco, L.; Papadia, P.; De Pascali, S.A.; Fanizzi, F.P. Harvest Year Effects on Apulian EVOOs Evaluated by 1H NMR Based Metabolomics. PeerJ 2016, 4, e2740. [Google Scholar] [CrossRef] [PubMed]
- De Pascali, S.A.; Coletta, A.; Del Coco, L.; Basile, T.; Gambacorta, G.; Fanizzi, F.P. Viticultural Practice and Winemaking Effects on Metabolic Profile of Negroamaro. Food Chem. 2014, 161, 112–119. [Google Scholar] [CrossRef] [PubMed]
- Worley, B.; Powers, R. PCA as a Practical Indicator of OPLS-DA Model Reliability. Curr. Metabolomics 2016, 4, 97–103. [Google Scholar] [CrossRef] [PubMed]
- Plata, C.; Millán, C.; Mauricio, J.C.; Ortega, J.M. Formation of Ethyl Acetate and Isoamyl Acetate by Various Species of Wine Yeasts. Food Microbiol. 2003, 20, 217–224. [Google Scholar] [CrossRef]
- Hopfer, H.; Nelson, J.; Ebeler, S.E.; Heymann, H. Correlating Wine Quality Indicators to Chemical and Sensory Measurements. Molecules 2015, 20, 8453–8483. [Google Scholar] [CrossRef]
- Paolini, M.; Tonidandel, L.; Larcher, R. Development, Validation and Application of a Fast GC-FID Method for the Analysis of Volatile Compounds in Spirit Drinks and Wine. Food Control 2022, 136, 108873. [Google Scholar] [CrossRef]
- Wine Flavours, Faults and Taints; The Australian Wine Research Institute: Adelaide, Australia; Available online: https://www.awri.com.au/industry_support/winemaking_resources/sensory_assessment/recognition-of-wine-faults-and-taints/wine_faults/ (accessed on 5 July 2024).
- Ali, K.; Maltese, F.; Toepfer, R.; Choi, Y.H.; Verpoorte, R. Metabolic Characterization of Palatinate German White Wines According to Sensory Attributes, Varieties, and Vintages using NMR Spectroscopy and Multivariate Data Analyses. J. Biomol. NMR 2011, 49, 255–266. [Google Scholar] [CrossRef]
- Coulter, A.D. Succinic Acid-How It Is Formed, What Is Its Effect on Titratable Acidity, and What Factors Influence Its Concentration in Wine? Wine Ind. J. 2004, 19, 16–25. [Google Scholar]
- Chidi, B.S.; Bauer, F.F.; Rossouw, D. Organic Acid Metabolism and the Impact of Fermentation Practices on Wine Acidity: A Review. S. Afr. J. Enol. Vitic. 2018, 39, 1–15. [Google Scholar] [CrossRef]
- Volschenk, H.; van Vuuren, H.J.J.; Viljoen-Bloom, M. Malic Acid in Wine: Origin, Function and Metabolism during Vinification. S. Afr. J. Enol. Vitic. 2006, 27, 123–136. [Google Scholar] [CrossRef]
- Hu, B.; Yue, Y.; Zhu, Y.; Wen, W.; Zhang, F.; Hardie, J.W. Proton Nuclear Magnetic Resonance-Spectroscopic Discrimination of Wines Reflects Genetic Homology of Several Different Grape (V. vinifera L.) Cultivars. PLoS ONE 2015, 10, e0142840. [Google Scholar] [CrossRef] [PubMed]
- Son, H.-S.; Hwang, G.-S.; Ahn, H.-J.; Park, W.-M.; Lee, C.-H.; Hong, Y.-S. Characterization of Wines from Grape Varieties Through Multivariate Statistical Analysis of 1H NMR Spectroscopic Data. Food Res. Int. 2009, 42, 1483–1491. [Google Scholar] [CrossRef]
- Lubbers, S.; Verret, C.; Voilley, A. The Effect of Glycerol on the Perceived Aroma of a Model Wine and a White Wine. LWT Food Sci. Technol. 2001, 34, 262–265. [Google Scholar] [CrossRef]
- Sun, Y.; Zhang, T.; Lü, H.; Yu, Z.; Li, X. Effect of Added Sulphur Dioxide Levels on the Fermentation Characteristics of Strawberry Wine. J. Inst. Brew. 2016, 122, 446–451. [Google Scholar] [CrossRef]
- Zhang, L.; Tao, Y.S.; Wen, Y.; Wang, H. Aroma Evaluation of Young Chinese Merlot Wines with Denomination of Origin. S. Afr. J. Enol. Vitic. 2013, 34, 46–53. [Google Scholar] [CrossRef]
- Cosme, F.; Inês, A.; Silva, D.; Filipe-Ribeiro, L.; Abrunhosa, L.; Nunes, F.M. Elimination of Ochratoxin A from White and Red Wines: Critical Characteristics of Activated Carbons and Impact on Wine Quality. LWT 2021, 140, 110838. [Google Scholar] [CrossRef]
- Varga, J.; Kocsubé, S.; Péteri, Z.; Vágvölgyi, C.; Tóth, B. Chemical, Physical and Biological Approaches to Prevent Ochratoxin Induced Toxicoses in Humans and Animals. Toxins 2010, 2, 1718–1750. [Google Scholar] [CrossRef]
- Quintela, S.; Villarán, M.C.; López de Armentia, I.; Elejalde, E. Ochratoxin A removal in wine: A review. Food Control 2013, 30, 439–445. [Google Scholar] [CrossRef]

| NMR ID | Treatment | Wine | Initial pH | Final pH * t |
|---|---|---|---|---|
| A | Control (absence of OTA) | Primitivo di Gioia | 3.74 | 3.74 |
| A | Control (absence of OTA) | Primitivo di Gioia | 3.66 | 3.74 |
| A | Control (absence of OTA) | Primitivo di Gioia | 3.62 | 3.72 |
| B | Repassage over fresh Primitivo pomace | Primitivo di Gioia | 3.69 | 3.72 |
| B | Repassage over fresh Primitivo pomace | Primitivo di Gioia | 3.68 | 3.74 |
| B | Repassage over fresh Primitivo pomace | Primitivo di Gioia | 3.68 | 3.73 |
| C | Repassage over stabilized Primitivo pomace | Primitivo di Gioia | 3.67 | 3.73 |
| C | Repassage over stabilized Primitivo pomace | Primitivo di Gioia | 3.69 | 3.75 |
| D | Repassage over fresh Aglianico pomace | Blend Primitivo di Gioia and Primitivo di Manduria | 3.55 | 3.75 |
| D | Repassage over fresh Aglianico pomace | Blend Primitivo di Gioia and Primitivo di Manduria | 3.59 | 3.74 |
| D | Repassage over fresh Aglianico pomace | Blend Primitivo di Gioia and Primitivo di Manduria | 3.61 | 3.74 |
| E | Activated carbon | Primitivo di Gioia | 3.83 | 3.74 |
| E | Activated carbon | Primitivo di Gioia | 3.70 | 3.74 |
| E | Activated carbon | Primitivo di Gioia | 3.90 | 3.74 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Del Coco, L.; Girelli, C.R.; Gambacorta, L.; Solfrizzo, M.; Fanizzi, F.P. 1H NMR Spectroscopy Primitivo Red Wine Screening After Grape Pomace Repassage for Possible Toxin Contamination Removal. Foods 2025, 14, 734. https://doi.org/10.3390/foods14050734
Del Coco L, Girelli CR, Gambacorta L, Solfrizzo M, Fanizzi FP. 1H NMR Spectroscopy Primitivo Red Wine Screening After Grape Pomace Repassage for Possible Toxin Contamination Removal. Foods. 2025; 14(5):734. https://doi.org/10.3390/foods14050734
Chicago/Turabian StyleDel Coco, Laura, Chiara Roberta Girelli, Lucia Gambacorta, Michele Solfrizzo, and Francesco Paolo Fanizzi. 2025. "1H NMR Spectroscopy Primitivo Red Wine Screening After Grape Pomace Repassage for Possible Toxin Contamination Removal" Foods 14, no. 5: 734. https://doi.org/10.3390/foods14050734
APA StyleDel Coco, L., Girelli, C. R., Gambacorta, L., Solfrizzo, M., & Fanizzi, F. P. (2025). 1H NMR Spectroscopy Primitivo Red Wine Screening After Grape Pomace Repassage for Possible Toxin Contamination Removal. Foods, 14(5), 734. https://doi.org/10.3390/foods14050734









