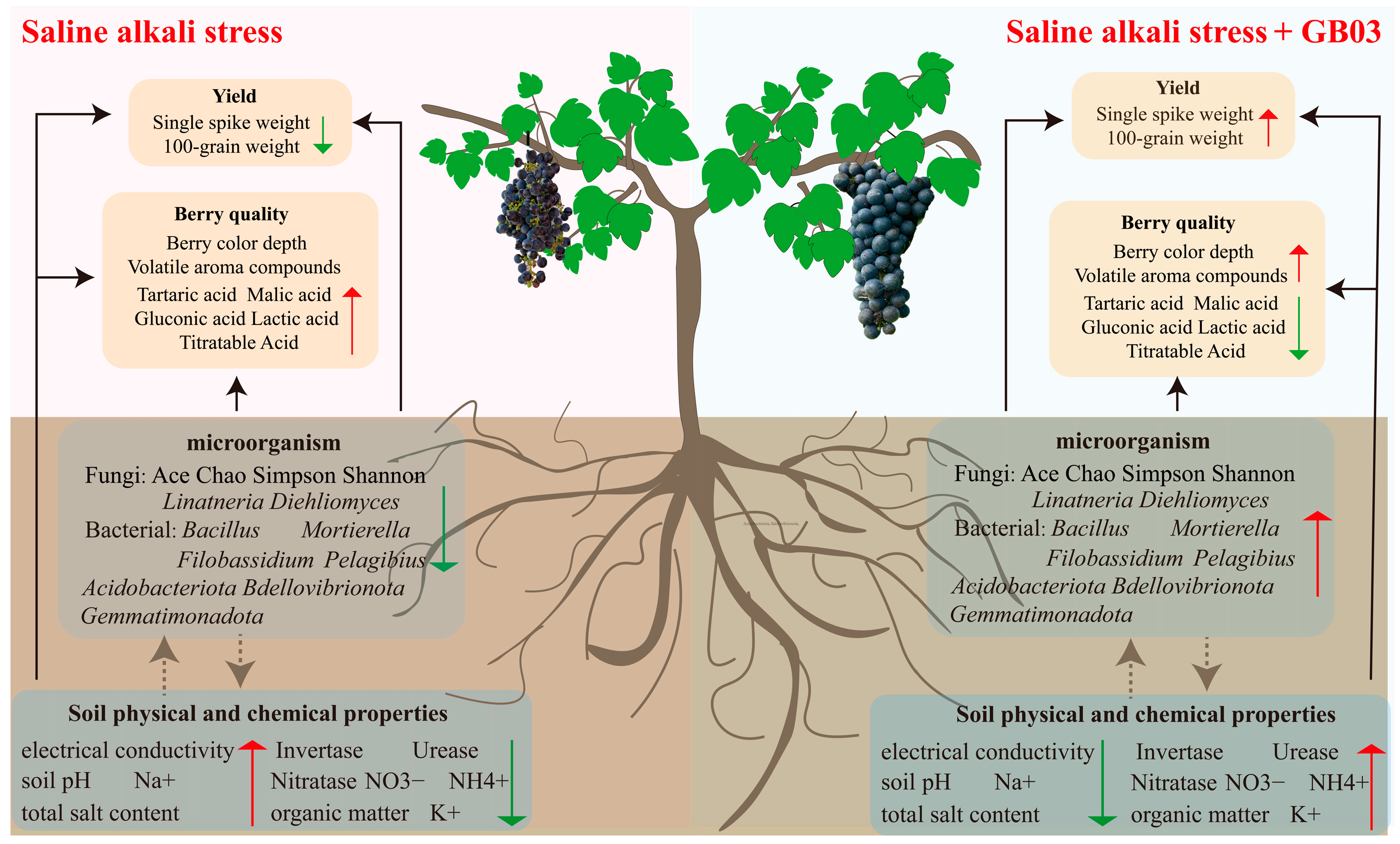
Microbial Inoculant GB03 Increased the Yield and Quality of Grape Fruit Under Salt-Alkali Stress by Changing Rhizosphere Microbial Communities
Abstract
1. Introduction
2. Materials and Methods
2.1. Field Conditions and Materials
2.2. Experimental Procedure and Treatments
2.3. Sample Collection
2.4. Determination of Parameters
2.4.1. Soil Physical and Chemical Indicators
2.4.2. Soil Enzyme Activity
2.4.3. Soil Microbial Parameters
2.4.4. Yield of Grape Berry
2.4.5. Reducing Sugar, Soluble Solid, Fructose, Glucose, Sugar-Acid Ratio, Total Tannin, Titratable Acidity Content, and pH Value in Grape Berry
2.4.6. Grape Berry Color
2.4.7. Anthocyanins Content in Grape Skins
2.4.8. Total Phenol Content in Grape Berry Skins
2.4.9. Volatile Substance Content in Grape Berry
2.5. Statistical Analysis
3. Results
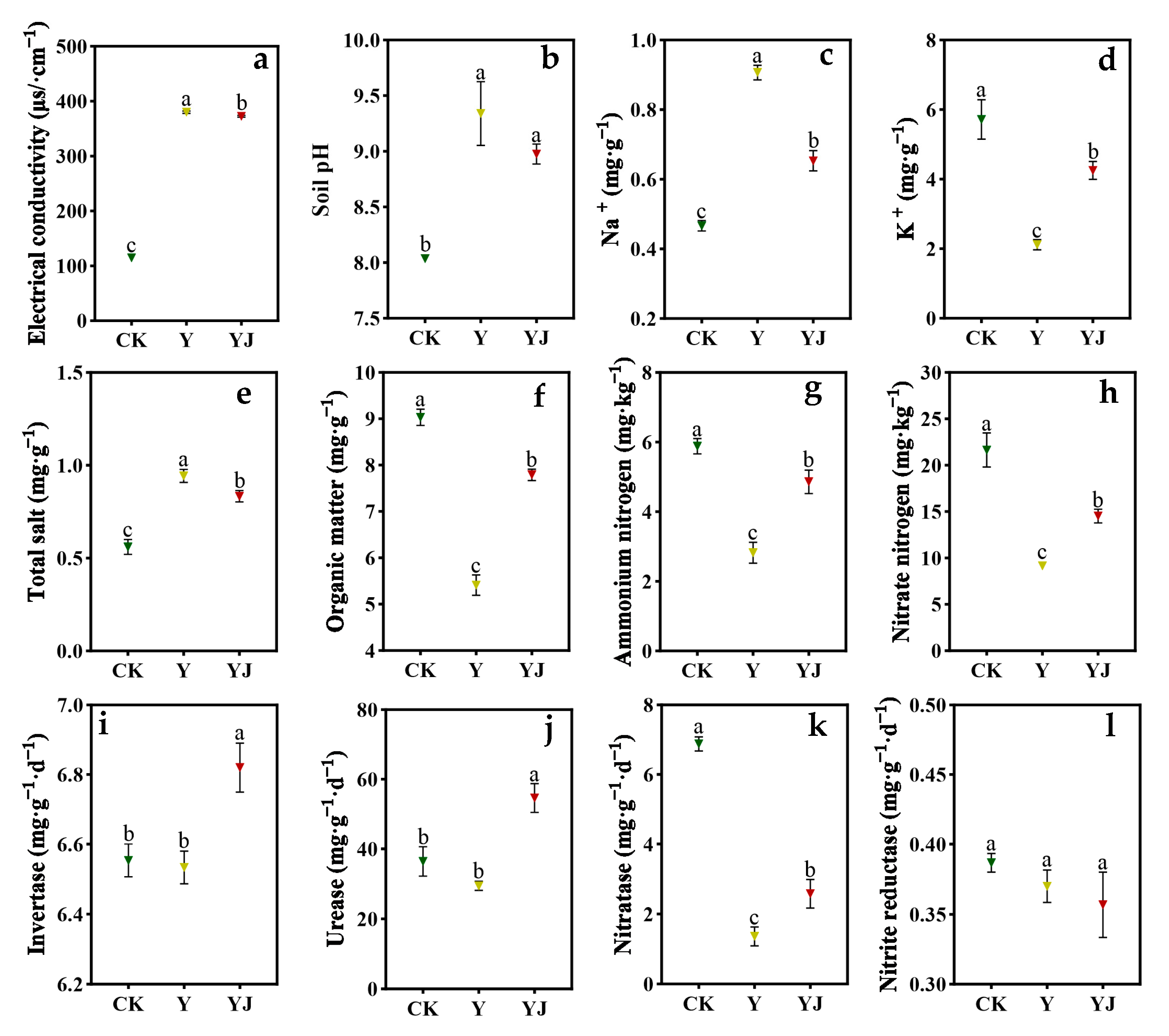
3.1. Effects of GB03 Microbial Inoculant Treatment on Soil Physicochemical Properties and Soil Enzyme Activities in Vineyard Under Salt Alkali Stress
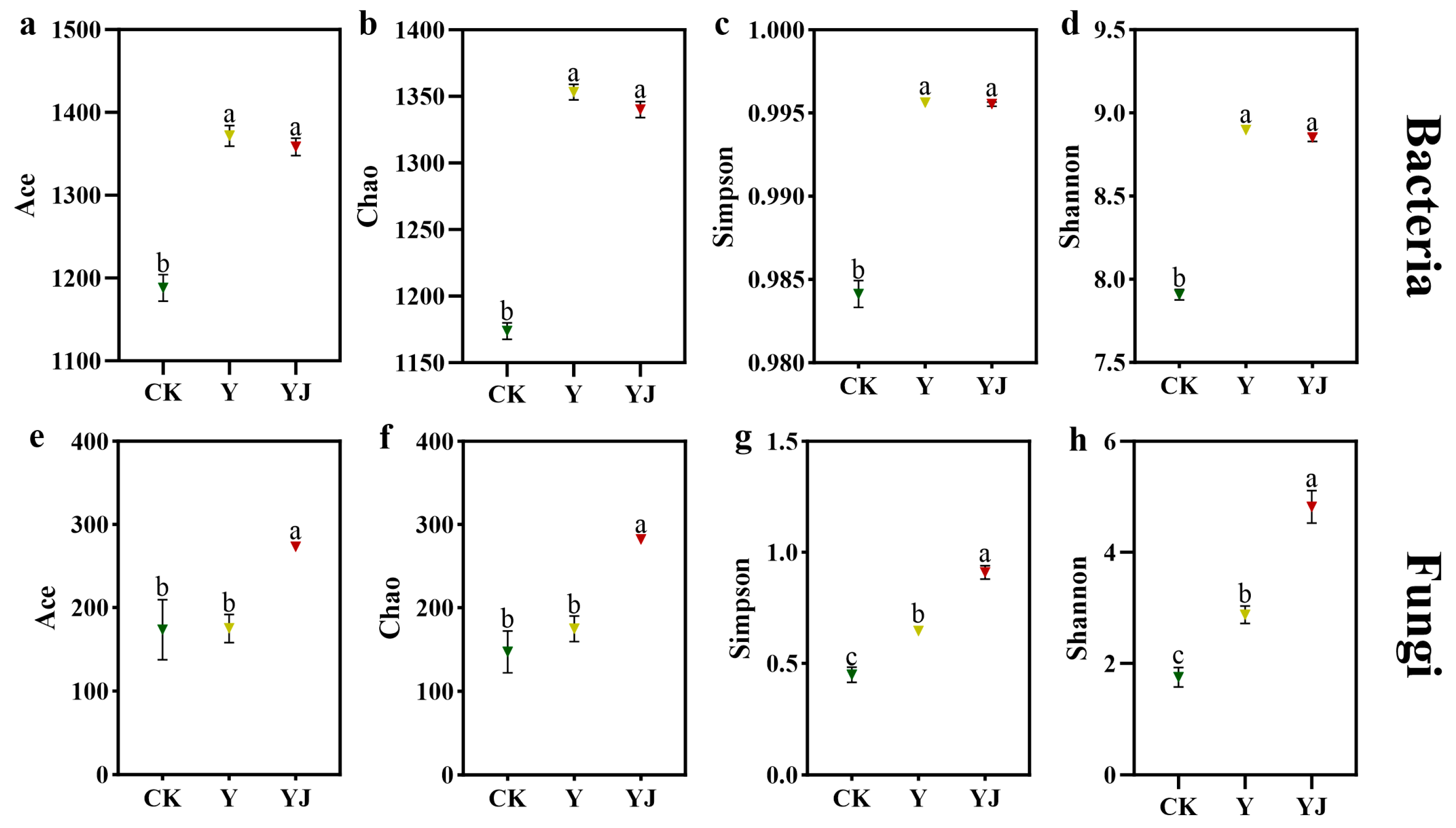
3.2. Effects of GB03 Microbial Agent Treatment on Soil Microbial Community α and β Diversity Under Salt Alkali Stress
3.3. Differences in the Horizontal Distribution of Microbial Bacteria and Fungi Phyal in Vineyard Soil Under Salt Alkali Stress After GB03 Microbial Agent Treatment
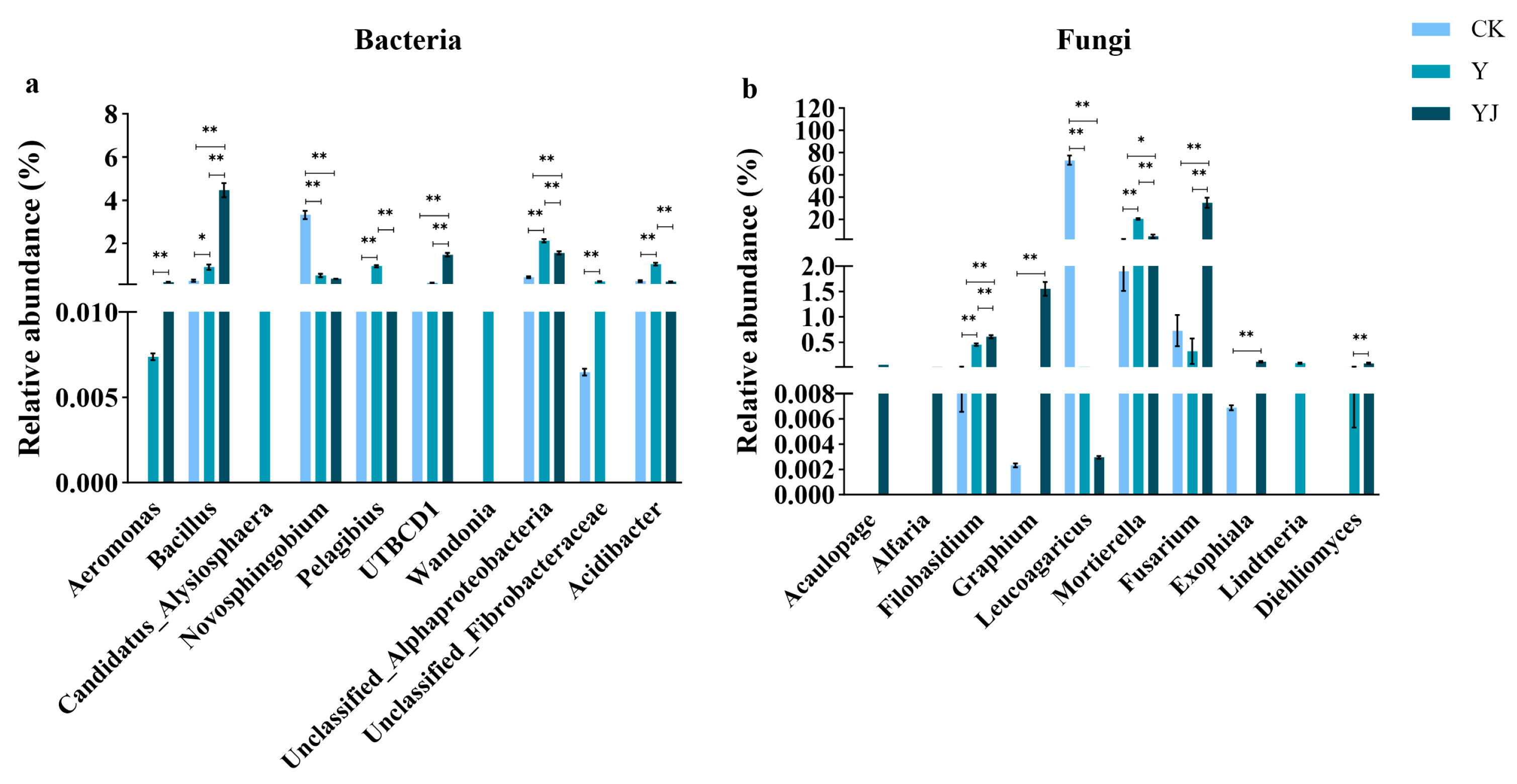
3.4. Differences in the Horizontal Distribution of Microbial Bacteria and Fungi Genera in Vineyard Soil Under Salt Alkali Stress After GB03 Microbial Agent Treatment
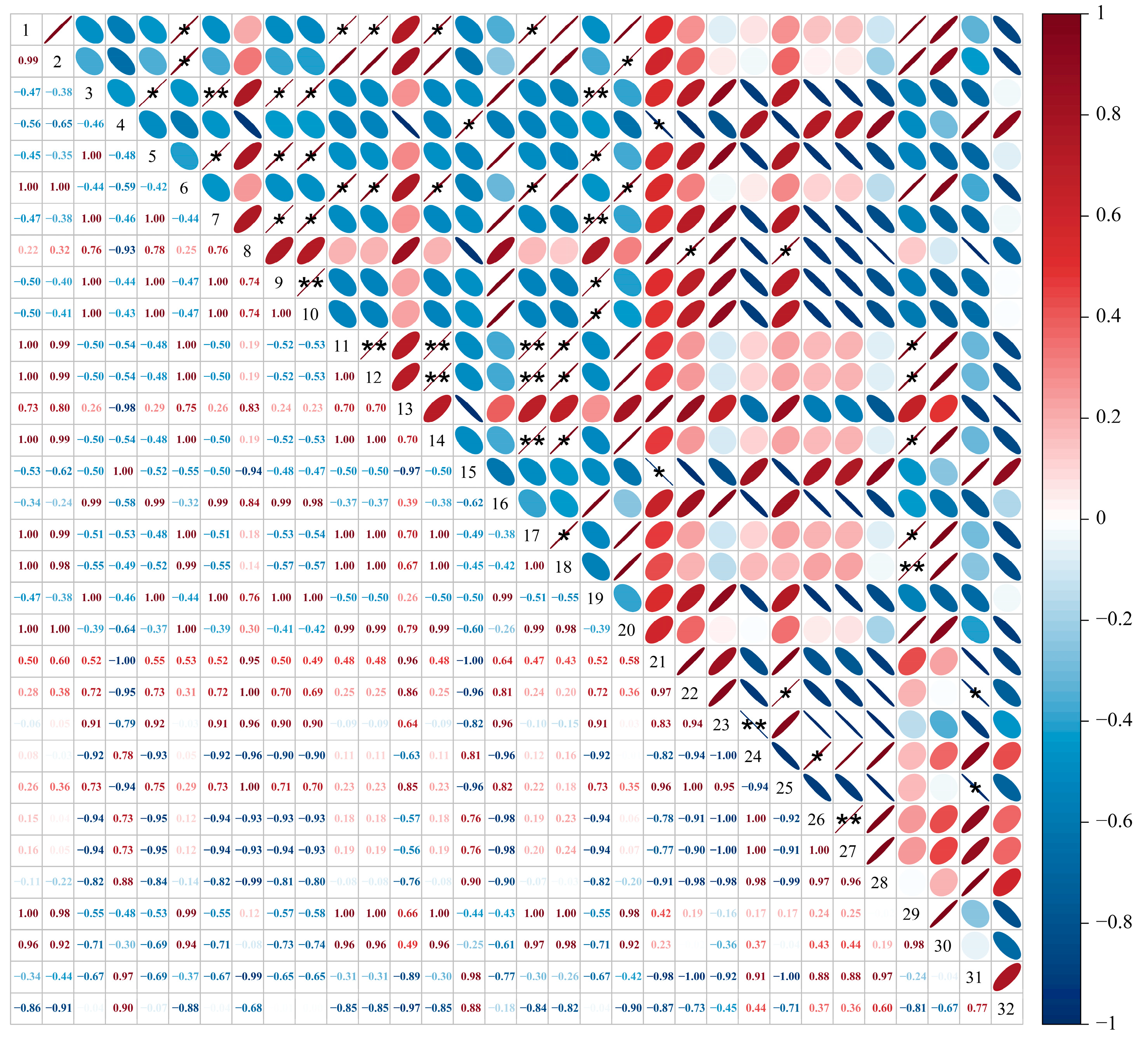
3.5. Correlation Analysis of GB03 Microbial Agent Treatment on Soil Microorganisms and Physicochemical Indexes in ‘Cabernet Sauvignon’ Vineyards Under Salt Alkali Stress
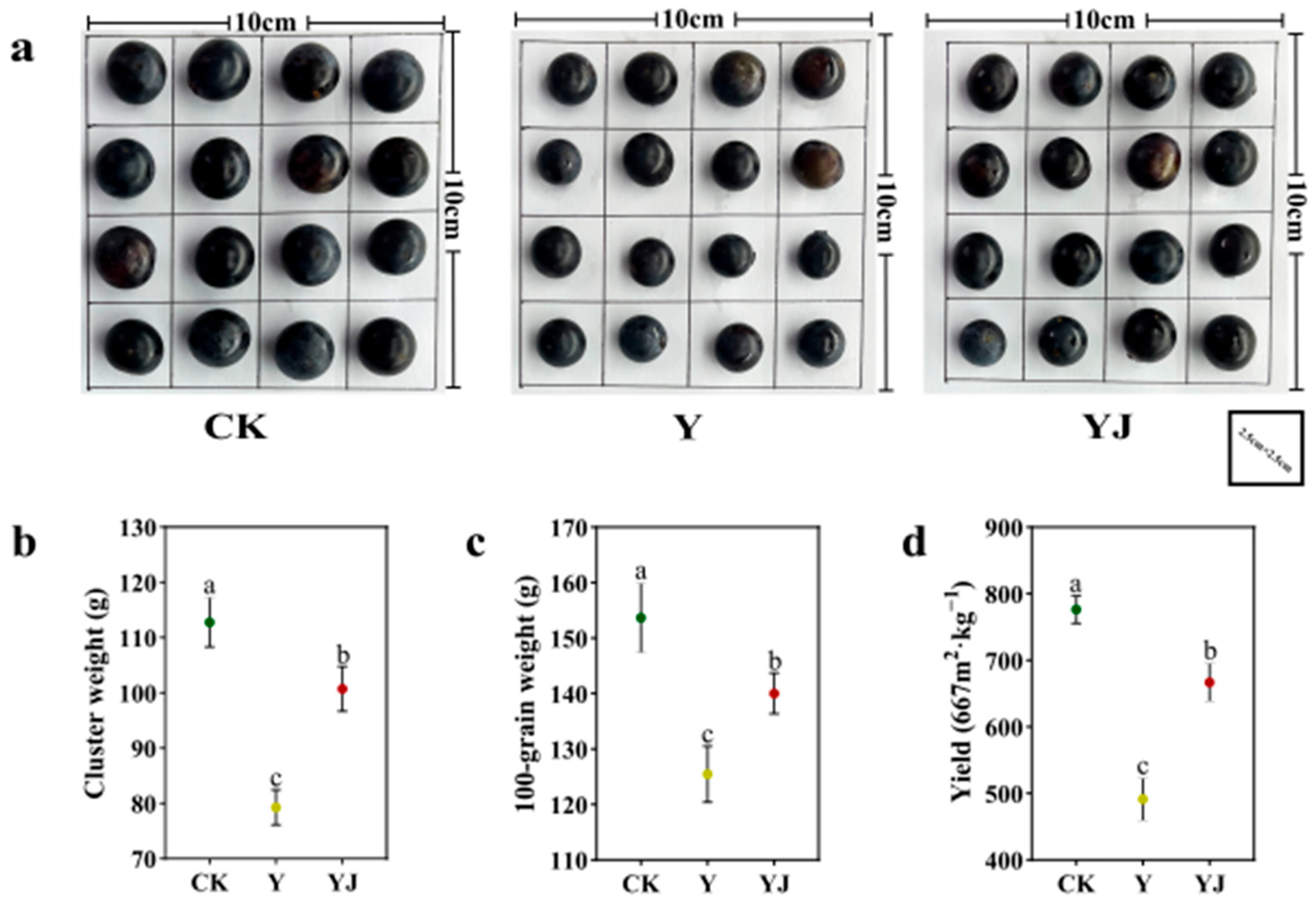
3.6. Effect of GB03 Microbial Inoculant Treatment on Phenotypic Characters and Yield of ‘Cabernet Sauvignon’ Grape Fruit Under Salt Alkali Stress
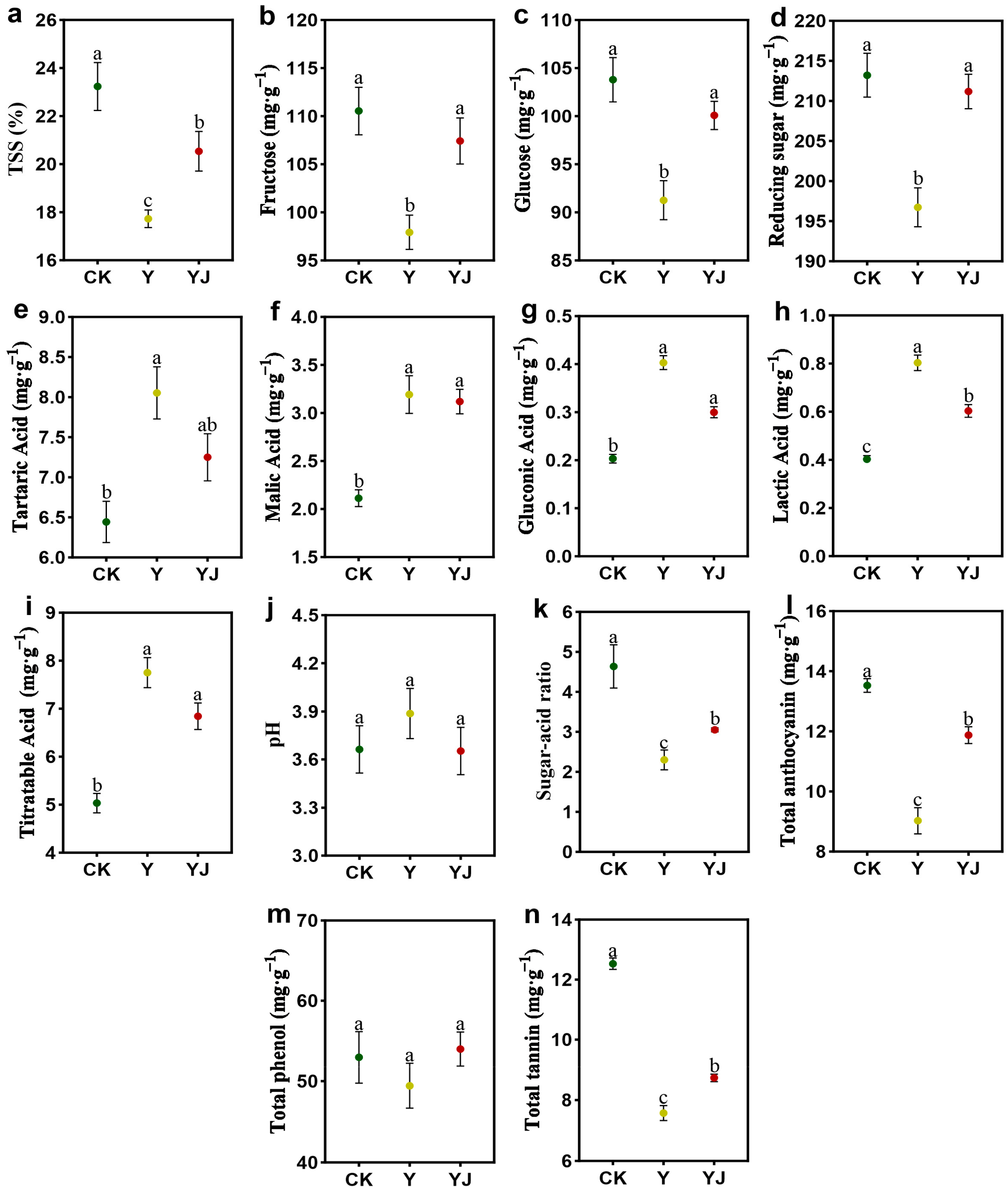
3.7. Effect of GB03 Microbial Inoculant Treatment on Fruit Quality of ‘Cabernet Sauvignon’ Grape Fruit Under Salt Alkali Stress
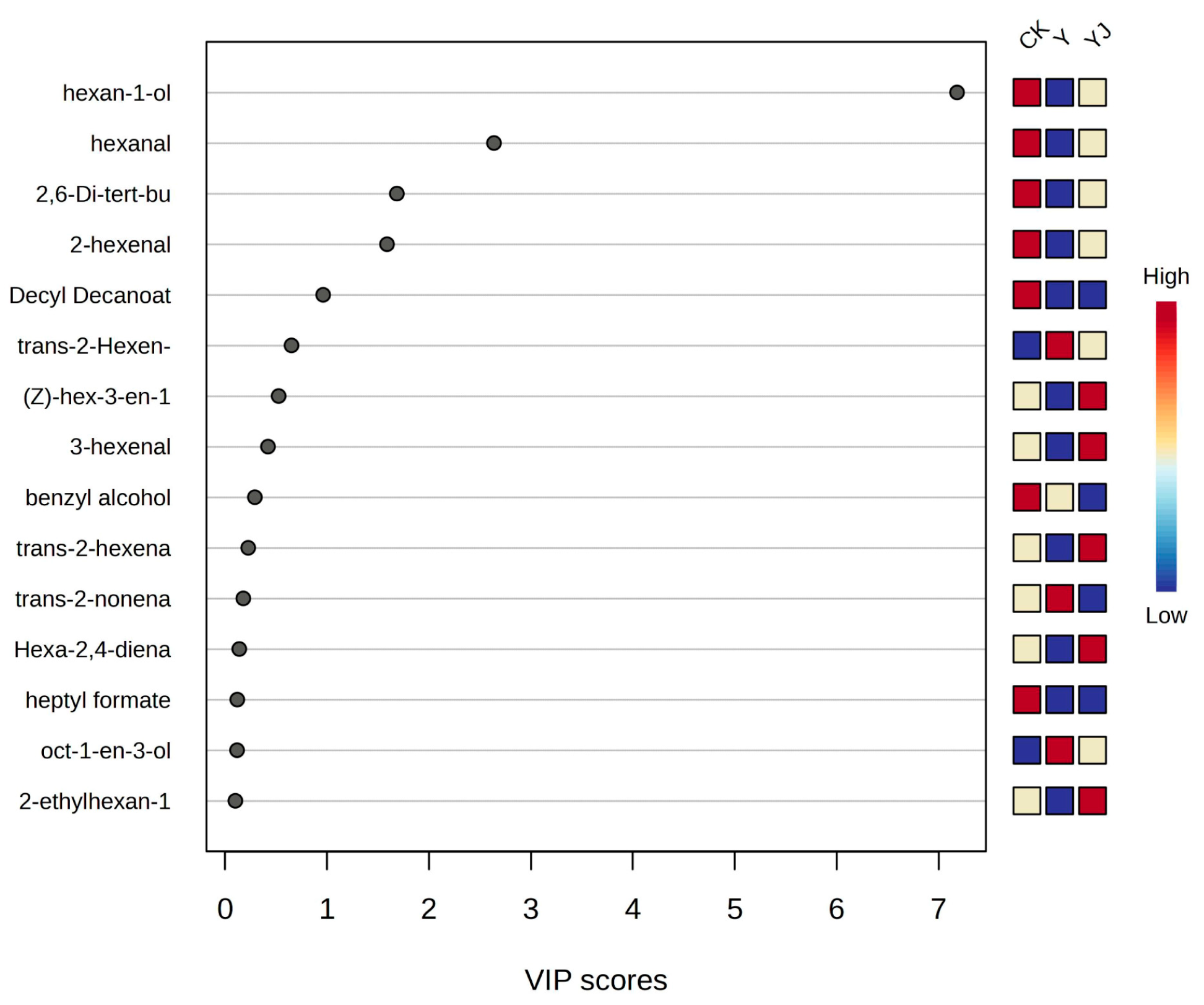
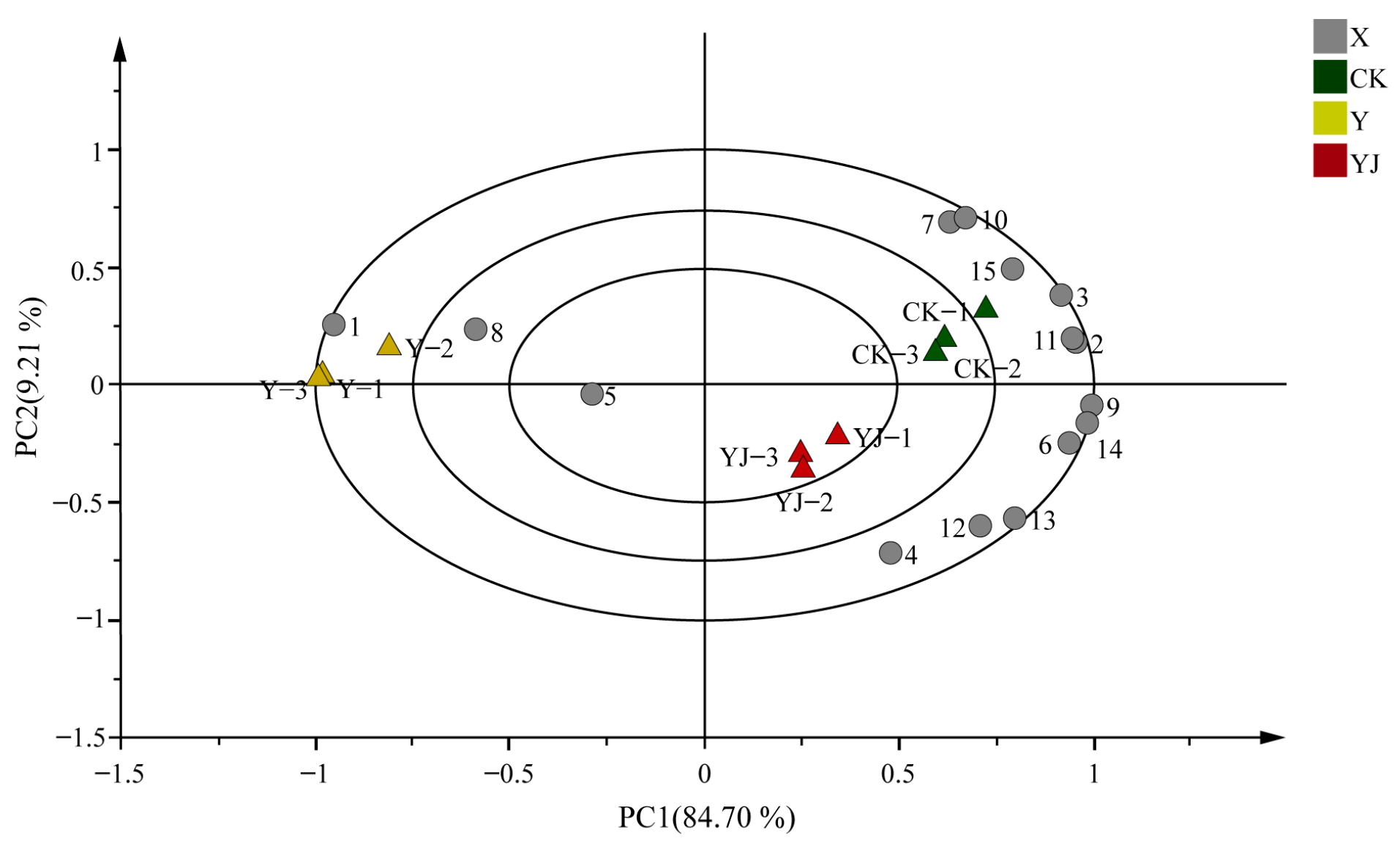
3.8. Partial Least Squares and Principal Component Analysis of Volatile Aroma Compounds in ’Cabernet Sauvignon’ Grape Fruit Under Salt Alkali Stress After Treatment with GB03 Microbial Inoculant
3.9. Correlation Analysis Between Soil Physicochemical Indicators and Fruit Quality in ‘Cabernet Sauvignon’ Vineyards Under Salt Alkali Stress After Treatment with GB03 Microbial Inoculant
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tao, Y.; Zhang, L. Intensity prediction of typical aroma characters of cabernet sauvignon wine in Changli County (China). LWT-Food Sci. Technol. 2010, 43, 1550–1556. [Google Scholar] [CrossRef]
- Ren, Y.; Sadeghnezhad, E.; Leng, X.; Pei, D.; Dong, T.; Zhang, P.; Gong, P.; Jia, H.; Fang, J. Assessment of ‘Cabernet Sauvignon’grape quality Half-Véraison to maturity for grapevines grown in different regions. Int. J. Mol. Sci. 2023, 24, 4670. [Google Scholar] [CrossRef] [PubMed]
- Yao, Y.; Chen, K.; Yang, X.; Li, J.; Li, X. Comparative study of the key aromatic compounds of Cabernet Sauvignon wine from the Xinjiang region of China. J. Food Sci. Technol. 2021, 58, 2109–2120. [Google Scholar] [CrossRef] [PubMed]
- Bouzas-Cid, Y.; Trigo-Córdoba, E.; Falqué, E.; Orriols, I.; Mirás-Avalos, J.M. Influence of supplementary irrigation on the amino acid and volatile composition of Godello wines from the Ribeiro Designation of Origin. Food Res. Int. 2018, 111, 715–723. [Google Scholar] [CrossRef]
- Wang, J.; Abbey, T.; Kozak, B.; Madilao, L.L.; Tindjau, R.; Del Nin, J.; Castellarin, S.D. Evolution over the growing season of volatile organic compounds in Viognier (Vitis vinifera L.) grapes under three irrigation regimes. Food Res. Int. 2019, 125, 108512. [Google Scholar] [CrossRef]
- Li, X.; Chu, C.; Ding, S.; Wei, H.; Wu, S.; Xie, B. Insight into how fertilization strategies increase quality of grape (Kyoho) and shift microbial community. Environ. Sci. Pollut. Res. 2022, 29, 27182–27194. [Google Scholar] [CrossRef]
- Mocali, S.; Kuramae, E.E.; Kowalchuk, G.A.; Fornasier, F.; Priori, S. Microbial functional diversity in vineyard soils: Sulfur metabolism and links with grapevine plants and wine quality. Front. Environ. Sci. 2020, 8, 75. [Google Scholar] [CrossRef]
- Poni, S.; Gatti, M.; Palliotti, A.; Dai, Z.; Duchêne, E.; Truong, T.-T.; Ferrara, G.; Matarrese, A.M.S.; Gallotta, A.; Bellincontro, A. Grapevine quality: A multiple choice issue. Sci. Hortic. 2018, 234, 445–462. [Google Scholar] [CrossRef]
- Soubeyrand, E.; Basteau, C.; Hilbert, G.; van Leeuwen, C.; Delrot, S.; Gomès, E. Nitrogen supply affects anthocyanin biosynthetic and regulatory genes in grapevine cv. Cabernet-Sauvignon berries. Phytochemistry 2014, 103, 38–49. [Google Scholar] [CrossRef]
- Delgado, R.; Martín, P.; Del Álamo, M.; González, M.R. Changes in the phenolic composition of grape berries during ripening in relation to vineyard nitrogen and potassium fertilisation rates. J. Sci. Food Agric. 2004, 84, 623–630. [Google Scholar] [CrossRef]
- Thomidis, T.; Zioziou, E.; Koundouras, S.; Karagiannidis, C.; Navrozidis, I.; Nikolaou, N. Effects of nitrogen and irrigation on the quality of grapes and the susceptibility to Botrytis bunch rot. Sci. Hortic. 2016, 212, 60–68. [Google Scholar] [CrossRef]
- Rogiers, S.Y.; Coetzee, Z.A.; Walker, R.R.; Deloire, A.; Tyerman, S.D. Potassium in the grape (Vitis vinifera L.) berry: Transport and function. Front. Plant Sci. 2017, 8, 1629. [Google Scholar] [CrossRef] [PubMed]
- Philippot, L.; Chenu, C.; Kappler, A.; Rillig, M.C.; Fierer, N. The interplay between microbial communities and soil properties. Nat. Rev. Microbiol. 2024, 22, 226–239. [Google Scholar] [CrossRef] [PubMed]
- Yin, X.; Feng, Q.; Li, Y.; Liu, W.; Zhu, M.; Xu, G.; Zheng, X.; Sindikubwabo, C. Induced soil degradation risks and plant responses by salinity and sodicity in intensive irrigated agro-ecosystems of seasonally-frozen arid regions. J. Hydrol. 2021, 603, 127036. [Google Scholar] [CrossRef]
- Tsiafouli, M.A.; Thébault, E.; Sgardelis, S.P.; De Ruiter, P.C.; Van Der Putten, W.H.; Birkhofer, K.; Hemerik, L.; De Vries, F.T.; Bardgett, R.D.; Brady, M.V. Intensive agriculture reduces soil biodiversity across Europe. Glob. Change Biol. 2015, 21, 973–985. [Google Scholar] [CrossRef]
- Tuck, S.L.; Winqvist, C.; Mota, F.; Ahnström, J.; Turnbull, L.A.; Bengtsson, J. Land-use intensity and the effects of organic farming on biodiversity: A hierarchical meta-analysis. J. Appl. Ecol. 2014, 51, 746–755. [Google Scholar] [CrossRef]
- Rousk, J.; Elyaagubi, F.K.; Jones, D.L.; Godbold, D.L. Bacterial salt tolerance is unrelated to soil salinity across an arid agroecosystem salinity gradient. Soil Biol. Biochem. 2011, 43, 1881–1887. [Google Scholar] [CrossRef]
- Xu, J.; Feng, Y.; Wang, Y.; Luo, X.; Tang, J.; Lin, X. The foliar spray of Rhodopseudomonas palustris grown under Stevia residue extract promotes plant growth via changing soil microbial community. J. Soils Sediments 2016, 16, 916–923. [Google Scholar] [CrossRef]
- Setia, R.; Gottschalk, P.; Smith, P.; Marschner, P.; Baldock, J.; Setia, D.; Smith, J. Soil salinity decreases global soil organic carbon stocks. Sci. Total Environ. 2013, 465, 267–272. [Google Scholar] [CrossRef]
- Singh, K. Microbial and enzyme activities of saline and sodic soils. Land Degrad. Dev. 2016, 27, 706–718. [Google Scholar] [CrossRef]
- Pan, C.; Liu, C.; Zhao, H.; Wang, Y. Changes of soil physico-chemical properties and enzyme activities in relation to grassland salinization. Eur. J. Soil Biol. 2013, 55, 13–19. [Google Scholar] [CrossRef]
- Yang, D.; Tang, L.; Cui, Y.; Chen, J.; Liu, L.; Guo, C. Saline-alkali stress reduces soil bacterial community diversity and soil enzyme activities. Ecotoxicology 2022, 31, 1356–1368. [Google Scholar] [CrossRef] [PubMed]
- Jia, J.; Bai, J.; Gao, H.; Wang, W.; Yin, S.; Wang, D.; Han, L. Effects of salinity and moisture on sediment net nitrogen mineralization in salt marshes of a Chinese estuary. Chemosphere 2019, 228, 174–182. [Google Scholar] [CrossRef] [PubMed]
- Walker, R.R.; Blackmore, D.H.; Clingeleffer, P.R.; Correll, R.L. Rootstock effects on salt tolerance of irrigated field-grown grapevines (Vitis vinifera L. cv. Sultana).: 1. Yield and vigour inter-relationships. Aust. J. Grape Wine Res. 2002, 8, 3–14. [Google Scholar] [CrossRef]
- Kloepper, J.; Schroth, M. Relationship of in vitro antibiosis of plant growth-promoting rhizobacteria to plant growth and the displacement of root microflora. Phytopathology 1981, 71, 1020–1024. [Google Scholar] [CrossRef]
- Kumawat, K.C.; Sharma, B.; Nagpal, S.; Kumar, A.; Tiwari, S.; Nair, R.M. Plant growth-promoting rhizobacteria: Salt stress alleviators to improve crop productivity for sustainable agriculture development. Front. Plant Sci. 2023, 13, 1101862. [Google Scholar] [CrossRef]
- Kumar, M.; Giri, V.P.; Pandey, S.; Gupta, A.; Patel, M.K.; Bajpai, A.B.; Jenkins, S.; Siddique, K.H. Plant-growth-promoting rhizobacteria emerging as an effective bioinoculant to improve the growth, production, and stress tolerance of vegetable crops. Int. J. Mol. Sci. 2021, 22, 12245. [Google Scholar] [CrossRef]
- Berger, B.; Baldermann, S.; Ruppel, S. The plant growth-promoting bacterium Kosakonia radicincitans improves fruit yield and quality of Solanum lycopersicum. J. Sci. Food Agric. 2017, 97, 4865–4871. [Google Scholar] [CrossRef]
- Chen, Y.; Li, S.; Liu, N.; He, H.; Cao, X.; Lv, C.; Zhang, K.; Dai, J. Effects of different types of microbial inoculants on available nitrogen and phosphorus, soil microbial community, and wheat growth in high-P soil. Environ. Sci. Pollut. Res. 2021, 28, 23036–23047. [Google Scholar] [CrossRef]
- Sarabia, M.; Cazares, S.; González-Rodríguez, A.; Mora, F.; Carreón-Abud, Y.; Larsen, J. Plant growth promotion traits of rhizosphere yeasts and their response to soil characteristics and crop cycle in maize agroecosystems. Rhizosphere 2018, 6, 67–73. [Google Scholar] [CrossRef]
- Julia, I.; Oscar, M.; Analía, L.; Zocolo Guilherme, J.; Virginia, L. Biofertilization with Macrocystis pyrifera algae extracts combined with PGPR-enhanced growth in Lactuca sativa seedlings. J. Appl. Phycol. 2020, 32, 4361–4371. [Google Scholar] [CrossRef]
- Fan, B.; Blom, J.; Klenk, H.-P.; Borriss, R. Bacillus amyloliquefaciens, Bacillus velezensis, and Bacillus siamensis form an “operational group B. amyloliquefaciens” within the B. subtilis species complex. Front. Microbiol. 2017, 8, 22. [Google Scholar] [CrossRef] [PubMed]
- Mullins, A.J.; Li, Y.; Qin, L.; Hu, X.; Xie, L.; Gu, C.; Mahenthiralingam, E.; Liao, X.; Webster, G. Reclassification of the biocontrol agents Bacillus subtilis BY-2 and Tu-100 as Bacillus velezensis and insights into the genomic and specialized metabolite diversity of the species. Microbiology 2020, 166, 1121–1128. [Google Scholar] [CrossRef] [PubMed]
- Jang, S.; Choi, S.-K.; Zhang, H.; Zhang, S.; Ryu, C.-M.; Kloepper, J.W. History of a model plant growth-promoting rhizobacterium, Bacillus velezensis GB03: From isolation to commercialization. Front. Plant Sci. 2023, 14, 1279896. [Google Scholar] [CrossRef]
- Han, Q.-Q.; Lü, X.-P.; Bai, J.-P.; Qiao, Y.; Paré, P.W.; Wang, S.-M.; Zhang, J.-L.; Wu, Y.-N.; Pang, X.-P.; Xu, W.-B. Beneficial soil bacterium Bacillus subtilis (GB03) augments salt tolerance of white clover. Front. Plant Sci. 2014, 5, 525. [Google Scholar] [CrossRef]
- Zhao, Q.; Wu, Y.-N.; Fan, Q.; Han, Q.-Q.; Paré, P.W.; Xu, R.; Wang, Y.-Q.; Wang, S.-M.; Zhang, J.-L. Improved growth and metabolite accumulation in Codonopsis pilosula (Franch.) Nannf. by inoculation of Bacillus amyloliquefaciens GB03. J. Agric. Food Chem. 2016, 64, 8103–8108. [Google Scholar] [CrossRef]
- Wang, Q.; Ou, E.-L.; Wang, P.-C.; Chen, Y.; Wang, Z.-Y.; Wang, Z.-W.; Fang, X.-W.; Zhang, J.-L. Bacillus amyloliquefaciens GB03 augmented tall fescue growth by regulating phytohormone and nutrient homeostasis under nitrogen deficiency. Front. Plant Sci. 2022, 13, 979883. [Google Scholar] [CrossRef]
- Yan, H.K.; Ma, S.; Lu, X.; Zhang, C.C.; Ma, L.; Li, K.; Wei, Y.C.; Gong, M.S.; Li, S. College of Life Science and Technology, Gansu Agricultural University, Lanzhou 730070, China. Hortscience 2022, 57, 1593–1599. [Google Scholar] [CrossRef]
- Zhang, C.; Liu, G.; Xue, S.; Song, Z. Rhizosphere soil microbial activity under different vegetation types on the Loess Plateau, China. Geoderma 2011, 161, 115–125. [Google Scholar] [CrossRef]
- Li, W.-H.; Liu, Q.-Z.; Peng, C. Effect of long-term continuous cropping of strawberry on soil bacterial community structure and diversity. J. Integr. Agric. 2018, 17, 2570–2582. [Google Scholar] [CrossRef]
- Devitt, D.; Jarrell, W.; Stevens, K. Sodium-potassium ratios in soil solution and plant response under saline conditions. Soil Sci. Soc. Am. J. 1981, 45, 80–86. [Google Scholar] [CrossRef]
- Pasković, I.; Herak Ćustić, M.; Pecina, M.; Bronić, J.; Ban, D.; Radić, T.; Pošćić, F.; Jukić Špika, M.; Soldo, B.; Palčić, I. Manganese soil and foliar fertilization of olive plantlets: The effect on leaf mineral and phenolic content and root mycorrhizal colonization. J. Sci. Food Agric. 2019, 99, 360–367. [Google Scholar] [CrossRef] [PubMed]
- Alhassan, A.R.M.; Ma, W.; Li, G.; Jiang, Z.; Wu, J.; Chen, G. Response of soil organic carbon to vegetation degradation along a moisture gradient in a wet meadow on the Qinghai–Tibet Plateau. Ecol. Evol. 2018, 8, 11999–12010. [Google Scholar] [CrossRef]
- Hosseini Bai, S.; Blumfield, T.J.; Xu, Z.; Chen, C.; Wild, C. Soil organic matter dynamics and nitrogen availability in response to site preparation and management during revegetation in tropical Central Queensland, Australia. J. Soils Sediments 2012, 12, 386–395. [Google Scholar] [CrossRef][Green Version]
- Bai, L.; Cui, J.; Jie, W.; Cai, B. Analysis of the community compositions of rhizosphere fungi in soybeans continuous cropping fields. Microbiol. Res. 2015, 180, 49–56. [Google Scholar] [CrossRef]
- Acevedo-Opazo, C.; Ortega-Farias, S.; Fuentes, S. Effects of grapevine (Vitis vinifera L.) water status on water consumption, vegetative growth and grape quality: An irrigation scheduling application to achieve regulated deficit irrigation. Agric. Water Manag. 2010, 97, 956–964. [Google Scholar] [CrossRef]
- Xiao, Q.; Ye, S.; Wang, H.; Xing, S.; Zhu, W.; Zhang, H.; Zhu, J.; Pu, C.; Zhao, D.; Zhou, Q. Soluble sugar, organic acid and phenolic composition and flavor evaluation of plum fruits. Food Chem. X 2024, 24, 101790. [Google Scholar] [CrossRef]
- Zhang, N.; Liu, X.; Jin, X.; Li, C.; Wu, X.; Yang, S.; Ning, J.; Yanne, P. Determination of total iron-reactive phenolics, anthocyanins and tannins in wine grapes of skins and seeds based on near-infrared hyperspectral imaging. Food Chem. 2017, 237, 811–817. [Google Scholar] [CrossRef]
- Shi, X.; Liu, F.; Cheng, C.; Wang, X.; Ji, X.; Wang, B.; Zheng, X.; Wang, H. Effects of different new shoots spacing on canopy light environment and fruit quality of grapevine under protected cultivation. Acta Hortic. Sin. 2018, 45, 436. [Google Scholar]
- Giacosa, S.; Ferrero, L.; Paissoni, M.A.; Segade, S.R.; Gerbi, V.; Rolle, L. Grape skin anthocyanin extraction from red varieties during simulated maceration: Influence of grape seeds and pigments adsorption on their surface. Food Chem. 2023, 424, 136463. [Google Scholar] [CrossRef]
- Li, W.; Li, W.; Yang, S.; Ma, Z.; Zhou, Q.; Mao, J.; Han, S.; Chen, B. Transcriptome and metabolite conjoint analysis reveals that exogenous methyl jasmonate regulates monoterpene synthesis in grape berry skin. J. Agric. Food Chem. 2020, 68, 5270–5281. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Q.; Tang, J.; Li, Z.; Yang, W.; Duan, Y. The influence of soil physico-chemical properties and enzyme activities on soil quality of saline-alkali agroecosystems in Western Jilin Province, China. Sustainability 2018, 10, 1529. [Google Scholar] [CrossRef]
- Azadi, N.; Raiesi, F. Salinization depresses soil enzyme activity in metal-polluted soils through increases in metal mobilization and decreases in microbial biomass. Ecotoxicology 2021, 30, 1071–1083. [Google Scholar] [CrossRef]
- Morrissey, E.M.; Gillespie, J.L.; Morina, J.C.; Franklin, R.B. Salinity affects microbial activity and soil organic matter content in tidal wetlands. Glob. Change Biol. 2014, 20, 1351–1362. [Google Scholar] [CrossRef]
- Adesemoye, A.; Torbert, H.; Kloepper, J. Plant growth-promoting rhizobacteria allow reduced application rates of chemical fertilizers. Microb. Ecol. 2009, 58, 921–929. [Google Scholar] [CrossRef]
- Kuzyakov, Y.; Friedel, J.; Stahr, K. Review of mechanisms and quantification of priming effects. Soil Biol. Biochem. 2000, 32, 1485–1498. [Google Scholar] [CrossRef]
- Zhang, K.; Shi, Y.; Cui, X.; Yue, P.; Li, K.; Liu, X.; Tripathi, B.M.; Chu, H. Salinity is a key determinant for soil microbial communities in a desert ecosystem. Msystems 2019, 4. [Google Scholar] [CrossRef]
- Pan, Y.; Kang, P.; Tan, M.; Hu, J.; Zhang, Y.; Zhang, J.; Song, N.; Li, X. Root exudates and rhizosphere soil bacterial relationships of Nitraria tangutorum are linked to k-strategists bacterial community under salt stress. Front. Plant Sci. 2022, 13, 997292. [Google Scholar] [CrossRef]
- Cappellari, L.d.R.; Banchio, E. Microbial volatile organic compounds produced by Bacillus amyloliquefaciens GB03 ameliorate the effects of salt stress in Mentha piperita principally through acetoin emission. J. Plant Growth Regul. 2020, 39, 764–775. [Google Scholar] [CrossRef]
- Lu, X.; Ma, L.; Zhang, C.; Yan, H.; Bao, J.; Gong, M.; Wang, W.; Li, S.; Ma, S.; Chen, B. Grapevine (Vitis vinifera) responses to salt stress and alkali stress: Transcriptional and metabolic profiling. BMC Plant Biol. 2022, 22, 528. [Google Scholar] [CrossRef]
- Walker, R.R.; Blackmore, D.H.; Clingeleffer, P.R.; Correll, R.L. Rootstock effects on salt tolerance of irrigated field-grown grapevines (Vitis vinifera L. cv. Sultana) 2. Ion concentrations in leaves and juice. Aust. J. Grape Wine Res. 2004, 10, 90–99. [Google Scholar] [CrossRef]
- Li, X.-L.; Wang, C.-R.; Li, X.-Y.; Yao, Y.-X.; Hao, Y.-J. Modifications of Kyoho grape berry quality under long-term NaCl treatment. Food Chem. 2013, 139, 931–937. [Google Scholar] [CrossRef] [PubMed]
- Lu, S.; Li, T.; Jiang, J. Effects of salinity on sucrose metabolism during tomato fruit development. Afr. J. Biotechnol. 2010, 9, 842–849. [Google Scholar]
- Zhang, H.; Xie, X.; Kim, M.S.; Kornyeyev, D.A.; Holaday, S.; Paré, P.W. Soil bacteria augment Arabidopsis photosynthesis by decreasing glucose sensing and abscisic acid levels in planta. Plant J. 2008, 56, 264–273. [Google Scholar] [CrossRef]
- Zhang, J.-L.; Aziz, M.; Qiao, Y.; Han, Q.-Q.; Li, J.; Wang, Y.-Q.; Shen, X.; Wang, S.-M.; Paré, P.W. Soil microbe Bacillus subtilis (GB03) induces biomass accumulation and salt tolerance with lower sodium accumulation in wheat. Crop Pasture Sci. 2014, 65, 423–427. [Google Scholar] [CrossRef]
- Treutter, D. Significance of flavonoids in plant resistance: A review. Environ. Chem. Lett. 2006, 4, 147–157. [Google Scholar] [CrossRef]
- Baudry, A.; Heim, M.A.; Dubreucq, B.; Caboche, M.; Weisshaar, B.; Lepiniec, L. TT2, TT8, and TTG1 synergistically specify the expression of BANYULS and proanthocyanidin biosynthesis in Arabidopsis thaliana. Plant J. 2004, 39, 366–380. [Google Scholar] [CrossRef]
- Matus, J.; Poupin, M.; Cañón, P.; Bordeu, E.; Alcalde, J.; Arce-Johnson, P. Isolation of WDR and bHLH genes related to flavonoid synthesis in grapevine (Vitis vinifera L.). Plant Mol. Biol. 2010, 72, 607–620. [Google Scholar] [CrossRef]
- Xiao, J.; Cao, M.; Lai, K.; Sun, K.; Zhang, L.; Gao, P.; Zhang, Y.; Yan, B.; Guo, L. Unveiling key metabolic pathways in Bacillus subtilis-mediated salt tolerance enhancement in Glycyrrhiza uralensis Fisch. through multi-omics analysis. Environ. Exp. Bot. 2024, 219, 105631. [Google Scholar] [CrossRef]
- Zhang, B.; Wang, X.-Q.; Yang, B.; Li, N.-N.; Niu, J.-M.; Shi, X.; Han, S.-Y. Copigmentation evidence of phenolic compound: The effect of caffeic and rosmarinic acids addition on the chromatic quality and phenolic composition of Cabernet Sauvignon red wine from the Hexi Corridor region (China). J. Food Compos. Anal. 2021, 102, 104037. [Google Scholar] [CrossRef]
- Zang, X.; Du, Q.; Qu, R.; Ye, D.; Lu, Y.; Liu, Y. Analysis of volatile aroma compounds and sensory characteristics contributing to regional style of red wines from Hexi corridor based on sixteen grape varieties/clones. Fermentation 2022, 8, 501. [Google Scholar] [CrossRef]

| Treatments | L* | a* | b* | C* |
|---|---|---|---|---|
| CK | 32.07 ± 0.53 c | 2.35 ± 0.17 a | 3.15 ± 0.13 a | 15.47 ± 0.06 a |
| Y | 37.79 ± 1.62 a | 2.08 ± 0.14 a | 3.01 ± 0.11 a | 13.44 ± 1.08 a |
| YJ | 34.78 ± 0.92 b | 2.13 ± 0.14 a | 2.92 ± 0.22 a | 13.17 ± 1.87 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yan, H.-K.; Zhang, C.-C.; Nai, G.-J.; Ma, L.; Lai, Y.; Pu, Z.-H.; Ma, S.-Y.; Li, S. Microbial Inoculant GB03 Increased the Yield and Quality of Grape Fruit Under Salt-Alkali Stress by Changing Rhizosphere Microbial Communities. Foods 2025, 14, 711. https://doi.org/10.3390/foods14050711
Yan H-K, Zhang C-C, Nai G-J, Ma L, Lai Y, Pu Z-H, Ma S-Y, Li S. Microbial Inoculant GB03 Increased the Yield and Quality of Grape Fruit Under Salt-Alkali Stress by Changing Rhizosphere Microbial Communities. Foods. 2025; 14(5):711. https://doi.org/10.3390/foods14050711
Chicago/Turabian StyleYan, Hao-Kai, Cong-Cong Zhang, Guo-Jie Nai, Lei Ma, Ying Lai, Zhi-Hui Pu, Shao-Ying Ma, and Sheng Li. 2025. "Microbial Inoculant GB03 Increased the Yield and Quality of Grape Fruit Under Salt-Alkali Stress by Changing Rhizosphere Microbial Communities" Foods 14, no. 5: 711. https://doi.org/10.3390/foods14050711
APA StyleYan, H.-K., Zhang, C.-C., Nai, G.-J., Ma, L., Lai, Y., Pu, Z.-H., Ma, S.-Y., & Li, S. (2025). Microbial Inoculant GB03 Increased the Yield and Quality of Grape Fruit Under Salt-Alkali Stress by Changing Rhizosphere Microbial Communities. Foods, 14(5), 711. https://doi.org/10.3390/foods14050711





