Evaluation of Functional Quality of Maize with Different Grain Colors and Differences in Enzymatic Properties of Anthocyanin Metabolism
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Site Description
2.2. Material
2.3. Experimental Design
2.4. Measurements
2.4.1. Carotenoid Content
2.4.2. Soluble Sugar Content
2.4.3. Vitamin C Content
2.4.4. Anthocyanin Content
2.4.5. Mineral Element Content
2.4.6. Phenylalanine Content
2.4.7. Enzyme Activity in Anthocyanin Synthesis
2.5. Statistical Analysis
3. Results
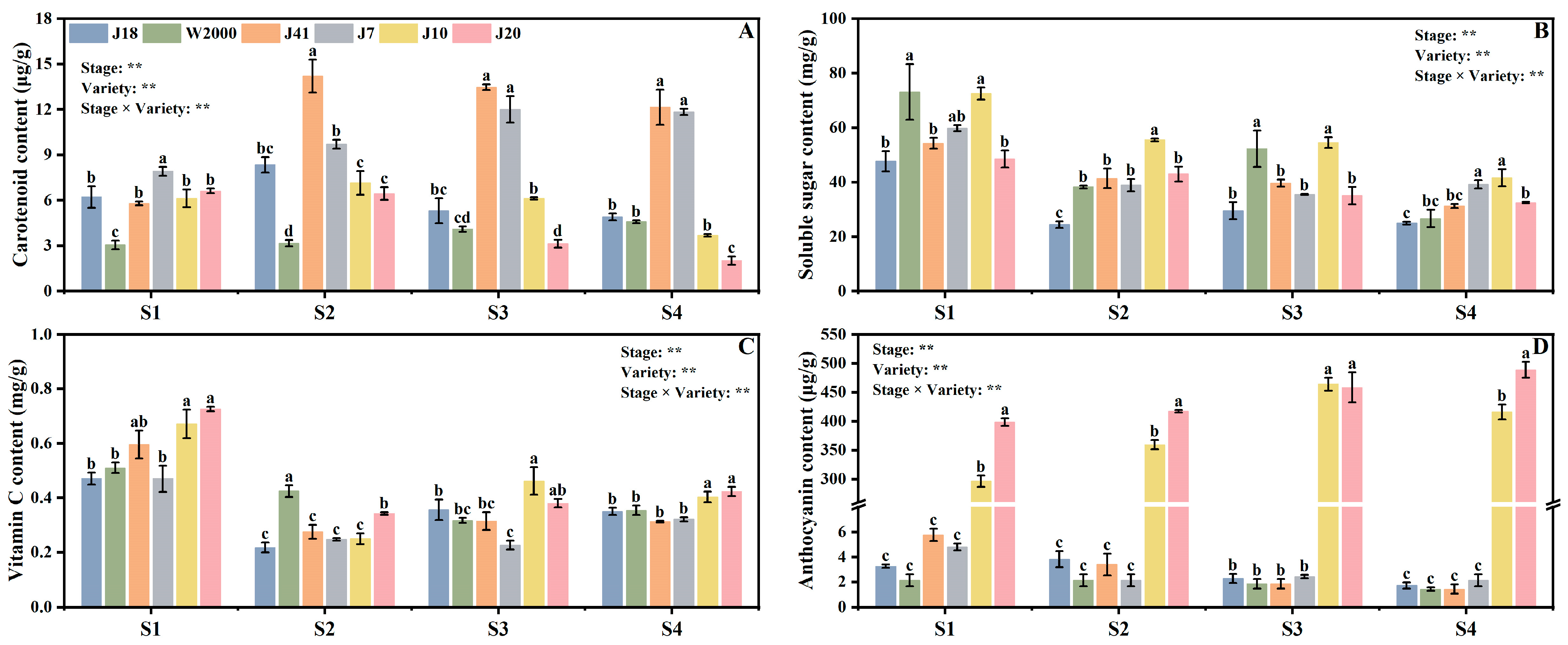
3.1. Functional Quality
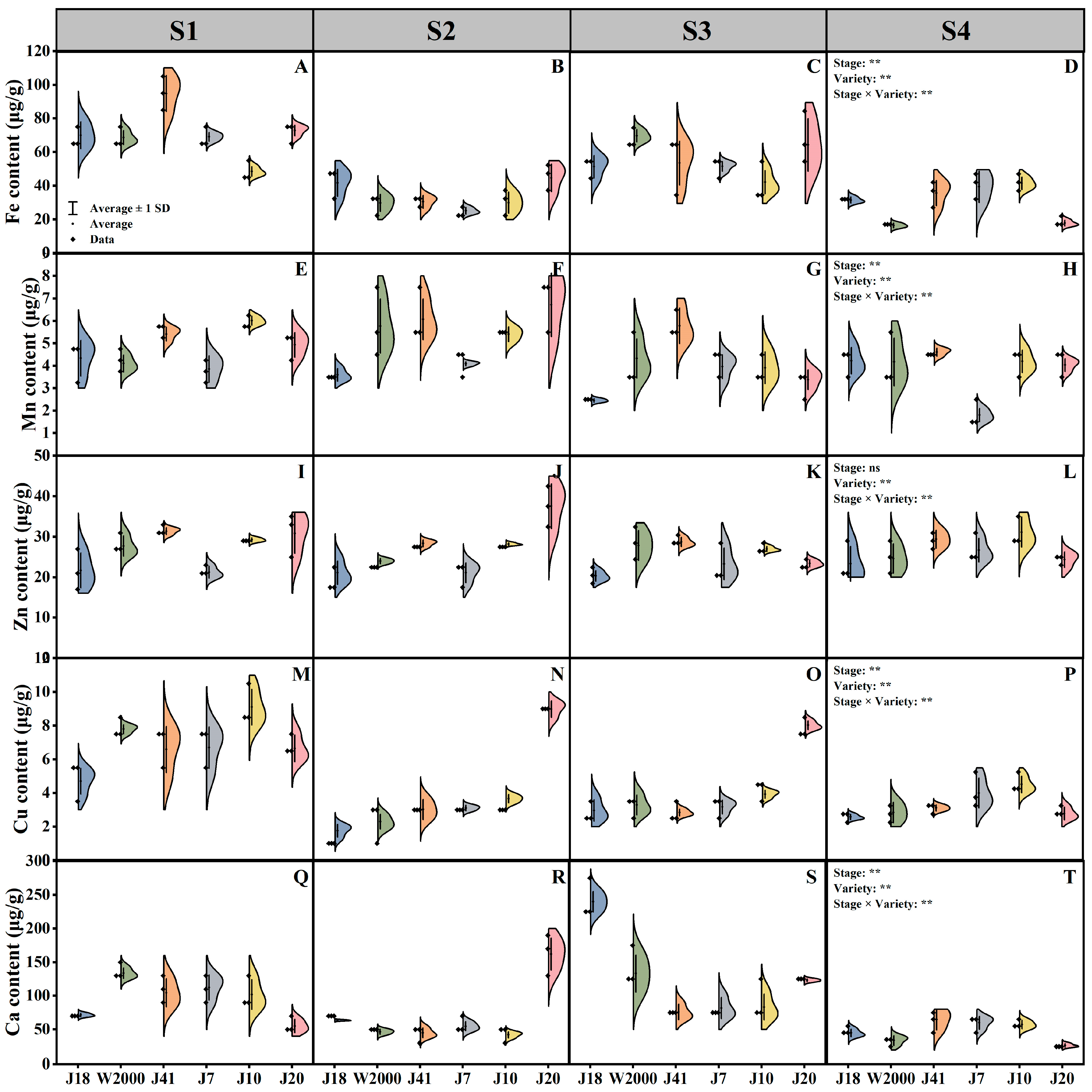
3.2. Mineral Element
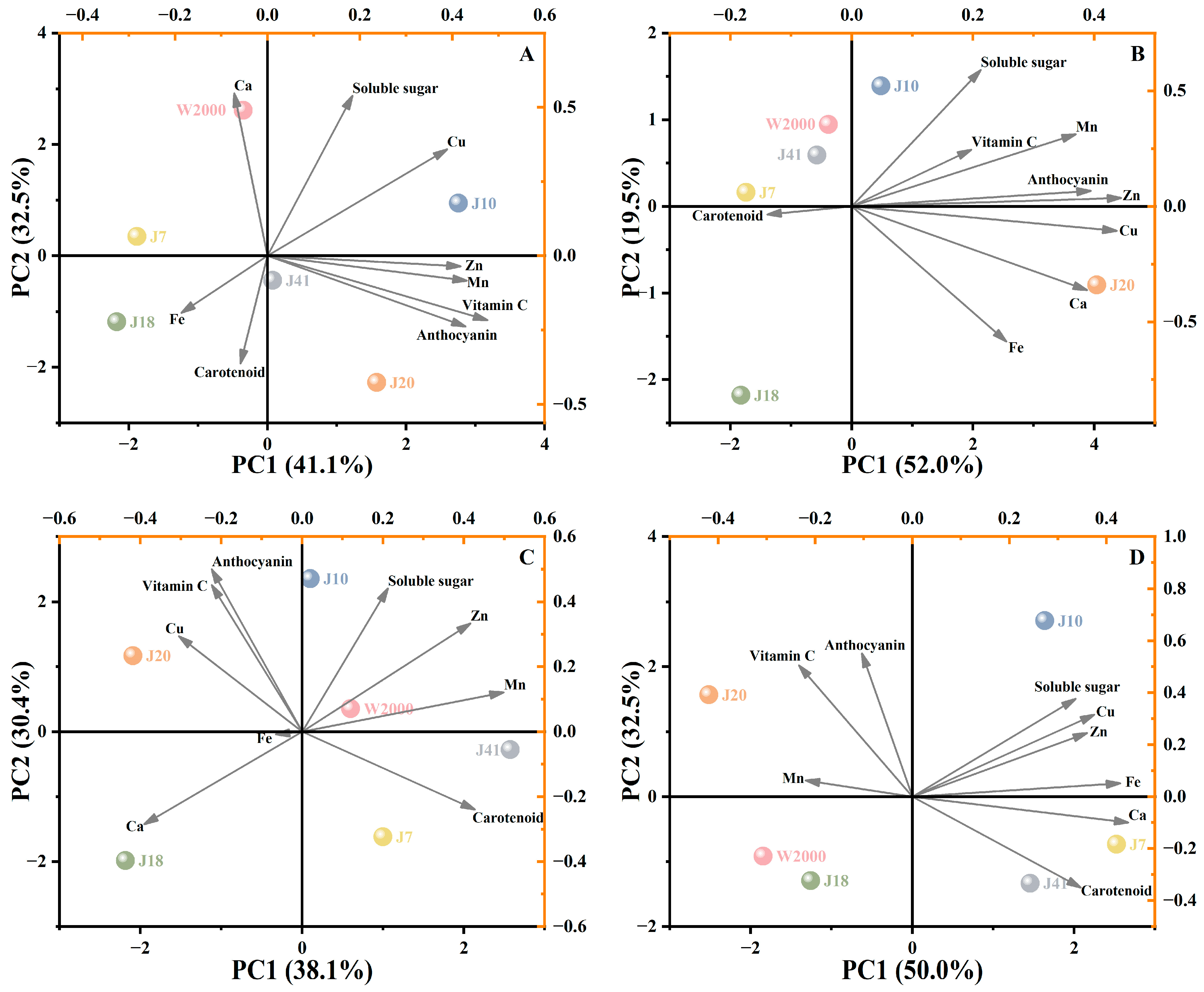
3.3. Principal Component Analysis
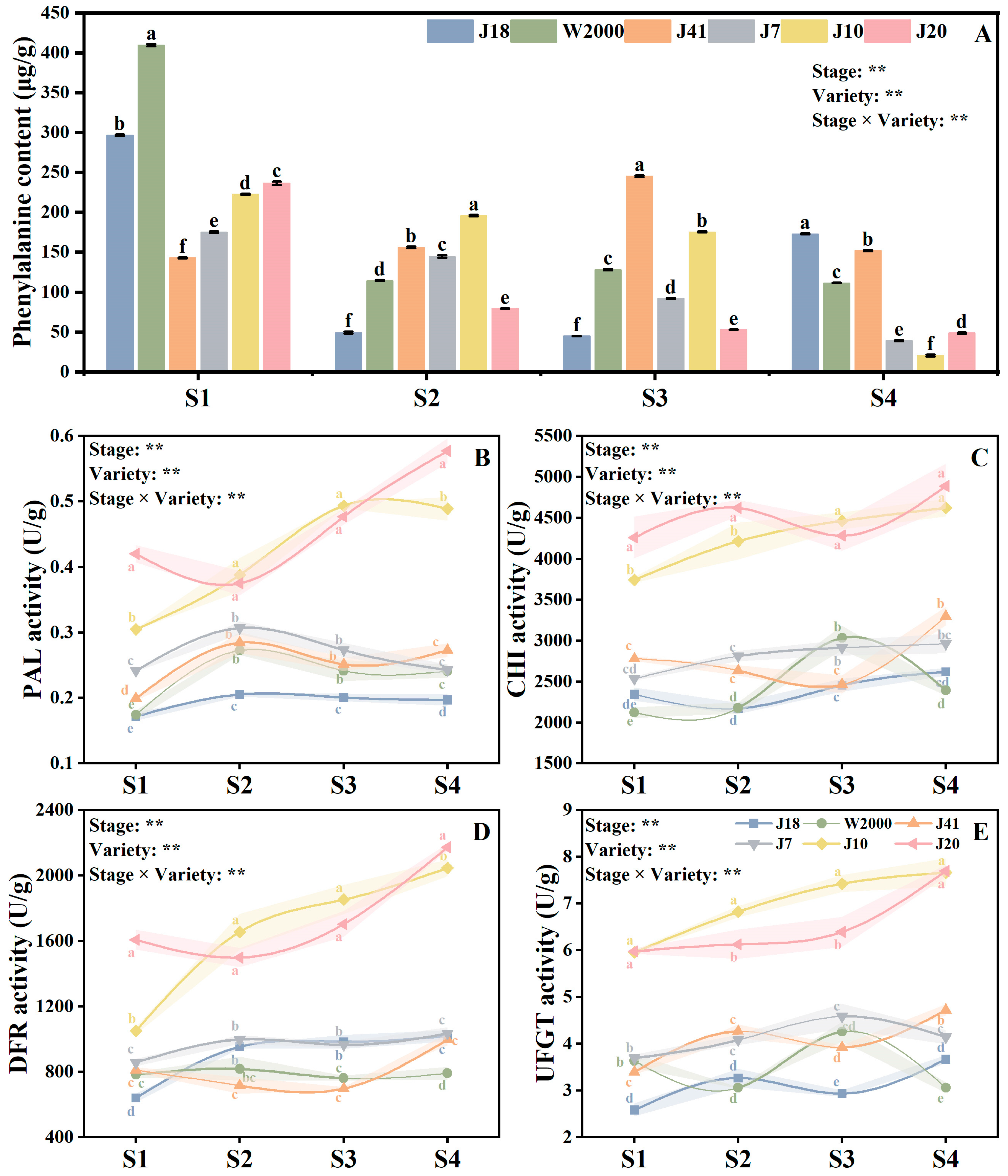
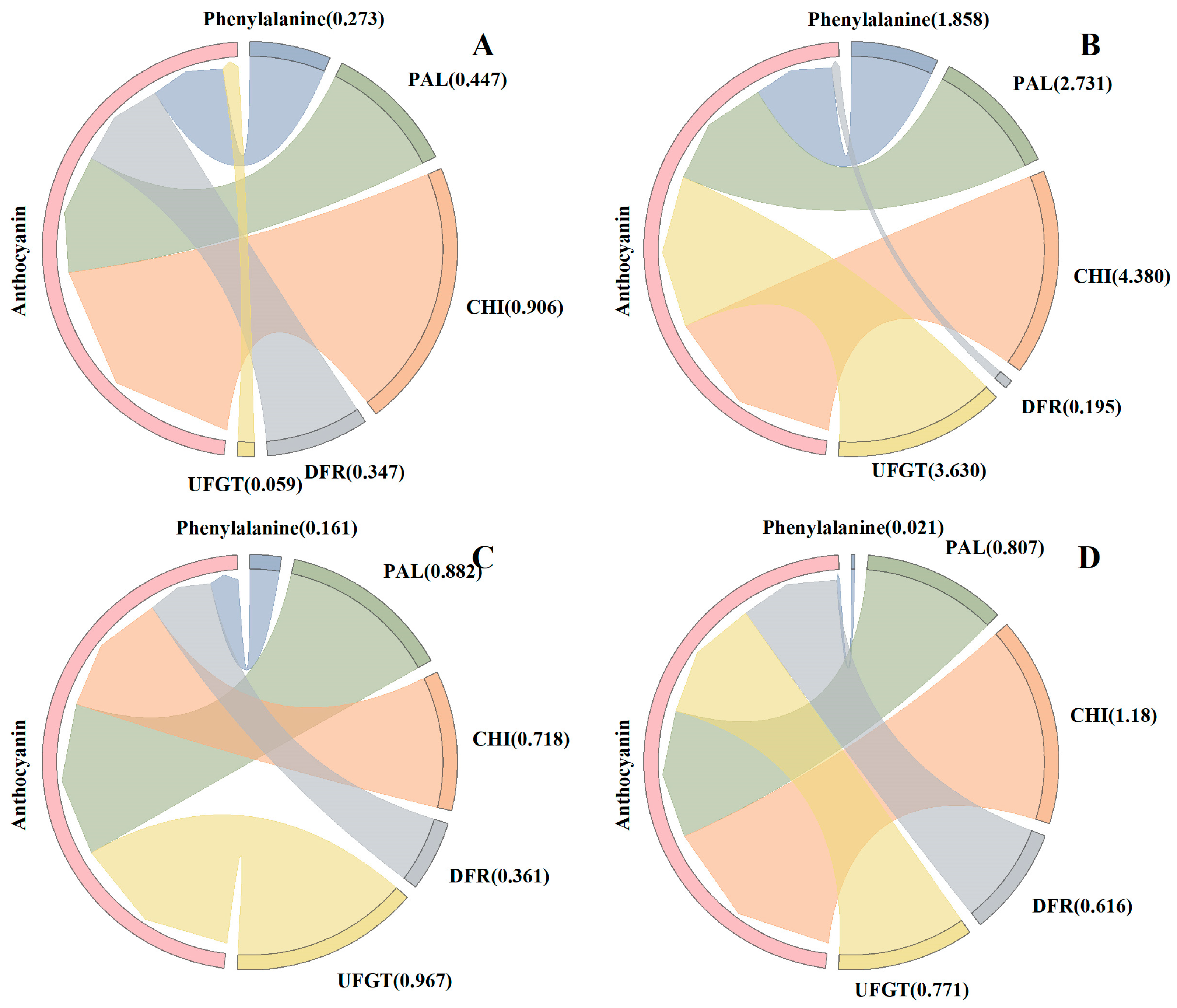
3.4. Anthocyanin Synthesis
3.5. Correlation Analysis
3.6. Regression Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Petroni, K.; Pilu, R.; Tonelli, C. Anthocyanin in corn: A wealth of genes for human health. Planta 2014, 240, 901–911. [Google Scholar] [CrossRef]
- Acosta-Estrada, B.A.; Serna-Saldívar, S.O.; Chuck-Hernández, C. Quality assessment of maize tortillas produced from landraces and high yield hybrids and varieties. Front. Nutr. 2023, 10, 1105619. [Google Scholar] [CrossRef] [PubMed]
- Colombo, R.; Ferron, L.; Papetti, A. Colored corn: An up-date on metabolites extraction, health implication, and potential use. Molecules 2021, 26, 199. [Google Scholar] [CrossRef] [PubMed]
- Erenstein, O.; Jaleta, M.; Sonder, K.; Mottaleb, K.; Prasanna, B.M. Global maize production, consumption and trade: Trends and R&D implications. Food Secur. 2022, 14, 1295–1319. [Google Scholar] [CrossRef]
- Lu, B.; An, H.; Song, X.; Yang, B.; Jian, Z.; Cui, F.; Xue, J.; Gao, Z.; Du, T. Enhancement of nutritional substance, trace elements, and pigments in waxy maize grains through foliar application of selenite. Foods 2024, 13, 1337. [Google Scholar] [CrossRef] [PubMed]
- Bhornchai, H.; Bhalang, S.; Ratchada, T.; Marvin, P.S.; Kamol, L. Anthocyanin, phenolics and antioxidant activity changes in purple waxy corn as affected by traditional cooking. Food Chem. 2014, 164, 510–517. [Google Scholar] [CrossRef]
- Reyes-Pavón, D.; Soto-Sigala, K.S.; Cano-Sampedro, E.; Méndez-Trujillo, V.; Navarro-Ibarra, M.J.; Pérez-Pasten-Borja, R.; Olvera-Sandoval, C.; Torres-Maravilla, E. Pigmented Native Maize: Unlocking the Potential of Anthocyanins and Bioactive Compounds from Traditional to Functional Beverages. Beverages 2024, 10, 69. [Google Scholar] [CrossRef]
- Suriano, S.; Balconi, C.; Valoti, P.; Redaelli, R. Comparison of total polyphenols, profile anthocyanin, color analysis, carotenoids and tocols in pigmented maize. LWT 2021, 144, 111257. [Google Scholar] [CrossRef]
- Jiang, Y.; Yang, L.; Xie, H.; Qin, L.Q.; Wang, L.Q.; Xie, X.D.; Zhou, H.Y.; Tan, X.J.; Zhou, J.G.; Cheng, W.D. Metabolomics and transcriptomics strategies to reveal the mechanism of diversity of maize kernel color and quality. BMC Genom. 2023, 24, 194. [Google Scholar] [CrossRef]
- Dragičević, V.; Brankov, M.; Stoiljković, M.; Tolimir, M.; Kanatas, P.; Travlos, I.; Simić, M. Kernel color and fertilization as factors of enhanced maize quality. Front. Plant Sci. 2022, 13, 1027618. [Google Scholar] [CrossRef] [PubMed]
- Rouf Shah, T.; Prasad, K.; Kumar, P. Maize—A potential source of human nutrition and health: A review. Cogent Food Agric. 2016, 2, 1166995. [Google Scholar] [CrossRef]
- Menkir, A.; Liu, W.P.; White, W.S.; Maziya-Dixon, B.; Rocheford, T. Carotenoid diversity in tropical-adapted yellow maize inbred lines. Food Chem. 2008, 109, 521–529. [Google Scholar] [CrossRef]
- Kljak, K.; Zurak, D.; Svečnjak, Z.; Grbeša, D. Relationship of physical properties and macronutrient composition with carotenoid profile in maize hybrids. Agriculture 2024, 14, 384. [Google Scholar] [CrossRef]
- Beswa, D.; Siwela, M.; Amonsou, E.O.; Kolanisi, U. Grain quality, provitamin a carotenoid profiles, and sensory quality of provitamin a-biofortified maize stiff porridges. Foods 2020, 9, 1909. [Google Scholar] [CrossRef]
- Ye, D.; Chen, J.; Yu, Z.; Sun, Y.; Gao, W.; Wang, X.; Zhang, R.; Zaib-Un-Nisa; Su, D.; Atif Muneer, M. Optimal plant density improves sweet maize fresh ear yield without compromising grain carbohydrate concentration. Agronomy 2023, 13, 2830. [Google Scholar] [CrossRef]
- Grosso, G.; Bei, R.; Mistretta, A.; Marventano, S.; Calabrese, G.; Masuelli, L.; Giganti, M.G.; Modesti, A.; Galvano, F.; Gazzolo, D. Effects of vitamin C on health: A review of evidence. Front. Biosci. (Landmark Ed.) 2013, 18, 1017–1029. [Google Scholar] [CrossRef]
- Shan, Q.J.; Liu, J.H.; Li, W.; Wang, H.; Hu, X.D.; Li, T.; Hu, J.G.; Guo, X.B.; Liu, R.H. Comprehensive evaluation of biosynthesis, accumulation, regulation of folate and vitamin C in waxy maize (Zea mays L. var. ceratina) with kernel development. J. Cereal Sci. 2019, 87, 215–224. [Google Scholar] [CrossRef]
- Paulsmeyer, M.N.; Vermillion, K.E.; Juvik, J.A. Assessing the diversity of anthocyanin composition in various tissues of purple corn (Zea mays L.). Phytochemistry 2022, 201, 113263. [Google Scholar] [CrossRef]
- Sanahuja, G.; Farré, G.; Bassie, L.; Zhu, C.F.; Christou, P.; Capell, T. Ascorbic acid synthesis and metabolism in maize are subject to complex and genotype-dependent feedback regulation during endosperm developshahment. Biotechnol. J. 2013, 8, 1221–1230. [Google Scholar] [CrossRef]
- Anirban, A.; Hong, H.T.; O’Hare, T.J. Profiling and quantification of anthocyanin in purple-pericarp sweetcorn and purple-pericarp maize. Molecules 2023, 28, 2665. [Google Scholar] [CrossRef] [PubMed]
- Lim, S.; Yi, G. Investigating seed mineral composition in Korean landrace maize (Zea mays L.) and its kernel texture specificity. J. Integr. Agric. 2019, 18, 1996–2005. [Google Scholar] [CrossRef]
- Ma, K.; Zhao, X.; Lu, B.; Wang, Y.; Yue, Z.; Zhang, L.; Diao, X.; Yuan, X. Effect of ecological factors on nutritional quality of foxtail millet (Setaria italica L.). Agronomy 2024, 14, 387. [Google Scholar] [CrossRef]
- Yao, B.M.; Chen, P.; Sun, G.X. Distribution of elements and their correlation in bran, polished rice, and whole grain. Food Sci. Nutr. 2019, 8, 982–992. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Shi, Y.L.; Xu, R.C.; Lu, D.L.; Lu, W.P. Effects of shading after pollination on kernel filling and physicochemical quality traits of waxy maize. Crop J. 2016, 12, 235–245. [Google Scholar] [CrossRef]
- Chachar, Z.; Lai, R.; Ahmed, N.; Lingling, M.; Chachar, S.; Paker, N.P.; Qi, Y. Cloned genes and genetic regulation of anthocyanin biosynthesis in maize, a comparative review. Front. Plant Sci. 2024, 15, 1310634. [Google Scholar] [CrossRef] [PubMed]
- Sharma, M.; Cortes-Cruz, M.; Ahern, K.R.; McMullen, M.; Brutnell, T.P.; Chopra, S. Identification of the Pr1 Gene product completes the anthocyanin biosynthesis pathway of maize. Genetics 2011, 188, 69–79. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Liu, J.; Shan, Q.; Bai, S.; Li, W.; Wen, T.; Guo, X.; Hu, J. The accumulation and biosynthesis of anthocyanin in black, white, and yellow waxy corns (Zea mays L. sinensis kulesh) during kernel maturation. Foods 2023, 12, 1486. [Google Scholar] [CrossRef]
- Boss, P.K.; Davies, C.; Robinson, S.P. Analysis of the expression of anthocyanin pathway genes in developing Vitis vinifera L. cv shiraz grape berries and the-implications for pathway regdation. Plant Physiol. 1996, 111, 1059–1066. [Google Scholar] [CrossRef] [PubMed]
- Irani, N.G. Cellular and Molecular Aspects of the Transport and Sequestration of Anthocyanin in Maize and Arabidopsis (Order No. 3220955). Ph.D. Thesis, The Ohio State University, Columbus, OH, USA, 2006. [Google Scholar]
- Chaves-Silva, S.; Santos, A.L.D.; Chalfun-Júnior, C.; Zhao, J.; Peres, L.E.P.; Benedito, V.A. Understanding the genetic regulation of anthocyanin biosynthesis in plants-Tools for breeding purple varieties of fruits and vegetables. Phytochemistry 2018, 153, 11–27. [Google Scholar] [CrossRef] [PubMed]
- Francavilla, A.; Joye, I.J. Anthocyanin in whole grain cereals and their potential effect on health. Nutrients 2020, 12, 2922. [Google Scholar] [CrossRef] [PubMed]
- Cui, L.N.; Gao, R.Q.; Dong, S.T.; Zhang, J.W.; Liu, P.; Zhang, H.Y.; Meng, J.J.; Shi, D.Y. Effects of ear shading on the anthocyanin contents and quality of kernels in various genotypes of maize. Aust. J. Crop Sci. 2012, 6, 704–710. [Google Scholar]
- Tian, X.M.; Han, D.; Sun, L.L.; Guo, Y.Q.; Liu, K.C.; Chen, L.R.; Gong, K.J. A multi-omics-based investigation into the regulation of phenolics in fresh maize kernels at different developmental stages. J. Cereal Sci. 2024, 119, 103962. [Google Scholar] [CrossRef]
- Cao, T.X.; Wang, S.L.; Ali, A.; Shan, N.; Sun, J.Y.; Chen, X.; Wang, P.T.; Zhu, Q.L.; Xiao, Y.; Luo, S.; et al. Transcriptome and metabolome analysis reveals the potential mechanism of tuber dynamic development in yam (Dioscorea polystachya Turcz.). LWT 2023, 181, 114764. [Google Scholar] [CrossRef]
- Cai, S.; Mao, Y.; Gu, Y.; Huang, B.; He, Z.; Zeng, M.; Wang, Z.; Chen, Q.; Tang, M.; Chen, J. Carotenoid and Phenolic Compositions and Antioxidant Activity of 23 Cultivars of Corn Grain and Corn Husk Extract. Foods 2024, 13, 3375. [Google Scholar] [CrossRef]
- Shen, S.; Li, B.B.; Deng, T.; Xiao, Z.D.; Chen, X.M.; Hu, N.; Zhang, B.C.; Wu, G.; Li, F.; Zhao, X.; et al. The equilibrium between sugars and ethylene is involved in shading- and drought-induced kernel abortion in maize. Plant Growth Regul. 2020, 91, 101–111. [Google Scholar] [CrossRef]
- Hu, Q.P.; Xu, J.G. Profiles of carotenoids, anthocyanin, phenolics, and antioxidant activity of selected color waxy corn grains during maturation. J. Agric. Food Chem. 2011, 59, 2026–2033. [Google Scholar] [CrossRef]
- Paesani, C.; Moiraghi, M.; Bustos, M.C.; Navarro, J.L.; Perez, G.T. Purple maize arabinoxylan could protect antioxidant compounds during digestion. Int. J. Food Sci. Nutr. 2024, 75, 774–785. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Yu, Y.; Xuan, Y.; Kari, A.; Yang, C.; Wang, C.; Zhang, C.; Gu, W.; Wang, H.; Hu, Y.; et al. Integrative transcriptome and metabolome analysis reveals the mechanisms of light-induced pigmentation in purple waxy maize. Front. Plant Sci. 2023, 14, 1203284. [Google Scholar] [CrossRef]
- Cheng, X.H.; Wang, P.P.; Chen, Q.Y.; Ma, T.T.; Wang, R.; Gao, Y.J.; Zhu, H.D.; Liu, Y.; Liu, B.C.; Sun, X.Y.; et al. Enhancement of anthocyanin and chromatic profiles in ‘Cabernet Sauvignon’ (Vitis vinifera L.) by foliar nitrogen fertilizer during veraison. J. Sci. Food Agric. 2022, 102, 383–395. [Google Scholar] [CrossRef] [PubMed]
- Cai, T.; Ge-Zhang, S.; Song, M. Anthocyanins in metabolites of purple corn. Front. Plant Sci. 2023, 14, 1154535. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, J.; Chen, Z.; Su, B.; Zhang, Y.; Wang, Z.; Ma, K.; Lu, B.; Ren, J.; Xue, J. Evaluation of Functional Quality of Maize with Different Grain Colors and Differences in Enzymatic Properties of Anthocyanin Metabolism. Foods 2025, 14, 544. https://doi.org/10.3390/foods14040544
Li J, Chen Z, Su B, Zhang Y, Wang Z, Ma K, Lu B, Ren J, Xue J. Evaluation of Functional Quality of Maize with Different Grain Colors and Differences in Enzymatic Properties of Anthocyanin Metabolism. Foods. 2025; 14(4):544. https://doi.org/10.3390/foods14040544
Chicago/Turabian StyleLi, Jing, Zhanqiang Chen, Baojie Su, Yanan Zhang, Zhiping Wang, Ke Ma, Boyu Lu, Jianhong Ren, and Jianfu Xue. 2025. "Evaluation of Functional Quality of Maize with Different Grain Colors and Differences in Enzymatic Properties of Anthocyanin Metabolism" Foods 14, no. 4: 544. https://doi.org/10.3390/foods14040544
APA StyleLi, J., Chen, Z., Su, B., Zhang, Y., Wang, Z., Ma, K., Lu, B., Ren, J., & Xue, J. (2025). Evaluation of Functional Quality of Maize with Different Grain Colors and Differences in Enzymatic Properties of Anthocyanin Metabolism. Foods, 14(4), 544. https://doi.org/10.3390/foods14040544






