Effect of Ultrasonic Intensity Treatment on the Physicochemical and Functional Properties of Coregonus peled Protamine
Abstract
1. Introduction
2. Materials and Methods
2.1. Coregonus Peled Nesting Material
2.2. Extraction
2.3. Protein Yield
2.4. Secondary Structure
2.5. Tertiary Structure
2.6. Ultraviolet (UV) Absorption Spectra
2.7. Scanning Electron Microscope (SEM)
2.8. The Functional Properties of CPP
2.8.1. Solubility
2.8.2. Carbonyl, Sulfhydryl, and Surface Hydrophobicity
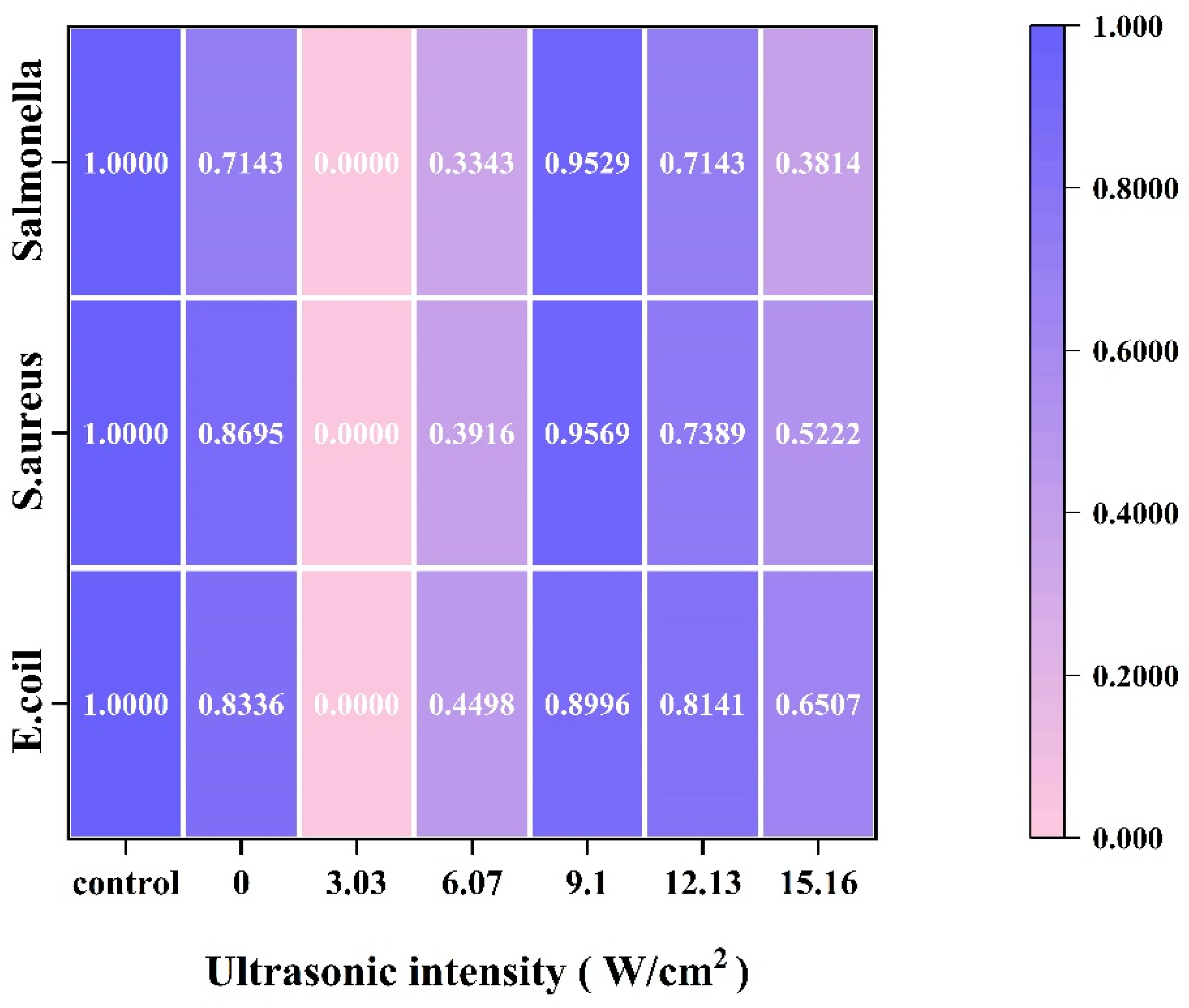
2.8.3. Zone of Inhibition
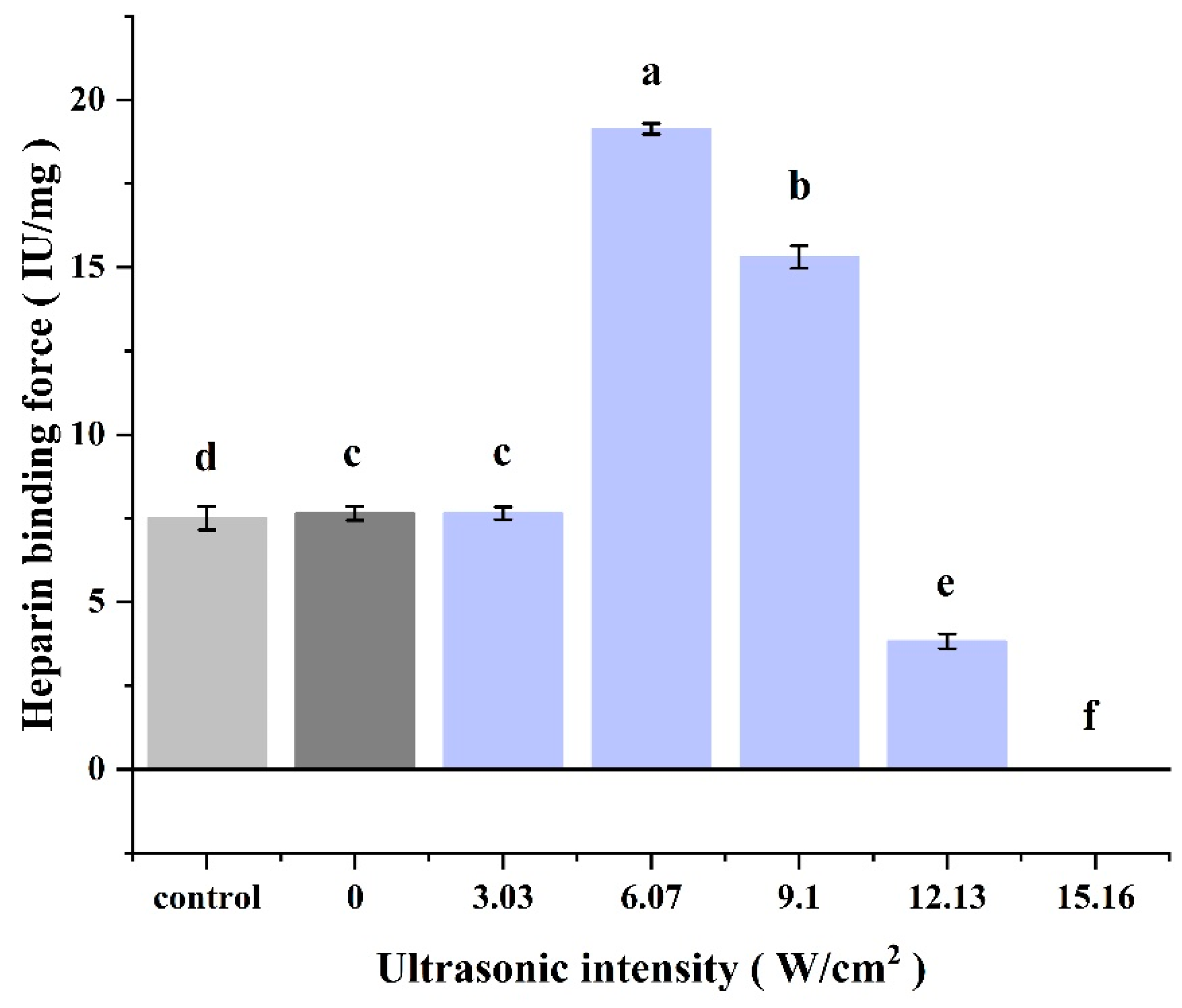
2.8.4. Heparin Binding
2.9. Statistical Analysis
3. Results and Discussion
3.1. Physical and Chemical Properties
3.1.1. Protein Yield
3.1.2. Secondary Structure
3.1.3. Tertiary Structure
3.1.4. UV Full-Wavelength Scanning
3.1.5. SEM Surface Morphology Analysis
3.2. Functional Nature of the CPP
3.2.1. Solubility
3.2.2. Carbonyl, Sulfhydryl, and Surface Hydrophobicity
3.2.3. Zone of Inhibition
3.2.4. Heparin Binding
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Liu, S.; Zhang, Y.; Chen, Y.; Su, Y.; Chen, B.; Wang, Y.; Xu, M.; Qiao, K.; Li, S.; Liu, Z. Isolation and Purification of Protamine from the Cultured Takifugu flavidus and Its Physicochemical Properties. Molecules 2024, 29, 263. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Li, L.; Li, X.; Tao, D.; Zhang, P.; Gong, J. Aptamer-protamine-siRNA nanoparticles in targeted therapy of ErbB3 positive breast cancer cells. Int. J. Pharm. 2020, 590, 119963. [Google Scholar] [CrossRef] [PubMed]
- Truelstrup Hansen, L.; Austin, J.W.; Gill, T.A. Antibacterial effect of protamine in combination with EDTA and refrigeration. Int. J. Food Microbiol. 2001, 66, 149–161. [Google Scholar] [CrossRef]
- Mckay, D.J.; Renaux, B.S.; Dixon, G.H. Rainbow trout protamines: Amino acid sequences of six distinct proteins from a single testis. Eur. J. Biochem. 1986, 158, 361–366. [Google Scholar] [CrossRef]
- Gusse, M.; Sautiere, P.; Chauviere, M.; Chevaillier, P. Extraction purification and characterization of the sperm protamines of the dog-fish Scylliorhinus caniculus. Biochim. Biophys. Acta (BBA)-Protein Struct. Mol. Enzymol. 1983, 748, 93–98. [Google Scholar] [CrossRef]
- Liu, J.; Yuan, J.; Chang, T. Application of micelles extraction in the purification of protamine from adult carp sperm. J. Biotechnol. 2008, 136, S475. [Google Scholar] [CrossRef]
- Dabbour, M.; Hamoda, A.; Mintah, B.K.; Wahia, H.; Betchem, G.; Xu, H.; He, R.; Ma, H. Ultrasonic-aided extraction and degossypolization of cottonseed meal protein: Optimization and characterization of functional traits and molecular structure. Ind. Crops Prod. 2023, 204, 117261. [Google Scholar] [CrossRef]
- Kaewbangkerd, K.; Hamzeh, A.; Yongsawatdigul, J. Ultrasound-assisted extraction of collagen from broiler chicken trachea and its biochemical characterization. Ultrason. Sonochem. 2023, 95, 106372. [Google Scholar] [CrossRef]
- Li, N.; Zhang, K.-X.; Du, J.-Y.; Tan, Z.-F.; Xu, Y.-P.; Liu, X.-Y.; Zhou, D.-Y.; Li, D.-Y. High-intensity ultrasound improved the physicochemical and gelling properties of Litopenaeus vannamei myofibrillar protein. Ultrason. Sonochem. 2022, 90, 106217. [Google Scholar] [CrossRef] [PubMed]
- Suchintita Das, R.; Tiwari, B.K.; Chemat, F.; Garcia-Vaquero, M. Impact of ultrasound processing on alternative protein systems: Protein extraction, nutritional effects and associated challenges. Ultrason. Sonochem. 2022, 91, 106234. [Google Scholar] [CrossRef] [PubMed]
- Duan, Y.; Yang, X.; Deng, D.; Zhang, L.; Ma, X.; He, L.; Zhu, X.; Zhang, X. Effects of ultrasonic waves of different powers on the physicochemical properties, functional characteristics, and ultrastructure of bovine liver peptides. Ultrason. Sonochem. 2024, 110, 107031. [Google Scholar] [CrossRef] [PubMed]
- Pan, H.; Zhang, X.; Ni, J.; Liang, Q.; Jiang, X.; Zhou, Z.; Shi, W. Effects of Ultrasonic Power on the Structure and Rheological Properties of Skin Collagen from Albacore (Thunnus alalunga). Marine Drugs 2024, 22, 84. [Google Scholar] [CrossRef]
- Ni, X.; Chen, C.; Li, R.; Liu, Q.; Duan, C.; Wang, X.; Xu, M. Effects of ultrasonic treatment on the structure and functional characteristics of myofibrillar proteins from black soldier fly. Int. J. Biol. Macromol. 2024, 278, 135057. [Google Scholar] [CrossRef]
- Wang, F.; Guo, X.; Wei, Y.; Liu, P.; Deng, X.; Lei, Y.; Zhao, Y.; Zhang, J. Ultrasound-assisted acid extraction of Coregonus peled protamine: Extraction, physicochemical and functional properties. LWT 2024, 201, 116256. [Google Scholar] [CrossRef]
- Li, Y.; Zeng, Q.-H.; Liu, G.; Peng, Z.; Wang, Y.; Zhu, Y.; Liu, H.; Zhao, Y.; Wang, J.J. Effects of ultrasound-assisted basic electrolyzed water (BEW) extraction on structural and functional properties of Antarctic krill (Euphausia superba) proteins. Ultrason. Sonochem. 2021, 71, 105364. [Google Scholar] [CrossRef] [PubMed]
- Jiang, W.; He, Y.; Xiong, S.; Liu, Y.; Yin, T.; Hu, Y.; You, J. Effect of mild ozone oxidation on structural changes of silver carp (Hypophthalmichthys molitrix) myosin. Food Bioprocess Technol. 2017, 10, 370–378. [Google Scholar] [CrossRef]
- Mach, H.; Volkin, D.B.; Burke, C.J.; Russell Middaugh, C. Ultraviolet Absorption Spectroscopy. Humana Press Ebooks 1995, 40, 91–114. [Google Scholar] [CrossRef]
- Wei, W.; Lu, M.; Xu, W.; Polyakov, N.E.; Dushkin, A.V.; Su, W.-k. Preparation of protamine-hyaluronic acid coated core-shell nanoparticles for enhanced solubility, permeability, and oral bioavailability of decoquinate. Int. J. Biol. Macromol. 2022, 218, 346–355. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Sun, Z.; Li, X.; Kong, B.; Sun, F.; Cao, C.; Chen, Q.; Zhang, H.; Liu, Q. Ultrasound-assisted alkaline extraction of protein from Tenebrio molitor larvae: Extraction kinetics, physiochemical, and functional traits. Ultrason. Sonochem. 2023, 95, 106379. [Google Scholar] [CrossRef] [PubMed]
- Tao, Y.; Zhao, D.-M.; Wen, Y. Expression, purification and antibacterial activity of the channel catfish hepcidin mature peptide. Protein Expr. Purif. 2014, 94, 73–78. [Google Scholar] [CrossRef] [PubMed]
- Wen, C.; Zhang, J.; Yao, H.; Zhou, J.; Duan, Y.; Zhang, H.; Ma, H. Advances in renewable plant-derived protein source: The structure, physicochemical properties affected by ultrasonication. Ultrason. Sonochem. 2019, 53, 83–98. [Google Scholar] [CrossRef] [PubMed]
- Jaques, L. Protamine—Antagonist to heparin. Can. Med. Assoc. J. 1973, 108, 1291–1297. [Google Scholar] [PubMed]
- Xie, C.; Du, J.; Xing, C.; Zhang, X.; Wang, L.; Chen, H.; Lin, T. Improving the extraction efficiency and functional properties of wheat germ protein by ultrasound-assisted. Czech J. Food Sci. 2023, 41, 118–126. [Google Scholar] [CrossRef]
- Dong, Z.Y.; Li, M.Y.; Tian, G.; Zhang, T.H.; Ren, H.; Quek, S.Y. Effects of ultrasonic pretreatment on the structure and functionality of chicken bone protein prepared by enzymatic method. Food Chem. 2019, 299, 125103. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.; Liu, P.; Wang, S.; Zhong, C.; Zhao, X. Ultrasonic Microwave-assisted micelle combined with fungal pretreatment of Eucommia ulmoides leaves significantly improved the extraction efficiency of total flavonoids and gutta-percha. Foods 2021, 10, 2399. [Google Scholar] [CrossRef]
- Fan, M.; Hu, T.; Zhao, S.; Xiong, S.; Xie, J.; Huang, Q. Gel characteristics and microstructure of fish myofibrillar protein/cassava starch composites. Food Chem. 2017, 218, 221–230. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Liu, L. Structural and functional properties of soy protein isolates modified by soy soluble polysaccharides. J. Agric. Food Chem. 2016, 64, 7275–7284. [Google Scholar] [CrossRef] [PubMed]
- Sun, W.; Zhou, F.; Sun, D.-W.; Zhao, M. Effect of oxidation on the emulsifying properties of myofibrillar proteins. Food Bioprocess Technol. 2013, 6, 1703–1712. [Google Scholar] [CrossRef]
- Vila, J.A.; Ripoll, D.R.; Scheraga, H.A. Physical reasons for the unusual α-helix stabilization afforded by charged or neutral polar residues in alanine-rich peptides. Biophys. Comput. Biol. 2000, 97, 13075–13079. [Google Scholar] [CrossRef]
- Wang, Z.; Zhao, H.; Cui, B.; Wang, Y. Ultrasound improves the physicochemical and foam properties of whey protein microgel. Front. Nutr. 2023, 10, 1140737. [Google Scholar] [CrossRef] [PubMed]
- Xiong, W.; Wang, Y.; Zhang, C.; Wan, J.; Shah, B.R.; Pei, Y.; Zhou, B.; Li, J.; Li, B. High intensity ultrasound modified ovalbumin: Structure, interface and gelation properties. Ultrason. Sonochem. 2016, 31, 302–309. [Google Scholar] [CrossRef] [PubMed]
- Xue, S.; Xu, X.; Shan, H.; Wang, H.; Yang, J.; Zhou, G. Effects of high-intensity ultrasound, high-pressure processing, and high-pressure homogenization on the physicochemical and functional properties of myofibrillar proteins. Innov. Food Sci. Emerg. Technol. 2018, 45, 354–360. [Google Scholar] [CrossRef]
- Zhang, Q.; Tu, Z.; Wang, H.; Huang, X.; Sha, X.; Xiao, H. Structural changes of ultrasonicated bovine serum albumin revealed by hydrogen-deuterium exchange and mass spectrometry. Anal. Bioanal. Chem. 2014, 406, 7243–7251. [Google Scholar] [CrossRef] [PubMed]
- Jiang, L.; Wang, J.; Li, Y.; Wang, Z.; Liang, J.; Wang, R.; Chen, Y.; Ma, W.; Qi, B.; Zhang, M. Effects of ultrasound on the structure and physical properties of black bean protein isolates. Food Res. Int. 2014, 62, 595–601. [Google Scholar] [CrossRef]
- Veeruraj, A.; Arumugam, M.; Ajithkumar, T.; Balasubramanian, T. Isolation and characterization of collagen from the outer skin of squid (Doryteuthis singhalensis). Food Hydrocoll. 2015, 43, 708–716. [Google Scholar] [CrossRef]
- O’sullivan, J.; Park, M.; Beevers, J. The effect of ultrasound upon the physicochemical and emulsifying properties of wheat and soy protein isolates. J. Cereal Sci. 2016, 69, 77–84. [Google Scholar] [CrossRef]
- Hu, S.; Wu, J.; Zhu, B.; Du, M.; Wu, C.; Yu, C.; Song, L.; Xu, X. Low oil emulsion gel stabilized by defatted Antarctic krill (Euphausia superba) protein using high-intensity ultrasound. Ultrason. Sonochem. 2021, 70, 105294. [Google Scholar] [CrossRef]
- Pan, J.; Lian, H.; Jia, H.; Li, S.; Hao, R.; Wang, Y.; Zhang, X.; Dong, X. Ultrasound treatment modified the functional mode of gallic acid on properties of fish myofibrillar protein. Food Chem. 2020, 320, 126637. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Zhong, M.; Wu, L.; Huang, Y.; Li, Y.; Qi, B. Effects of ultrasound-assisted salt (NaCl) extraction method on the structural and functional properties of Oleosin. Food Chem. 2022, 372, 131238. [Google Scholar] [CrossRef]
- Chen, Y.N.; Li, H.L.; Huang, J.J.; Li, M.J.; Liao, T.; Zu, X.Y. Antimicrobial activities and mechanism of sturgeon spermary protein extracts against Escherichia coli. Front. Nutr. 2022, 9, 11. [Google Scholar] [CrossRef] [PubMed]
- Lesmes, L.P.; Bohorquez, M.Y.; Carreño, L.F.; Patarroyo, M.E.; Lozano, J.M. A C-terminal cationic fragment derived from an arginine-rich peptide exhibits in vitro antibacterial and anti-plasmodial activities governed by its secondary structure properties. Peptides 2009, 30, 2150–2160. [Google Scholar] [CrossRef]







Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, F.; Shu, D.; Wei, Y.; Guo, X.; Liu, P.; Deng, X.; Zhao, Y.; Lei, Y.; Zhang, J. Effect of Ultrasonic Intensity Treatment on the Physicochemical and Functional Properties of Coregonus peled Protamine. Foods 2025, 14, 481. https://doi.org/10.3390/foods14030481
Wang F, Shu D, Wei Y, Guo X, Liu P, Deng X, Zhao Y, Lei Y, Zhang J. Effect of Ultrasonic Intensity Treatment on the Physicochemical and Functional Properties of Coregonus peled Protamine. Foods. 2025; 14(3):481. https://doi.org/10.3390/foods14030481
Chicago/Turabian StyleWang, Feifei, Dong Shu, Yabo Wei, Xin Guo, Pingping Liu, Xiaorong Deng, Yunfeng Zhao, Yongdong Lei, and Jian Zhang. 2025. "Effect of Ultrasonic Intensity Treatment on the Physicochemical and Functional Properties of Coregonus peled Protamine" Foods 14, no. 3: 481. https://doi.org/10.3390/foods14030481
APA StyleWang, F., Shu, D., Wei, Y., Guo, X., Liu, P., Deng, X., Zhao, Y., Lei, Y., & Zhang, J. (2025). Effect of Ultrasonic Intensity Treatment on the Physicochemical and Functional Properties of Coregonus peled Protamine. Foods, 14(3), 481. https://doi.org/10.3390/foods14030481




