Novel Fermented Plant-Based Functional Beverage: Biological Potential and Impact on the Human Gut Microbiota †
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Preparation
2.2. Microorganisms, Growth Conditions, and Inoculation
2.3. Fermentation Process and Enumeration of Microorganisms
2.4. Biological Activity of the Fermented Product
2.4.1. Total Phenolic Content and Antioxidant Activity
2.4.2. Antidiabetic Activity—α-Glucosidase Inhibitory Activity
2.5. In Vitro Simulation of Gastrointestinal Digestion of the Fermented Product
2.5.1. Simulated Digestion Fluids
- -
- Salivary α-amylase (α-amylase from human saliva Type XIII-A, lyophilized powder, 300–1500 U/mg protein, Sigma) solution, at 75 U/mL in the final mixture, was made up in SSF electrolyte stock solution.
- -
- Porcine pepsin (pepsin from porcine gastric mucosa, powder, ≥250 U/mg solid, Sigma), at 2000 U/mL in the final mixture, was made up in SGF electrolyte stock solution.
- -
- Pancreatin (pancreatin from porcine pancreas, powder solution), at 100 U/mL, and bile salts (Oxoid Limited, Thermo Fisher (Heysham) Limited, Lancaster, United Kingdom), at 12 g/L, were made up in SIF electrolyte stock solution.
2.5.2. Oral, Gastric, and Intestinal Phases
2.6. Faecal Fermentations
2.6.1. Recruitment of Participants and Collection of Samples
2.6.2. Faecal Fermentation Conditions and Procedure
- (1)
- The digested fermented PBFB.
- (2)
- A positive control (C+), FOS from chicory root, purity: >95%, degree of polymerization between 2 and 8 (Megazyme, Bray, Ireland).
- (3)
- A negative control (C−) which had no source of carbon added (instead of the sample, the basal medium was added).
2.6.3. Acidification and Organic Acids Production
2.7. Bacterial Enumeration of the Gut Microbiota
2.7.1. DNA Extraction and Quantification
2.7.2. Bacterial Enumeration Using Real-Time Quantitative-PCR
2.8. Statistical Analysis
3. Results and Discussion
3.1. Fermentation of the Wholegrain Finger Millet Slurry
3.2. Biological Activity of the Fermented Product
3.2.1. Total Phenolic Content (TPC) and Antioxidant Activity
3.2.2. Antidiabetic Activity—α-Glucosidase Inhibitory Activity
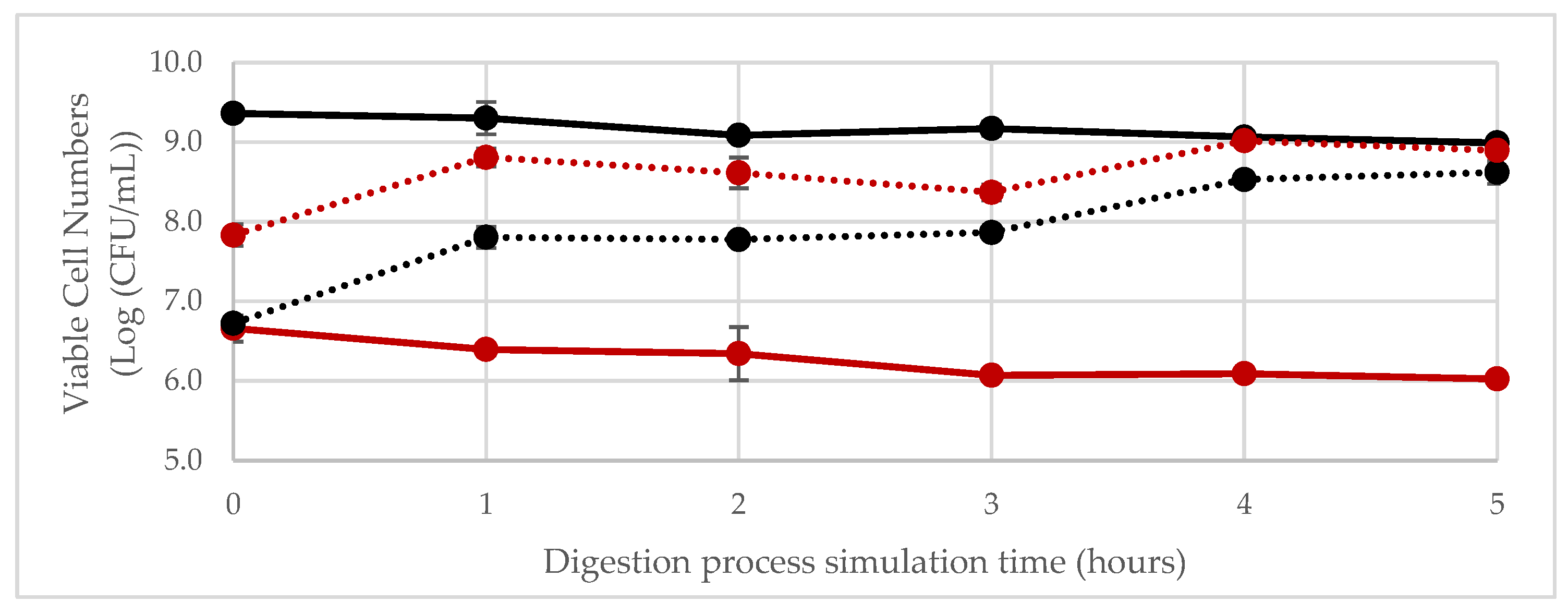
3.3. In Vitro Simulation of Gastrointestinal Digestion of the Fermented Product
Simulation of Digestion in the Gastrointestinal Tract
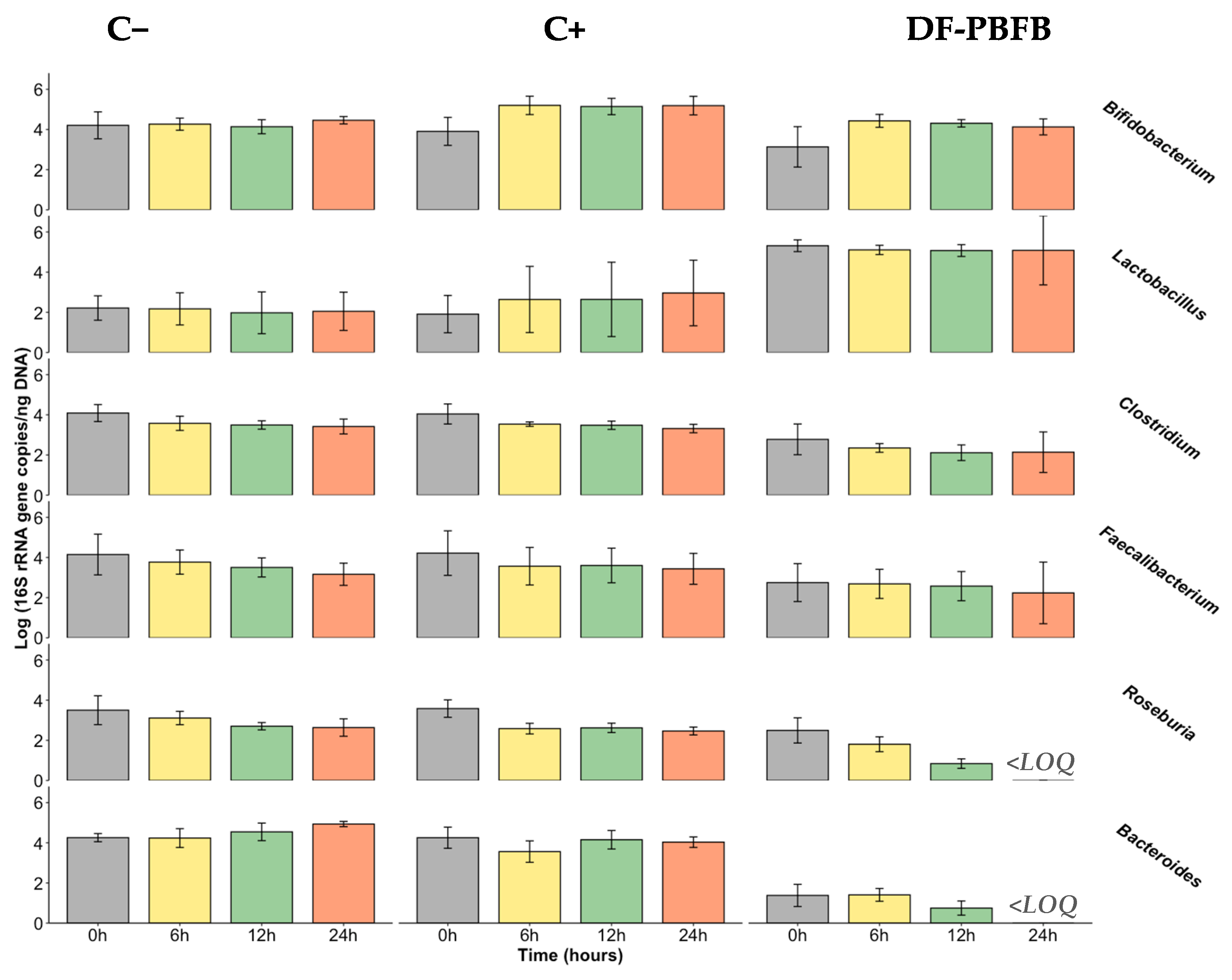
3.4. Fermentability Assay Using Human Faecal Samples
- Bifidobacterium spp.
- Lactobacillus spp.
- Clostridium leptum subgroup and Faecalibacterium prausnitzii
- Roseburia spp.
- Bacteroides spp.
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Valero-Cases, E.; Cerdá-Bernad, D.; Pastor, J.J.; Frutos, M.J. Non-Dairy Fermented Beverages as Potential Carriers to Ensure Probiotics, Prebiotics, and Bioactive Compounds Arrival to the Gut and Their Health Benefits. Nutrients 2020, 12, 1666. [Google Scholar] [CrossRef] [PubMed]
- Vasudha, S.; Mishra, H.N. Non Dairy Probiotic Beverages. Int. Food Res. J. 2013, 20, 7–15. [Google Scholar]
- Prado, F.C.; Parada, J.L.; Pandey, A.; Soccol, C.R. Trends in Non-Dairy Probiotic Beverages. Food Res. Int. 2008, 41, 111–123. [Google Scholar] [CrossRef]
- Grand View Research. Functional Foods Market Size, Share & Trends Analysis Report by Ingredient (Carotenoids, Prebiotics & Probiotics, Fatty Acids, Dietary Fibers), by Product, by Application, by Region, and Segment Forecasts, 2022–2030; Market Analysis Report; Grand View Research: San Francisco, CA, USA, 2019; 100p, Available online: https://www.grandviewresearch.com/industry-analysis/functional-food-market (accessed on 27 November 2024).
- Tangyu, M.; Muller, J.; Bolten, C.J.; Wittmann, C. Fermentation of Plant-Based Milk Alternatives for Improved Flavour and Nutritional Value. Appl. Microbiol. Biotechnol. 2019, 103, 9263–9275. [Google Scholar] [CrossRef]
- Waters, D.M.; Mauch, A.; Coffey, A.; Arendt, E.K.; Zannini, E. Lactic Acid Bacteria as a Cell Factory for the Delivery of Functional Biomolecules and Ingredients in Cereal-Based Beverages: A Review. Crit. Rev. Food Sci. Nutr. 2015, 55, 503–520. [Google Scholar] [CrossRef]
- Ogunremi, O.R.; Banwo, K.; Sanni, A.I. Starter-Culture to Improve the Quality of Cereal-Based Fermented Foods: Trends in Selection and Application. Curr. Opin. Food Sci. 2017, 13, 38–43. [Google Scholar] [CrossRef]
- Dlamini, N.R.; Taylor, J.R.N.; Rooney, L.W. The Effect of Sorghum Type and Processing on the Antioxidant Properties of African Sorghum-Based Foods. Food Chem. 2007, 105, 1412–1419. [Google Scholar] [CrossRef]
- Taylor, J.R.N.; Duodu, K.G. Effects of Processing Sorghum and Millets on Their Phenolic Phytochemicals and the Implications of This to the Health-Enhancing Properties of Sorghum and Millet Food and Beverage Products. J. Sci. Food Agric. 2015, 95, 225–237. [Google Scholar] [CrossRef]
- Dordević, T.M.; Šiler-Marinković, S.S.; Dimitrijević-Branković, S.I. Effect of Fermentation on Antioxidant Properties of Some Cereals and Pseudo Cereals. Food Chem. 2010, 119, 957–963. [Google Scholar] [CrossRef]
- Ghosh, K.; Ray, M.; Adak, A.; Halder, S.K.; Das, A.; Jana, A.; Parua (Mondal), S.; Vágvölgyi, C.; Das Mohapatra, P.K.; Pati, B.R.; et al. Role of Probiotic Lactobacillus Fermentum KKL1 in the Preparation of a Rice Based Fermented Beverage. Bioresour. Technol. 2015, 188, 161–168. [Google Scholar] [CrossRef]
- Nionelli, L.; Coda, R.; Curiel, J.A.; Poutanen, K.; Gobbetti, M.; Rizzello, C.G. Manufacture and Characterization of a Yogurt-like Beverage Made with Oat Flakes Fermented by Selected Lactic Acid Bacteria. Int. J. Food Microbiol. 2014, 185, 17–26. [Google Scholar] [CrossRef]
- Rasane, P.; Jha, A.; Kumar, A.; Sharma, N. Reduction in Phytic Acid Content and Enhancement of Antioxidant Properties of Nutricereals by Processing for Developing a Fermented Baby Food. J. Food Sci. Technol. 2015, 52, 3219–3234. [Google Scholar] [CrossRef] [PubMed]
- Yeap, S.K.; Mohd Ali, N.; Mohd Yusof, H.; Alitheen, N.B.; Beh, B.K.; Ho, W.Y.; Koh, S.P.; Long, K. Antihyperglycemic Effects of Fermented and Nonfermented Mung Bean Extracts on Alloxan-Induced-Diabetic Mice. J. Biomed. Biotechnol. 2012, 2012, 285430. [Google Scholar] [CrossRef] [PubMed]
- Kwon, D.Y.; Daily, J.W.; Kim, H.J.; Park, S. Antidiabetic Effects of Fermented Soybean Products on Type 2 Diabetes. Nutr. Res. 2010, 30, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Keishing, S.; Banu, T. Effect of Fermentation on the Nutrient Content, Antioxidant and Antidiabetic Activities of Hawaijar, an Indigeneous Fermented Soya of Manipur, India. J. Hum. Nutr. Food Sci. 2015, 3, 1066. [Google Scholar]
- Park, H.; Kwon, J.; Kim, H.; Choi, Y.; Choi, J.; Song, S. Antioxidant and Antidiabetic Activity of Fermented Youngia sonchifolia Maxim Leaf Tea. Planta Medica J. Med. Plant Nat. Prod. Res. 2013, 79, PJ35. [Google Scholar] [CrossRef]
- Aisoni, J.E.; Yusha, M.; Orole, O.O. Antimicrobial and Antidiabetic Potentials of Processed Finger Millet (Eleusine coracana). Int. J. Biol. Res. 2018, 6, 18–22. [Google Scholar] [CrossRef]
- Chaudhary, J.K.; Mudgal, S. Effect of Incorporation of Finger Millet (Eleusine coracana) on the Antimicrobial, ACE Inhibitory, Antioxidant and Antidiabetic Potential of a Milkmillet Composite Probiotic Fermented Product. Indian J. Dairy Sci. 2020, 73, 222–230. [Google Scholar] [CrossRef]
- Mota de Carvalho, N.; Costa, E.M.; Silva, S.; Pimentel, L.; Fernandes, T.H.; Pintado, M.E. Fermented Foods and Beverages in Human Diet and Their Influence on Gut Microbiota and Health. Fermentation 2018, 4, 90. [Google Scholar] [CrossRef]
- Cai, X.; Wijesekara, T.; Xu, B. New Insights into Recent Development, Health Benefits, Emerging Technologies, and Future Trends of Cereal-Based Fermented Products. Process Biochem. 2024, 147, 433–439. [Google Scholar] [CrossRef]
- Singh, V.; Lee, G.D.; Son, H.W.; Amani, S.; Baunthiyal, M.; Shin, J.H. Anti-Diabetic Prospects of Dietary Bio-Actives of Millets and the Significance of the Gut Microbiota: A Case of Finger Millet. Front. Nutr. 2022, 9, 1056445. [Google Scholar] [CrossRef] [PubMed]
- Bell, V.; Ferrão, J.; Pimentel, L.; Pintado, M.; Fernandes, T. One Health, Fermented Foods, and Gut Microbiota. Foods 2018, 7, 195. [Google Scholar] [CrossRef] [PubMed]
- Wilson, A.S.; Koller, K.R.; Ramaboli, M.C.; Nesengani, L.T.; Ocvirk, S.; Chen, C.; Flanagan, C.A.; Sapp, F.R.; Merritt, Z.T.; Bhatti, F.; et al. Diet and the Human Gut Microbiome: An International Review. Dig. Dis. Sci. 2020, 65, 723–740. [Google Scholar] [CrossRef] [PubMed]
- Ruan, W.; Engevik, M.A.; Spinler, J.K.; Versalovic, J. Healthy Human Gastrointestinal Microbiome: Composition and Function After a Decade of Exploration. Dig. Dis. Sci. 2020, 65, 695–705. [Google Scholar] [CrossRef]
- Gibson, G.R.; Roberfroid, M.B. Dietary Modulation of the Human Colonic Microbiota: Introducing the Concept of Prebiotics. J. Nutr. 1995, 125, 1401–1412. [Google Scholar] [CrossRef]
- Thursby, E.; Juge, N. Introduction to the Human Gut Microbiota. Biochem. J. 2017, 474, 1823–1836. [Google Scholar] [CrossRef]
- Morrison, D.J.; Preston, T. Formation of Short Chain Fatty Acids by the Gut Microbiota and Their Impact on Human Metabolism. Gut Microbes 2016, 7, 189–200. [Google Scholar] [CrossRef]
- Nagai, M.; Obata, Y.; Takahashi, D.; Hase, K. Fine-Tuning of the Mucosal Barrier and Metabolic Systems Using the Diet-Microbial Metabolite Axis. Int. Immunopharmacol. 2016, 37, 79–86. [Google Scholar] [CrossRef]
- Sharma, S.; Tripathi, P. Gut Microbiome and Type 2 Diabetes: Where We Are and Where to Go? J. Nutr. Biochem. 2019, 63, 101–108. [Google Scholar] [CrossRef]
- Marchesi, J.R.; Dutilh, B.E.; Hall, N.; Peters, W.H.M.; Roelofs, R.; Boleij, A.; Tjalsma, H. Towards the Human Colorectal Cancer Microbiome. PLoS ONE 2011, 6, e20447. [Google Scholar] [CrossRef]
- Halfvarson, J.; Brislawn, C.J.; Lamendella, R.; Vázquez-Baeza, Y.; Walters, W.A.; Bramer, L.M.; D’Amato, M.; Bonfiglio, F.; McDonald, D.; Gonzalez, A.; et al. Dynamics of the Human Gut Microbiome in Inflammatory Bowel Disease. Nat. Microbiol. 2017, 2, 17004. [Google Scholar] [CrossRef] [PubMed]
- Flores, G.E.; Caporaso, J.G.; Henley, J.B.; Rideout, J.R.; Domogala, D.; Chase, J.; Leff, J.W.; Vázquez-Baeza, Y.; Gonzalez, A.; Knight, R.; et al. Temporal Variability Is a Personalized Feature of the Human Microbiome. Genome Biol. 2014, 15, 531. [Google Scholar] [CrossRef] [PubMed]
- Gentile, C.L.; Weir, T.L. The Gut Microbiota at the Intersection of Diet and Human Health. Science 2018, 362, 776–780. [Google Scholar] [CrossRef] [PubMed]
- Vila-Real, C. Nutritional Value of African Indigenous Whole Grain Cereals Millet and Sorghum. Nutr. Food Sci. Int. J. 2017, 4, 1. [Google Scholar] [CrossRef]
- Lee, H.M.; Lee, Y. A Differential Medium for Lactic Acid-Producing Bacteria in a Mixed Culture. Lett. Appl. Microbiol. 2008, 46, 676–681. [Google Scholar] [CrossRef]
- Sousa, S.; Pinto, J.; Pereira, C.; Xavier Malcata, F.; Bertoldo Pacheco, M.T.; Gomes, A.M.; Pintado, M. In Vitro Evaluation of Yacon (Smallanthus sonchifolius) Tuber Flour Prebiotic Potential. Food Bioprod. Process. 2015, 95, 96–105. [Google Scholar] [CrossRef]
- Mendes, M.; Carvalho, A.P.; Magalhães, J.M.C.S.; Moreira, M.; Guido, L.; Gomes, A.M.; Delerue-Matos, C. Response Surface Evaluation of Microwave-Assisted Extraction Conditions for Lycium Barbarum Bioactive Compounds. Innov. Food Sci. Emerg. Technol. 2016, 33, 319–326. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant Activity Applying an Improved Abts Radical Cation Decolorization Assay. Free. Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Gião, M.; Gónzalez-Sanjosé, M.; Rivero-Pérez, M.; Pereira, C.; Pintado, M.; Malcata, F. Infusions of Portuguese Medicinal Plants: Dependence of Final Antioxidant Capacity and Phenol Content on Extraction Features. J. Sci. Food Agric. 2007, 87, 2638–2647. [Google Scholar] [CrossRef]
- Bondet, V.; Brand-Williams, W.; Berset, C. Kinetics and Mechanisms of Antioxidant Activity Using the DPPH. Free Radical Method. LWT-Food Sci. Technol. 1997, 30, 609–615. [Google Scholar] [CrossRef]
- Kwon, Y.; Apostolidis, E.; Shetty, K. In Vitro Studies of Eggplant (Solanum melongena) Phenolics as Inhibitors of Key Enzymes Relevant for Type 2 Diabetes and Hypertension. Bioresour. Technol. 2008, 99, 2981–2988. [Google Scholar] [CrossRef] [PubMed]
- Minekus, M.; Alminger, M.; Alvito, P.; Ballance, S.; Bohn, T.; Bourlieu, C.; Carrière, F.; Boutrou, R.; Corredig, M.; Dupont, D.; et al. A Standardised Static In Vitro Digestion Method Suitable for Food-an International Consensus. Food Funct. 2014, 5, 1113–1124. [Google Scholar] [CrossRef] [PubMed]
- Brodkorb, A.; Egger, L.; Alminger, M.; Alvito, P.; Assunção, R.; Ballance, S.; Bohn, T.; Bourlieu-lacanal, C.; Boutrou, R.; Carrière, F. INFOGEST Static in Vitro Simulation of Gastrointestinal Food Digestion. Nat. Protoc. 2019, 14, 991–1014. [Google Scholar] [CrossRef] [PubMed]
- Mota de Carvalho, N.; Walton, G.E.; Poveda, C.G.; Silva, S.N.; Amorim, M.; Madureira, A.R.; Pintado, M.E.; Gibson, G.R.; Jauregi, P. Study of in Vitro Digestion of Tenebrio Molitor Flour for Evaluation of Its Impact on the Human Gut Microbiota. J. Funct. Foods 2019, 59, 101–109. [Google Scholar] [CrossRef]
- Nzytech Genes & Enzymes. NZY Tissue gDNA Isolation Kit-Protocol. Available online: https://www.nzytech.com/media/dds/brochurescertificates/mb135_ifu_en_v2401.pdf (accessed on 27 November 2024).
- Vila-Real, C.; Pimenta-Martins, A.; Mbugua, S.; Hagrétou, S.-L.; Katina, K.; Maina, N.H.; Pinto, E.; Gomes, A.M.P. Novel Synbiotic Fermented Finger Millet-Based Yoghurt-like Beverage: Nutritional, Physicochemical, and Sensory Characterization. J. Funct. Foods 2022, 99, 105324. [Google Scholar] [CrossRef]
- Gomes, A.M.; Andrade, J.C.; Freitas, A.C. The Use of Probiotics in the Food Industry. In Strategies for Obtaining Healthier Foods; Rodriguez, J.M.L., Carballo García, F.J., Eds.; Nova Science Publishers, Inc.: Hauppauge, NY, USA, 2017; pp. 129–182. ISBN 978-1-5361-2159-9. [Google Scholar]
- Gabaza, M.; Shumoy, H.; Muchuweti, M.; Vandamme, P.; Raes, K. Effect of Fermentation and Cooking on Soluble and Bound Phenolic Profiles of Finger Millet Sour Porridge. J. Agric. Food Chem. 2016, 64, 7615–7621. [Google Scholar] [CrossRef]
- Adebo, O.A.; Medina-Meza, I.G. Impact of Fermentation on the Phenolic Compounds and Antioxidant Activity of Whole Cereal Grains: A Mini Review. Molecules 2020, 25, 927. [Google Scholar] [CrossRef]
- Rizzello, C.G.; Lorusso, A.; Montemurro, M.; Gobbetti, M. Use of Sourdough Made with Quinoa (Chenopodium quinoa) Flour and Autochthonous Selected Lactic Acid Bacteria for Enhancing the Nutritional, Textural and Sensory Features of White Bread. Food Microbiol. 2016, 56, 1–13. [Google Scholar] [CrossRef]
- Sharma, S.; Kandasamy, S.; Kavitake, D.; Shetty, P.H. Probiotic Characterization and Antioxidant Properties of Weissella Confusa KR780676, Isolated from an Indian Fermented Food. LWT-Food Sci. Technol. 2018, 97, 53–60. [Google Scholar] [CrossRef]
- Adebayo-Tayo, B.; Ishola, R.; Oyewunmi, T. Characterization, Antioxidant and Immunomodulatory Potential on Exopolysaccharide Produced by Wild Type and Mutant Weissella Confusa Strains. Biotechnol. Rep. 2018, 19, e00271. [Google Scholar] [CrossRef]
- Kim, J.S.; Hyun, T.K.; Kim, M.J. The Inhibitory Effects of Ethanol Extracts from Sorghum, Foxtail Millet and Proso Millet on α-Glucosidase and α-Amylase Activities. Food Chem. 2010, 124, 1647–1651. [Google Scholar] [CrossRef]
- Han, S.M.; Lee, J.S. Production and Its Anti-Hyperglycemic Effects of γ-Aminobutyric Acid from the Wild Yeast Strain Pichia Silvicola UL6-1 and Sporobolomyces Carnicolor 402-JB-1. Mycobiology 2017, 45, 199–203. [Google Scholar] [CrossRef] [PubMed]
- Hariyanto, I.; Hsieh, C.-W.; Hsu, Y.-H.; Chen, L.-G.; Chu, C.; Weng, B.B.-C. In Vitro and In Vivo Assessments of Anti-Hyperglycemic Properties of Soybean Residue Fermented with Rhizopus oligosporus and Lactiplantibacillus plantarum. Life 2022, 12, 1716. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.; Zhang, Q.; Dang, H.; Liu, X.; Tian, F.; Zhao, J.; Chen, Y.; Zhang, H.; Chen, W. Screening for Potential New Probiotic Based on Probiotic Properties and α-Glucosidase Inhibitory Activity. Food Control 2014, 35, 65–72. [Google Scholar] [CrossRef]
- Ramchandran, L.; Shah, N.P. Effect of Exopolysaccharides and Inulin on the Proteolytic, Angiotensin-I-Converting Enzyme- and α-Glucosidase-Inhibitory Activities as Well as on Textural and Rheological Properties of Low-Fat Yogurt during Refrigerated Storage. Dairy Sci. Technol. 2009, 89, 583–600. [Google Scholar] [CrossRef]
- Borzelleca, J.F.; Pariza, M.W.; Heimbach, J.T. GRAS Notification for Lactobacillus Plantarum Strain 299v; Probi AB and JHeimbach LLC: Washington, DC, USA, 2016. [Google Scholar]
- Johansson, M.L.; Nobaek, S.; Berggren, A.; Nyman, M.; Björck, I.; Ahrné, S.; Jeppsson, B.; Molin, G. Survival of Lactobacillus Plantarum DSM 9843 (299v), and Effect on the Short-Chain Fatty Acid Content of Faeces after Ingestion of a Rose-Hip Drink with Fermented Oats. Int. J. Food Microbiol. 1998, 42, 29–38. [Google Scholar] [CrossRef]
- Patel, A.; Prajapati, J.B.; Holst, O.; Ljungh, A. Determining Probiotic Potential of Exopolysaccharide Producing Lactic Acid Bacteria Isolated from Vegetables and Traditional Indian Fermented Food Products. Food Biosci. 2014, 5, 27–33. [Google Scholar] [CrossRef]
- Kalui, C.M.; Mathara, J.M.; Kutima, P.M. Probiotic Potential of Spontaneously Fermented Cereal Based Foods—A Review. Afr. J. Biotechnol. 2010, 9, 2490–2498. [Google Scholar]
- Patel, H.M.; Pandiella, S.S.; Wang, R.H.; Webb, C. Influence of Malt, Wheat, and Barley Extracts on the Bile Tolerance of Selected Strains of Lactobacilli. Food Microbiol. 2004, 21, 83–89. [Google Scholar] [CrossRef]
- Thomas, V.; Clark, J.; Doré, J. Fecal Microbiota Analysis: An Overview of Sample Collection Methods and Sequencing Strategies. Future Microbiol. 2015, 10, 1485–1504. [Google Scholar] [CrossRef]
- Dimidi, E.; Cox, S.R.; Rossi, M.; Whelan, K. Fermented Foods: Definitions and Characteristics, Impact on the Gut Microbiota and Effects on Gastrointestinal Health and Disease. Nutrients 2019, 11, 1806. [Google Scholar] [CrossRef] [PubMed]
- Tsafrakidou, P.; Michaelidou, A.M.; Biliaderis, C.G. Fermented Cereal-Based Products: Nutritional Aspects, Possible Impact on Gut Microbiota and Health Implications. Foods 2020, 9, 734. [Google Scholar] [CrossRef] [PubMed]
- Sori, N.; Chundanga Poyil, N.; Khan, M. In Vitro Fermentation of Kodo and Kutki Millets by Human Gut Microbiota: Gut Microbiota and Metabolomic Analysis. Food Biosci. 2023, 56, 103343. [Google Scholar] [CrossRef]
- Liu, Y.; Xue, K.; Iversen, K.N.; Qu, Z.; Dong, C.; Jin, T.; Hallmans, G.; Åman, P.; Johansson, A.; He, G.; et al. The Effects of Fermented Rye Products on Gut Microbiota and Their Association with Metabolic Factors in Chinese Adults—An Explorative Study. Food Funct. 2021, 12, 9141–9150. [Google Scholar] [CrossRef]
- Kristek, A.; Wiese, M.; Heuer, P.; Kosik, O.; Schär, M.Y.; Soycan, G.; Alsharif, S.; Kuhnle, G.G.C.; Walton, G.; Spencer, J.P.E. Oat Bran, but Not Its Isolated Bioactive β-Glucans or Polyphenols, Have a Bifidogenic Effect in an in Vitro Fermentation Model of the Gut Microbiota. Br. J. Nutr. 2019, 121, 549–559. [Google Scholar] [CrossRef]
- Yan, J.; Xue, Q.; Chen, W.; Wang, K.; Peng, D.; Jiang, J.; Li, P.; Du, B. Probiotic-Fermented Rice Buckwheat Alleviates High-Fat Diet-Induced Hyperlipidemia in Mice by Suppressing Lipid Accumulation and Modulating Gut Microbiota. Food Res. Int. 2022, 155, 111125. [Google Scholar] [CrossRef]
- Fabiano, G.A.; Shinn, L.M.; Antunes, A.E.C. Relationship between Oat Consumption, Gut Microbiota Modulation, and Short-Chain Fatty Acid Synthesis: An Integrative Review. Nutrients 2023, 15, 3534. [Google Scholar] [CrossRef]
- Taylor, B.C.; Lejzerowicz, F.; Poirel, M.; Shaffer, J.P.; Jiang, L.; Aksenov, A.; Litwin, N.; Humphrey, G.; Martino, C.; Miller-Montgomery, S.; et al. Consumption of Fermented Foods Is Associated with Systematic Differences in the Gut Microbiome and Metabolome. mSystems 2020, 5, e00901-19. [Google Scholar] [CrossRef]
- Campos, D.A.; Coscueta, E.R.; Vilas-Boas, A.A.; Silva, S.; Teixeira, J.A.; Pastrana, L.M.; Pintado, M.M. Impact of Functional Flours from Pineapple By-Products on Human Intestinal Microbiota. J. Funct. Foods 2020, 67, 103830. [Google Scholar] [CrossRef]
- Ribeiro, T.B.; Costa, C.M.; Bonifácio-Lopes, T.; Silva, S.; Veiga, M.; Monforte, A.R.; Nunes, J.; Vicente, A.A.; Pintado, M. Prebiotic Effects of Olive Pomace Powders in the Gut: In Vitro Evaluation of the Inhibition of Adhesion of Pathogens, Prebiotic and Antioxidant Effects. Food Hydrocoll. 2020, 112, 106312. [Google Scholar] [CrossRef]
- Lynch, K.M.; Coffey, A.; Arendt, E.K. Exopolysaccharide Producing Lactic Acid Bacteria: Their Techno-Functional Role and Potential Application in Gluten-Free Bread Products. Food Res. Int. 2018, 110, 52–61. [Google Scholar] [CrossRef] [PubMed]
- Amaretti, A.; Bottari, B.; Morreale, F.; Savo Sardaro, M.L.; Angelino, D.; Raimondi, S.; Rossi, M.; Pellegrini, N. Potential Prebiotic Effect of a Long-Chain Dextran Produced by Weissella cibaria: An in Vitro Evaluation. Int. J. Food Sci. Nutr. 2020, 71, 563–571. [Google Scholar] [CrossRef] [PubMed]
- Olano-Martin, E.; Mountzouris, K.C.; Gibson, G.R.; Rastall, R.A. In Vitro Fermentability of Dextran, Oligodextran and Maltodextrin by Human Gut Bacteria. Br. J. Nutr. 2000, 83, 247–255. [Google Scholar] [CrossRef] [PubMed]
- Heeney, D.D.; Gareau, M.G.; Marco, M.L. Intestinal Lactobacillus in Health and Disease, a Driver or Just along for the Ride? Curr. Opin. Biotechnol. 2018, 49, 140–147. [Google Scholar] [CrossRef] [PubMed]
- Grimaldi, R.; Cela, D.; Swann, J.R.; Vulevic, J.; Gibson, G.R.; Tzortzis, G.; Costabile, A. In Vitro Fermentation of B-GOS: Impact on Faecal Bacterial Populations and Metabolic Activity in Autistic and Non-Autistic Children. FEMS Microbiol. Ecol. 2017, 93, fiw233. [Google Scholar] [CrossRef]
- Mills, D.J.S.; Tuohy, K.M.; Booth, J.; Buck, M.; Crabbe, M.J.C.; Gibson, G.R.; Ames, J.M. Dietary Glycated Protein Modulates the Colonic Microbiota towards a More Detrimental Composition in Ulcerative Colitis Patients and Non-Ulcerative Colitis Subjects. J. Appl. Microbiol. 2008, 105, 706–714. [Google Scholar] [CrossRef]
- Connolly, M.L.; Lovegrove, J.A.; Tuohy, K.M. In Vitro Fermentation Characteristics of Whole Grain Wheat Flakes and the Effect of Toasting on Prebiotic Potential. J. Med. Food 2012, 15, 33–43. [Google Scholar] [CrossRef]
- Kabeerdoss, J.; Sankaran, V.; Pugazhendhi, S.; Ramakrishna, B.S. Clostridium Leptum Group Bacteria Abundance and Diversity in the Fecal Microbiota of Patients with Inflammatory Bowel Disease: A Case-Control Study in India. BMC Gastroenterol. 2013, 13, 1. [Google Scholar] [CrossRef]
- Mao, B.; Li, D.; Zhao, J.; Liu, X.; Gu, Z.; Chen, Y.Q.; Zhang, H.; Chen, W. Metagenomic Insights into the Effects of Fructo-Oligosaccharides (FOS) on the Composition of Fecal Microbiota in Mice. J. Agric. Food Chem. 2015, 63, 856–863. [Google Scholar] [CrossRef]
- Ramirez-Farias, C.; Slezak, K.; Fuller, Z.; Duncan, A.; Holtrop, G.; Louis, P. Anti-Inflammatory Effects of Long-Chain n-3 PUFA in Rhinovirus-Infected Cultured Airway Epithelial Cells. Br. J. Nutr. 2009, 101, 533–540. [Google Scholar] [CrossRef]
- Vanegas, S.M.; Meydani, M.; Barnett, J.B.; Goldin, B.; Kane, A.; Rasmussen, H.; Brown, C.; Vangay, P.; Knights, D.; Jonnalagadda, S.; et al. Substituting Whole Grains for Refined Grains in a 6-Wk Randomized Trial Has a Modest Effect on Gut Microbiota and Immune and Inflammatory Markers of Healthy Adults. Am. J. Clin. Nutr. 2017, 105, 635–650. [Google Scholar] [CrossRef] [PubMed]
- Umu, O.C.O.; Rudi, K.; Diep, D.B. Modulation of the Gut Microbiota by Prebiotic Fibres and Bacteriocins. Microb. Ecol. Health Dis. 2017, 28, 1348886. [Google Scholar] [CrossRef] [PubMed]
- Lordan, C.; Thapa, D.; Ross, R.P.; Cotter, P.D. Potential for Enriching Next-Generation Health-Promoting Gut Bacteria through Prebiotics and Other Dietary Components. Gut Microbes 2020, 11, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Zhu, C.; Guan, Q.; Song, C.; Zhong, L.; Ding, X.; Zeng, H.; Nie, P.; Song, L. Regulatory Effects of Lactobacillus Fermented Black Barley on Intestinal Microbiota of NAFLD Rats. Food Res. Int. 2021, 147, 110467. [Google Scholar] [CrossRef]
- Garrote, G.L.; Abraham, A.G.; Rumbo, M. Is Lactate an Undervalued Functional Component of Fermented Food Products? Front. Microbiol. 2015, 6, 629. [Google Scholar] [CrossRef]
- Hernández, M.A.G.; Canfora, E.E.; Jocken, J.W.E.; Blaak, E.E. The Short-Chain Fatty Acid Acetate in Body Weight Control and Insulin Sensitivity. Nutrients 2019, 11, 1943. [Google Scholar] [CrossRef]
- Chambers, E.S.; Preston, T.; Frost, G.; Morrison, D.J. Role of Gut Microbiota-Generated Short-Chain Fatty Acids in Metabolic and Cardiovascular Health. Curr. Nutr. Rep. 2018, 7, 198–206. [Google Scholar] [CrossRef]
- Gomes, S.D.; Oliveira, C.S.; Azevedo-Silva, J.; Casanova, M.R.; Barreto, J.; Pereira, H.; Chaves, S.R.; Rodrigues, L.R.; Casal, M.; Côrte-Real, M.; et al. The Role of Diet Related Short-Chain Fatty Acids in Colorectal Cancer Metabolism and Survival: Prevention and Therapeutic Implications. Curr. Med. Chem. 2020, 27, 4087–4108. [Google Scholar] [CrossRef]
- Baxter, N.T.; Schmidt, A.W.; Venkataraman, A.; Kim, K.S.; Waldron, C.; Schmidt, T.M. Dynamics of Human Gut Microbiota and Short-Chain Fatty Acids in Response to Dietary Interventions with Three Fermentable Fibers. mBio 2019, 10, e02566-18. [Google Scholar] [CrossRef]
- De Vadder, F.; Kovatcheva-Datchary, P.; Zitoun, C.; Duchampt, A.; Bäckhed, F.; Mithieux, G. Microbiota-Produced Succinate Improves Glucose Homeostasis via Intestinal Gluconeogenesis. Cell Metab. 2016, 24, 151–157. [Google Scholar] [CrossRef]
- Fernández-Veledo, S.; Vendrell, J. Gut Microbiota-Derived Succinate: Friend or Foe in Human Metabolic Diseases? Rev. Endocr. Metab. Disord. 2019, 20, 439–447. [Google Scholar] [CrossRef] [PubMed]
- Louis, P.; Flint, H.J. Diversity, Metabolism and Microbial Ecology of Butyrate-Producing Bacteria from the Human Large Intestine. FEMS Microbiol. Lett. 2009, 294, 1–8. [Google Scholar] [CrossRef] [PubMed]
- De Filippo, C.; Cavalieri, D.; Di Paola, M.; Ramazzotti, M.; Poullet, J.B.; Massart, S.; Collini, S.; Pieraccini, G.; Lionetti, P. Impact of Diet in Shaping Gut Microbiota Revealed by a Comparative Study in Children from Europe and Rural Africa. Proc. Natl. Acad. Sci. USA 2010, 107, 14691–14696. [Google Scholar] [CrossRef] [PubMed]
- Rinninella, E.; Raoul, P.; Cintoni, M.; Franceschi, F.; Miggiano, G.A.D.; Gasbarrini, A.; Mele, M.C. What Is the Healthy Gut Microbiota Composition? A Changing Ecosystem across Age, Environment, Diet, and Diseases. Microorganisms 2019, 7, 14. [Google Scholar] [CrossRef] [PubMed]
| Target Group | Primers Sequence (5′-3′) (F: Forward; R: Reverse) | Optimal Annealing Temperature (°C) | Microorganism | Strain Reference | Genome Size (Base Pairs) | Copies of 16S RNA Gene |
|---|---|---|---|---|---|---|
| Bifidobacterium spp. | F: CGC GTC YGG TGT GAA AGR: CCC CAC ATC CAG CAT CCA | 62 | Bifidobacterium longum subsp. infantis | DSM 20088 (S12) | 2,832,748 | 4 |
| Lactobacillus spp. 1 | F: CAC CGC TAC ACA TGG AG R: AGC AGT AGG GAA TCT TCC A | 59 | Lacticaseibacillus rhamnosus | Lcr35 | 2,937,400 | 5 |
| Clostridium leptum subgroup | F: GCA CAA GCA GTG GAG TR: CTT CCT CCG TTT TGT CAA | 57.5 | Clostridium leptum | DSM 753 (VPI T7-24-1) | 3,270,109 | 2 |
| Roseburia spp. | F: TAC TGC ATT GGA AAC TGT CG R: CGG CAC CGA AGA GCA AT | 60 | Roseburia hominis | DSM 16839 (A2-183) | 3,592,125 | 4 |
| Faecalibacterium prausnitzii | F: GGA GGA AGA AGG TCT TCG G R: AAT TCC GCC TAC CTC TGC ACT | 60 | Faecalibacterium prausnitzii | DSM 17677 (A2-165) | 3,214,418 2 | 6 2 |
| Bacteroides spp. | F: ATA GCC TTT CGA AAG RAA GAT R: CCA GTA TCA ACT GCA ATT TTA | 54.5 | Phocaeicola vulgatus (former Bacteroides vulgatus) | DSM 1447 | 5,163,189 | 7 |
| Unfermented Slurry | Fermented PBFB (F-PBFB) | |
|---|---|---|
| TPC (mg GAE 1/kg PBFB) | 181 ± 11 a | 244 ± 11 b |
| DPPH (mg TE 2/kg PBFB) | 180 ± 11 a | 153 ± 11 b |
| ABTS (mg AAE 3/kg PBFB) | 239 ± 15 a | 238 ± 8 a |
| α-glucosidase inhibitory activity (%) | 14 ± 2 a | 21 ± 2 b |
| Log (16S rRNA Gene Copies/ng DNA) | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| C− | C+ (FOS) | DF-PBFB | ||||||||||||||
| Absolute Concentration | Increment (+)/Reduction (−) | Absolute Concentration | Increment (+)/Reduction (−) | Absolute Concentration | Increment (+)/Reduction (−) | |||||||||||
| Bifidobacterium spp. | 0 h | 4.3 | ± | 0.7 | a,x | n.a. 1 | 3.9 | ± | 0.7 | a,x,y | n.a. | 3.1 | ± | 0.9 | a,y | n.a. |
| 6 h | 4.3 | ± | 0.3 | a,x | +1.4 | 5.2 | ± | 0.5 | b,x | +33.1 | 4.2 | ± | 0.3 | b,x | +41.3 | |
| 12 h | 4.1 | ± | 0.3 | a,x | −1.6 | 5.1 | ± | 0.4 | b,x | +31.7 | 4.3 | ± | 0.2 | b,x | +37.5 | |
| 24 h | 4.5 | ± | 0.2 | a,x,y | +6.0 | 5.2 | ± | 0.5 | b,x | +32.8 | 4.1 | ± | 0.2 | a,b,y | +31.7 | |
| Lactobacillus spp. | 0 h | 2.2 | ± | 0.6 | a,x | n.a. | 1.9 | ± | 0.9 | a,x | n.a. | 5.3 | ± | 0.2 | a,y | n.a. |
| 6 h | 2.2 | ± | 0.8 | a,x | −1.9 | 2.6 | ± | 1.6 | a,x | +38.2 | 5.1 | ± | 0.2 | a,y | −3.9 | |
| 12 h | 2.0 | ± | 1.0 | a,x | −10.7 | 2.6 | ± | 1.6 | a,x | −13.9 | 5.1 | ± | 0.3 | a,y | −4.5 | |
| 24 h | 2.1 | ± | 0.9 | a,x | −7.4 | 3.0 | ± | 1.6 | a,x,y | −18.0 | 5.1 | ± | 0.2 | a,y | −4.3 | |
| Clostridium leptum subgroup | 0 h | 4.1 | ± | 0.4 | a,x | n.a. | 4.0 | ± | 0.5 | a,x | n.a. | 2.8 | ± | 0.7 | b,y | n.a. |
| 6 h | 3.6 | ± | 0.3 | a,x | −12.5 | 3.5 | ± | 0.1 | a,x | −12.5 | 2.4 | ± | 0.2 | b,y | −15.4 | |
| 12 h | 3.5 | ± | 0.2 | a,x | −14.5 | 3.5 | ± | 0.2 | a,x | −13.9 | 2.1 | ± | 0.4 | b,y | −24.0 | |
| 24 h | 3.4 | ± | 0.4 | a,x | −16.4 | 3.3 | ± | 0.2 | a,x | −18.0 | 2.1 | ± | 0.5 | b,y | −23.0 | |
| Roseburia spp. | 0 h | 3.4 | ± | 0.7 | a,x | n.a. | 3.5 | ± | a,x | n.a. | 2.3 | ± | 0.6 | a,y | n.a. | |
| 6 h | 2.9 | ± | 0.4 | a,x | −11.1 | 2.6 | ± | 0.2 | a,b,x,y | −27.9 | 1.7 | ± | 0.4 | a,b,y | −27.7 | |
| 12 h | 2.5 | ± | 0.4 | a,x | −22.8 | 2.6 | ± | 0.2 | a,b,x | −26.8 | 0.7 | ± | 0.5 | b,y | −66.3 | |
| 24 h | 2.4 | ± | 0.6 | a,x | −24.8 | 2.4 | ± | 0.2 | b,x | −31.2 | <LOQ 2 | n.a. | ||||
| Feecalibacterium praunitzii | 0 h | 4.1 | ± | 1.0 | a,x | n.a. | 4.2 | ± | 1.1 | a,x | n.a. | 2.8 | ± | 1.0 | a,x | n.a. |
| 6 h | 3.8 | ± | 0.6 | a,x | −9.1 | 3.6 | ± | 0.9 | a,x | −15.5. | 2.7 | ± | 0.7 | a,x | −2.3 | |
| 12 h | 3.5 | ± | 0.5 | a,x | −15.5 | 3.6 | ± | 0.9 | a,x | −14.6 | 2.6 | ± | 0.7 | a,x | −6.4 | |
| 24 h | 3.2 | ± | 0.5 | a,x | −23.7 | 3.4 | ± | 0.7 | a,x | −18.6 | 2.2 | ± | 0.9 | a,x | −18.7 | |
| Bacteroides spp. | 0 h | 3.9 | ± | 0.7 | a,x | n.a. | 4.1 | ± | 0.6 | a,x | n.a. | 1.3 | ± | 0.6 | b,y | n.a. |
| 6 h | 3.9 | ± | 0.8 | a,x | −0.5 | 3.4 | ± | 0.6 | a,x | −16.3 | 1.3 | ± | 0.3 | b,y | 2.0 | |
| 12 h | 4.3 | ± | 0.6 | a,x | +6.8 | 3.9 | ± | 0.8 | a,x | −2.4 | 0.5 | ± | 0.3 | b,y | −45.7 | |
| 24 h | 4.7 | ± | 0.6 | a,x | +15.9 | 3.7 | ± | 0.8 | a,x | −5.2 | <LOQ | n.a. | ||||
| Time (h) | C− | C+ (FOS) | DF-PBFB | ||||
|---|---|---|---|---|---|---|---|
| pH | 0 | 6.947 ± 0.008 | a,x | 6.94 ± 0.02 | ax | 6.61 ± 0.02 | ax |
| 6 | 6.6 ± 0.1 | a,x | 4.5 ± 0.3 | b,y | 4.8 ± 0.4 | b,y | |
| 12 | 6.58 ± 0.07 | a,x | 4.2 ± 0.3 | b,y | 4.6 ± 0.3 | b,y | |
| 24 | 6.6 ± 0.1 | a,x | 4.0 ± 0.3 | b,y | 4.5 ± 0.3 | b,z | |
| Sugars and organic acids (g/kg) | |||||||
| Sucrose | 0 | <LOD | <LOD | 3.1 ± 0.1 | a | ||
| 6 | <LOD | <LOD | 2.1 ± 0.3 | b | |||
| 12 | <LOD | <LOD | 2.1 ± 0.5 | b | |||
| 24 | <LOD | <LOD | 1.9 ± 0.3 | b | |||
| Glucose | 0 | <LOD | <LOD | 2.9 ± 0.1 | a | ||
| 6 | <LOD | <LOD | 2.3 ± 0.3 | b | |||
| 12 | <LOD | <LOD | 2.1 ± 0.3 | b | |||
| 24 | <LOD | <LOD | 2.0 ± 1.4 | b | |||
| Fructose | 0 | <LOD | 2.1 ± 0.3 | a,x | 1.8 ± 0.2 | a,x | |
| 6 | <LOD | 2.4 ± 1.0 | a,x | 1.3 ± 0.5 | a,x | ||
| 12 | <LOD | 2.4 ± 0.9 | a,x | 1.0 ± 0.4 | a,y | ||
| 24 | <LOD | 2.6 ± 1.2 | a,x | 1.0 ± 0.3 | a,y | ||
| Succinic acid | 0 | 0.20 ± 0.04 | a,x | <LOD | 0.7 ± 0.1 | a,y | |
| 6 | 0.17 ± 0.03 | a,x | 0.6 ± 0.3 | a,y | 0.8 ± 0.1 | a,y | |
| 12 | 0.13 ± 0.04 | a,x | 0.6 ± 0.4 | a,y | 0.8 ± 0.1 | a,y | |
| 24 | <LOD | 0.6 ± 0.2 | a,x | 0.8 ± 0.2 | a,x | ||
| Lactic acid | 0 | <LOD | <LOD | 0.9 ± 0.1 | a | ||
| 6 | <LOD | 1.8 ± 0.6 | a,x | 1.6 ± 0.3 | a,x | ||
| 12 | <LOD | 2.0 ± 0.9 | a,x | 2.3 ± 0.8 | b,x | ||
| 24 | <LOD | 2.2 ± 0.9 | a,x | 2.3 ± 0.7 | b,x | ||
| Acetic acid | 0 | 0.0485 ± 0.007 | a | <LOD | <LOD | ||
| 6 | 0.17 ± 0.06 | a,b,x | 1.0 ± 0.5 | b,y | 0.59 ± 0.07 | b,x,y | |
| 12 | 0.40 ± 0.07 | a,b,x | 0.9 ± 0.3 | b,x | 0.8 ± 0.2 | b,x | |
| 24 | 0.59 ± 0.06 | b,x | 1.0 ± 0.3 | b,x | 0..8 ± 0.2 | b,x | |
| Propionic acid | 0 | 0.04 ± 0.03 | a | <LOD | <LOD | ||
| 6 | 0.16 ± 0.03 | a,x | 0.3 ± 0.1 | a,y | 0.4 ± 0.2 | a,z | |
| 12 | 0.25 ± 0.05 | b,x | 0.27 ± 0.09 | a,x | 0.4 ± 0.1 | a,x | |
| 24 | 0.37 ± 0.05 | b,x | 0.26 ± 0.07 | a,x | 0.4 ± 0.1 | a,x | |
| Butyric acid | 0 | <LOD | <LOD | <LOD | |||
| 6 | 0.16 ± 0.07 | a,x | 0.3 ± 0.1 | a,y | 0.19 ± 0.07 | a,y | |
| 12 | 0.29 ± 0.09 | a,b,x | 0.2 ± 0.1 | a,x | 0.20± 0.05 | a,x | |
| 24 | 0.42 ± 0.09 | b,x | 0.2 ± 0.1 | a,y | 0.20 ± 0.06 | a,y | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vila-Real, C.; Costa, C.; Pimenta-Martins, A.; Mbugua, S.; Hagrétou, S.-L.; Katina, K.; Maina, N.H.; Pinto, E.; Gomes, A.M.P. Novel Fermented Plant-Based Functional Beverage: Biological Potential and Impact on the Human Gut Microbiota. Foods 2025, 14, 433. https://doi.org/10.3390/foods14030433
Vila-Real C, Costa C, Pimenta-Martins A, Mbugua S, Hagrétou S-L, Katina K, Maina NH, Pinto E, Gomes AMP. Novel Fermented Plant-Based Functional Beverage: Biological Potential and Impact on the Human Gut Microbiota. Foods. 2025; 14(3):433. https://doi.org/10.3390/foods14030433
Chicago/Turabian StyleVila-Real, Catarina, Célia Costa, Ana Pimenta-Martins, Samuel Mbugua, Sawadogo-Lingani Hagrétou, Kati Katina, Ndegwa H. Maina, Elisabete Pinto, and Ana M. P. Gomes. 2025. "Novel Fermented Plant-Based Functional Beverage: Biological Potential and Impact on the Human Gut Microbiota" Foods 14, no. 3: 433. https://doi.org/10.3390/foods14030433
APA StyleVila-Real, C., Costa, C., Pimenta-Martins, A., Mbugua, S., Hagrétou, S.-L., Katina, K., Maina, N. H., Pinto, E., & Gomes, A. M. P. (2025). Novel Fermented Plant-Based Functional Beverage: Biological Potential and Impact on the Human Gut Microbiota. Foods, 14(3), 433. https://doi.org/10.3390/foods14030433








