Near-Infrared Spectroscopy as a Tool for the Traceability Control of High-Quality Iberian Dry-Cured Meat Products
Abstract
1. Introduction
2. Materials and Methodology
2.1. Iberian Dry-Cured Tenderloin Samples
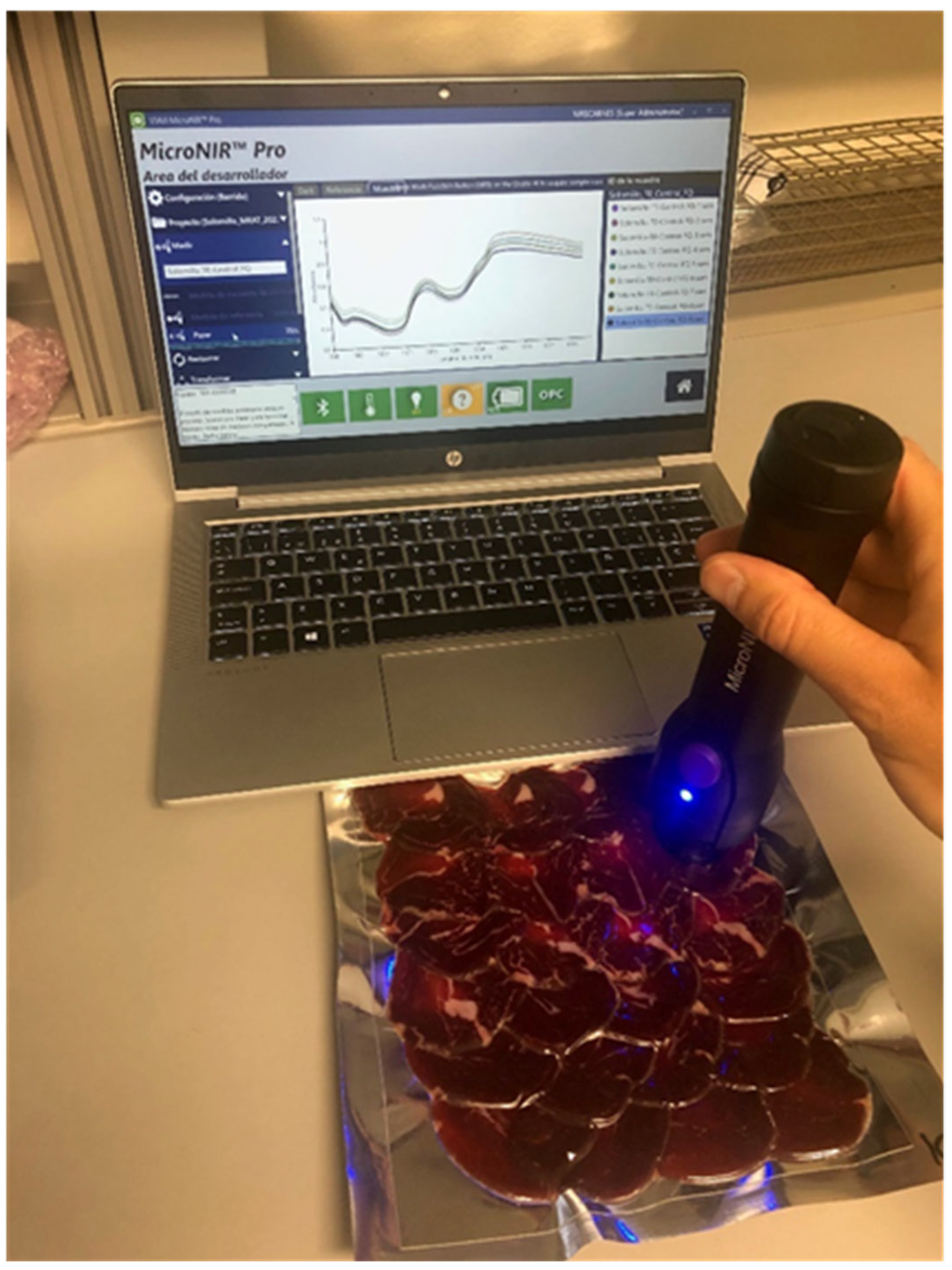
2.2. NIR Spectra Acquisition
2.3. Chemometric Analysis
2.4. Evaluation of Qualitative Prediction Models
2.5. Reference Analysis and Statistical Analysis
3. Results
3.1. Spectra Information
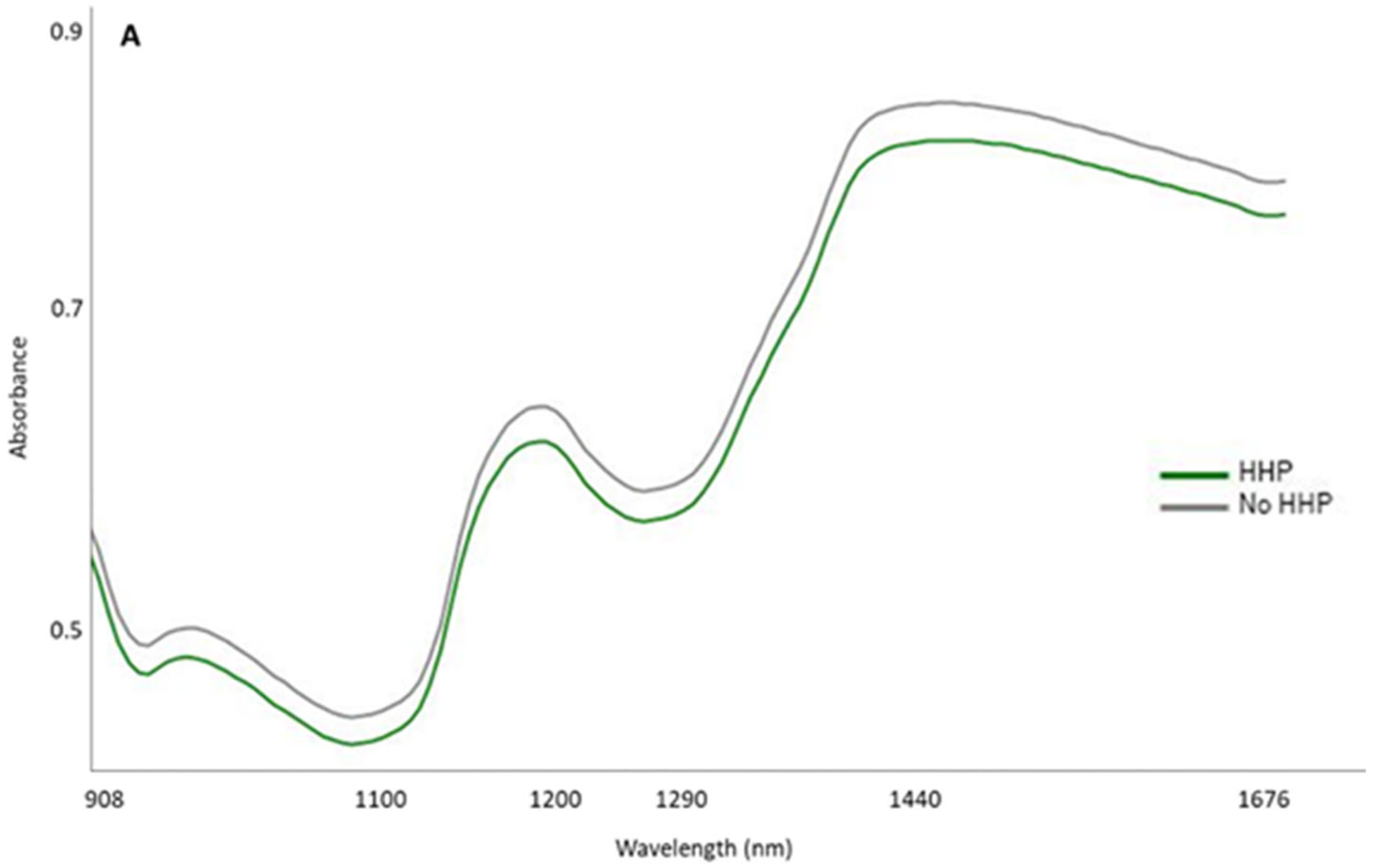
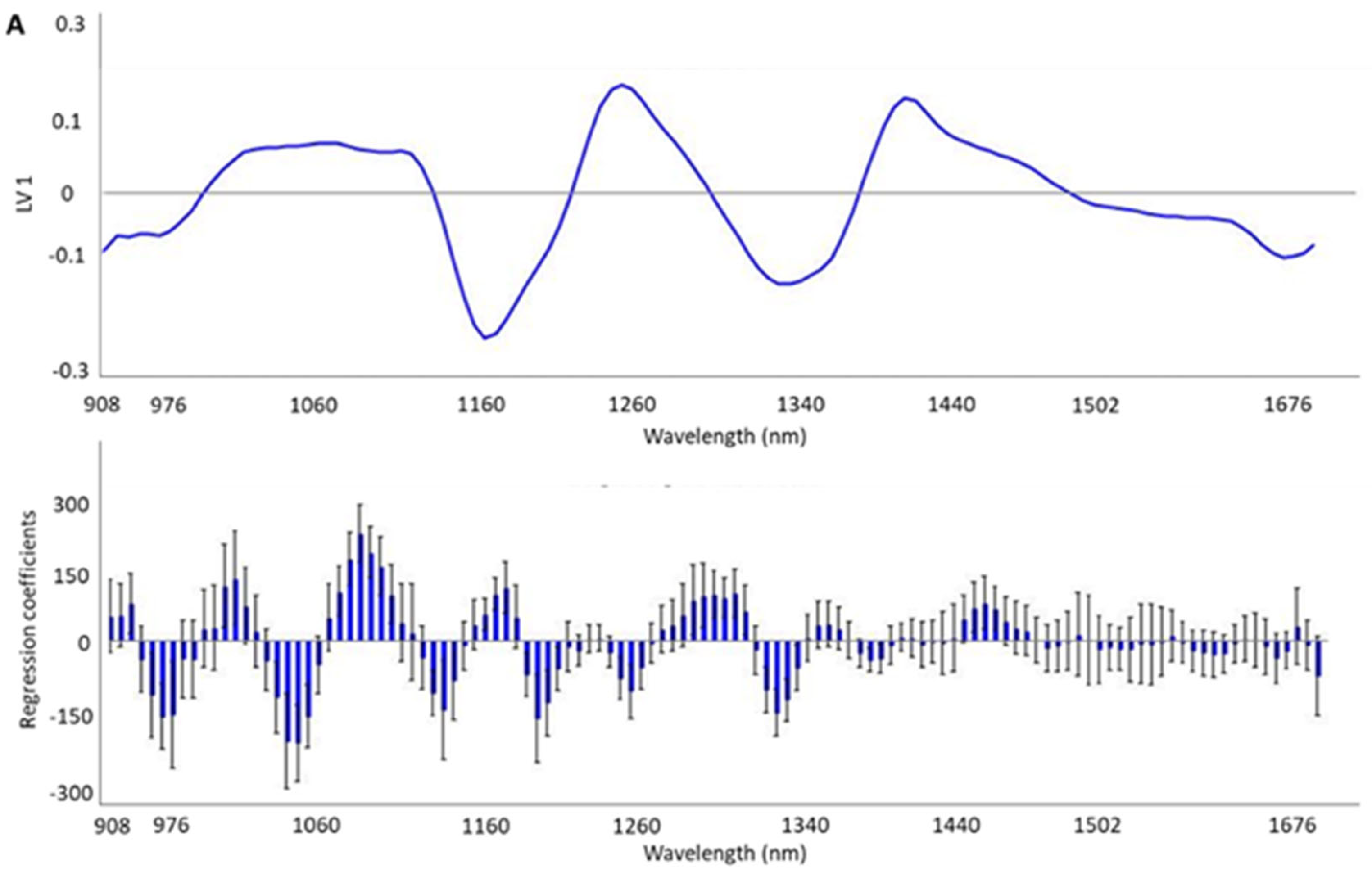
3.2. Development and Validation Models for Discrimination of the HHP Treatment
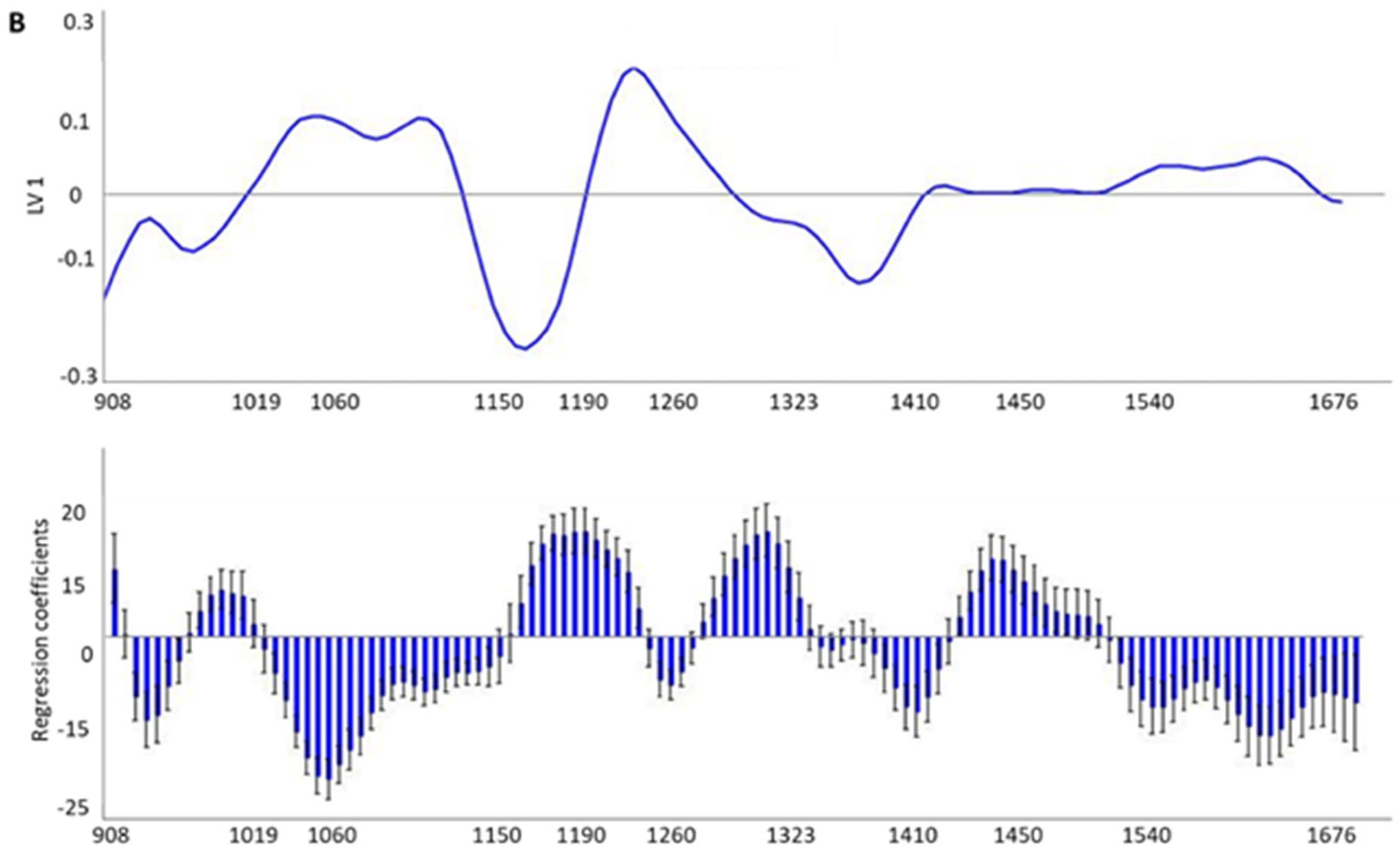
3.3. Development and Validation Models for Discrimination of the Preservation Temperature
3.4. Effect of the HHP Processing and Preservation Temperature on the α- and γ-Tocopherol, Fatty Acid Profile, and Oxidative Status
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Correction Statement
References
- MAPA. Informe Del Volumen de Productos Ibéricos Certificados Comercializados; MAPA: Madrid, Spain, 2019. [Google Scholar]
- Ortiz, A.; Díaz-Caro, C.; Tejerina, D.; Escribano, M.; Crespo, E.; Gaspar, P. Consumption of Fresh Iberian Pork: Two-Stage Cluster for the Identification of Segments of Consumers According to Their Habits and Lifestyles. Meat Sci. 2021, 173, 108373. [Google Scholar] [CrossRef] [PubMed]
- Martillanes, S.; Rocha-Pimienta, J.; Ramírez, R.; García-Parra, J.; Delgado-Adámez, J. Effect of an Active Packaging with Rice Bran Extract and High-Pressure Processing on the Preservation of Sliced Dry-Cured Ham from Iberian Pigs. LWT 2021, 151, 112128. [Google Scholar] [CrossRef]
- USDA. Available online: https://www.fsis.usda.gov/guidelines/2014-0001 (accessed on 4 December 2024).
- Carrapiso, A.I.; Trejo-Álvarez, A.; Martín-Mateos, M.J.; Delgado-Adámez, J.; García-Parra, J.; Ramírez, R. New Insights into the Application of High-Pressure Processing and Storage Time and Temperature to Sliced Iberian Chorizo. Foods 2023, 12, 472. [Google Scholar] [CrossRef] [PubMed]
- Martín, M.J.; García-Parra, J.; Trejo, A.; Gómez-Quintana, A.; Miguel-Pintado, C.; Riscado, A.; Paulo, L.; Ramírez Bernabé, R. Comparative Effect of High Hydrostatic Pressure Treatment on Spanish and Portuguese Traditional Chorizos and Evolution at Different Storage Temperatures. J. Food Process. Preserv. 2021, 45, e15082. [Google Scholar] [CrossRef]
- Ramírez, R.; Trejo, A.; Delgado-Adámez, J.; Martín-Mateos, M.J.; García-Parra, J. Effect of High-Hydrostatic-Pressure Processing and Storage Temperature on Sliced Iberian Dry-Cured Sausage (“Salchichón”) from Pigs Reared in Montanera System. Foods 2022, 11, 1338. [Google Scholar] [CrossRef]
- Cava, R.; Higuero, N.; Ladero, L. High-Pressure Processing and Storage Temperature on Listeria Monocytogenes, Microbial Counts and Oxidative Changes of Two Traditional Dry-Cured Meat Products. Meat Sci. 2021, 171, 108273. [Google Scholar] [CrossRef]
- Horcada, A.; Valera, M.; Juárez, M.; Fernández-Cabanás, V.M. Authentication of Iberian Pork Official Quality Categories Using a Portable near Infrared Spectroscopy (NIRS) Instrument. Food Chem. 2020, 318, 126471. [Google Scholar] [CrossRef]
- Cáceres-Nevado, J.M.; Garrido-Varo, A.; De Pedro-Sanz, E.; Tejerina-Barrado, D.; Pérez-Marín, D.C. Non-Destructive Near Infrared Spectroscopy for the Labelling of Frozen Iberian Pork Loins. Meat Sci. 2021, 175, 108440. [Google Scholar] [CrossRef]
- RD 4/2014; Real Decreto Por El Que Se Aprueba La Norma de Calidad Para La Carne, El Jamón, La Paleta y La Caña de Lomo Ibérico. Ministry of Agriculture, Fisheries and Food: Madrid, Spain, 2014.
- Tejerina, D.; Contador, R.; Ortiz, A. Near Infrared Spectroscopy (NIRS) as Tool for Classification into Official Commercial Categories and Shelf-Life Storage Times of Pre-Sliced Modified Atmosphere Packaged Iberian Dry-Cured Loin. Food Chem. 2021, 356, 129733. [Google Scholar] [CrossRef]
- León, L.; Ortiz, A.; Ezquerro, S.; Tejerina, D. NIRS (Near Infrared Spectroscopy) Classification of Sliced Duroc Dry-Cured Ham under Various Packaging Systems and Storage Temperature and Time. Meat Sci. 2023, 206, 109348. [Google Scholar] [CrossRef]
- Naes, T.; Isaksson, T.; Fearn, T.; Davies, T. A User Friendly Guide to Multivariate Calibration and Classification; NIR Publications: Chichester, UK, 2002. [Google Scholar]
- Faber, N.K.M. A Closer Look at the Bias–Variance Trade off in Multivariate Calibration. J. Chemom. 1999, 13, 185–192. [Google Scholar] [CrossRef]
- Oliveri, P.; Malegori, C.; Casale, M. Multivariate Classification Techniques. In Reference Module in Chemistry, Molecular Sciences and Chemical Engineering; Elsevier: Amsterdam, The Netherlands, 2018. [Google Scholar]
- Liu, Q.; Scheller, K.; Schaeffer, D. Technical Note: A Simplified Procedure for Vitamin E Determination in Beef Muscle. J. Anim. Sci. 1996, 74, 2406–2410. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ortiz, A.; León, L.; Sánchez, M.; Ezquerro, S.; Polo, M.; Ramírez, M.R.; Freire, M.; Martín-Mateos, M.J.; Tejerina, D. Effect of Storage Temperature and High-Pressure Processing on the Preservation of Sliced Duroc Vacuum-Packed Dry-Cured Ham. Food Packag. Shelf Life 2023, 40, 101199. [Google Scholar] [CrossRef]
- Folch, J.; Lees, M.; Sloane-Stanley, G. A Simple Method for the Isolation and Purification of Total Lipids from Animal Tissues. J. Biol. Chem. 1957, 193, 265–275. [Google Scholar]
- Salih, A.M.; Smith, D.M.; Price, J.F.; Dawson, L.E. Modified Extraction 2-Thiobarbituric Acid Method for Measuring Lipid Oxidation in Poultry. Poult. Sci. 1987, 66, 1483–1488. [Google Scholar] [CrossRef]
- Oliver, C.N.; Ahn, B.W.; Moerman, E.J.; Goldstein, S.; Satadtman, E.R. Aged-Related Changes in Oxidized Proteins. J. Biol. Chem. 1987, 262, 5488–5491. [Google Scholar] [CrossRef]
- Barbin, D.F.; Felicio, A.L.D.S.M.; Sun, D.-W.; Nixdorf, S.L.; Hirooka, E.Y. Application of Infrared Spectral Techniques on Quality and Compositional Attributes of Coffee: An Overview. Food Res. Int. 2014, 61, 23–32. [Google Scholar] [CrossRef]
- Osborne, B.; Fearn, T.; Hindle, P. Practical NIR Spectroscopywith Applications in Food and Beverage Analysis; Longman Scientific & Technical: Harlow, UK, 1993. [Google Scholar]
- Guyon, C.; Meynier, A.; de Lamballerie, M. Protein and Lipid Oxidation in Meat: A Review with Emphasis on High-Pressure Treatments. Trends Food Sci. Technol. 2016, 50, 131–143. [Google Scholar] [CrossRef]
- Cheah, P.B.; Ledward, D.A. Catalytic Mechanism of Lipid Oxidation Following High Pressure Treatment in Pork Fat and Meat. J. Food Sci. 1997, 62, 1135–1139. [Google Scholar] [CrossRef]
- Rivas-Cañedo, A.; Martínez-Onandi, N.; Gaya, P.; Nuñez, M.; Picon, A. Effect of High-Pressure Processing and Chemical Composition on Lipid Oxidation, Aminopeptidase Activity and Free Amino Acids of Serrano Dry-Cured Ham. Meat Sci. 2021, 172, 108349. [Google Scholar] [CrossRef]
- Karastogiannidou, C.; Ryley, J. The Recovery of Added Malondialdehyde from Rancid Chicken by the Distillation Method for Thiobarbituric Acid-Reactive Substances. Int. J. Food Sci. Technol. 2007, 29, 19–22. [Google Scholar] [CrossRef]
- Bramley, P.; Elmadfa, I.; Kafatos, A.; Kelly, F.; Manios, Y.; Roxborough, H.; Schuch, W.; Sheehy, P.; Wagner, K.-H. Vitamin E. J. Sci. Food Agric. 2000, 80, 913–938. [Google Scholar] [CrossRef]
- Ventanas, S.; Estevez, M.; Tejeda, J.F.; Ruiz, J. Protein and Lipid Oxidation in Longissimus Dorsi and Dry Cured Loin from Iberian Pigs as Affected by Crossbreeding and Diet. Meat Sci. 2006, 72, 647–655. [Google Scholar] [CrossRef]
- Pérez-Marín, D.; Fearn, T.; Riccioli, C.; De Pedro, E.; Garrido, A. Probabilistic Classification Models for the in Situ Authentication of Iberian Pig Carcasses Using near Infrared Spectroscopy. Talanta 2021, 222, 121511. [Google Scholar] [CrossRef]

| Time (Months) | Pressurization Treatment | Temperature | Calibration | External Validation | Total |
|---|---|---|---|---|---|
| 0 | HHP | 4 °C/20 °C | 21 | 9 | 30 |
| No HHP | 4 °C/20 °C | 20 | 8 | 28 | |
| 4 | HHP | 4 °C | 21 | 9 | 30 |
| 20 °C | 21 | 9 | 30 | ||
| No HHP | 4 °C | 21 | 9 | 30 | |
| 20 °C | 21 | 9 | 30 | ||
| 8 | HHP | 4 °C | 21 | 9 | 30 |
| 20 °C | 21 | 9 | 30 | ||
| No HHP | 4 °C | 21 | 9 | 30 | |
| 20 °C | 21 | 9 | 30 | ||
| Total | 209 | 89 | 298 | ||
| Treatment | Pre-Treatment | Range (nm) | LVs | Cross-Validation | External Validation | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| N | 1-VR | RMSECV | N | SE | SP | Accuracy | ||||
| HHP | SNV + DE + SG 1,4,4,1 | 908.1–1676.5 | 16 | 161 | 0.78 | 0.233 | 89 | 60.00 | 61.36 | 60.67 |
| Storage Temperature | Pre-treatment | Range (nm) | LVs | Cross-Validation | External Validation | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| n | 1-VR | RMSECV | N | SE | SP | Accuracy | ||||
| 4 °C | SNV + DE + SG 1,4,4,1 | 908.1–1676.5 | 7 | 149 | 0.94 | 0.122 | 72 | 97.22 | 100.00 | 98.61 |
| Treatment | Antioxidants | Main Fatty Acids Groups (g/100 g FAMEs) | Oxidative Status | ||||
|---|---|---|---|---|---|---|---|
| α Tocoferol ug/g | ɣ Tocoferol ug/g | SFA | MUFA | PUFA | µg MDA/g | nmol Carbonyls/mg Protein | |
| Control | 12.25 ± 0.21 | 1.47 ± 0.04 | 39.02 ± 0.14 | 54.31 ± 0.18 | 6.53 ± 0.14 | 1.37 ± 0.04 | 3.49 ± 0.08 |
| HHP | 12.70 ± 0.22 | 1.40 ± 0.02 | 38.81 ± 0.13 | 54.57 ± 0.15 | 6.46 ± 0.13 | 1.52 ± 0.03 | 3.91 ± 0.11 |
| p value | 0.147 | 0.111 | 0.283 | 0.279 | 0.739 | 0.004 | 0.003 |
| Storage Temperature | Antioxidants | Main Fatty Acids Groups (g/100 g FAMEs) | Oxidative Status | ||||
|---|---|---|---|---|---|---|---|
| α Tocoferol ug/g | ɣ Tocoferol ug/g | SFA | MUFA | PUFA | µg MDA/g | nmol Carbonyls/mg Protein | |
| 4 °C | 12.05 ± 0.15 | 1.44 ± 0.03 | 38.67 ± 0.15 | 54.98 ± 0.13 | 6.30 ± 0.08 | 1.30 ± 0.03 | 3.84 ± 0.07 |
| 20 °C | 11.18 ± 0.18 | 1.28 ± 0.03 | 39.37 ± 0.16 | 54.50 ± 0.13 | 5.93 ± 0.09 | 1.41 ± 0.03 | 3.68 ± 0.08 |
| p value | 0.000 | 0.000 | 0.002 | 0.013 | 0.003 | 0.026 | 0.117 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ortiz, A.; León, L.; Ramírez, M.R.; Tejerina, D. Near-Infrared Spectroscopy as a Tool for the Traceability Control of High-Quality Iberian Dry-Cured Meat Products. Foods 2025, 14, 432. https://doi.org/10.3390/foods14030432
Ortiz A, León L, Ramírez MR, Tejerina D. Near-Infrared Spectroscopy as a Tool for the Traceability Control of High-Quality Iberian Dry-Cured Meat Products. Foods. 2025; 14(3):432. https://doi.org/10.3390/foods14030432
Chicago/Turabian StyleOrtiz, Alberto, Lucía León, María Rosario Ramírez, and David Tejerina. 2025. "Near-Infrared Spectroscopy as a Tool for the Traceability Control of High-Quality Iberian Dry-Cured Meat Products" Foods 14, no. 3: 432. https://doi.org/10.3390/foods14030432
APA StyleOrtiz, A., León, L., Ramírez, M. R., & Tejerina, D. (2025). Near-Infrared Spectroscopy as a Tool for the Traceability Control of High-Quality Iberian Dry-Cured Meat Products. Foods, 14(3), 432. https://doi.org/10.3390/foods14030432








