Baijiu–Peanut Pairing In Vitro and In Vivo: The Decreased but Prolonged Aftertaste of Baijiu Under the Effect of Mouth Coating Formed by Peanut Lipid
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Materials
2.2. Effect of Peanut Lipid on the Release Intensity of Baijiu Flavor Compounds
2.3. Equilibrium Constant Detection Experiment
2.4. Intraoral SPME Experiment
2.5. GC-MS Operating Conditions
2.6. Molecular Dynamics Simulation
2.7. Data Processing
3. Results and Discussion
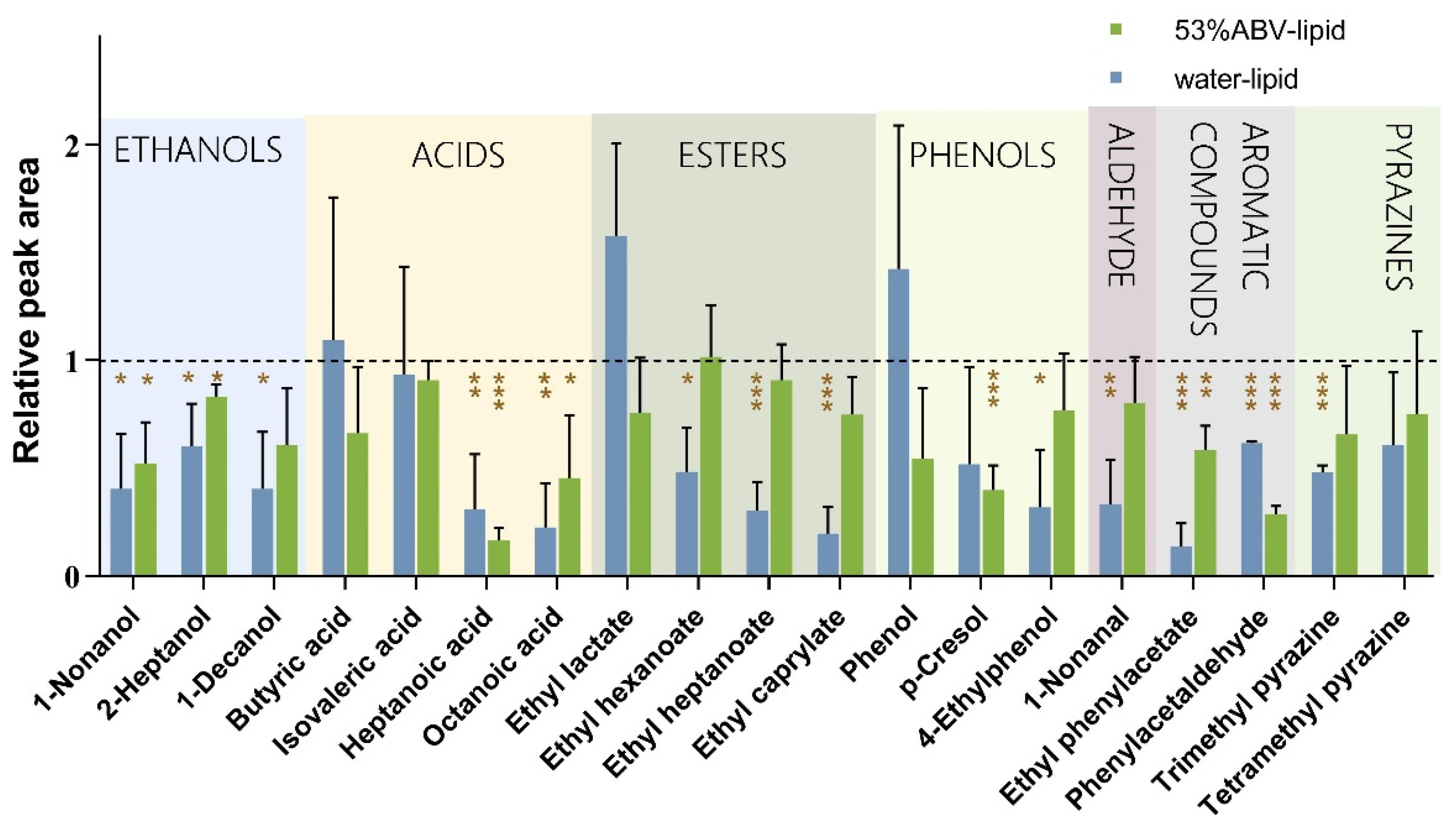
3.1. Influence of Lipid on the Release of Baijiu Flavor Substances
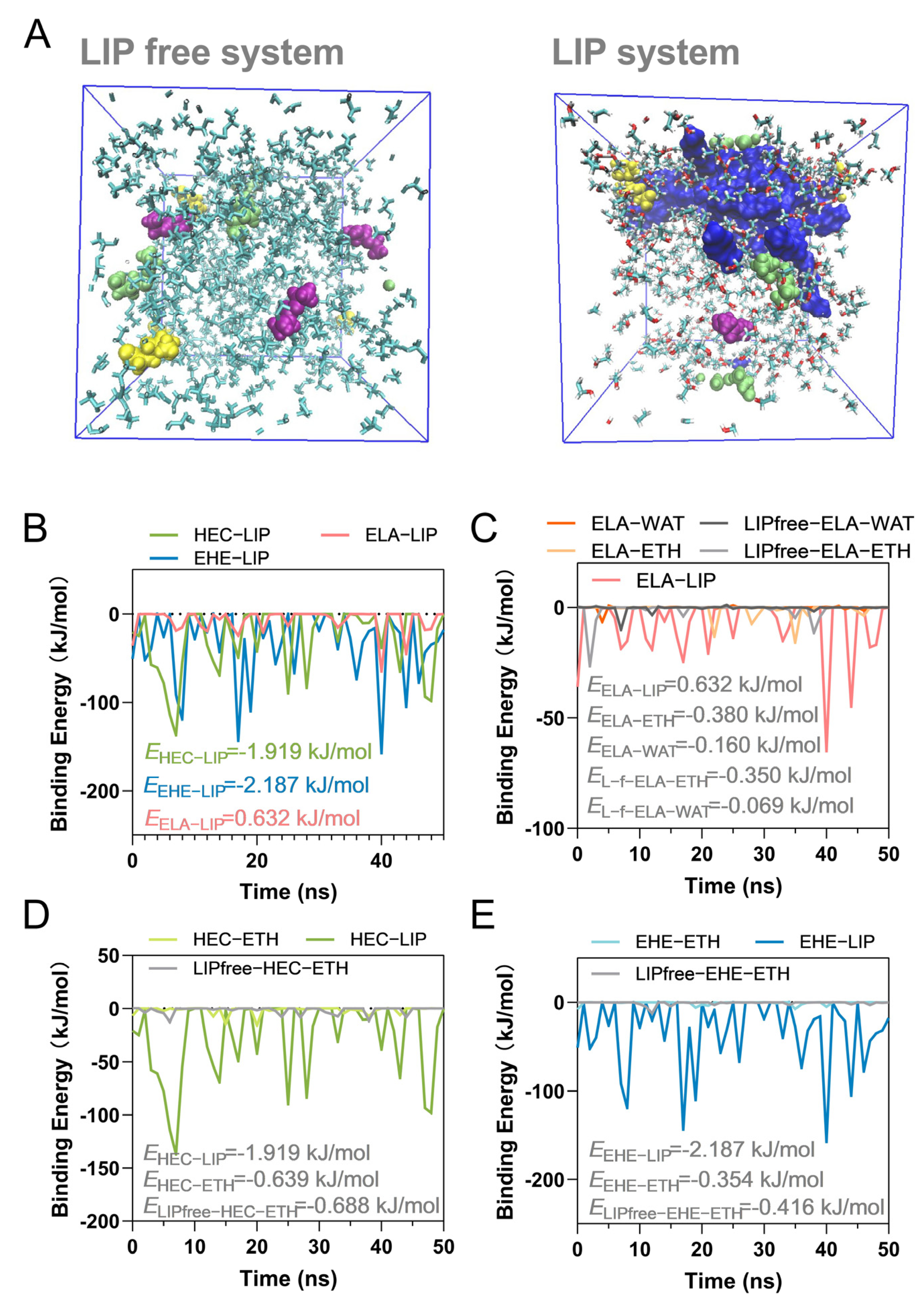
3.2. Molecular Dynamics Simulation of the Interaction Between Lipid Molecules and Ethanol Molecules
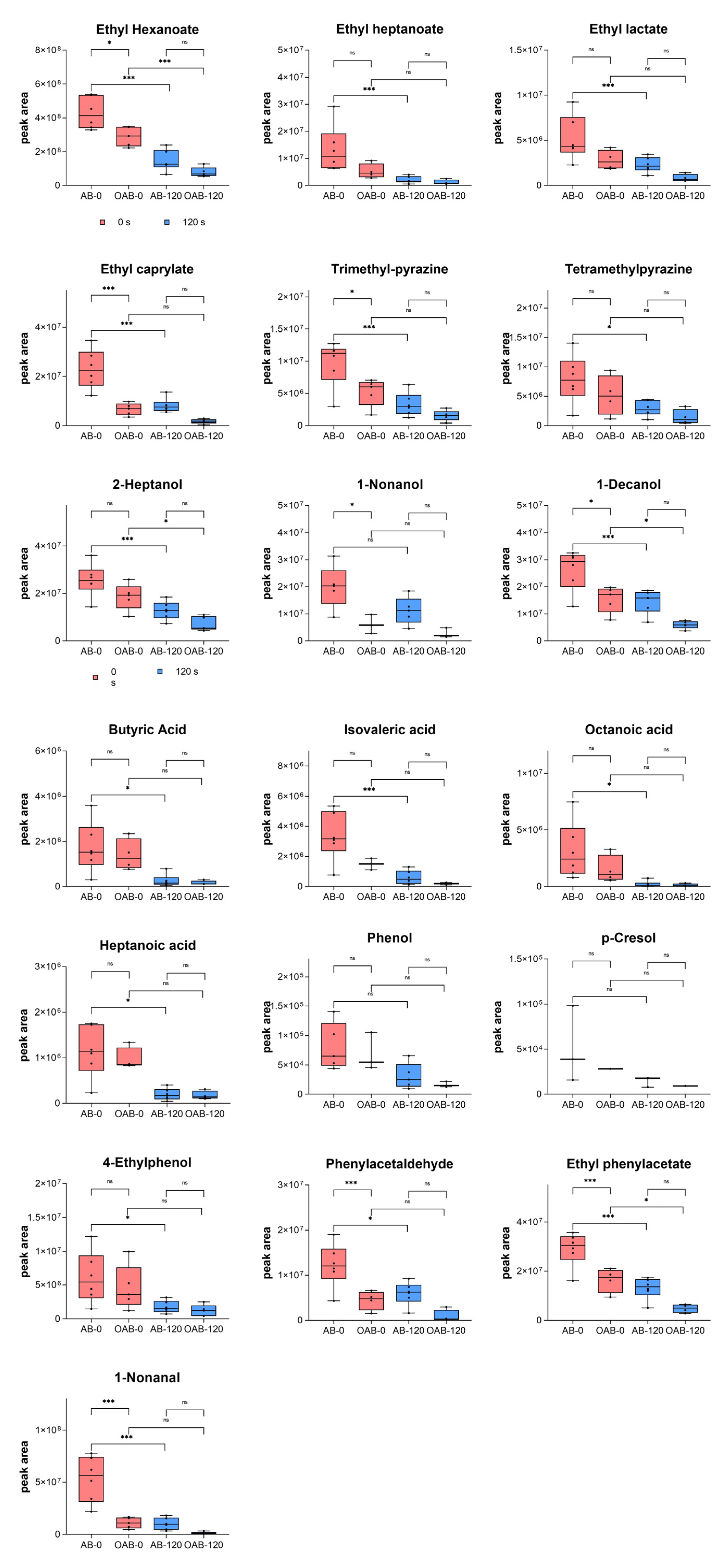
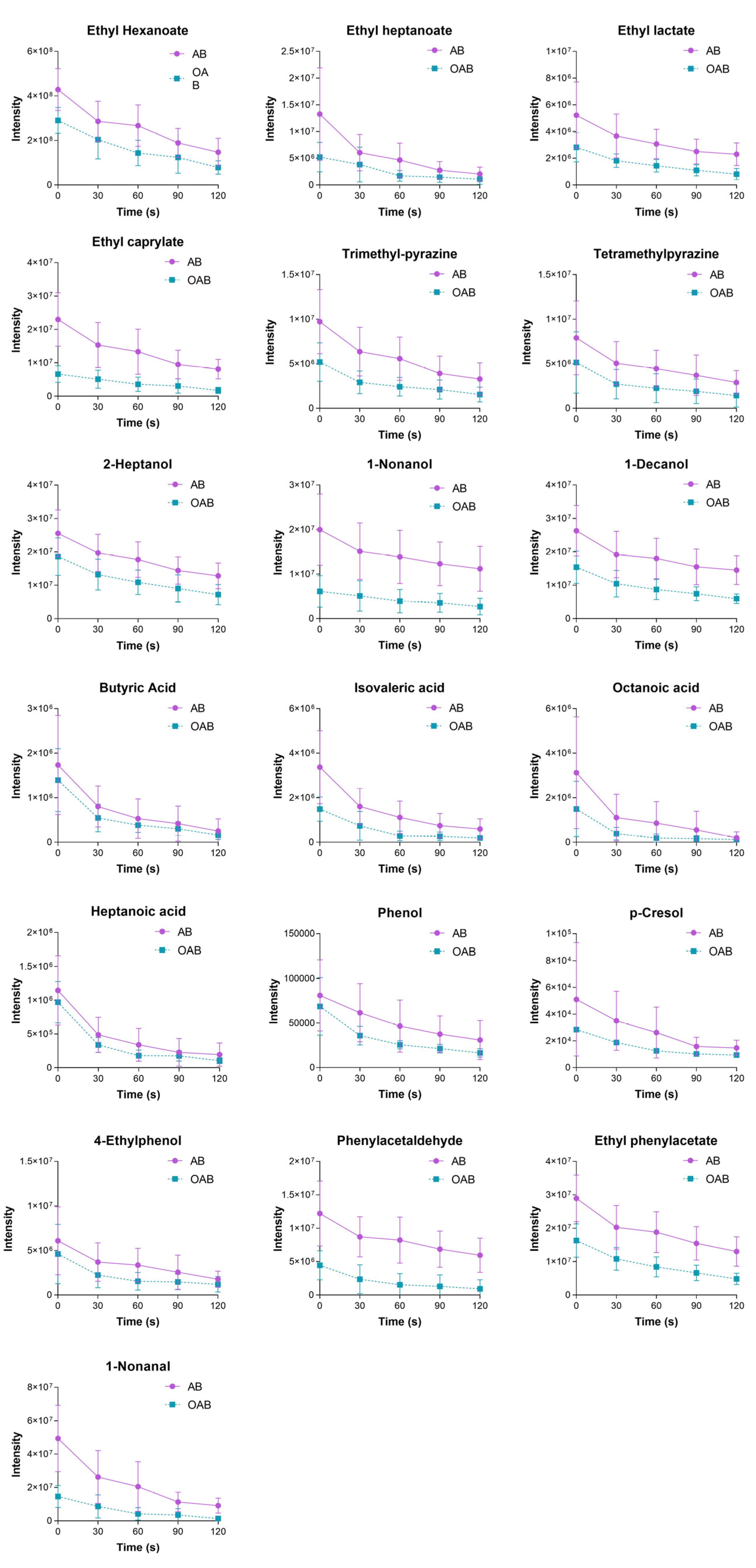
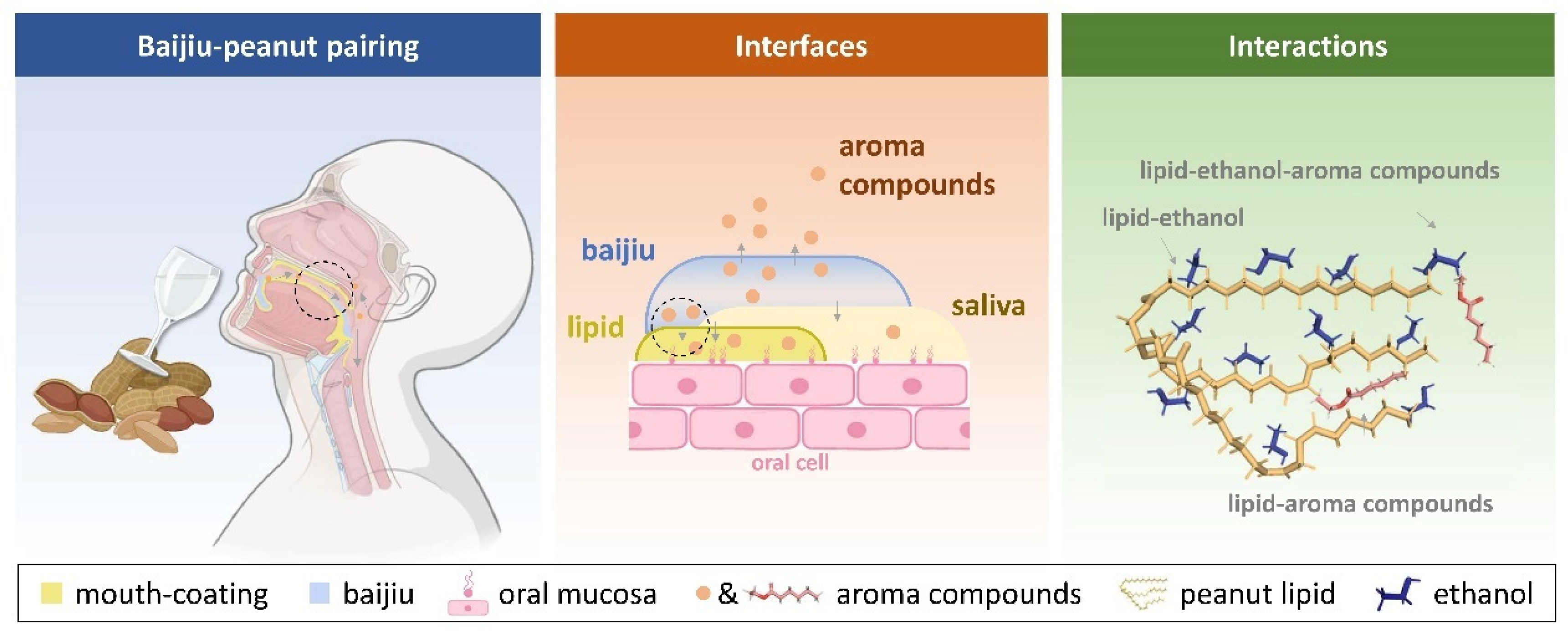
3.3. Influence of the Oral Coating Formed by Peanut Lipid on Baijiu Aftertaste
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Liu, H.; Sun, B. Effect of Fermentation Processing on the Flavor of Baijiu. J. Agric. Food Chem. 2018, 66, 5425–5432. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Zhao, Y.; Chen, X.; Zhang, Y.; Li, H.; Zhao, D.; Wang, B.; Ye, X.; Sun, B.; Sun, J. Peanut Pairing Baijiu: To Enhance Retronasal Aroma Intensity while Reducing Baijiu Aftertaste. J. Agric. Food Chem. 2024, 72, 14851–14864. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Yang, Y.; Hu, X.; Li, H.; Zhao, D.; Wang, B.; Ye, X.; Zhang, Y.; Sun, B.; Sun, J. Unraveling the role of peanut protein in baijiu-peanut pairing flavor complexity: A focus on ethanol-induced denaturation. Food Chem. 2025, 463. [Google Scholar] [CrossRef] [PubMed]
- Carrín, M.E.; Carelli, A.A. Peanut oil: Compositional data. Eur. J. Lipid Sci. Technol. 2010, 112, 697–707. [Google Scholar] [CrossRef]
- Tarrega, A.; Yven, C.; Sémon, E.; Salles, C. In-mouth aroma compound release during cheese consumption: Relationship with food bolus formation. Int. Dairy J. 2011, 21, 358–364. [Google Scholar] [CrossRef]
- Guichard, E. Interactions between flavor compounds and food ingredients and their influence on flavor perception. Food Rev. Int. 2002, 18, 49–70. [Google Scholar] [CrossRef]
- de Roos, K.B. How lipids influence flavor perception. In Food Lipids: Chemistry, Flavor, and Texture; Shahidi, F., Weenen, H., Eds.; ACS: Columbus, OH, USA, 2006; Volume 920, pp. 145–158. [Google Scholar]
- Guichard, E.; Galindo-Cuspinera, V.; Feron, G. Physiological mechanisms explaining human differences in fat perception and liking in food spreads-a review. Trends Food Sci. Technol. 2018, 74, 46–55. [Google Scholar] [CrossRef]
- van Ruth, S.M.; King, C.; Giannouli, P. Influence of lipid fraction, emulsifier fraction, and mean particle diameter of oil-in-water emulsions on the release of 20 aroma compounds. J. Agric. Food Chem. 2002, 50, 2365–2371. [Google Scholar] [CrossRef]
- Seuvre, A.M.; Voilley, A. Physico-Chemical Interactions in the Flavor-Release Process; Springer: Berlin/Heidelberg, Germany, 2017; pp. 35–36. [Google Scholar]
- Mao, L.; Roos, Y.H.; Biliaderis, C.G.; Miao, S. Food emulsions as delivery systems for flavor compounds: A review. Crit. Rev. Food Sci. Nutr. 2017, 57, 3173–3187. [Google Scholar] [CrossRef]
- Camacho, S.; van Riel, V.; de Graaf, C.; van de Velde, F.; Stieger, M. Physical and sensory characterizations of oral coatings of oil/water emulsions. J. Agric. Food Chem. 2014, 62, 5789–5795. [Google Scholar] [CrossRef] [PubMed]
- Boisard, L.; Andriot, I.; Arnould, C.; Achilleos, C.; Salles, C.; Guichard, E. Structure and composition of model cheeses influence sodium NMR mobility, kinetics of sodium release and sodium partition coefficients. Food Chem. 2013, 136, 1070–1077. [Google Scholar] [CrossRef]
- Tsachaki, M.; Linforth, R.S.T.; Taylor, A.J. Dynamic Headspace Analysis of the Release of Volatile Organic Compounds from Ethanolic Systems by Direct APCI-MS. J. Agric. Food Chem. 2005, 53, 8328–8333. [Google Scholar] [CrossRef] [PubMed]
- Taylor, A.J.; Tsachaki, M.; Lopez, R.; Morris, C.; Ferreira, V.; Wolf, B. Odorant Release from Alcoholic Beverages. In Flavors in Noncarbonated Beverages; ACS: Columbus, OH, USA, 2010; pp. 161–175. [Google Scholar]
- Chen, L.; Wang, Z.; Liao, P.; Li, A.; Zhang, Y.; Li, H.; Sun, J. The effect of saliva on the aroma release of esters in simulated baijiu under the impact of high ethanol concentration. J. Food Compos. Anal. 2021, 104, 104134. [Google Scholar] [CrossRef]
- Lesme, H.; Alleaume, C.; Bouhallab, S.; Famelart, M.H.; Marzin, C.; Lopez-Torres, L.; Prost, C.; Rannou, C. Aroma-retention capacities of functional whey protein aggregates: Study of a strawberry aroma in solutions and in fat-free yogurts. Food Res. Int. 2020, 136, 109491. [Google Scholar] [CrossRef]
- Savary, G.; Hucher, N.; Petibon, O.; Grisel, M. Study of interactions between aroma compounds and acacia gum using headspace measurements. Food Hydrocoll. 2014, 37, 1–6. [Google Scholar] [CrossRef]
- Yu, Y.; Chen, S.; Nie, Y.; Xu, Y. Optimization of an intra-oral solid-phase microextraction (SPME) combined with comprehensive two-dimensional gas chromatography-time-of-flight mass spectrometry (GC x GC-TOFMS) method for oral aroma compounds monitoring of Baijiu. Food Chem. 2022, 385, 132502. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Nie, Y.; Chen, S.; Xu, Y. Characterization of the dynamic retronasal aroma perception and oral aroma release of Baijiu by progressive profiling and an intra-oral SPME combined with GC × GC-TOFMS method. Food Chem. 2023, 405, 134854. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Yan, R.; Zhao, Y.; Sun, J.; Zhang, Y.; Li, H.; Zhao, D.; Wang, B.; Ye, X.; Sun, B. Characterization of the aroma release from retronasal cavity and flavor perception during baijiu consumption by Vocus-PTR-MS, GC×GC-MS, and TCATA analysis. LWT 2023, 174, 114430. [Google Scholar] [CrossRef]
- Linforth, R.; Martin, F.; Carey, M.; Jim, D.; Taylor, A. Retronasal Transport of Aroma Compounds. J. Agric. Food Chem. 2003, 50, 1111. [Google Scholar] [CrossRef]
- Lyu, J.; Fu, J.; Chen, S.; Xu, Y.; Nie, Y.; Tang, K. Impact of tannins on intraoral aroma release and retronasal perception, including detection thresholds and temporal perception by taste, in model wines. Food Chem. 2022, 375, 131890. [Google Scholar] [CrossRef] [PubMed]
- Munoz-Gonzalez, C.; Perez-Jimenez, M.; Criado, C.; Pozo-Bayon, M.A. Effects of Ethanol Concentration on Oral Aroma Release After Wine Consumption. Molecules 2019, 24, 3253. [Google Scholar] [CrossRef] [PubMed]
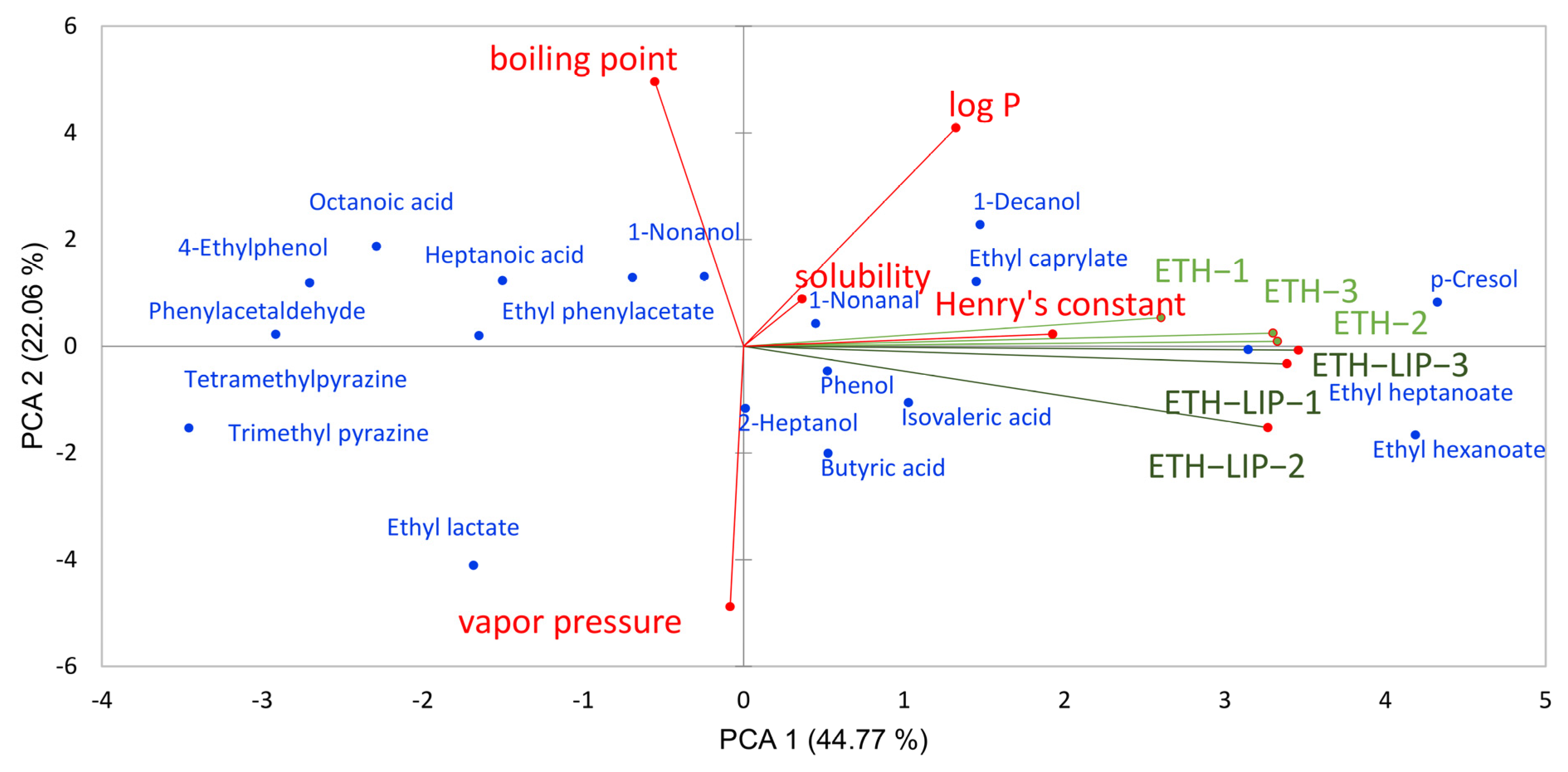
| CAS | Aroma | Log p | Boiling Point | Vapor Pressure | Water Solubility | Henery’s Law Constant | Concentration of the In Vitro Experiment (mg/L) | Concentration of Added Aromatic Substances in Baijiu (mg/L) | |
|---|---|---|---|---|---|---|---|---|---|
| Ethyl hexanoate | 123-66-0 | sweet, fruity | 2.83 | 167 | 1.80 | 309 | 7.23 × 10−4 | 50 | - |
| 2-Heptanol | 543-49-7 | lemon, floral | 2.29 | 161 | 1.23 | 3.57 × 103 | 2.34 × 10−5 | 100 | 50 |
| Ethyl heptanoate | 106-30-9 | pineapple, rum | 3.37 | 187 | 0.68 | 102 | 9.60 × 10−4 | 50 | - |
| Ethyl lactate | 97-64-3 | fruity, buttery | −0.19 | 154 | 3.75 | 4.73 × 10−5 | 4.82 × 10−5 | 300 | 200 |
| 1-Nonanal | 124-19-6 | floral, lemon | 3.27 | 191 | 0.37 | 132 | 4.93 × 10−4 | 100 | 50 |
| Ethyl caprylate | 106-32-1 | fruity, wine | 3.81 | 209 | 0.24 | 33.4 | 1.27 × 10−3 | 50 | - |
| Trimethyl pyrazine | 14667-55-1 | potato, roast | 0.95 | 172 | 1.45 | 1.52 × 10−4 | 3.92 × 10−6 | 10 | - |
| Tetramethyl pyrazine | 1124-11-4 | coffee, roast | 1.56 | 190 | 0.15 | 7.01 × 103 | 4.33 × 10−6 | 10 | - |
| Butyric acid | 107-92-6 | cheese, butter | 1.07 | 164 | 1.65 | 6.61 × 10−4 | 5.35 × 10−7 | 700 | 100 |
| Phenylacetaldehyde | 122-78-1 | green, floral | 1.78 | 195 | 0.35 | 3.03 × 103 | 5.48 × 10−6 | 500 | 50 |
| 1-Nonanol | 124-19-6 | floral, lemon | 3.27 | 191 | 0.37 | 132 | 4.93 × 10−4 | 100 | 20 |
| Isovaleric acid | 503-74-2 | cheese, pungent | 1.16 | 177 | 0.44 | 2.92 × 10−4 | 1.28 × 10−6 | 700 | 100 |
| 1-Decanol | 112-30-1 | fatty, floral | 4.57 | 231 | 0.01 | 28.2 | 5.47 × 10−5 | 200 | 50 |
| Ethyl phenylacetate | 101-97-3 | honey, rose | 2.50 | 227 | 0.09 | 729 | 1.88 × 10−5 | 100 | - |
| Phenol | 108-95-2 | plastic, rubber | 1.48 | 182 | 0.35 | 2.62 × 10−4 | 5.61 × 10−7 | 100 | - |
| Heptanoic acid | 111-14-8 | sour, sweat | 2.37 | 222 | 0.12 | 1.96 × 103 | 2.26 × 10−6 | 400 | 50 |
| Octanoic acid | 124-07-2 | fatty, waxy | 3.03 | 239 | 0.05 | 496 | 3.00 × 10−6 | 400 | 50 |
| p-Cresol | 106-44-5 | pungent, stable | 1.94 | 202 | 0.11 | 9.25 × 103 | 6.19 × 10−7 | 100 | - |
| 4-Ethylphenol | 123-07-9 | leather, spice | 2.47 | 218 | 0.04 | 2.35 × 103 | 8.21 × 10−7 | 100 | 20 |
| Water Solution | 53% ABV | 53% ABV + 4% Lipid | 53% ABV + 8% Lipid | |
|---|---|---|---|---|
| Ethyl caproate | 0.1494 ± 0.0345 c | 0.0574 ± 0.0025 a | 0.0267 ± 0.0035 b | 0.0179 ± 0.0013 b |
| 2-Heptanol | 0.0405 ± 0.0034 a | 0.0375 ± 0.0035 a | 0.0283 ± 0.0034 ab | 0.0212 ± 0.0020 b |
| Ethyl heptanoate | 0.1669 ± 0.0438 a | 0.1356 ± 0.0146 b | 0.0252 ± 0.0030 c | 0.0193 ± 0.0014 c |
| Ethyl lactate | 0.0050 ± 0.0004 c | 0.1004 ± 0.0089 a | 0.0362 ± 0.0047 b | 0.0100 ± 0.0005 c |
| 1-Nonanal | 0.1818 ± 0.0038 a | 0.1143 ± 0.0110 b | 0.0581 ± 0.0065 c | 0.0181 ± 0.0018 d |
| Ethyl caprylate | 0.1193 ± 0.0145 a | 0.1064 ± 0.0083 a | 0.0429 ± 0.0048 b | 0.0151 ± 0.0013 c |
| Trimethyl pyrazine | 0.0087 ± 0.0010 c | 0.0651 ± 0.0038 a | 0.0482 ± 0.0052 b | 0.0327 ± 0.0030 b |
| Tetramethyl pyrazine | 0.0092 ± 0.0010 c | 0.0738 ± 0.0043 a | 0.0423 ± 0.0043 b | 0.0456 ± 0.0024 b |
| Butyric acid | 0.0180 ± 0.0021 c | 0.1029 ± 0.0082 a | 0.0705 ± 0.0056 b | 0.0227 ± 0.0038 c |
| Phenylacetaldehyde | 0.0015 ± 0.0002 c | 0.2681 ± 0.0261 a | 0.0513 ± 0.0050 b | 0.0113 ± 0.0011 c |
| 1-Nonanol | 0.0150 ± 0.0003 c | 0.1166 ± 0.0179 a | 0.0742 ± 0.0087 b | 0.0179 ± 0.0023 c |
| Isovaleric acid | 0.0152 ± 0.0002 b | 0.0440 ± 0.0064 a | 0.0358 ± 0.0034 a | 0.0090 ± 0.0007 b |
| 1-Decanol | 0.0350 ± 0.0003 a | 0.0608 ± 0.0071 a | 0.0357 ± 0.0029 b | 0.0182 ± 0.0022 c |
| Ethyl phenylacetate | 0.0014 ± 0.0001 c | 0.0560 ± 0.0078 a | 0.0369 ± 0.0038 b | 0.0450 ± 0.0024 ab |
| Phenol | 0.0300 ± 0.0035 a | 0.0220 ± 0.0018 ab | 0.0168 ± 0.0020 ab | 0.0094 ± 0.0009 b |
| Heptanoic acid | 0.0136 ± 0.0005 b | 0.0540 ± 0.0052 a | 0.0268 ± 0.0028 a | 0.0095 ± 0.0003 b |
| Octanoic acid | 0.0125 ± 0.0015 c | 0.0638 ± 0.0058 a | 0.0355 ± 0.0017 b | 0.0138 ± 0.0005 c |
| p-Cresol | 0.0004 ± 0.0001 c | 0.0499 ± 0.0038 a | 0.0299 ± 0.0032 b | 0.0102 ± 0.0006 c |
| 4-Ethylphenol | 0.0333 ± 0.0028 a | 0.0208 ± 0.0020 ab | 0.0158 ± 0.0012 b | 0.0138 ± 0.0009 b |
| Esolv, polar | Esolv, nonpolar | EMM | Eele | Evdw | |
|---|---|---|---|---|---|
| ELA-OOL | 2.322 | −1.437 | −5.020 | −0.298 | −4.722 |
| ELA-OLL | 2.651 | −1.148 | −5.693 | −0.485 | −5.208 |
| ELA-POL | 1.154 | −1.053 | −2.793 | −0.064 | −2.729 |
| EHE-OOL | 4.750 | −5.604 | −21.408 | −0.386 | −19.001 |
| EHE-OLL | 4.595 | −5.103 | −19.605 | −0.408 | −19.197 |
| EHE-POL | 2.775 | −3.340 | −11.735 | −0.199 | −11.536 |
| HEC-OOL | 3.679 | −4.554 | −14.615 | −0.122 | −14.493 |
| HEC-OLL | 3.629 | −4.661 | −15.458 | −0.326 | −15.132 |
| HEC-POL | 4.901 | −4.701 | −16.679 | −0.372 | −16.307 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, L.; Sun, J.; Zhang, Y.; Li, H.; Zhao, D.; Wang, B.; Ye, X.; Sun, B. Baijiu–Peanut Pairing In Vitro and In Vivo: The Decreased but Prolonged Aftertaste of Baijiu Under the Effect of Mouth Coating Formed by Peanut Lipid. Foods 2025, 14, 423. https://doi.org/10.3390/foods14030423
Chen L, Sun J, Zhang Y, Li H, Zhao D, Wang B, Ye X, Sun B. Baijiu–Peanut Pairing In Vitro and In Vivo: The Decreased but Prolonged Aftertaste of Baijiu Under the Effect of Mouth Coating Formed by Peanut Lipid. Foods. 2025; 14(3):423. https://doi.org/10.3390/foods14030423
Chicago/Turabian StyleChen, Lu, Jinyuan Sun, Yanyan Zhang, Hehe Li, Dongrui Zhao, Bowen Wang, Xingqian Ye, and Baoguo Sun. 2025. "Baijiu–Peanut Pairing In Vitro and In Vivo: The Decreased but Prolonged Aftertaste of Baijiu Under the Effect of Mouth Coating Formed by Peanut Lipid" Foods 14, no. 3: 423. https://doi.org/10.3390/foods14030423
APA StyleChen, L., Sun, J., Zhang, Y., Li, H., Zhao, D., Wang, B., Ye, X., & Sun, B. (2025). Baijiu–Peanut Pairing In Vitro and In Vivo: The Decreased but Prolonged Aftertaste of Baijiu Under the Effect of Mouth Coating Formed by Peanut Lipid. Foods, 14(3), 423. https://doi.org/10.3390/foods14030423





