Synergistic Anti-Helicobacter pylori Effects of Takifugu obscurus Skin Peptides and Lactobacillus plantarum: A Potential Gastric Health Dietary Supplement
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
2.2. Preparation and Isolation of TSP
2.3. Bacterial Culture and Experimental Grouping
2.4. Analysis of the Amino Acid Distribution
2.5. SEM Morphological Characterization
2.6. H. pylori Inhibition Assay
2.7. H. pylori UA Assay
2.8. Nucleic Acid Leakage Level
2.9. NPN Absorption Assay
2.10. H. pylori IR Spectra Data Acquisition
2.11. Statistical Analysis
3. Results
3.1. Anti-H. pylori Activity of TSP
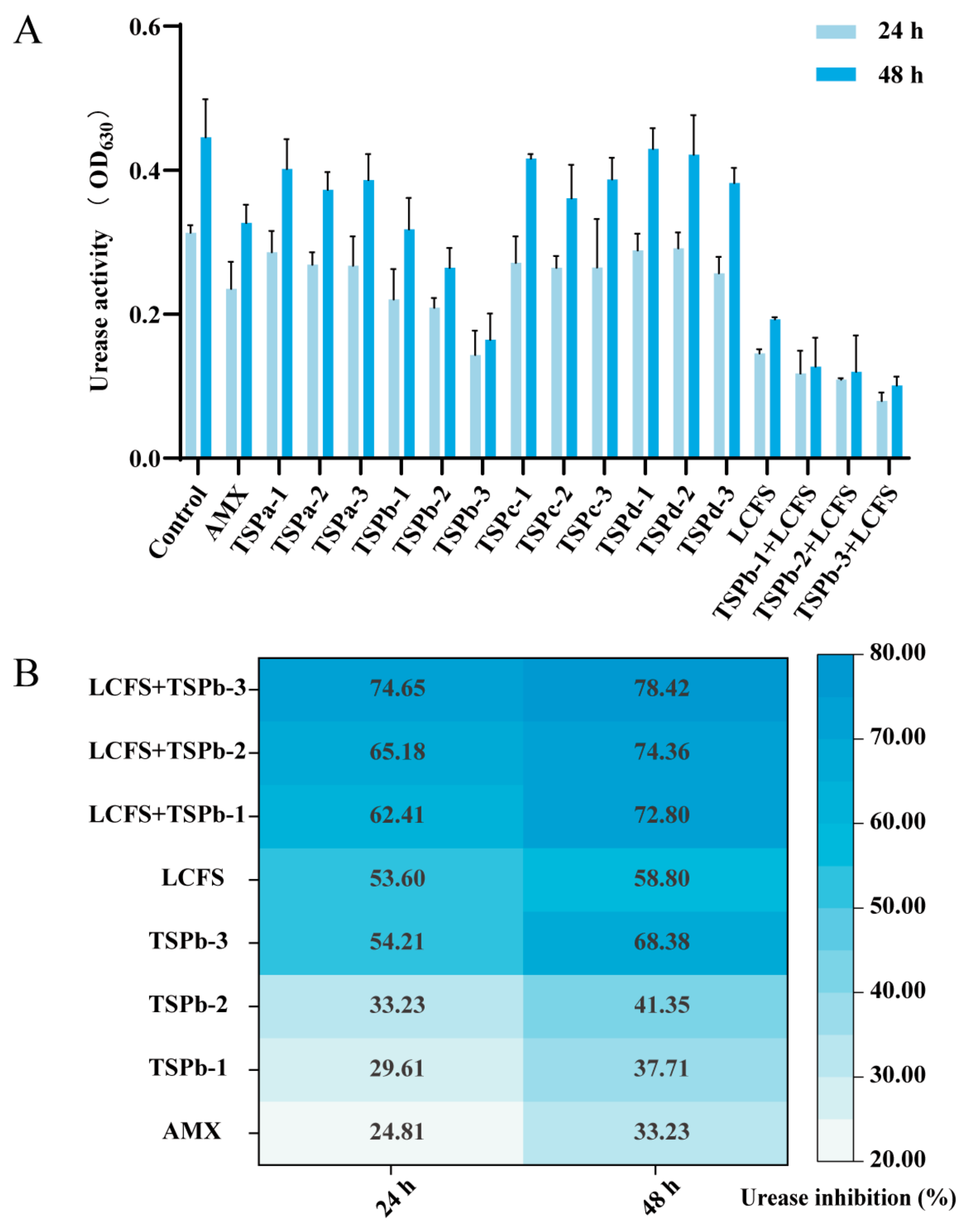
3.2. UA Inhibition Rate of H. pylori
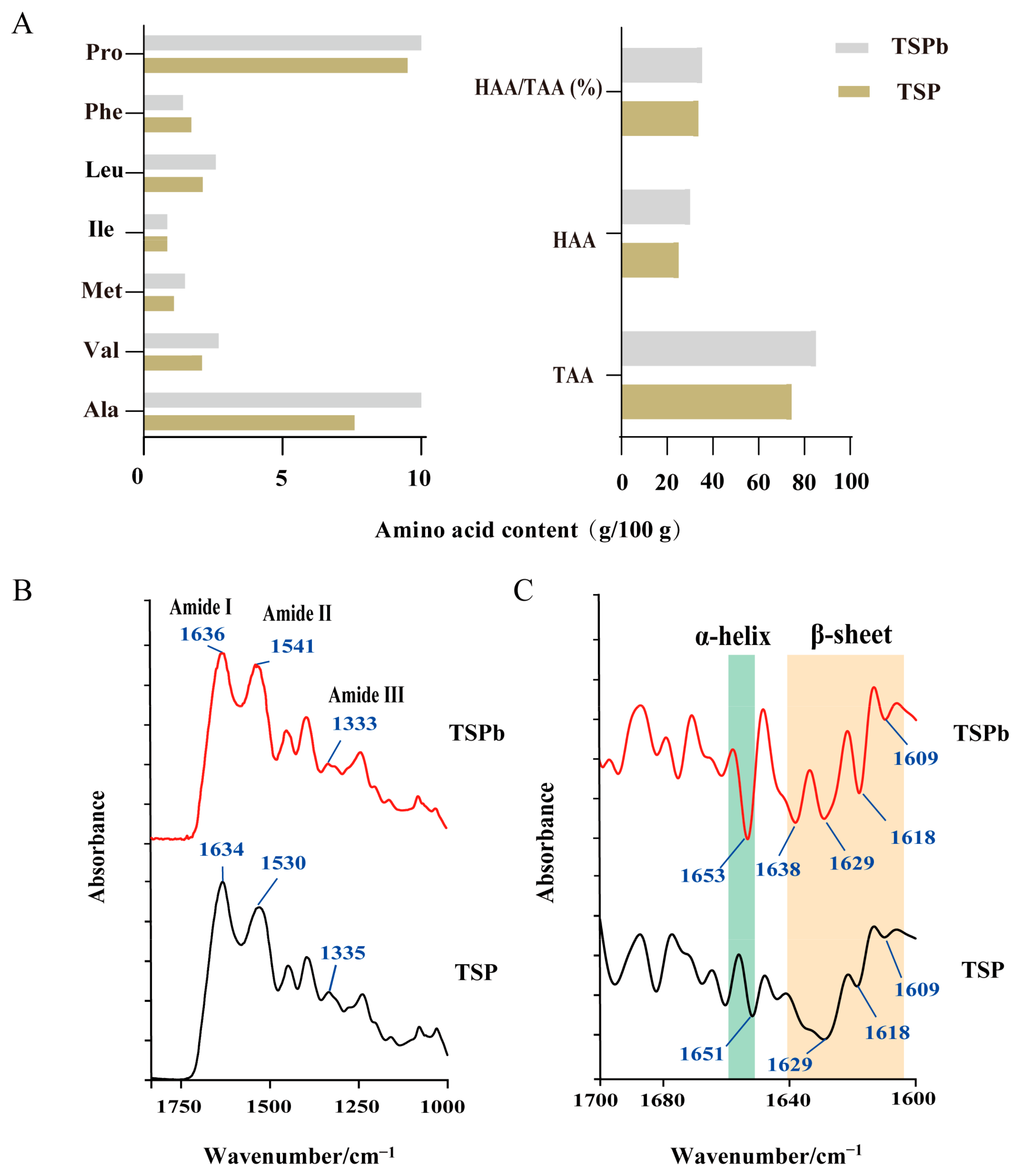
3.3. Characterization of TSPb
3.4. Impacts on H. pylori Membrane Integrity and Nucleic Acid Leakage
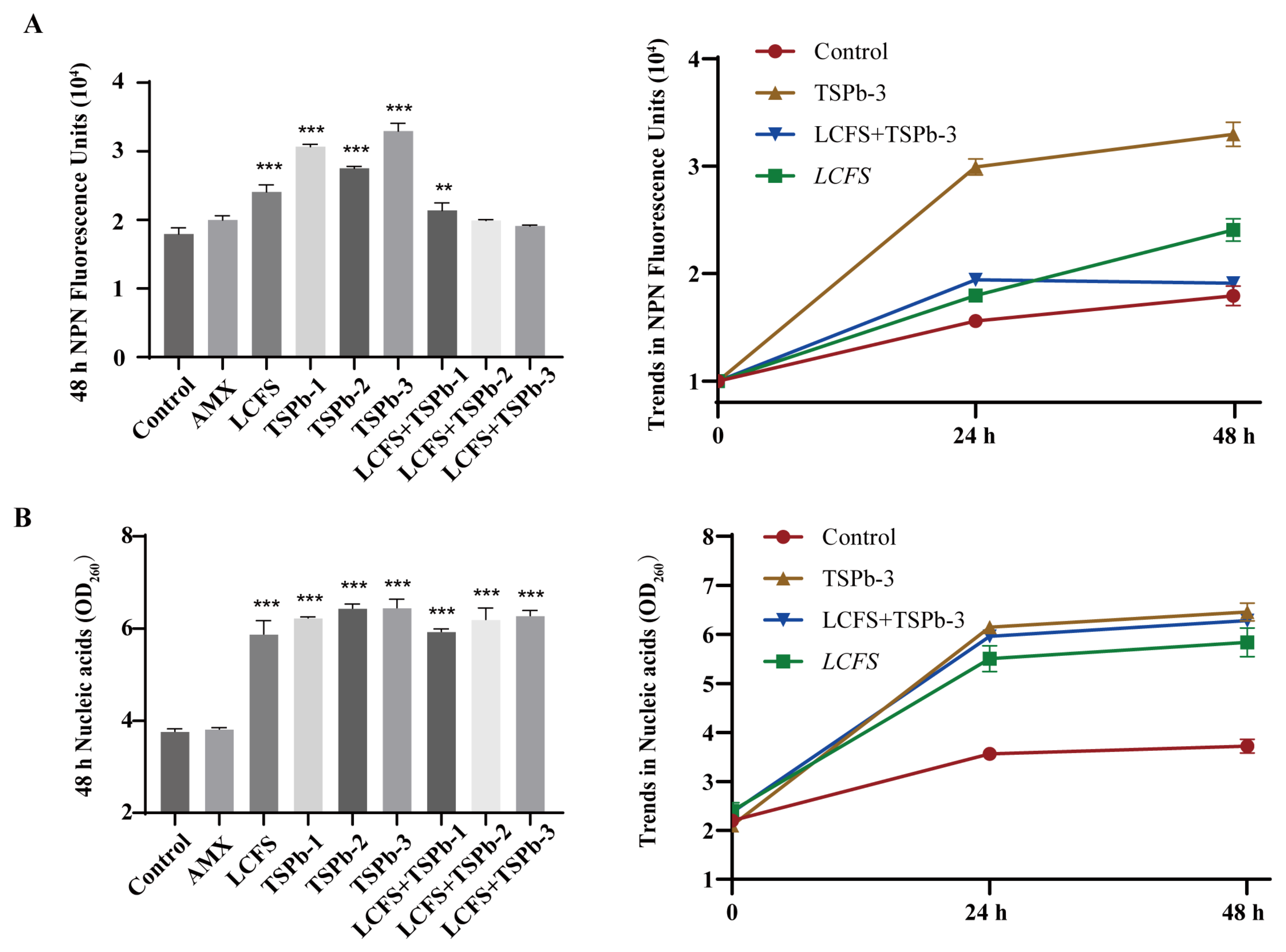
3.4.1. Membrane Integrity
3.4.2. Nucleic Acid Leakage
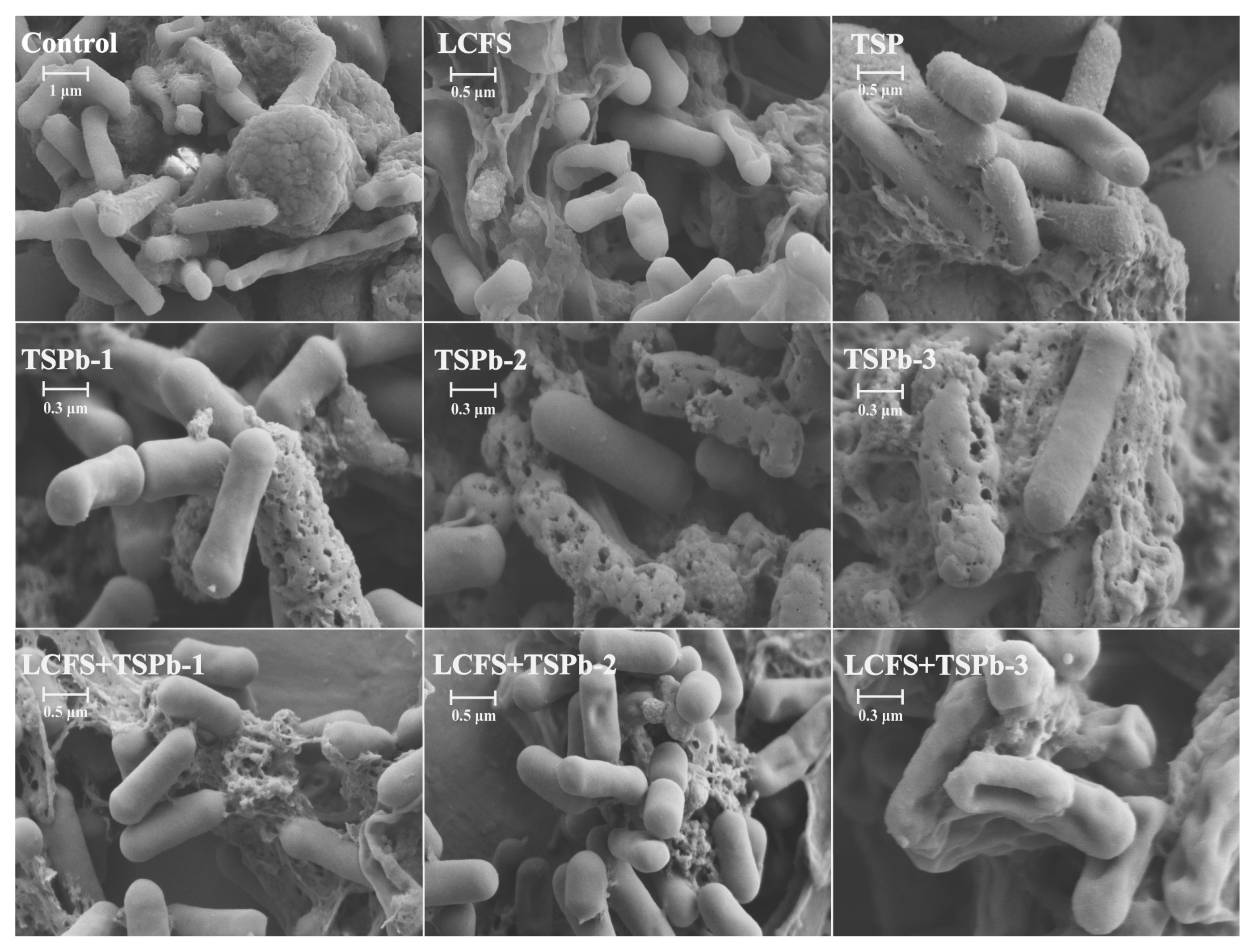
3.5. Biofilm Morphology of H. pylori
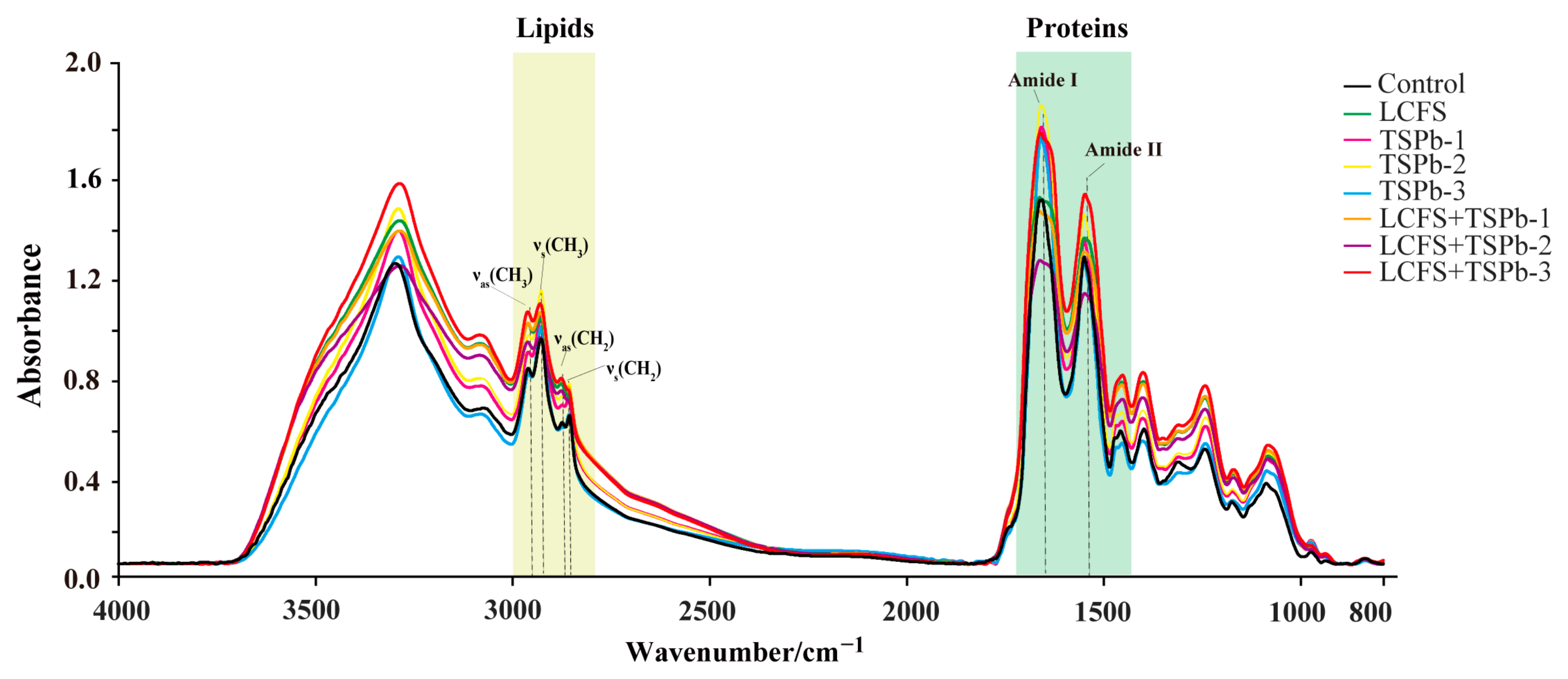
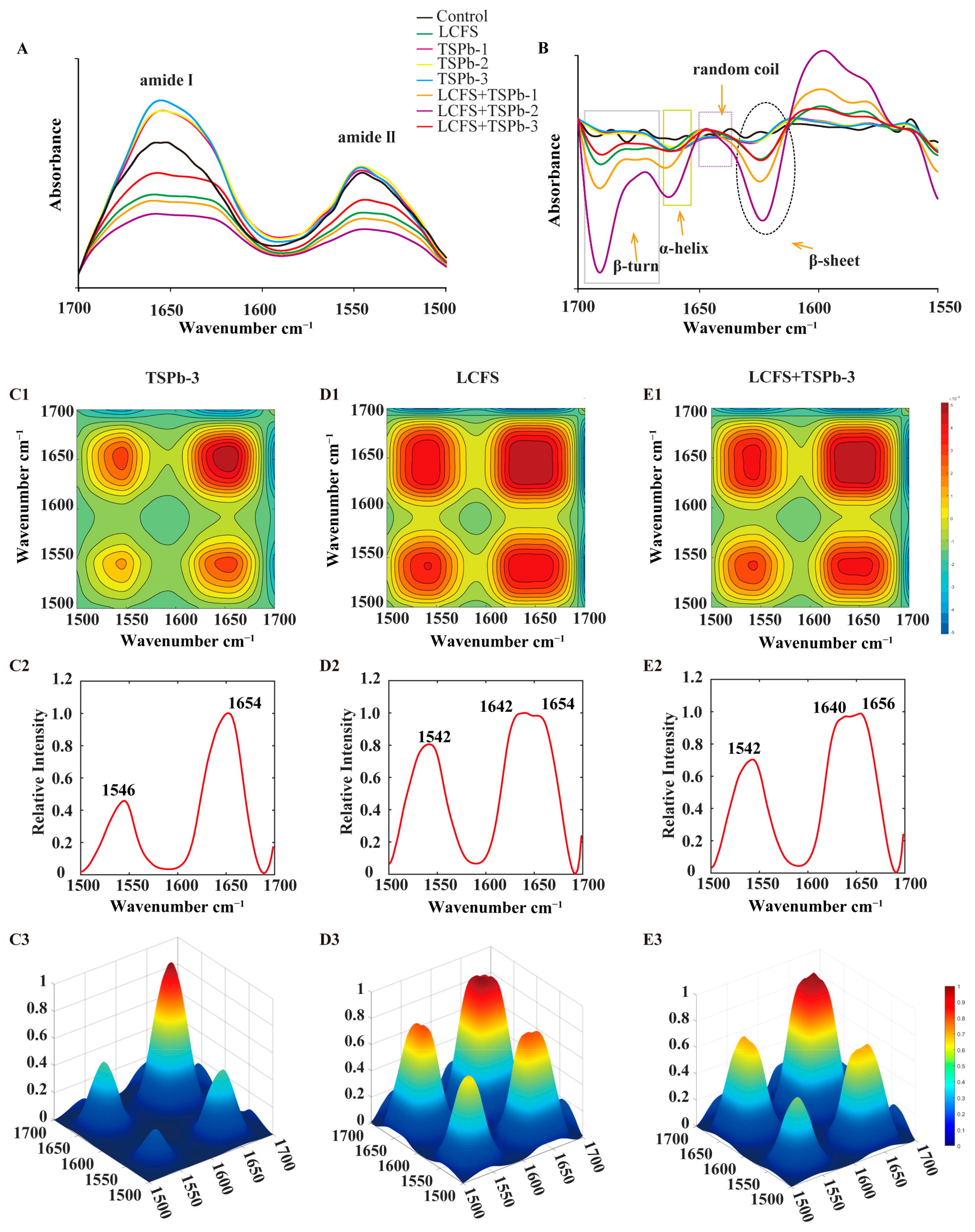
3.6. Bacterial Composition Analysis
3.6.1. Proteins
3.6.2. Lipids
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Chmiela, M.; Kupcinskas, J. Review: Pathogenesis of Helicobacter pylori Infection. Helicobacter 2019, 24, e12638. [Google Scholar] [CrossRef] [PubMed]
- Parsonnet, J.; Friedman, G.D.; Vandersteen, D.P.; Chang, Y.; Vogelman, J.H.; Orentreich, N.; Sibley, R.K. Helicobacter pylori Infection and the Risk of Gastric Carcinoma. N. Engl. J. Med. 1991, 325, 1127–1131. [Google Scholar] [CrossRef] [PubMed]
- Masaoka, T.; Suzuki, H.; Kurabayashi, K.; Nomoto, Y.; Nishizawa, T.; Mori, M.; Hibi, T. Could Frameshift Mutations in the FrxA and RdxA Genes of Helicobacter pylori Be a Marker for Metronidazole Resistance? Aliment. Pharmacol. Ther. 2006, 24, 81–87. [Google Scholar] [CrossRef]
- Hooi, J.K.Y.; Lai, W.Y.; Ng, W.K.; Suen, M.M.Y.; Underwood, F.E.; Tanyingoh, D.; Malfertheiner, P.; Graham, D.Y.; Wong, V.W.S.; Wu, J.C.Y.; et al. Global Prevalence of Helicobacter Pylori Infection: Systematic Review and Meta-Analysis. Gastroenterology 2017, 153, 420–429. [Google Scholar] [CrossRef]
- Gené, E.; Calvet, X.; Azagra, R.; Gisbert, J.P. Triple vs. Quadruple Therapy for Treating Helicobacter pylori Infection: A Meta-analysis. Aliment. Pharmacol. Ther. 2003, 17, 1137–1143. [Google Scholar] [CrossRef]
- Theuretzbacher, U.; Outterson, K.; Engel, A.; Karlén, A. The Global Preclinical Antibacterial Pipeline. Nat. Rev. Microbiol. 2020, 18, 275–285. [Google Scholar] [CrossRef]
- Dadashzadeh, K.; Milani, M.; Rahmati, M.; Akbarzadeh, A. Real-Time PCR Detection of 16S RRNA Novel Mutations Associated with Helicobacter Pylori Tetracycline Resistance in Iran. Asian Pac. J. Cancer Prev. 2014, 15, 8883–8886. [Google Scholar] [CrossRef]
- Dunne, C. Factors That Mediate Colonization of the Human Stomach by Helicobacter pylori. World J. Gastroenterol. 2014, 20, 5610. [Google Scholar] [CrossRef]
- Ashida, H.; Ogawa, M.; Kim, M.; Mimuro, H.; Sasakawa, C. Bacteria and Host Interactions in the Gut Epithelial Barrier. Nat. Chem. Biol. 2012, 8, 36–45. [Google Scholar] [CrossRef]
- Aleman, R.S.; Yadav, A. Systematic Review of Probiotics and Their Potential for Developing Functional Nondairy Foods. Appl. Microbiol. 2023, 4, 47–69. [Google Scholar] [CrossRef]
- Gao, X.W.; Mubasher, M.; Fang, C.Y.; Reifer, C.; Miller, L.E. Dose–Response Efficacy of a Proprietary Probiotic Formula of Lactobacillus Acidophilus CL1285 and Lactobacillus Casei LBC80R for Antibiotic-Associated Diarrhea and Clostridium Difficile-Associated Diarrhea Prophylaxis in Adult Patients. Am. J. Gastroenterol. 2010, 105, 1636–1641. [Google Scholar] [CrossRef] [PubMed]
- Sunanliganon, C. Lactobacillus plantarum B7 Inhibits Helicobacter pylori Growth and Attenuates Gastric Inflammation. World J. Gastroenterol. 2012, 18, 2472. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Guo, L.; Zhang, X.; Mu, H.; Sha, S.; Lin, Y.; Hou, H.; Wang, L. Whole-Genome Sequencing Combined with Mass Spectrometry to Identify Bacteriocin and Mine Silent Genes. LWT 2022, 169, 113975. [Google Scholar] [CrossRef]
- Narayana, J.L.; Huang, H.-N.; Wu, C.-J.; Chen, J.-Y. Epinecidin-1 Antimicrobial Activity: In Vitro Membrane Lysis and In Vivo Efficacy against Helicobacter Pylori Infection in a Mouse Model. Biomaterials 2015, 61, 41–51. [Google Scholar] [CrossRef] [PubMed]
- Lapis, K. Physiological and Pathophysiological Significance of the Antimicrobial (Host Defensive) Small Peptides. Orv. Hetil. 2008, 149, 2419–2424. [Google Scholar] [CrossRef]
- Hirakura, Y.; Kobayashi, S.; Matsuzaki, K. Specific Interactions of the Antimicrobial Peptide Cyclic β-Sheet Tachyplesin I with Lipopolysaccharides. Biochim. Biophys. Acta (BBA)—Biomembr. 2002, 1562, 32–36. [Google Scholar] [CrossRef]
- Luo, Y.; Song, Y. Mechanism of Antimicrobial Peptides: Antimicrobial, Anti-Inflammatory and Antibiofilm Activities. Int. J. Mol. Sci. 2021, 22, 11401. [Google Scholar] [CrossRef]
- Narayana, J.L.; Huang, H.-N.; Wu, C.-J.; Chen, J.-Y. Efficacy of the Antimicrobial Peptide TP4 against Helicobacter pylori Infection: In Vitro Membrane Perturbation via Micellization and in Vivo Suppression of Host Immune Responses in a Mouse Model. Oncotarget 2015, 6, 12936–12954. [Google Scholar] [CrossRef]
- Ma, X.; Aminov, R.; Franco, O.L.; de la Fuente-Nunez, C.; Wang, G.; Wang, J. Editorial: Antimicrobial Peptides and Their Druggability, Bio-Safety, Stability, and Resistance. Front. Microbiol. 2024, 15, 1425952. [Google Scholar] [CrossRef]
- Majura, J.J.; Cao, W.; Chen, Z.; Htwe, K.K.; Li, W.; Du, R.; Zhang, P.; Zheng, H.; Gao, J. The Current Research Status and Strategies Employed to Modify Food-Derived Bioactive Peptides. Front. Nutr. 2022, 9, 950823. [Google Scholar] [CrossRef]
- Lu, S.; Kong, S.; Wang, Y.; Hu, Z.; Zhang, L.; Liao, M. Gastric Acid-Response Chitosan/Alginate/Tilapia Collagen Peptide Composite Hydrogel: Protection Effects on Alcohol-Induced Gastric Mucosal Injury. Carbohydr. Polym. 2022, 277, 118816. [Google Scholar] [CrossRef] [PubMed]
- Vijayan, D.K.; Sreerekha, P.R.; Dara, P.K.; Ganesan, B.; Mathew, S.; Anandan, R.; Ravisankar, C.N. Antioxidant Defense of Fish Collagen Peptides Attenuates Oxidative Stress in Gastric Mucosa of Experimentally Ulcer-Induced Rats. Cell Stress. Chaperones 2022, 27, 45–54. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Jiang, W.; Chen, X.; Chen, X.; Wu, J.; Huang, J.; Cai, X.; Wang, S. Identification of Novel Antifreeze Peptides from Takifugu obscurus Skin and Molecular Mechanism in Inhibiting Ice Crystal Growth. J. Agric. Food Chem. 2022, 70, 14148–14156. [Google Scholar] [CrossRef] [PubMed]
- Jung, J.-I.; Baek, S.-M.; Nguyen, T.H.; Kim, J.W.; Kang, C.-H.; Kim, S.; Imm, J.-Y. Effects of Probiotic Culture Supernatant on Cariogenic Biofilm Formation and RANKL-Induced Osteoclastogenesis in RAW 264.7 Macrophages. Molecules 2021, 26, 733. [Google Scholar] [CrossRef]
- Ji, J.; Yang, H. In Vitro Effects of Lactobacillus Plantarum LN66 and Antibiotics Used Alone or in Combination on Helicobacter Pylori Mature Biofilm. Microorganisms 2021, 9, 424. [Google Scholar] [CrossRef]
- Techo, S.; Visessanguan, W.; Vilaichone, R.; Tanasupawat, S. Characterization and Antibacterial Activity Against Helicobacter Pylori of Lactic Acid Bacteria Isolated from Thai Fermented Rice Noodle. Probiotics Antimicrob. Proteins 2019, 11, 92–102. [Google Scholar] [CrossRef]
- Chen, W.; Tang, H.; Jiang, N.; Zhong, Q.; Hu, Y.; Chen, H.; Chen, W. Antibacterial Effect of Black Pepper Petroleum Ether Extract against Listeria monocytogenes and Salmonella typhimurium. J. Food Qual. 2019, 2019, 2356161. [Google Scholar] [CrossRef]
- Helander, I.M.; Mattila-Sandholm, T. Fluorometric Assessment of Gram-Negative Bacterial Permeabilization. J. Appl. Microbiol. 2000, 88, 213–219. [Google Scholar] [CrossRef]
- Zhou, C.; Jiang, M.; Ye, X.; Liu, X.; Zhao, W.; Ma, L.; Zhou, C. Antibacterial Activities of Peptide HF-18 Against Helicobacter Pylori and Its Virulence Protein CagA. Int. J. Pept. Res. Ther. 2022, 28, 63. [Google Scholar] [CrossRef]
- Li, S.; Wang, Y.; Xue, Z.; Jia, Y.; Li, R.; He, C.; Chen, H. The Structure-Mechanism Relationship and Mode of Actions of Antimicrobial Peptides: A Review. Trends Food Sci. Technol. 2021, 109, 103–115. [Google Scholar] [CrossRef]
- Xue, C.; Zhang, L.; Li, H.; Wang, S.; Li, Q.; Luo, X.; Liu, W.; Du, M.; Yi, H.; Han, X. Functionality of the S-Layer Proteins from Lactobacillus in the Competitive against Enteropathogens Infection. Eur. Food Res. Technol. 2013, 236, 249–255. [Google Scholar] [CrossRef]
- Tang, H.; Chen, W.; Dou, Z.-M.; Chen, R.; Hu, Y.; Chen, W.; Chen, H. Antimicrobial Effect of Black Pepper Petroleum Ether Extract for the Morphology of Listeria Monocytogenes and Salmonella Typhimurium. J. Food Sci. Technol. 2017, 54, 2067–2076. [Google Scholar] [CrossRef]
- Hou, S.-W.; He, S.-Y.; Xie, J.; Li, M.-Y.; Hong, M.-S.; Guan, F.-L.; Hu, Y.-L.; Huang, Y.-L.; Xu, C.-H. Integral Characterization of Normal and Alopecic Hair at Different Degeneration Stages by In-Situ Visible and Chemical Imaging. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2020, 235, 118315. [Google Scholar] [CrossRef] [PubMed]
- Song, S.Y.; Lee, Y.K.; Kim, I.-J. Sugar and Acid Content of Citrus Prediction Modeling Using FT-IR Fingerprinting in Combination with Multivariate Statistical Analysis. Food Chem. 2016, 190, 1027–1032. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Xu, C.-H.; Wang, P.; Sun, S.-Q.; Chen, J.-B.; Li, J.; Chen, T.; Wang, J.-B. Analysis and Identification of Different Animal Horns by a Three-Stage Infrared Spectroscopy. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2011, 83, 265–270. [Google Scholar] [CrossRef] [PubMed]
- Chan, K.L.A.; Kazarian, S.G.; Mavraki, A.; Williams, D.R. Fourier Transform Infrared Imaging of Human Hair with a High Spatial Resolution without the Use of a Synchrotron. Appl. Spectrosc. 2005, 59, 149–155. [Google Scholar] [CrossRef]
- Aboualizadeh, E.; Ranji, M.; Sorenson, C.M.; Sepehr, R.; Sheibani, N.; Hirschmugl, C.J. Retinal Oxidative Stress at the Onset of Diabetes Determined by Synchrotron FTIR Widefield Imaging: Towards Diabetes Pathogenesis. Analyst 2017, 142, 1061–1072. [Google Scholar] [CrossRef]
- Patel, A.; Shah, N.; Prajapati, J.B. Clinical Application of Probiotics in the Treatment of Helicobacter Pylori Infection—A Brief Review. J. Microbiol. Immunol. Infect. 2014, 47, 429–437. [Google Scholar] [CrossRef]
- Wan, G.-Y.; Lam, K.-M.; Wong, I.-I.; FONG, P.; Meng, L.-R. Extraction of Antibacterial Peptides against H. pylori from Bovine Milk Casein. Arch. Med. Sci. 2021, 18, 376. [Google Scholar] [CrossRef]
- Li, G.; Miao, Z.; Liu, X.; Wang, Q.; Zheng, X. Four Novel Anti-Adhesive Activity Peptides against Helicobacter pylori Derived from Rice Bran Protein: Release, Identification and Anti-Adhesive Mechanisms Elucidation. Food Funct. 2024, 15, 8418–8431. [Google Scholar] [CrossRef]
- Chi, C.-F.; Wang, B.; Wang, Y.-M.; Zhang, B.; Deng, S.-G. Isolation and Characterization of Three Antioxidant Peptides from Protein Hydrolysate of Bluefin Leatherjacket (Navodon septentrionalis) Heads. J. Funct. Foods 2015, 12, 1–10. [Google Scholar] [CrossRef]
- Ansari, S.; Yamaoka, Y. Survival of Helicobacter pylori in Gastric Acidic Territory. Helicobacter 2017, 22, e12386. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Q.; Xue, B.; Gu, R.; Li, P.; Gu, Q. Lactobacillus plantarum ZJ316 Attenuates Helicobacter pylori -Induced Gastritis in C57BL/6 Mice. J. Agric. Food Chem. 2021, 69, 6510–6523. [Google Scholar] [CrossRef]
- Parsons, J.B.; Yao, J.; Frank, M.W.; Jackson, P.; Rock, C.O. Membrane Disruption by Antimicrobial Fatty Acids Releases Low-Molecular-Weight Proteins from Staphylococcus Aureus. J. Bacteriol. 2012, 194, 5294–5304. [Google Scholar] [CrossRef]
- Loh, B.; Grant, C.; Hancock, R.E. Use of the Fluorescent Probe 1-N-Phenylnaphthylamine to Study the Interactions of Aminoglycoside Antibiotics with the Outer Membrane of Pseudomonas Aeruginosa. Antimicrob. Agents Chemother. 1984, 26, 546–551. [Google Scholar] [CrossRef]
- Arts, M.T.; Kohler, C.C. Health and Condition in Fish: The Influence of Lipids on Membrane Competency and Immune Response. In Lipids in Aquatic Ecosystems; Springer: New York, NY, USA, 2009; pp. 237–256. [Google Scholar]
- Hufton, J.; Harding, J.; Smith, T.; Romero-González, M.E. The Importance of the Bacterial Cell Wall in Uranium(VI) Biosorption. Phys. Chem. Chem. Phys. 2021, 23, 1566–1576. [Google Scholar] [CrossRef]


| Group | Diameter of Inhibition Zone (cm) |
|---|---|
| AMX (0.125 mg/mL) | 1.76 ± 0.25 |
| TSP-1 (1.000 mg/mL) | 0.50 ± 0.10 |
| TSP-2 (2.000 mg/mL) | 0.70 ± 0.10 |
| TSP-3 (3.000 mg/mL) | 0.94 ± 0.05 |
| Peak Position (cm−1) | Main Functional Groups |
|---|---|
| ~3289 | υ (NH3) |
| 3000–2800 | υas (CH3), υas (CH2);υs (CH3), υs (CH2) |
| 1700~1600 | Amide I |
| 1600~1500 | Amide II |
| ~1460 | υas (CH3), υ (CH2) |
| ~1395 | δs (CH3), υs (COO-) |
| 1085, 1240 | υs (PO2−), υas (PO2−) |
| 1085–1041 | δ (C-C), υ (C-O) |
| 980–930 | δout-plane (C-H), δ (C-O), υ (C-C) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gu, L.; Tang, Y.; Zhang, J.; Tao, N.; Wang, X.; Wang, L.; Xu, C. Synergistic Anti-Helicobacter pylori Effects of Takifugu obscurus Skin Peptides and Lactobacillus plantarum: A Potential Gastric Health Dietary Supplement. Foods 2025, 14, 406. https://doi.org/10.3390/foods14030406
Gu L, Tang Y, Zhang J, Tao N, Wang X, Wang L, Xu C. Synergistic Anti-Helicobacter pylori Effects of Takifugu obscurus Skin Peptides and Lactobacillus plantarum: A Potential Gastric Health Dietary Supplement. Foods. 2025; 14(3):406. https://doi.org/10.3390/foods14030406
Chicago/Turabian StyleGu, Lei, Yiying Tang, Jieshuai Zhang, Ningping Tao, Xichang Wang, Liping Wang, and Changhua Xu. 2025. "Synergistic Anti-Helicobacter pylori Effects of Takifugu obscurus Skin Peptides and Lactobacillus plantarum: A Potential Gastric Health Dietary Supplement" Foods 14, no. 3: 406. https://doi.org/10.3390/foods14030406
APA StyleGu, L., Tang, Y., Zhang, J., Tao, N., Wang, X., Wang, L., & Xu, C. (2025). Synergistic Anti-Helicobacter pylori Effects of Takifugu obscurus Skin Peptides and Lactobacillus plantarum: A Potential Gastric Health Dietary Supplement. Foods, 14(3), 406. https://doi.org/10.3390/foods14030406





