Abstract
In this study, C. pyrenoidosa were cultured with seven different concentrations of Na2SeO4 (0–10 mg/L), and the effects of Na2SeO4 on the growth, Se-forms, and nutritional quality of C. pyrenoidosa were explored. The results showed that at the concentration of 0.5 mg/L Na2SeO4, the C. pyrenoidosa were plump and healthy; the contents of biomass, soluble protein, lipids, and TPUFA reached the highest level; the total Se content in C. pyrenoidosa increased with the increasing Na2SeO4 concentrations. However, the proportion of organic Se in C. pyrenoidosa. reached the highest value of 87.58% at the concentration of 0.5 mg/L Na2SeO4. Among organic Se forms, SeMet accounted for the largest proportion, while MeSeCys accounted for a relatively smaller proportion, but SeCys2 was not detected. The addition of Na2SeO4 (except for ≤0.5 mg/L) reduced the contents of photosynthetic pigments in C. pyrenoidosa. In addition, the antioxidant capacity of C. pyrenoidosa first increased and then decreased with the increasing Na2SeO4 concentrations, but different enzymes exhibited different tolerances to Na2SeO4. Based on the above research results, 0.5 mg/L Na2SeO4 concentration is recommended for the production of Se-rich C. pyrenoidosa. Our findings will provide a theoretical basis and practical references for the development of Se-rich C. pyrenoidosa health care products.
1. Introduction
Selenium (Se) is an essential trace element for human life activities. It plays important biological functions, like antioxidant, immune regulation, thyroid hormone metabolism participation, and reproductive support [1,2,3]. Generally, humans mainly replenish Se by taking in organic Se. Selenomethionine (SeMet), selenocysteine (SeCys), and selenocystine (SeCys2), which are formed due to Se, substitute the sulfur in methionine, cysteine, and cystine, respectively [4]. They are not only incorporated into protein synthesis but also serve as a source of Se for the synthesis of Selenoproteins, which play important roles in antioxidant and redox biology (methionine-sulfoxide reductase (MsrB1), thioredoxin reductases (Txnr) and glutathione peroxidases (GSH-Px) and modulate ROS levels (Selenoprotein T, Selenoprotein S and Selenoprotein P) [5,6]. However, Se mainly exists in the inorganic form in nature, which is highly toxic with low digestion and absorption. Therefore, inorganic Se needs to be converted into organic Se by organisms [7]. In recent years, the microalgae represented by C. pyrenoidosa have become a hot topic of research on Se-rich foods because the C. pyrenoidosa are rich in protein, polyunsaturated fatty acids, and carotenoids, and they can efficiently absorb inorganic Se in the environment and convert it into organic Se [8,9,10].
In current studies, the most common method for algal Se enrichment is to add exogenous sodium selenite (Na2SeO3) or Na2SeO4 as a Se source into algal growth environments [11,12]. The absorption rate and tolerance rate of different algae to Na2SeO3 and Na2SeO4 vary greatly. For example, Guimarães et al. [13] have found that the absorption and utilization rate of Na2SeO3 is four times as much as that of Na2SeO4 via Nannochloropsis oceanica. In contrast, several studies also found that the absorption and utilization rates of Na2SeO4 were higher than those of Na2SeO3 via Chlamydomonas reinhardtii and Chlorella vulgaris [14,15,16]. Although many studies have reported the absorption and conversion mechanisms of Na2SeO3 via C. pyrenoidosa [8,9,10,17], few reports on the absorption rate and conversion efficiency of Na2SeO4 via C. pyrenoidosa are available. Zhao et al. [9] found that both total Se and organic Se content increased with the increasing Na2SeO3 concentration in C. pyrenoidosa, but the organic Se conversion rate showed a gradual decrease trend. The Hubei Provincial Health Commission stipulated in 2022 that the proportion of organic Se in safe Se-rich products must be more than 80% [18]. Previous studies have shown that organic Se in algal cells mainly has three forms, namely, SeMet, SeCys, and SeCys2 [13,19]. Therefore, when producing Se-rich products, the total Se content in the products, especially the form, content, and proportion of organic Se, should be taken into account. However, so far, the effect of Na2SeO4 on the C. pyrenoidosa has not been studied.
The growth status and nutritional quality of the algae are very important for the production of Se-rich foods. Previous studies showed that Se has a dual effect on the growth of algae. High concentrations of Se will inhibit algae growth, as well as reduce its’ antioxidant capacity by decreasing the activities of the superoxide dismutase (SOD) and catalase (CAT), and GSH-Px [8,9]. Excessive concentrations of Se can also reduce the nutritional quality of algae by reducing the synthesis of the protein, total unsaturated fatty acids (TUFAs), photosynthetic pigments, and others in algae [8,19]. In this study, for the first time, C. pyrenoidosa was used as a Se-rich carrier; Na2SeO4 was used as an exogenous Se; and the BG11 nutrient solution containing different concentrations of Na2SeO4 was employed as a medium to culture C. pyrenoidosa so as to explore the effects of different Na2SeO4 concentrations on the growth, health status, and nutritional quality of C. pyrenoidosa. Our findings will provide a theoretical basis for the development of nutritious and healthy Se-rich algal.
2. Materials and Methods
2.1. Experimental Materials and Reagents
C. pyrenoidosa (FACHB-9) and BG11 nutrient solutions were purchased from the Institute of Hydrobiology, Chinese Academy of Sciences (Wuhan, China). The Na2SeO4 was purchased from Shandong West Asia Chemical Co., Ltd. (Liaocheng, China). (MDA, BC0020), (SOD, BC0170), (POD, BC0090), (CAT, BC0200), and (GSH-Px, BC1170) detection kits were obtained from Solaibao Technology Co., Ltd. (Beijing, China).
2.2. Culture and Collection of C. pyrenoidosa
The nutrient solution with a glucose concentration of 5 g/L and a carbon–nitrogen ratio of 5:1 was prepared by adding glucose and sodium nitrate to the BG11 basic nutrient solution. The nutrient solution was poured into a 250 mL triangular flask and added with the Na2SeO4 solution to pre-prepare the final volume of the 100 mL of culture solution containing 0, 0.2, 0.5, 1, 3, 5, and 10 mg/L Na2SeO4, respectively. Each treatment has three replicates, and three independent experiments were carried out. The culture solution with the initial pH 6.0 was sterilized in a sterilizing pot at 121 °C for 20 min and inoculated with C. pyrenoidosa at the exponential growth phase (initial optical density OD680 = 0.1, dry weight ≈ 0.0195 g/L) on a sterile operating table. The C. pyrenoidosa was incubated at 28 °C in a horizontal constant-temperature shaker (180 r/min) with a light intensity of 8000 lux and a photo period of 24 h light: 0 h dark for 5 d. The C. pyrenoidosa were collected at the plateau stage, and its relevant indicators were detected.
2.3. Determination of C. pyrenoidosa Biomass and Growth Inhibition Rate
The biomass of C. pyrenoidosa was measured by dry weight method, as described by Zhao et al. [20]. The collected fresh C. pyrenoidosa were washed three times with ultrapure water. When the Se was undetectable in the supernatant by a hydride generation atomic fluorescence spectrometer (the method detection limit was 0.01 μg/L), the C. pyrenoidosa were freeze-dried, and the dry weight was weighed with an analytical balance. The growth inhibition rate of C. pyrenoidosa was calculated according to formula (1): growth inhibition rate (%) = (Nc − Nt)/Nc × 100%. In the formula, Nc represents the final biomass of the C. pyrenoidosa in the control group, and Nt indicates the final biomass of C. pyrenoidosa under Se-addition treatments.
2.4. Determination of Se Forms in C. pyrenoidosa
In total, 0.5 g of dry C. pyrenoidosa was microwave-digested with HNO3–HClO4 (4:1) mixed acid. The total Se content was measured using a hydride generation atomic fluorescence spectrometer via the previously reported method in our lab [21]. The contents of SeMet, MeSeCys, and SeCys2 in C. pyrenoidosa were determined by Stander Kechuang Pharmaceutical Technology Co., LTD. (Qingdao, China) using a liquid chromatograph-mass spectrometer. Briefly, the 0.5 g of dry C. pyrenoidosa were mixed with 5 mL of the pepsin XIV solution and subjected to ultrasonic treatment in a 37 °C water bath for 1 h. After extraction, the supernatant was obtained via centrifugation and filtered by a 0.45 μm filter membrane before analysis. The contents of MeSeCys, SeMet, and SeCys2 in C. pyrenoidosa were determined using standard curves prepared under the same conditions, with the known concentrations of MeSeCys, SeMet, and SeCys2, respectively. The volatile Se content was determined using the following equation, as reported in the study of Wang et al. [19]: Cvolatile = Cinitial − Cmedium − Calgae. Cinitial represent the initial concentration of the Se added, Cmedium represents the residue concentration of Se in the medium, and Calgae represents the total Se content in C. pyrenoidosa.
2.5. Microscopic and Ultrastructural Observation of C. pyrenoidosa
The microstructure observation of C. pyrenoidosa under the concentrations of 0, 0.5, and 1 mg/L Na2SeO4 was performed by using the method previously reported with a minor modification [22]. Specifically, the C. pyrenoidosa was fixed with Lugol’s reagent for 5 min and observed under a microscope at ×400 times magnification, and the diameter of the C. pyrenoidosa was measured. The scanning electron microscopy (SEM) samples of C. pyrenoidosa were pre-processed, referring to Zheng et al. [23]. The apparent morphology of the processed C. pyrenoidosa sample was observed under a biological scanning electron microscope (JSM-6390LV) at ×8000 times magnification. The C. pyrenoidosa transmission electron microscopy (TEM) samples were pre-processed, referring to [20]. The processed C. pyrenoidosa TEM samples were observed with a 100 KV transmission electron microscope (H-7650) at ×15,000 times magnification.
2.6. Analysis of Nutritional Components of C. pyrenoidosa
The C. pyrenoidosa extract was obtained by using the method previously reported [24]. Briefly, 0.1 g of dry C. pyrenoidosa was mixed with 3 mL of the 3% potassium hydroxide solution, subjected to a 90 min boiling water bath, and C. pyrenoidosa were ultrasonicated for 9 min with an interval of 2 s between 4 s ultrasonication, and then C. pyrenoidosa extract was centrifuged. Subsequently, the soluble protein content was determined by using the Coomassie brilliant blue method. The content of total polysaccharides was determined by using the phenol-sulfuric acid method. The total lipid of C. pyrenoidosa was determined by using the method previously reported with slight modifications [25]. Specifically, 0.2 g of dry C. pyrenoidosa was resuspended in 3 mL of the mixed solution (chloroform: methanol: water volume ratio = 1:2:0.8), ultrasonically extracted for 15 min, then added with 1 mL of chloroform and 1 mL of distilled water, vortexed, stood for 1 min, and centrifuged at 6580× g for 5 min to collect the chloroform layer. The extraction process was repeated once. The chloroform layers from two extractions were combined and dried with nitrogen at 45 °C, and the oil content in the chloroform layers was determined by using the differential method. The fatty acid composition and component contents of C. pyrenoidosa were determined by Stander Kechuang Pharmaceutical Technology Co., Ltd. (Qingdao, China) using the gas chromatograph mass spectrometer. Briefly, the 0.2 g C. pyrenoidosa dry samples were subjected to hydrolysis, fat extraction, fat saponification, and fatty acid methyl esterification before measurements. The determination methods were used according to the previous reference described by Honicky et al. [26].
2.7. Determination of Photosynthetic Pigments
The photosynthetic pigments Chla, Chlb, and carotenoids in C. pyrenoidosa were measured according to previously reported method [27]. Specifically, 5 mL of the fresh C. pyrenoidosa samples were centrifuged for 5 min at 4110× g, and the supernatant was removed. Afterward, the C. pyrenoidosa were washed three times with distilled water and added with 5 mL methanol to extract photosynthetic pigments in the dark at 4 °C for 24 h. After further centrifugation, the absorbance at 665, 652, and 470 nm of Chla, Chlb, and the carotenoid in the supernatant was measured with a UV-visible spectrophotometer, respectively, and the concentration of each pigment (mg/L) was calculated according to the following formulae: Chla (mg/L) = 15.65 × A665 − 7.340 × A652; Chlb (mg/L) = 34.09 × A652 − 15.28 × A665; Carotenoid (mg/L) = (1000 × A470 − 1.633 × Chla − 104.9 × Chlb)/221.
2.8. Determination of MDA and Antioxidant Enzyme Activity
The C. pyrenoidosa were centrifuged at 4110× g for 10 min, and the supernatant was discarded. The precipitate was rinsed repeatedly with deionized water until the medium attached to the algae was completely removed. The C. pyrenoidosa precipitate was placed in a pre-cooled phosphate buffer solution (PBS) and ultrasonically disrupted on ice, and the homogenate was centrifuged at 8220× g for 30 min. The supernatant was collected for enzyme activity measurement. The total protein concentration in the supernatant was determined using the Coomassie brilliant blue method. The MDA content and enzyme activity of SOD, POD, CAT, and GSH-Px in C. pyrenoidosa were detected according to the kit’s instructions.
2.9. Data Analysis
One-way analysis of variance of the obtained data was performed using GraphPad Prism 9.5 statistical software. Each experiment was repeated three times. All the data were expressed as a mean ± standard deviation (SD) of three biological replicates (n = 3). p < 0.05 was considered as statistically significant.
3. Results
3.1. Effect of Na2SeO4 on Growth of C. pyrenoidosa
With the increasing concentration of Na2SeO4, the color of the C. pyrenoidosa liquid gradually changed from dark green to light green. At a concentration of 10 mg/L Na2SeO4, algal liquid turned to light yellow, which was accompanied by the production of red substances (Figure 1A). As shown in Figure 1B,C, with the increasing concentration of Na2SeO4, the biomass of C. pyrenoidosa at 5 d first increased (0–0.5 mg/L) and then decreased (1–10 mg/L). The growth inhibition ratios of C. pyrenoidosa were −0.94%, −6.02%, 15.72%, 43.22%, 74.04%, and 84.10% under the concentrations of 0.2, 0.5, 1, 3, 5, and 10 mg/L Na2SeO4, respectively (Figure 2C). Theirs’ regression equation was obtained as follows:
y = −0.0118x2 + 0.2126x − 0.0652 (R2 = 0.9803)
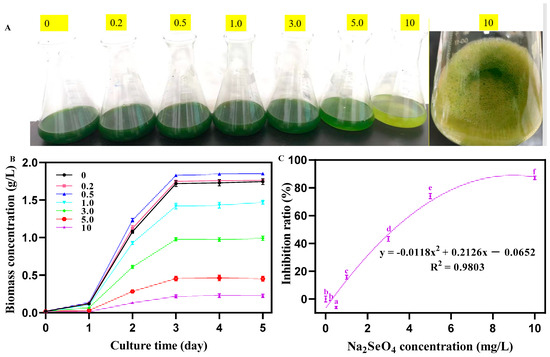
Figure 1.
Growth status of C. pyrenoidosa under different concentrations of Na2SeO4. (A) Color of the algal liquid under different concentrations of Na2SeO4. (B) Biomass of C. pyrenoidosa under different concentrations of Na2SeO4. (C) Growth inhibition curve of C. pyrenoidosa under different concentrations of Na2SeO4. Data are expressed as mean ± SD (standard deviation, n = 3). Different lowercase letters represent significant differences. p ≤ 0.05 is considered statistically significant.
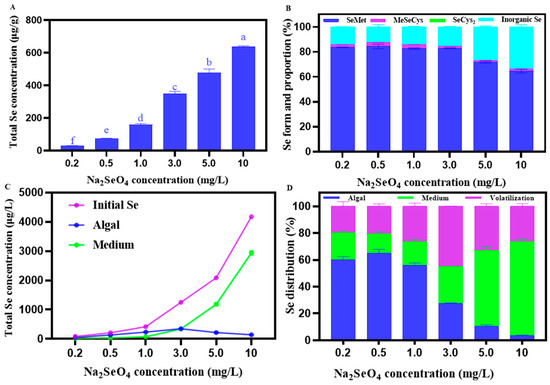
Figure 2.
(A) Total Se content in C. pyrenoidosa under different Na2SeO4 addition concentrations. (B) Different Se forms and their proportions in C. pyrenoidosa under different Na2SeO4 addition concentrations. (C) Total Se content in the solution before Se absorption by C. pyrenoidosa (blue), the remaining solution after C. pyrenoidosa extraction (purple), C. pyrenoidosa (green). (D) Se distribution in the algae-water system under different Na2SeO4 addition concentrations. The volatile Se content was determined using the following equation: C volatile = Cinitial − Cmedium − Calgae. Data are expressed as mean ± SD (standard deviation) of three biological replicates. Different lowercase letters represent significant differences. p ≤ 0.05 is considered as significantly different.
3.2. Effect of Na2SeO4 Concentrations on Se Forms in C. pyrenoidosa
The total Se content in C. pyrenoidosa increased with the increasing Na2SeO4 concentrations, reaching the highest value of 638.36 ± 4.45 μg/g at the concentration of 10 mg/L Na2SeO4 (Figure 2A). As shown in Figure 2B, Se mainly existed in the form of organic Se in C. pyrenoidosa. With the increasing concentrations of Na2SeO4, the proportion of organic Se remained stable at (0–1.0 mg/L) and significantly decreased when the addition of Na2SeO4 > 3.0 mg/L. At a concentration of 0.5 mg/L Na2SeO4, the proportion of organic Se in C. pyrenoidosa reached the highest value of 87.58%, while at the concentration of 10 mg/L Na2SeO4, the lowest value of 66.11% was observed. Among organic Se forms, SeMet accounted for the largest proportion in Se, while MeSeCys accounted for a relatively smaller proportion, but SeCys2 was not detected. For example, at the concentration of 0.5 mg/L Na2SeO4, SeMet, and MeSeCys, respectively, accounted for 84.35 ± 1.81% and 3.22 ± 0.12% in the total Se of C. pyrenoidosa, with their contents being 61.84 ± 3.75 μg/g and 2.36 ± 0.18 μg/g, respectively. As shown in Figure 2C, at the concentration of 0–3.0 mg/L Na2SeO4, more Na2SeO4 was absorbed by the C. pyrenoidosa, relative to the Na2SeO4 remaining in the solution, and at the concentration of 3.0–10 mg/L Na2SeO4, the opposite trend was observed. As shown in Figure 2D, the percentage of Na2SeO4 absorbed by C. pyrenoidosa showed an initial increase and then a decrease with the increase in the applied Na2SeO4 concentration, reaching the highest value of 64.93 ± 2.31% and the lowest value of 3.44 ± 0.27% at 0.5 mg/L and 10 mg/L Na2SeO4 of the addition concentrations, respectively. Similarly, the percentage of volatile Na2SeO4 in C. pyrenoidosa showed a trend of first increasing and then decreasing with the increasing Na2SeO4 addition concentration, reaching the highest value of 45.03 ± 0.07% and the lowest value of 19.75 ± 2.71% at 3 mg/L and 0.2 mg/L Na2SeO4 concentrations, respectively. In contrast, the percentage of Na2SeO4 remaining in the solution first showed a decreasing and then an increasing trend, with the increasing Na2SeO4 concentration reaching the lowest value of 14.62 ± 0.99%, and the highest value of 70.32 ± 1.58% at the concentrations of the 0.5 mg/L and 10 mg/L Na2SeO4 concentrations, respectively.
3.3. Microscopic and Ultrastructural Observations of C. pyrenoidosa
As shown in Figure 3A–C, the microscopic observation results showed that under no Na2SeO4 treatment, the number of C. pyrenoidosa with the diameter range of 1–3 μm and 3–5 μm was 47 and 11, respectively (Table 1); under the 0.5 mg/L Na2SeO4 treatment, the number of C. pyrenoidosa with diameter ranges of 1–3 μm and 3–5 μm was 12 and 37, respectively (Table 1). Under the 1.0 mg/L Na2SeO4 treatment, the number of C. pyrenoidosa with diameter ranges of 1–3 μm and 3–5 μm was 22 and 11, respectively (Table 1), accompanied by C. pyrenoidosa cell splitting (a), cell agglomeration (b), and cavity phenomena (c) of C. pyrenoidosa. SEM observation showed that the surface of C. pyrenoidosa under 0 or 0.5 mg/L Na2SeO4 treatments was smooth, exhibiting obvious clear ridges, and the space interval between C. pyrenoidosa was appropriate without the particle structure (Figure 3D–F). Under the 1 mg/L Na2SeO4 treatment, the surface of the C. pyrenoidosa became rough; the ridges on the surface were relatively blurred; the C. pyrenoidosa membrane began to indent inward; the cell morphology became distorted; and fragmented structures and agglomeration phenomenon appeared between C. pyrenoidosa (Figure 3F). As shown in Figure 3G–L, TEM observation showed that under the 0 or 0.5 mg/L Na2SeO4 treatment, the C. pyrenoidosa cell wall (cw) and cell membrane (cm) edges were relatively neat, smooth, and closely connected; chloroplasts (ch) occupied almost the entire cell, and leaf-shaped chloroplasts were close to the periphery of the C. pyrenoidosa; thylakoids (th), taking on lamellar layers, were distributed in the chloroplasts; in the cytoplasm, the pyrenoids (p) were clearly visible, which were surrounded by a layer of evenly distributed starch granules. As shown in Figure 3L, when C. pyrenoidosa were treated with a concentration of 1 mg/L Na2SeO4, the C. pyrenoidosa membrane gradually became rough, indented, or even disrupted; the cytoplasm–cell wall separation phenomenon gradually became serious; the starch (st) granules gradually became larger, thus gradually crowding out thylakoids and occupying their spatial position in chloroplasts; the lamellar structure of thylakoids became too fuzzy to be distinguished, and the shape was distorted; the pyrenoid became slightly larger; and the volume of lipid (li) droplets became larger.
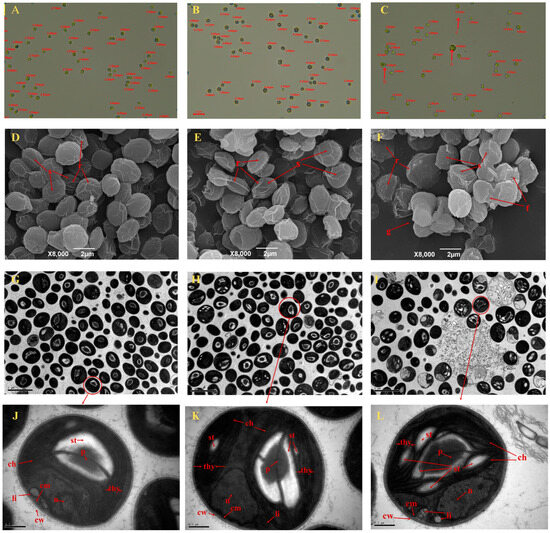
Figure 3.
Representative diagram of microscopic and ultrastructural observations of C. pyrenoidosa. (A–C) Microscopic observation of the diameter and structure of C. pyrenoidosa at different concentrations of Na2SeO4 under the optical microscope: a, splitting; b, agglomeration; c, cavity phenomenon. (D–F) Scanning electron microscopy photos of C. pyrenoidosa at concentrations of 0, 0.5, and 1.0 mg/L Na2SeO4, respectively. s, cell surface; r, ridge of the cell; f, cell fragment; g, cell aggregates. (G–I) Transmission electron microscopy photos of C. pyrenoidosa at concentrations of 0, 0.5, and 1.0 mg/L Na2SeO4, respectively (8000 times magnification). (J–L) Ultrastructure of C. pyrenoidosa at 0, 0.5, and 1.0 mg/L Na2SeO4 concentrations, respectively (15,000 times magnification). p, pyrenoids; st, starch plate; thy, thylakoids; ch, chloroplast; n, nucleus; li, lipid droplet; cw, cell wall; and cm, cell membrane.

Table 1.
Diameter of C. pyrenoidosa under different Na2SeO4 concentrations.
3.4. Nutritional Components of C. pyrenoidosa
The soluble protein content in C. pyrenoidosa first showed a trend of increasing (0–0.5 mg/L) and then decreasing (0.5–10 mg/L), with the increasing concentration of applied Na2SeO4 (Figure 4A). The polysaccharide content in C. pyrenoidosa showed an increasing trend with the increase in the concentration of Na2SeO4 applied (Figure 4B). The lipid content in C. pyrenoidosa exhibited a trend of first increasing (0–1.0 mg/L) and then decreasing (1.0–10 mg/L), with the increasing concentration of Na2SeO4 (Figure 4C). Table 2 showed that the relative contents of total unsaturated fatty acids (TUFAs), total poly unsaturated fatty acids (TPUFA)s, n-3 series unsaturated fatty acids, n-6 unsaturated fatty acids, eicosapentaenoic acid (EPA), and docosahexaenoic acid (DHA), in C. pyrenoidosa first increased and then decreased as the concentration of Na2SeO4 increased, reaching the highest at a concentration of 0.5 mg/L Na2SeO4. The relative contents of total monounsaturated fatty acids (TMUFAs) and total saturated fatty acids (TSFAs) exhibited an opposite change trend, reaching the lowest value at the 0.5 mg/L Na2SeO4 concentration.
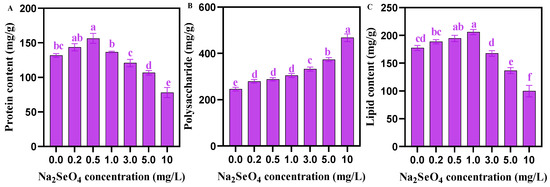
Figure 4.
Nutrients in C. pyrenoidosa under different Na2SeO4 treatments. (A) Soluble protein content of C. pyrenoidosa. (B) Total polysaccharide content of C. pyrenoidosa. (C) Total lipid content of C. pyrenoidosa. Data are expressed as mean ± SD (standard deviation) (n = 3). Different lowercase letters represent significant differences. p ≤ 0.05 is considered statistically significant.

Table 2.
Fatty acid composition and relative content (%) in C. pyrenoidosa under different concentrtions of Na2SeO4 treatments.
3.5. Analysis of Photosynthetic Pigment Content
The contents of Chla, Chlb, and carotenoids in C. pyrenoidosa showed no significant difference when the addition of Na2SeO4 under 0.5 mg/L. The addition of Na2SeO4 (1.0–10 mg/L) significantly reduced the contents of Chla, Chlb, and carotenoids in C. pyrenoidosa (Figure 5).
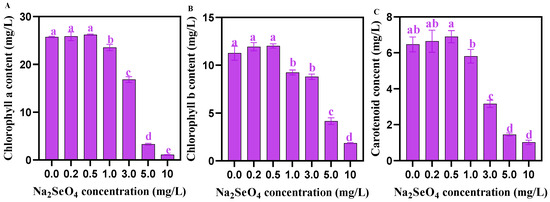
Figure 5.
Chlorophyll contents in C. pyrenoidosa under different Na2SeO4 treatments. (A) Chla content in C. pyrenoidosa. (B) Chlb content in C. pyrenoidosa. (C) Carotenoid content in C. pyrenoidosa. Data are expressed as mean ± SD (standard deviation, n = 3). Different lowercase letters represent significant differences. p ≤ 0.05 is considered statistically significant.
3.6. Activities of Antioxidant Enzymes in C. pyrenoidosa Under Different Concentrations of Na2SeO4 Treatments
As shown in Figure 6, at the Na2SeO4 addition concentration of 0–1.0 mg/L, the content of MDA did not change significantly, but when Na2SeO4 concentration was ≥1 mg/L, the content of MDA increased with the increase in Na2SeO4 concentration. The activity of GSH-Px in C. pyrenoidosa increased with the increase in Na2SeO4 concentration, but the activities of SOD, CAT, and POD first increased (0–3.0 mg/L Na2SeO4) and then decreased (3.0–10 mg/L Na2SeO4).
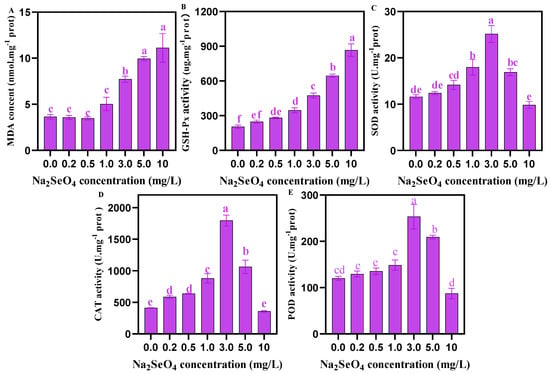
Figure 6.
Activities of antioxidant enzymes in C. pyrenoidosa under different concentrations of Na2SeO4 treatments. (A) MDA content in C. pyrenoidosa. (B) GSH-Px activity in C. pyrenoidosa. (C) SOD activity in C. pyrenoidosa. (D) CAT activity in C. pyrenoidosa. (E) POD activity in C. pyrenoidosa. Data are expressed as mean ± SD (standard deviation) (n = 3). Different lowercase letters represent significant differences. p ≤ 0.05 is considered statistically significant.
4. Discussion
Se has the dual functions of promoting growth and inhibiting toxicity of algae. In this study, we found that 0–0.5 mg/L Na2SeO4 promoted the growth of C. pyrenoidosa, while 1–10 mg/L Na2SeO4 inhibited its growth. Our microscopic and electron microscopic observations found that the addition of 0.5 mg/L Na2SeO4 resulted in plump C. pyrenoidosa. For example, the number of C. pyrenoidosa with the diameter range of 3–5 μm were 11, 37, and 11 under 0, 0.5, and 1.0 mg/L Na2SeO4 treatments, respectively (Figure 3A–C and Table 1). However, when the Na2SeO4 addition concentration was ≥1 mg/L, splitting, agglomeration, and cavity phenomena of C. pyrenoidosa were observed; the C. pyrenoidosa cell surface became rough; the C. pyrenoidosa membrane began to indent; and the thylakoid lamellar structure became blurred, which was accompanied by some other symptoms. The reason might be that the C. pyrenoidosa relieve Se toxicity and secrete some reducing substances that react with the Na2SeO4 on the C. pyrenoidosa cell surface, making C. pyrenoidosa surface rough, thus enhancing the light-shading effect on the algae body, ultimately affecting the structures of chloroplasts and the thylakoids [28]. The stable MDA level and the increased enzyme activities of GSH-Px, SOD, and CAT of C. pyrenoidosa under 0.5 mg/L Na2SeO4 treatment in the present study also supported the above results. In addition, when Na2SeO4 was ≥10 mg/L, red elemental Se appeared in the algae solution, which might be due to the reduction in oxidized Se to monomeric Se by C. pyrenoidosa, thus alleviating the oxidative damage caused by high Na2SeO4 concentration to the C. pyrenoidosa [29].
Although some studies on Se-rich C. pyrenoidosa have been conducted, most of them focus on total Se enrichment in C. pyrenoidosa [8,9,10], and report that the contents, Se-forms, and proportions of organic Se in C. pyrenoidosa are very limited. This study found that the total Se content of C. pyrenoidosa increased with the increase in the added Na2SeO4 concentration. However, as the concentration of Na2SeO4 increased, the proportion of organic Se in C. pyrenoidosa kept stable at (0–1.0 mg/L), and then decreased (3.0–10 mg/L); SeMet in organic Se accounted for the largest proportion; MeSeCys accounted for a smaller proportion; and SeCys2 was not detected. At the concentration of 0.5 mg/L Na2SeO4, the proportion of organic Se in C. pyrenoidosa was as high as 87.58%, and the content of SeMet was 61.84 μg/g. Studies reported that over 50% of dietary Se-supplementation for humans is in the form of SeMet [6]. In microalgae, SeMet, SeCys, and MeSeCys can be mutually converted through the Se metabolic pathway [19,30]. Usually, microalgae take up inorganic Se via sulfur-transport channels. Subsequently, they synthesize selenocysteine (SeCys) to fulfill their nutritional needs. Nevertheless, SeCys is further transformed into selenomethionine (SeMet). This process continues until methylation leads to the formation of volatile dimethyl selenide or dimethyl diselenide, which helps to reduce the accumulation of Se within the cells [31]. In this study, the content of SeMet was higher than that of SeCys2 and MeSeCys in the C. pyrenoidosa, which has also been reported in other studies by Mylenko et al. [7] and Guimarães et al. [32]. Similarly, Zhao et al. [9] found that the total Se content and organic Se content in C. pyrenoidosa increased with the increasingly applied Na2SeO3 concentration, but the organic Se conversion rate showed a gradual decreasing trend. Interestingly, under Na2SeO3 treatment, the organic Se form in Tribonema minus was mainly SeCys, followed by SeMet, which may be related to the mechanisms underlying different tolerances of different algae species to Se [19]. Our data also show that in the algae-water system, the proportion of Na2SeO4 in the remaining solution (after C. pyrenoidosa. extraction) showed a trend of first decreasing (0–0.5 mg/L) and then increasing (1.0–10 mg/L) as the Na2SeO4 concentration increased. Under the 0.5 mg/L Na2SeO4 treatment, the proportion of Se in the residual solution was reduced to 14.62%. These studies showed that the production of Se-rich C. pyrenoidosa, with a 0.5 mg/L concentration of Na2SeO4 can also reduce the Se residual solution pollution.
Proteins, lipids, and polysaccharides are the main organic nutrients in C. pyrenoidosa. This study found that compared with the control group, the protein and lipid contents in C. pyrenoidosa increased at a concentration of 1.0 mg/L Na2SeO4. This result was verified by our electron microscopic observation of the obvious increase in lipid droplets (Figure 3L). The possible reason for this result might be that the oxidative damage caused by Na2SeO4 needs to be repaired by antioxidant enzyme system, and thus, C. pyrenoidosa. have to synthesize more lipids to maintain the energy required for life activities [33]. Our data show that ≥ 3.0 mg/L Na2SeO4 concentration led to a significant decrease in protein and lipid contents, thus probably leading to the sub-health status or even death of C. pyrenoidosa. This result is consistent with previous research reports on other algae [19,20]. In contrast, accompanied by the significant decrease in protein and lipid contents, the polysaccharide content in C. pyrenoidosa continuously significantly increased with the increasing Na2SeO4 concentration, especially at the concentration of 3.0–10 mg/L Na2SeO4. The potential reason might be that the C. pyrenoidosa re-decomposed extra proteins other than those used for stress response and extra lipids, and other than those used for survival, reproduction, and membrane system construction into polysaccharides, so as to maintain the energy required for the life activities of the C. pyrenoidosa [34,35,36]. Previous research has indicated that C. pyrenoidosa can regulate the accumulation of its own proteins, polysaccharides, and lipids to adapt to different environments [37]. In addition, DHA- and EPA-rich algae oil is widely recognized as playing an important role in maintaining health and preventing cardiovascular and cerebrovascular diseases in organisms [38,39]. In this study, we observed the maximum relative contents of the TUFAs, essential fatty acids (EFA), n-3 series unsaturated fatty acids, n-6 series unsaturated fatty acids, unsaturated fatty acids, EPA, and DHA in C. pyrenoidosa cultured under the 0.5 mg/L Na2SeO4 treatment. Consistently, Pires et al. [16] have also found that the addition of appropriate amounts of Se can promote the synthesis of total unsaturated fatty acids in C. pyrenoidosa. In aquaculture, studies reported that C. pyrenoidosa are an excellent source of polyunsaturated fatty acids (PUFAs), particularly the n-3 series, which can replace fish oil and fish meal in diets [40,41].
Photosynthetic pigments are crucial for algae cells to absorb and convert light energy, and their contents are an important physiological indicator, reflecting the photosynthetic ability and the health status of algal cells [42]. In addition, photosynthetic pigments can also serve as a non-enzymatic antioxidant system to scavenge excited oxygen molecules and protect the photosynthetic membrane system [43]. In this study, we found that ≤0.5 mg/L exogenous Na2SeO4 concentration did not affect the synthesis of photosynthetic pigments in C. pyrenoidosa, while ≥1.0 mg/L Na2SeO4 concentration inhibited the synthesis of photosynthetic pigments in C. pyrenoidosa, and under ≥1.0 mg/L Na2SeO4 treatment, the changing trend of photosynthetic pigments in C. pyrenoidosa was highly consistent with that of C. pyrenoidosa biomass. These results are consistent with some previous reports [8,19]. Furthermore, our TEM results also showed that the chloroplast structure of C. pyrenoidosa was intact at ≤0.5 mg/L Na2SeO4 concentration, but it was destroyed at ≥1.0 mg/L Na2SeO4 concentration, further supporting that ≤0.5 mg/L exogenous Na2SeO4 was safe for C. pyrenoidosa.
MDA is an important parameter, reflecting the degree of peroxidative damage to C. pyrenoidosa. In this study, when the exogenous Na2SeO4 concentration was ≤0.5 mg/L, the content of MDA in C. pyrenoidosa did not change significantly. GSH-Px, SOD, CAT, and POD are the antioxidant enzymes which help to eliminate oxidative damage from the algae body. In this study, at ≤3.0 mg/L Na2SeO4 concentrations, the activities of GSH-Px, SOD, CAT, and POD in C. pyrenoidosa were increased with the increase in Na2SeO4 concentration, which might be because Se-stress will stimulate major enzymes represented by GSH-Px, SOD, CAT, and POD in the enzymatic antioxidant system to alleviate oxidative damage to C. pyrenoidosa caused by Na2SeO4. When Na2SeO4 was ≥5.0 mg/L, the activities of SOD, CAT, and POD enzymes in C. pyrenoidosa were decreased with the increasing concentration of Na2SeO4. The possible reason might be that excessive Na2SeO4 has exceeded the ultimate tolerance of the C. pyrenoidosa, thus resulting in the failure of the enzymatic antioxidant system. Our results are consistent with some previous reports [19,44,45]. Interestingly, when Na2SeO4 was ≥5.0 mg/L, GSH-Px enzyme activity increased with the increase in Na2SeO4 concentration, which might be explained by the higher Se tolerance of GSH-Px since Se is the enzyme active center of GSH-Px. Our results were supported by some previous findings that the higher Se stress intensity, the higher GSH-Px activity in C. pyrenoidosa [19].
5. Conclusions
In this study, for the first time, we explored the effects of Na2SeO4 on the C. pyrenoidosa growth, Se-form, and nutritional quality of C. pyrenoidosa. The results indicated that Na2SeO4 exerted a dose-dependent effect on the growth of C. pyrenoidosa. The growth of C. pyrenoidosa promoted under 0.5 mg/L Na2SeO4, while it inhibited ≥1 mg/L Na2SeO4. At the concentration of 0.5 mg/L Na2SeO4, C. pyrenoidosa were plump and healthy; the content and proportion of organic-Se reached 64.20 μg/g and 87.58%; the main component of organic-Se is SeMet; the contents of biomass, soluble protein, lipids, and TPUFA reached the highest level. On the other hand, a 0.5 mg/L concentration of Na2SeO4 can also reduce the Se residual solution pollution. However, the addition of high concentration Na2SeO4 could inhibit the growth of C. pyrenoidosa, reduce the conversion rate of organic Se, and decrease the contents of protein and lipid. Overall, we recommended 0.5 mg/L Na2SeO4 for the production of Se-rich C. pyrenoidosa, which can not only produce high-quality C. pyrenoidosa but also environmental protection. Our findings will provide a theoretical basis for the development of algal health care products.
Author Contributions
X.Z.: Formal analysis, investigation, methodology, and writing—original draft. J.J.: Investigation, methodology, and writing—original draft. S.Y., H.S. and Y.Z.: Investigation, formal analysis, and methodology. Q.Z., Z.Z. and D.Y.: Methodology, supervision, and writing—review and editing. M.Z.: Conceptualization, funding acquisition, project administration, supervision, and writing—review and editing. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by [the National Natural Science Foundation of China] grant number [32373156 and 42106147]. And the APC was funded by [32373156].
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The original contributions presented in this study are included in the article. Further inquiries can be directed to the corresponding author.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (Grant No. 32373156) and (Grant No. 42106147).
Conflicts of Interest
The authors declare that they have no competing interests.
Abbreviations
CAT, catalase; Chla, chlorophyll a; Chlb, chlorophyll b; C. pyrenoidosa, Chlorella pyrenoidosa; GSH-Px, glutathione peroxidase; MDA, malonaldehyde; MeSeCys, methylselenocysteine; Na2SeO4, sodium selenate; Na2SeO3, sodium selenite; POD, peroxidase; PBS, phosphate buffer solution; Se, Selenium; SEM, scanning electron microscopy; SeCys2, selenocystine; SeCys, selenocysteine; SOD, superoxide dismutase; SeMet, selenomethionine; TEM, transmission electron microscopy.
References
- Schweizer, U.; Fradejas-Villar, N. Why 21? The significance of selenoproteins for human health revealed by inborn errors of metabolism. FASEB J. 2016, 30, 3669–3681. [Google Scholar] [CrossRef] [PubMed]
- Schiavon, M.; Ertani, A.; Parrasia, S.; Vecchia, F.D. Selenium accumulation and metabolism in algae. Aquat. Toxicol. 2017, 189, 1–8. [Google Scholar] [CrossRef]
- Dabravolski, S.A.; Sukhorukov, V.N.; Melnichenko, A.A.; Khotina, V.A.; Orekhov, A.N. The Role of Selenium in Atherosclerosis Development, Progression, Prevention and Treatment. Biomedicines 2023, 11, 2010. [Google Scholar] [CrossRef] [PubMed]
- Gojkovic, Ž.; Vílchez, C.; Torronteras, R.; Vigara, J.; Gómez-Jacinto, V.; Janzer, N.; Gómez-Ariza, J.-L.; Márová, I.; Garbayo, I. Effect of Selenate on Viability and Selenomethionine Accumulation of Chlorella sorokiniana Grown in Batch Culture. Sci. World J. 2014, 2014, 401265. [Google Scholar] [CrossRef] [PubMed]
- Kryukov, G.V.; Castellano, S.; Novoselov, S.V.; Lobanov, A.V.; Zehtab, O.; Guigó, R.; Gladyshev, V.N. Characterization of Mammalian Selenoproteomes. Science 2003, 300, 1439–1443. [Google Scholar] [CrossRef]
- Schrauzer, G.N. The nutritional significance, metabolism and toxicology of selenomethionine. Adv. Food Nutr. Res. 2003, 47, 73–112. [Google Scholar]
- Mylenko, M.; Vu, D.L.; Kuta, J.; Ranglová, K.; Kubác, D.; Lakatos, G.; Grivalsky, T.; Caporgno, M.P.; Manoel, J.A.C.; Kopecky, J.; et al. Selenium Incorporation to Amino Acids in Chlorella Cultures Grown in Phototrophic and Heterotrophic Regimes. J. Agric. Food Chem. 2020, 68, 1654–1665. [Google Scholar] [CrossRef] [PubMed]
- Zhong, Y.; Cheng, J.J. Effects of Selenite on Unicellular Green Microalga Chlorella pyrenoidosa: Bioaccumulation of Selenium, Enhancement of Photosynthetic Pigments, and Amino Acid Production. J. Agric. Food Chem. 2017, 65, 10875–10883. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.F.; Song, X.S.; Cao, X.; Wang, Y.H.; Si, Z.H.; Chen, Y. Toxic effect and bioaccumulation of selenium in green alga Chlorella pyrenoidosa. J. Appl. Phycol. 2019, 31, 1733–1742. [Google Scholar] [CrossRef]
- Li, Z.P.; Li, Y.Q.; Li, Y.W.; Yue, Y.; Guo, G.X.; Hu, X.Q. Cultivation of Chlorella pyrenoidosa for Selenium Enrichment. J. Guangdong Ocean. Univ. 2020, 40, 35–42. [Google Scholar]
- Kieliszek, M.; Blazejak, S.; Gientka, I.; Bzducha-Wróbel, A. Accumulation and metabolism of selenium by yeast cells. Appl. Microbiol. Biotechnol. 2015, 99, 5373–5382. [Google Scholar] [CrossRef] [PubMed]
- Yoshinaga, M.; How, S.; Blanco, D.; Murdoch, I.S.; Grudny, M.; Powers, S.L.; Molina, N.; Rosen, B.P.; Welch, A.Z. Directed Evolution of Saccharomyces cerevisiae for Increased Selenium Accumulation. Microorganisms 2018, 6, 14. [Google Scholar] [CrossRef]
- Guimaraes, B.O.; de Boer, K.; Gremmen, P.; Drinkwaard, A.; Wieggers, R.; Wijffels, R.H.; Barbosa, M.J.; D’Adamo, S. Selenium enrichment in the marine microalga Nannochloropsis oceanica. Algal Res. 2021, 59, 8. [Google Scholar] [CrossRef]
- Morlon, H.; Fortin, C.; Adam, C.; Garnier-Laplace, J. Cellular quotas and induced toxicity of selenite in the unicellular green alga Chlamydomonas reinhardtii. Radioprotection 2005, 40, S101–S106. [Google Scholar] [CrossRef][Green Version]
- Geoffroy, L.; Gilbin, R.; Simon, O.; Floriani, M.; Adam, C.; Pradines, C.; Cournac, L.; Garnier-Laplace, J. Effect of selenate on growth and photosynthesis of Chlamydomonas reinhardtii. Aquat. Toxicol. 2007, 83, 149–158. [Google Scholar] [CrossRef] [PubMed]
- Pires, R.; Costa, M.; Silva, J.; Pedras, B.; Concórdio-Reis, P.; Lapa, N.; Ventura, M. Se-enrichment of Chlorella vulgaris grown under different trophic states for food supplementation. Algal Res. 2022, 68, 10. [Google Scholar] [CrossRef]
- Qin, B.L.; Wang, Y.B.; Shan, J.F.; Ding, C.L. Effects of sodium selenite on growth and antioxidant enzyme activity of Chlorella proteinosa. J. Dalian Ocean. Univ. 2020, 35, 839–846. [Google Scholar]
- DBS42/002-2022; Food Safety Local Standard Selenium Content Requirements for Organic Selenium-Rich Food. Hubei Provincial Health Commission: Wuhan, China, 2022.
- Wang, F.F.; Li, Y.H.; Yang, R.D.; Zhang, N.; Li, S.Y.; Zhu, Z.Z. Effects of sodium selenite on the growth, biochemical composition and selenium biotransformation of the filamentous microalga Tribonema minus. Bioresour. Technol. 2023, 384, 9. [Google Scholar] [CrossRef]
- Zhao, T.; Tan, L.J.; Huang, W.Q.; Wang, J.T. The interactions between micro polyvinyl chloride (mPVC) and marine dinoflagellate Karenia mikimotoi: The inhibition of growth, chlorophyll and photosynthetic efficiency. Environ. Pollut. 2019, 247, 883–889. [Google Scholar] [CrossRef] [PubMed]
- Deng, X.F.; Liu, K.Z.; Li, M.F.; Zhang, W.; Zhao, X.H.; Zhao, Z.Q.; Liu, X.W. Difference of selenium uptake and distribution in the plant and selenium form in the grains of rice with foliar spray of selenite or selenate at different stages. Field Crop. Res. 2017, 211, 165–171. [Google Scholar] [CrossRef]
- Ameen, F.; Al-Homaidan, A.A.; Almahasheer, H.; Dawoud, T.; Alwakeel, S.; AlMaarofi, S. Biomonitoring coastal pollution on the Arabian Gulf and the Gulf of Aden using macroalgae: A review. Mar. Pollut. Bull. 2022, 175, 13. [Google Scholar] [CrossRef]
- Zheng, S.M.; Zhou, Q.X.; Chen, C.H.; Yang, F.X.; Cai, Z.; Li, D.; Geng, Q.J.; Feng, Y.M.; Wang, H.Q. Role of extracellular polymeric substances on the behavior and toxicity of silver nanoparticles and ions to green algae Chlorella vulgaris. Sci. Total Environ. 2019, 660, 1182–1190. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.Y. Study of Lipid, Protein and Polysaccharide Extraction form Chlorella. Master’s Dissertation, Beijing University of Chemical Industry, Beijing, China, 2014. [Google Scholar]
- Meng, Y.Y.; Jiang, J.P.; Wang, H.T.; Cao, X.P.; Xue, S.; Yang, Q.; Wang, W.L. The characteristics of TAG and EPA accumulation in Nannochloropsis oceanica IMET1 under different nitrogen supply regimes. Bioresour. Technol. 2015, 179, 483–489. [Google Scholar] [CrossRef] [PubMed]
- Honicky, M.; Cardoso, S.M.; de Lima, L.R.A.; Ozcariz, S.G.I.; Vieira, F.G.K.; de Carlos Back, I.; Moreno, Y.M.F. Added sugar and trans fatty acid intake and sedentary behavior were associated with excess total-body and central adiposity in children and adolescents with congenital heart disease. Pediatr. Obes. 2020, 15, e12623. [Google Scholar] [CrossRef]
- Deng, X.Y.; Gao, K.; Sun, J.L. Physiological and biochemical responses of Synechococcus sp. PCC7942 to Irgarol 1051 and diuron. Aquat. Toxicol. 2012, 122, 113–119. [Google Scholar] [CrossRef] [PubMed]
- Feng, J. Study on the Effect of Typical Nanoparticles on Chloroform Formation during Pre-Chlorination of Chlorella Pyrenoidosa. Master’s Dissertation, Chongqing University, Chongqing, China, 2022. [Google Scholar]
- Babaei, A.; Ranglová, K.; Malapascua, J.R.; Masojídek, J. The synergistic effect of Selenium (selenite, −SeO32−) dose and irradiance intensity in Chlorella cultures. AMB Express 2017, 7, 14. [Google Scholar] [CrossRef]
- Schiavon, M.; Pilon-Smits, E.A.H. The fascinating facets of plant selenium accumulation—biochemistry, physiology, evolution and ecology. New Phytol. 2016, 213, 1582–1596. [Google Scholar] [CrossRef] [PubMed]
- Gojkovic, Ž.; Garbayo, I.; Ariza, J.L.G.; Márová, I.; Vílchez, C. Selenium bioaccumulation and toxicity in cultures of green microalgae. Algal Res. 2015, 7, 106–116. [Google Scholar] [CrossRef]
- Guimaraes, B.O.; Villarreal-Toribio, B.; García-Barrera, T.; Arias-Borrego, A.; Gremmen, P.; Wijffels, R.H.; Barbosa, M.J.; D’Adamo, S. Effect of sulphur on selenium accumulation and speciation in Nannochloropsis oceanica. J. Funct. Food. 2022, 96, 11. [Google Scholar] [CrossRef]
- Sabatini, S.E.; Juárez, A.B.; Eppis, M.R.; Bianchi, L.; Luquet, C.M.; de Molina, M.D.R. Oxidative stress and antioxidant defenses in two green microalgae exposed to copper. Ecotox. Environ. Safe. 2009, 72, 1200–1206. [Google Scholar] [CrossRef] [PubMed]
- Brügger, B. Membrane Biology: Disentangling Cellular Lipid Connections. Curr. Biol. 2020, 30, R1090–R1092. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.Y.; Guo, T.Y.; Zhou, Y.P.; Han, S.; Sun, S.G.; Luo, F.J. Biological functions of active ingredients in quinoa bran: Advance and prospective. Crit. Rev. Food Sci. Nutr. 2024, 64, 4101–4115. [Google Scholar] [CrossRef] [PubMed]
- Li, S.F.; Chen, X.L.; Wong, M.H.; Chen, H.R.; Tao, L.; Liufu, G.Y.; Cheng, J.J.; Yang, X.W. Mechanism study on the regulation of metabolite flux for producing promising bioactive substances in microalgae Desmodesmus sp. YT through salinity stress. Algal Res. 2022, 64, 11. [Google Scholar] [CrossRef]
- Ansari, F.A.; Ratha, S.K.; Renuka, N.; Ramanna, L.; Gupta, S.K.; Rawat, I.; Bux, F. Effect of microplastics on growth and biochemical composition of microalga Acutodesmus obliquus. Algal Res. 2021, 56, 13. [Google Scholar] [CrossRef]
- Chalima, A.; Taxeidis, G.; Topakas, E. Optimization of the production of docosahexaenoic fatty acid by the heterotrophic microalga Crypthecodinium cohnii utilizing a dark fermentation effluent. Renew. Energy 2020, 152, 102–109. [Google Scholar] [CrossRef]
- Kumari, A.; Garima; Bharadvaja, N. A comprehensive review on algal nutraceuticals as prospective therapeutic agent for different diseases. 3 Biotech 2023, 13, 17. [Google Scholar] [CrossRef]
- Roy, S.S.; Pal, R. Microalgae in Aquaculture: A Review with Special References to Nutritional Value and Fish Dietetics. Proc. Zool. Soc. 2014, 68, 1–8. [Google Scholar] [CrossRef]
- Chen, W.; Gao, S.; Huang, Y.; Chang, K.; Zhao, X. Addition of Chlorella sorokiniana meal in the diet of juvenile rainbow trout (Oncorhynchus mykiss): Influence on fish growth, gut histology, oxidative stress, immune response, and disease resistance against Aeromonas salmonicida. Fish Shellfish Immunol. 2022, 129, 243–250. [Google Scholar] [CrossRef] [PubMed]
- Zheng, X.Y.; Xu, Z.X.; Zhao, D.M.; Luo, Y.; Lai, C.C.; Huang, B.; Pan, X.J. Double-dose responses of Scenedesmus capricornus microalgae exposed to humic acid. Sci. Total Environ. 2022, 806, 11. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Zhong, Y.; Huang, Z.; Yang, Y.F. Selenium Accumulation in Unicellular Green Alga Chlorella vulgaris and Its Effects on Antioxidant Enzymes and Content of Photosynthetic Pigments. PLoS ONE 2014, 9, 8. [Google Scholar] [CrossRef]
- Rezayian, M.; Niknam, V.; Ebrahimzadeh, H. Oxidative damage and antioxidative system in algae. Toxicol. Rep. 2019, 6, 1309–1313. [Google Scholar] [CrossRef]
- Singh, P.; Singh, S.; Maurya, P.; Mohanta, A.; Dubey, H.; Khadim, S.R.; Singh, A.K.; Pandey, A.K.; Singh, A.K.; Asthana, R.K. Bioaccumulation of selenium in halotolerant microalga Dunaliella salina and its impact on photosynthesis, reactive oxygen species, antioxidative enzymes, and neutral lipids. Mar. Pollut. Bull. 2023, 190, 114842. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).