Microbial Level and Microbiota Change of Laver in Dried Laver Processing Line During Production Seasons
Abstract
1. Introduction
2. Materials and Methods
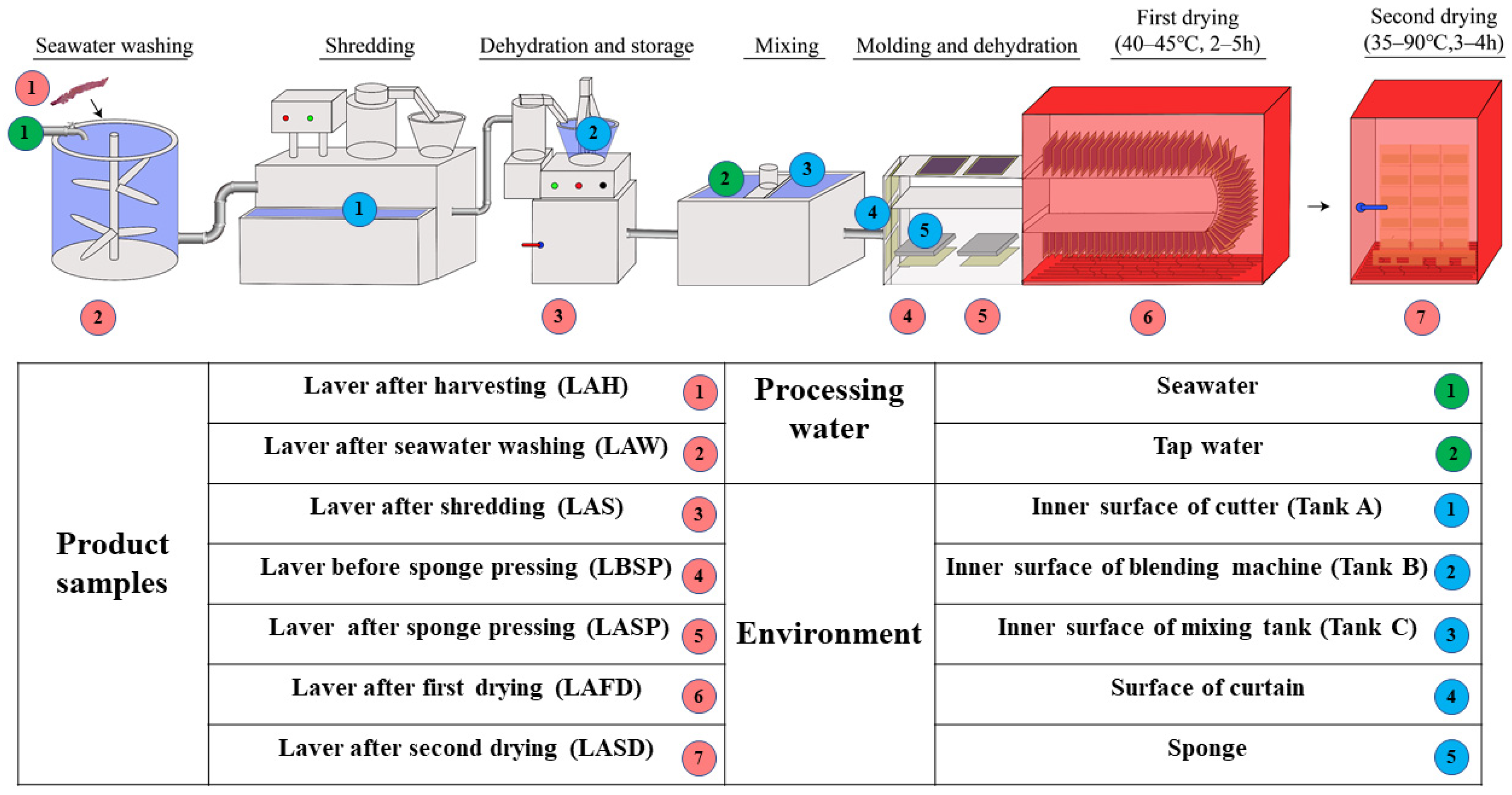
2.1. Study Design
2.2. Sample Collection
2.3. Culture-Dependent Microbial Analysis
2.4. 16S rRNA Sequencing and Bioinformatics Analysis
2.5. Statistical Analysis
3. Results
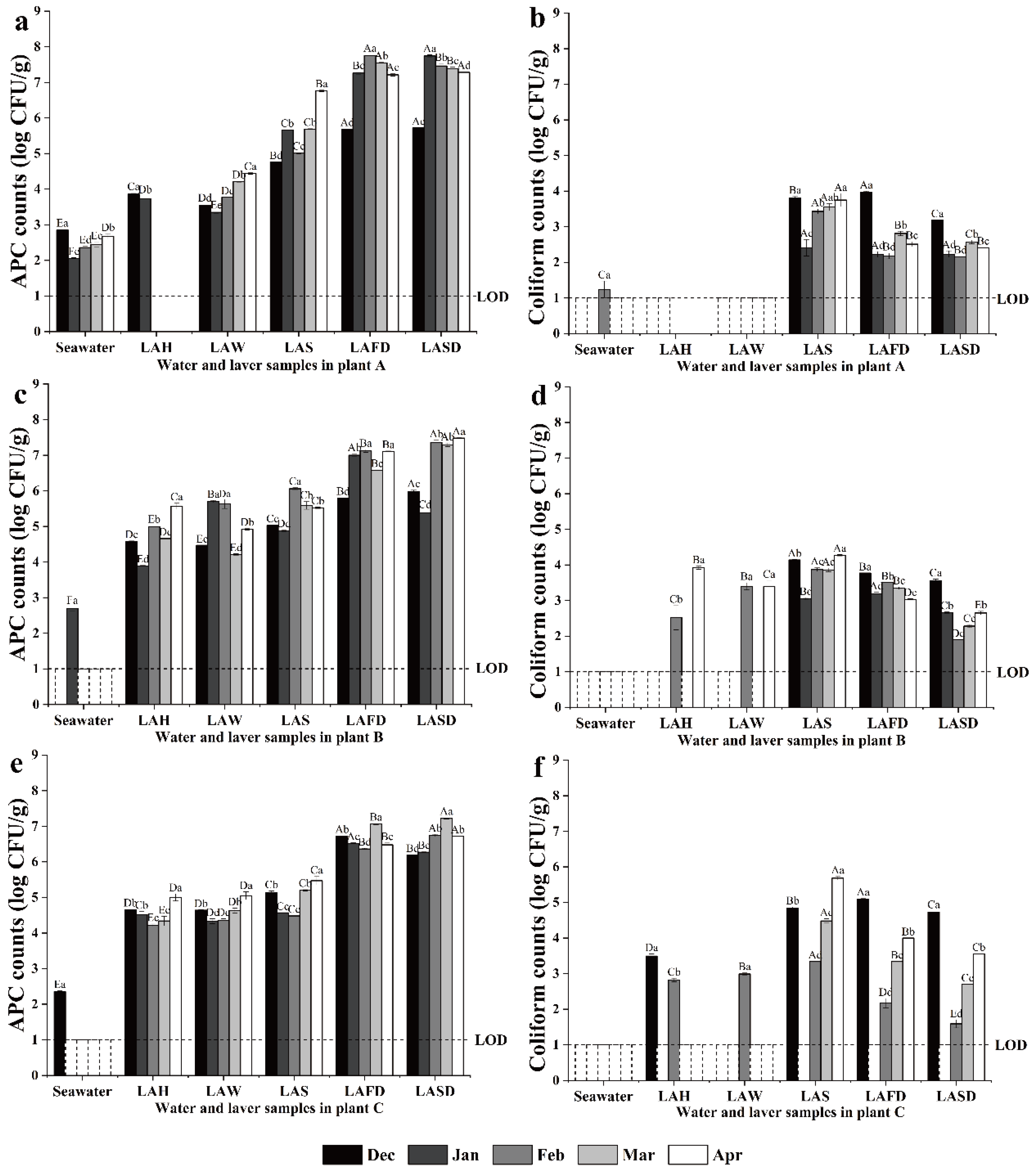
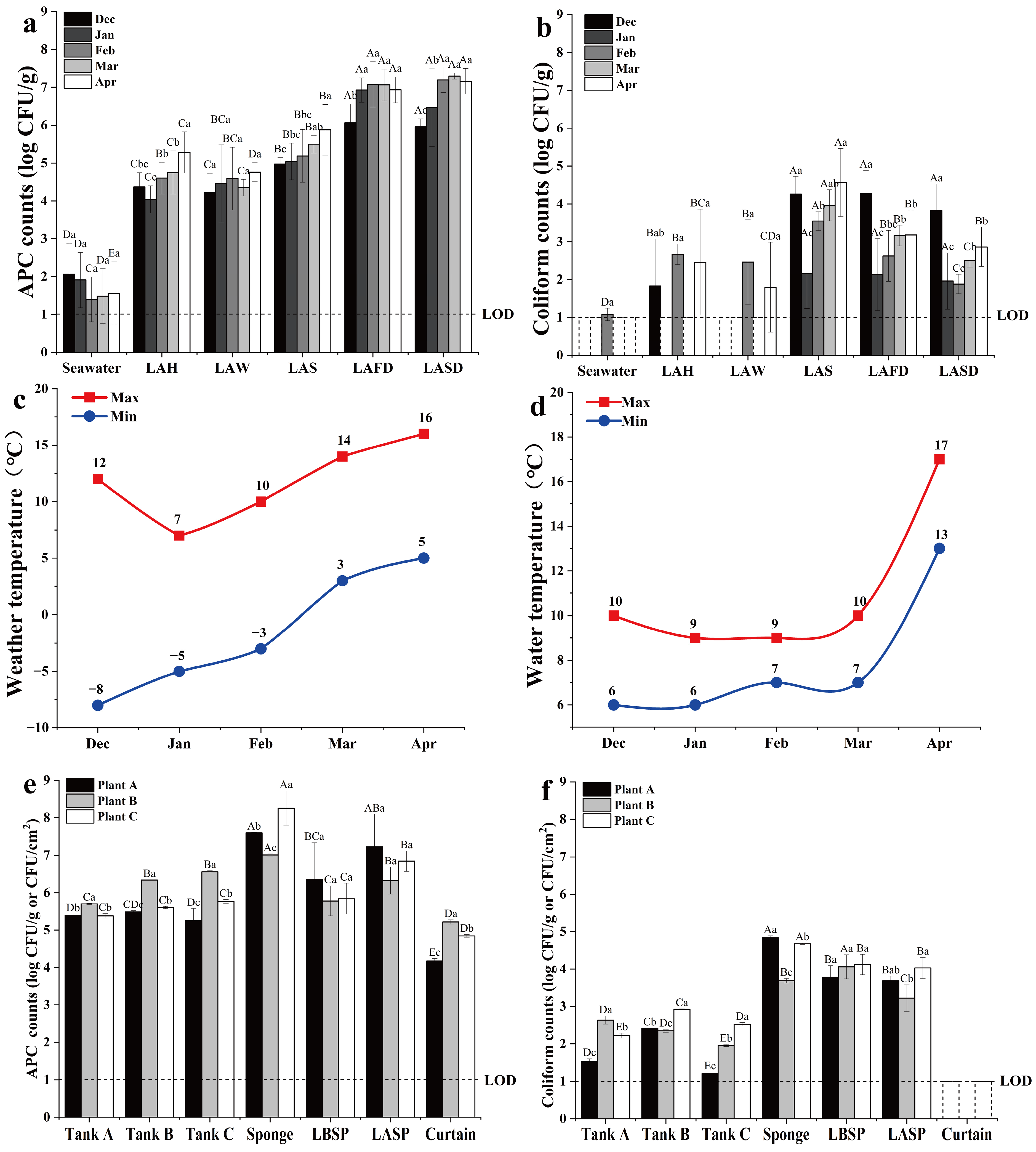
3.1. Microbiological Analysis of Processing Water, Materials, and FCS Samples
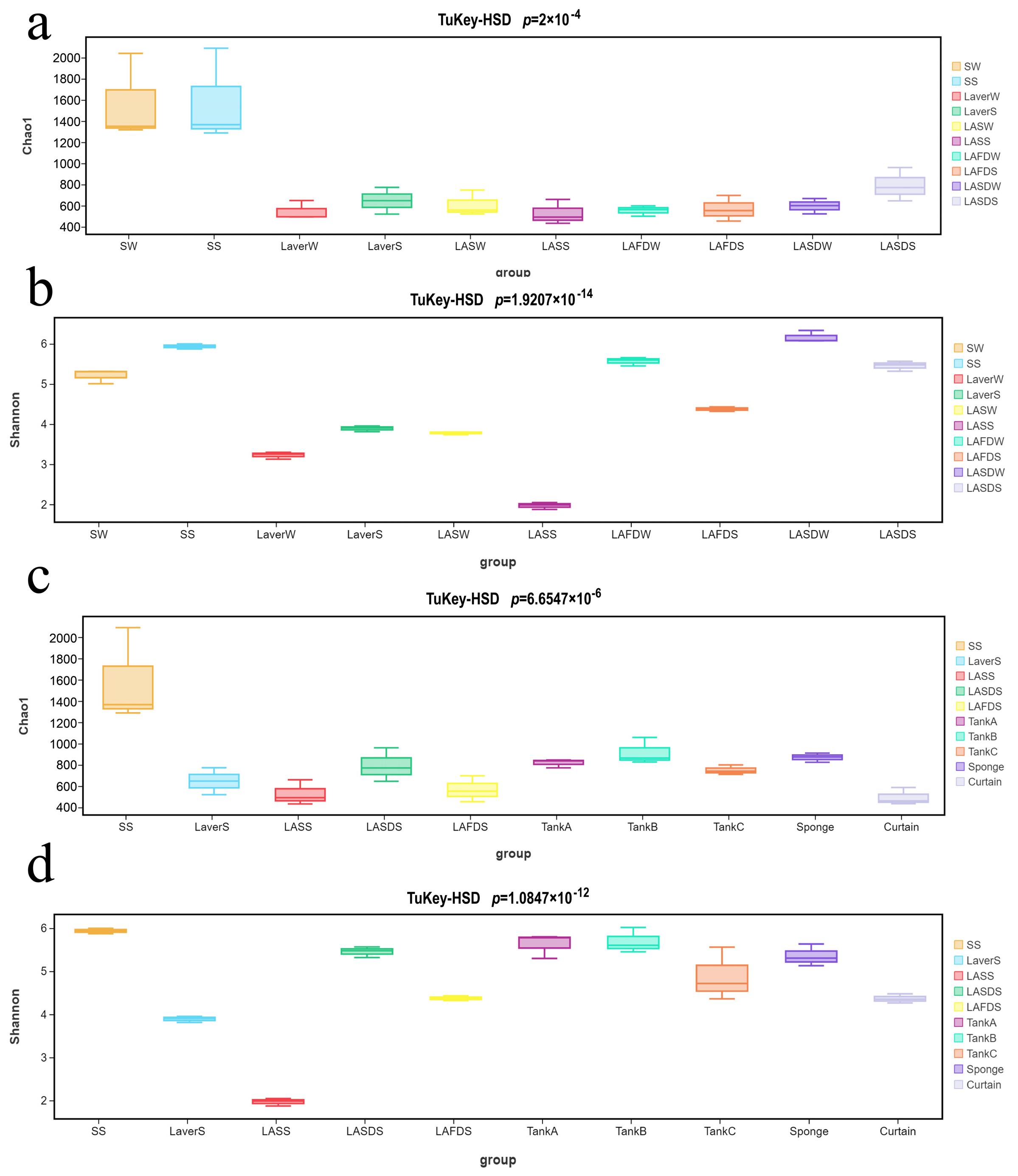
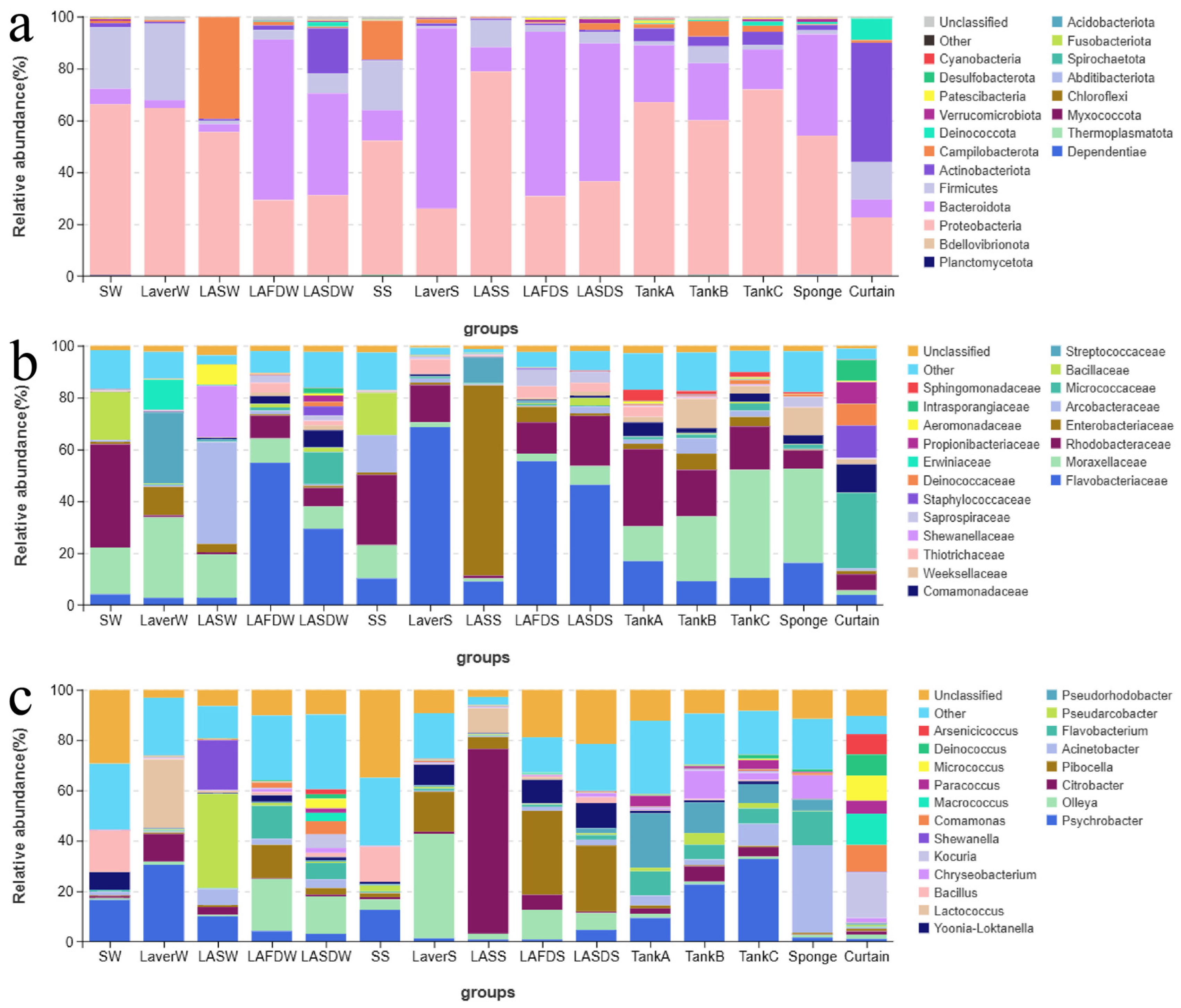
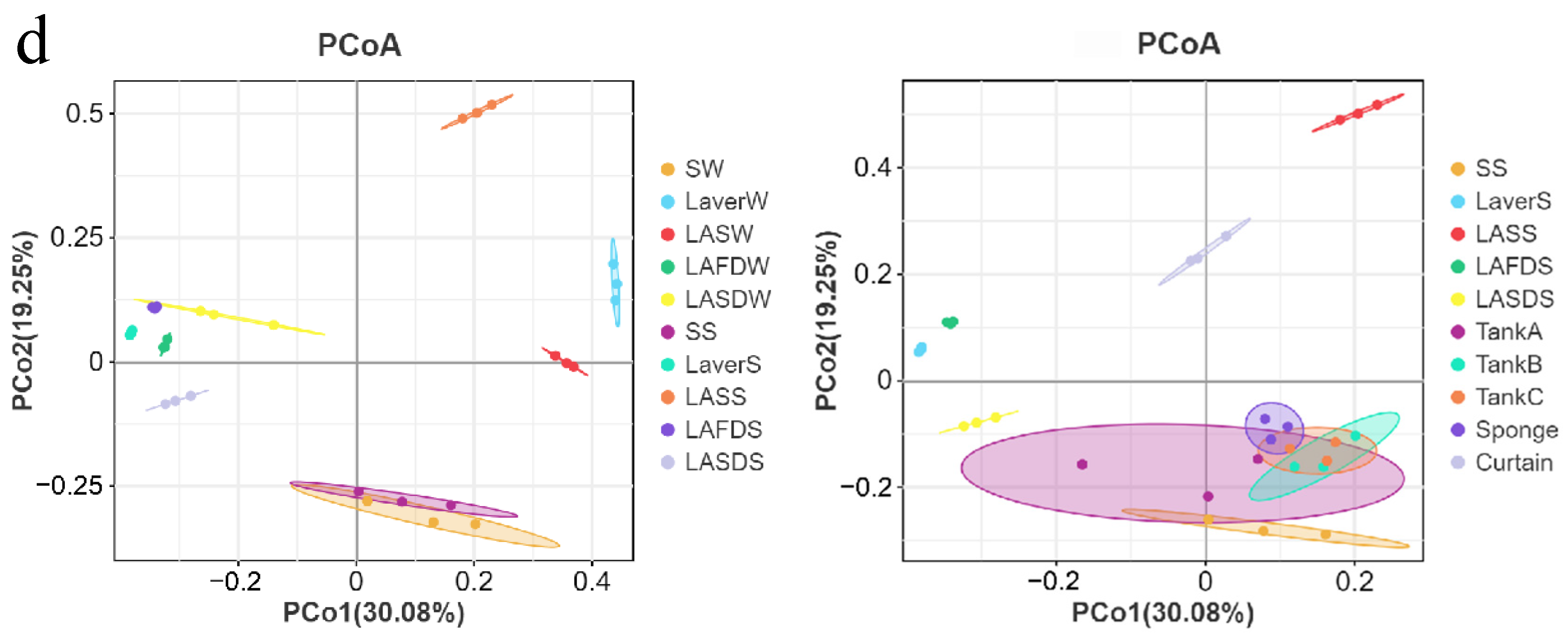
3.2. Microbiome Analysis of Processing Water, Laver Materials, and FCS Samples
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yan, X.; Li, H.; Yi, J.; Sun, C.; Yu, Q.; Wen, R. Unravelling the effects of drying techniques on Porphyra yezoensis: Morphology, rehydration properties, metabolomic profile, and taste formation. Food Chem. 2025, 464, 141562. [Google Scholar] [CrossRef]
- Tomić Maksan, M.; Matulić, D.; Mesić, Ž.; Memery, J. Segmenting and profiling seaweed consumers: A cross-cultural comparison of Australia, the United Kingdom and Croatia. Food Qual. Prefer. 2025, 122, 105305. [Google Scholar] [CrossRef]
- Cavallo, G.; Lorini, C.; Garamella, G.; Bonaccorsi, G. Seaweeds as a “Palatable” Challenge between Innovation and Sustainability: A Systematic Review of Food Safety. Sustainability 2021, 13, 7652. [Google Scholar] [CrossRef]
- Løvdal, T.; Lunestad, B.T.; Myrmel, M.; Rosnes, J.T.; Skipnes, D. Microbiological Food Safety of Seaweeds. Foods 2021, 10, 2719. [Google Scholar] [CrossRef]
- Pastrana-Pastrana, Á.J.; Rodríguez-Herrera, R.; Solanilla-Duque, J.F.; Flores-Gallegos, A.C. Plant proteins, insects, edible mushrooms and algae: More sustainable alternatives to conventional animal protein. J. Future Foods 2025, 5, 248–256. [Google Scholar] [CrossRef]
- Zhou, F.; Zhu, Z.; Wang, C.; Zhao, S.; Han, S.; Chen, L.; Ding, Y.; Liao, C.; Li, X.; Xu, Y.; et al. Microbial analysis of the production line for roasted seaweed snacks. LWT 2023, 187, 115372. [Google Scholar] [CrossRef]
- Choi, E.S.; Kim, N.H.; Kim, H.W.; Kim, S.A.; Jo, J.I.; Kim, S.H.; Lee, S.H.; Ha, S.D.; Rhee, M.S. Microbiological Quality of Seasoned Roasted Laver and Potential Hazard Control in a Real Processing Line. J. Food Prot. 2014, 77, 2069–2075. [Google Scholar] [CrossRef]
- Noh, B.-Y.; Hwang, S.-H.; Cho, Y.-S. Microbial Contamination Levels in Porphyra sp. Distributed in Korea Korean J. Fish. Aquat. Sci. 2019, 52, 180–184. [Google Scholar] [CrossRef]
- Wang, W.; Zhu, Z.; Wang, C.; Zhou, F.; Yu, H.; Zhang, Y.; Zhou, W.; Yang, J.; Zhu, Q.; Chen, Y.; et al. Post-drying decontamination of laver by dielectric barrier discharge plasma, UV radiation, ozonation, and hot air treatments. LWT 2023, 176, 114518. [Google Scholar] [CrossRef]
- Kim, J.-W.; Puligundla, P.; Mok, C. Microbial decontamination of dried laver using corona discharge plasma jet (CDPJ). J. Food Eng. 2015, 161, 24–32. [Google Scholar] [CrossRef]
- Kim, J.-W.; Puligundla, P.; Mok, C. Dielectric barrier discharge plasma for microbial decontamination of dried laver: Effects on physicochemical characteristics. Int. J. Food Sci. Technol. 2015, 50, 2630–2638. [Google Scholar] [CrossRef]
- Wu, H.; Huo, Y.; Zhang, J.; Liu, Y.; Zhao, Y.; He, P. Bioremediation efficiency of the largest scale artificial Porphyra yezoensis cultivation in the open sea in China. Mar. Pollut. Bull. 2015, 95, 289–296. [Google Scholar] [CrossRef]
- Son, K.-T.; Lach, T.; Jung, Y.J.; Kang, S.-K.; Eom, S.-H.; Lee, D.S.; Lee, M.-S.; Kim, Y.-M.J.F.; Sciences, A. Food Hazard Analysis During Dried-laver Processing. Fish. Aquat. Sci. 2014, 17, 197–201. [Google Scholar] [CrossRef]
- Kwon, K.; Ryu, D.-G.; Jeong, M.-C.; Kang, E.-H.; Jang, Y.; Kwon Ji, Y.; Kim, J.-M.; Shin, I.-S.; Kim, Y.-M. Analysis of Microbial Contaminants and Microbial Changes during Dried-laver Pyropia spp. Processing. Korean J. Fish. Aquat. Sci. 2018, 51, 8–14. [Google Scholar] [CrossRef]
- Li, X.; Xiong, Q.; Xu, B.; Wang, H.; Zhou, H.; Sun, Y. Bacterial community dynamics during different stages of processing of smoked bacon using the 16S rRNA gene amplicon analysis. Int. J. Food Microbiol. 2021, 351, 109076. [Google Scholar] [CrossRef]
- Zwirzitz, B.; Wetzels, S.U.; Dixon, E.D.; Stessl, B.; Zaiser, A.; Rabanser, I.; Thalguter, S.; Pinior, B.; Roch, F.F.; Strachan, C.; et al. The sources and transmission routes of microbial populations throughout a meat processing facility. NPJ Biofilms Microbiomes 2020, 6, 26. [Google Scholar] [CrossRef]
- Guo, M.; Wu, F.; Hao, G.; Qi, Q.; Li, R.; Li, N.; Wei, L.; Chai, T. Bacillus subtilis Improves Immunity and Disease Resistance in Rabbits. Front. Immunol. 2017, 8, 354. [Google Scholar] [CrossRef]
- Chen, S.; Zhou, Y.; Chen, Y.; Gu, J. fastp: An ultra-fast all-in-one FASTQ preprocessor. Bioinformatics 2018, 34, i884–i890. [Google Scholar] [CrossRef]
- Magoč, T.; Salzberg, S.L. FLASH: Fast length adjustment of short reads to improve genome assemblies. Bioinformatics 2011, 27, 2957–2963. [Google Scholar] [CrossRef] [PubMed]
- Edgar, R.C. UPARSE: Highly accurate OTU sequences from microbial amplicon reads. Nat. Methods 2013, 10, 996–998. [Google Scholar] [CrossRef] [PubMed]
- Edgar, R.C.; Haas, B.J.; Clemente, J.C.; Quince, C.; Knight, R. UCHIME improves sensitivity and speed of chimera detection. Bioinformatics 2011, 27, 2194–2200. [Google Scholar] [CrossRef] [PubMed]
- Beckers, B.; Op De Beeck, M.; Thijs, S.; Truyens, S.; Weyens, N.; Boerjan, W.; Vangronsveld, J. Performance of 16s rDNA primer pairs in the study of rhizosphere and endosphere bacterial microbiomes in metabarcoding studies. Front. Microbiol. 2016, 7, 650. [Google Scholar] [CrossRef]
- Yu, Y.; Wang, Y.; Okonkwo, C.E.; Chen, L.; Zhou, C. Multimode ultrasonic-assisted decontamination of fruits and vegetables: A review. Food Chem. 2024, 450, 139356. [Google Scholar] [CrossRef] [PubMed]
- Cho, W.-I.; Song, S.-H. Optimal non-thermal inactivation methods by study of the microbial control effect in laver (Porphya sp.). Food Sci. Biotechnol. 2022, 31, 1189–1196. [Google Scholar] [CrossRef] [PubMed]
- Zheng, S.; Wu, A.; Wang, H.; Chen, L.; Song, J.; Zhang, H.; He, M.; Wang, C.; Chen, H.; Wang, Q. Purification efficiency of Pyropia-processing wastewater and microalgal biomass production by the combination of Chlorella sp. C2 cultivated at different culture temperatures and chitosan. Bioresour. Technol. 2023, 373, 128730. [Google Scholar] [CrossRef] [PubMed]
- Snyder, A.B.; Martin, N.; Wiedmann, M. Microbial food spoilage: Impact, causative agents and control strategies. Nat. Rev. Microbiol. 2024, 22, 528–542. [Google Scholar] [CrossRef]
- Pakdel, M.; Olsen, A.; Bar, E.M.S. A Review of Food Contaminants and Their Pathways Within Food Processing Facilities Using Open Food Processing Equipment. J. Food Prot. 2023, 86, 100184. [Google Scholar] [CrossRef]
- Xu, Z.S.; Ju, T.; Yang, X.; Gänzle, M. A Meta-Analysis of Bacterial Communities in Food Processing Facilities: Driving Forces for Assembly of Core and Accessory Microbiomes across Different Food Commodities. Microorganisms 2023, 11, 1575. [Google Scholar] [CrossRef]
- Gu, G.; Ottesen, A.; Bolten, S.; Ramachandran, P.; Reed, E.; Rideout, S.; Luo, Y.; Patel, J.; Brown, E.; Nou, X. Shifts in spinach microbial communities after chlorine washing and storage at compliant and abusive temperatures. Food Microbiol. 2018, 73, 73–84. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Sheng, L.; Canakapalli, S.S.; Wang, L. Evaluation of the microbial control efficacies of commonly used home-drying and storage practices of dried peaches. LWT 2022, 165, 113734. [Google Scholar] [CrossRef]
- Khalid, N.I.; Saulaiman, U.S.; Nasiruddin, N.A.; Hatdran, M.M.; Ab Aziz, N.; Nor Khaizura, M.A.R.; Hasnan, N.Z.N.; Taip, F.S.; Sobri, S. Integrating cleaning studies with industrial practice: Case study of an effective cleaning program for a frozen meat patties SME factory. J. Clean. Prod. 2019, 235, 688–700. [Google Scholar] [CrossRef]
- Agüeria, D.A.; Libonatti, C.; Civit, D. Cleaning and disinfection programmes in food establishments: A literature review on verification procedures. J. Appl. Microbiol. 2021, 131, 23–35. [Google Scholar] [CrossRef] [PubMed]
- Carvalheira, A.; Silva, J.; Teixeira, P. Acinetobacter spp. in food and drinking water—A review. Food Microbiol. 2021, 95, 103675. [Google Scholar] [CrossRef]
- Xu, J.-G.; Huang, X.-N.; Meng, J.; Chen, J.-Y.; Han, B.-Z. Characterization and comparison of the bacterial community on environmental surfaces through a fresh-cut vegetables processing line in China. Food Res. Int. 2022, 155, 111075. [Google Scholar] [CrossRef] [PubMed]
- Singh, J.K.; Adams, F.G.; Brown, M.H. Diversity and Function of Capsular Polysaccharide in Acinetobacter baumannii. Front. Microbiol. 2019, 9, 3301. [Google Scholar] [CrossRef]
- Crandall, P.G.; O’Bryan, C.A.; Wang, D.; Gibson, K.E.; Obe, T. Environmental monitoring in food manufacturing: Current perspectives and emerging frontiers. Food Control 2024, 159, 110269. [Google Scholar] [CrossRef]
- Cho, T.J.; Rhee, M.S. Health Functionality and Quality Control of Laver (Porphyra, Pyropia): Current Issues and Future Perspectives as an Edible Seaweed. Mar. Drugs 2020, 18, 14. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ding, Y.; Zhou, F.; Zhou, R.; Wang, Q.; Pan, S.; Wang, W. Microbial Level and Microbiota Change of Laver in Dried Laver Processing Line During Production Seasons. Foods 2025, 14, 399. https://doi.org/10.3390/foods14030399
Ding Y, Zhou F, Zhou R, Wang Q, Pan S, Wang W. Microbial Level and Microbiota Change of Laver in Dried Laver Processing Line During Production Seasons. Foods. 2025; 14(3):399. https://doi.org/10.3390/foods14030399
Chicago/Turabian StyleDing, Yi, Feifei Zhou, Renjie Zhou, Qiqi Wang, Saikun Pan, and Wenbin Wang. 2025. "Microbial Level and Microbiota Change of Laver in Dried Laver Processing Line During Production Seasons" Foods 14, no. 3: 399. https://doi.org/10.3390/foods14030399
APA StyleDing, Y., Zhou, F., Zhou, R., Wang, Q., Pan, S., & Wang, W. (2025). Microbial Level and Microbiota Change of Laver in Dried Laver Processing Line During Production Seasons. Foods, 14(3), 399. https://doi.org/10.3390/foods14030399





