Using Pathogenic Escherichia coli Type III Secreted Effectors espK and espV as Markers to Reduce the Risk of Potentially Enterohemorrhagic Shiga Toxin-Producing Escherichia coli in Beef
Abstract
1. Introduction
2. Materials and Methods
2.1. Molecular Screening Assays
2.2. Screening of E. coli Isolates
2.3. Inoculation Study
2.4. Aerobic Plate Count (APC)
2.5. Natural Beef Broths
2.6. Culture Isolation
2.7. Statistical Analysis
3. Results and Discussion
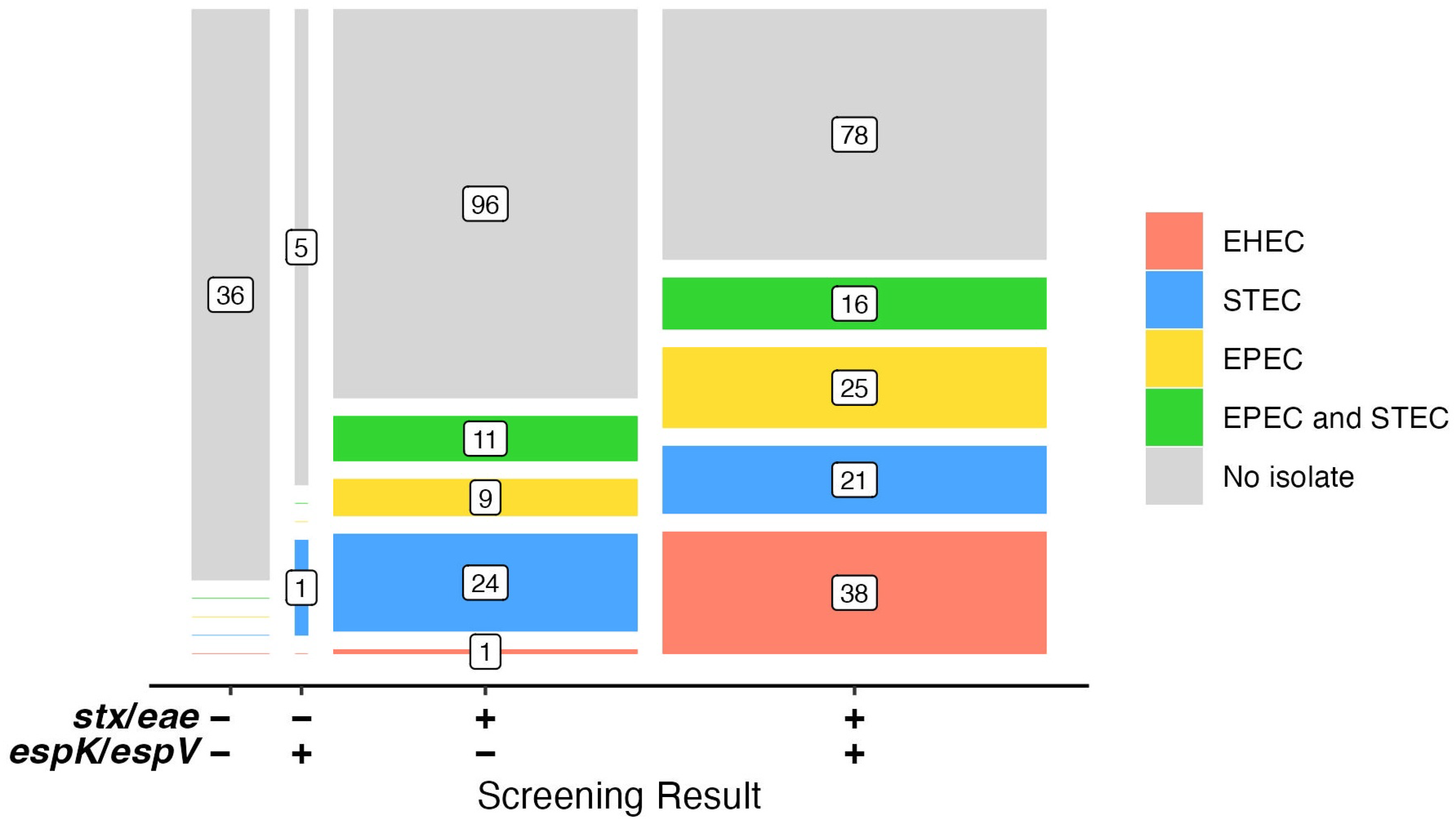
3.1. Examination of E. coli Strains for espK/espV
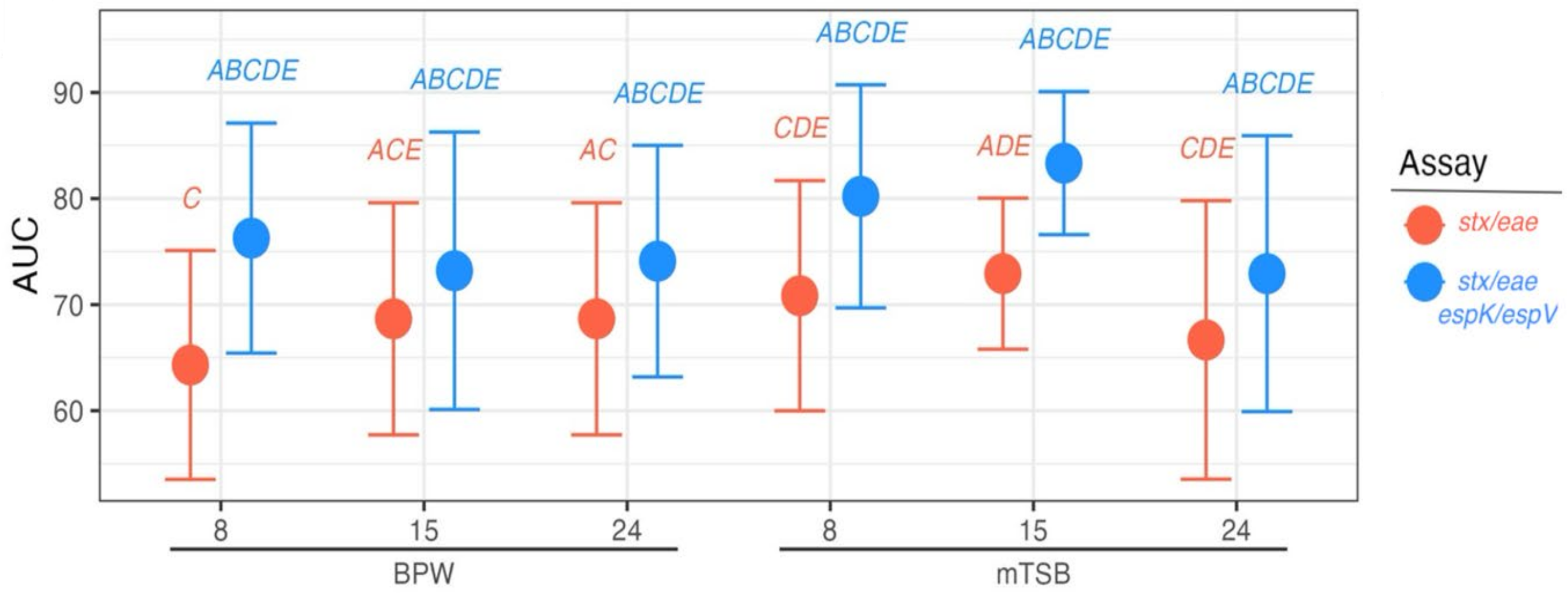
3.2. Distinguishing Beef Samples Inoculated with EHEC from Those Inoculated with STEC and EPEC
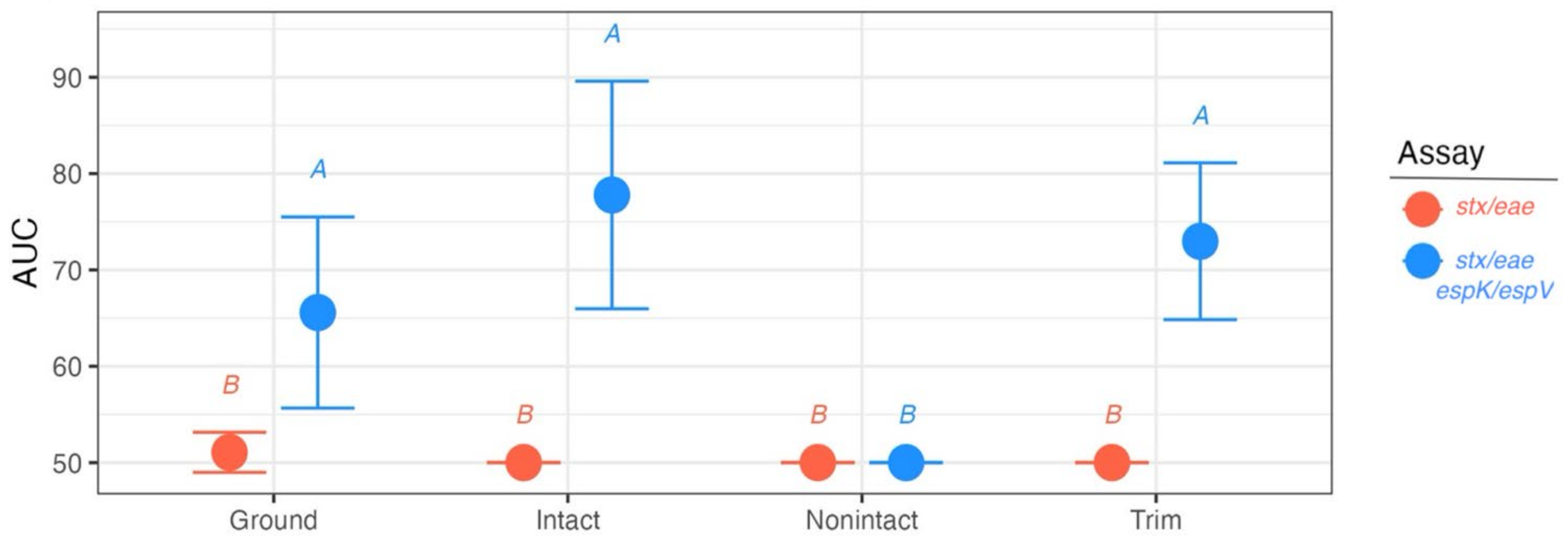
3.3. Analysis of Suspect Beef Samples
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| STEC | Shiga toxin-producing E. coli |
| EHEC | enterohemorrhagic E. coli |
| EPEC | enteropathogenic E. coli |
| AUC | Area Under the Receiver Operating Characteristic curve |
| LEE | locus of enterocyte effacement |
| T3SS | type III secretion system |
| nle | non-LEE located effector |
| OI | O-islands |
| PEC | Pathogenic E. coli |
| BPW | Buffered peptone water |
| TSB | Tryptic soy broth |
| MSD | Manual sampling device |
| SMAC | Sorbitol MacConkey Agar |
| mTSBca | modified TSB containing casamino acids |
| WBAM | washed blood agar containing mitomycin C |
| mRBA | modified Rainbow agar |
| FSL | Field Service Laboratories |
| USMARC | US Meat Animal Research Center |
References
- Nataro, J.P.; Kaper, J.B. Diarrheagenic Escherichia coli. Clin. Microbiol. Rev. 1998, 11, 142–201. [Google Scholar] [CrossRef] [PubMed]
- Perna, N.T.; Mayhew, G.F.; Pósfai, G.; Elliott, S.; Donnenberg, M.S.; Kaper, J.B.; Blattner, F.R. Molecular evolution of a pathogenicity island from enterohemorrhagic Escherichia coli O157:H7. Infect. Immun. 1998, 66, 3810–3817. [Google Scholar] [CrossRef]
- Hacker, J.; Blum-Oehler, G.; Hochhut, B.; Dobrindt, U. The molecular basis of infectious diseases: Pathogenicity islands and other mobile genetic elements. Acta Microbiol. Immunol. Hung. 2003, 50, 321–330. [Google Scholar] [CrossRef]
- Vlisidou, I.; Marchés, O.; Dziva, F.; Mundy, R.; Frankel, G.; Stevens, M.P. Identification and characterization of EspK, a type III secreted effector protein of enterohaemorrhagic Escherichia coli O157:H7. FEMS Microbiol. Lett. 2006, 263, 32–40. [Google Scholar] [CrossRef] [PubMed]
- Tobe, T.; Beatson, S.A.; Taniguchi, H.; Abe, H.; Bailey, C.M.; Fivian, A.; Younis, R.; Matthews, S.; Marches, O.; Frankel, G.; et al. An extensive repertoire of type III secretion effectors in Escherichia coli O157 and the role of lambdoid phages in their dissemination. Proc. Natl. Acad. Sci. USA 2006, 103, 14941–14946. [Google Scholar] [CrossRef] [PubMed]
- Arbeloa, A.; Oates, C.V.; Marchès, O.; Hartland, E.L.; Frankel, G. Enteropathogenic and enterohemorrhagic Escherichia coli type III secretion effector EspV induces radical morphological changes in eukaryotic cells. Infect. Immun. 2011, 79, 1067–1076. [Google Scholar] [CrossRef] [PubMed]
- Delannoy, S.; Beutin, L.; Fach, P. Discrimination of enterohemorrhagic Escherichia coli (EHEC) from non-EHEC strains based on detection of various combinations of type III effector genes. J. Clin. Microbiol. 2013, 51, 3257–3262. [Google Scholar] [CrossRef]
- Brooks, J.T.; Sowers, E.G.; Wells, J.G.; Greene, K.D.; Griffin, P.M.; Hoekstra, R.M.; Strockbine, N.A. Non-O157 Shiga toxin-producing Escherichia coli infections in the United States, 1983–2002. J. Infect. Dis. 2005, 192, 1422–1429. [Google Scholar] [CrossRef]
- USDA; FSIS. Expansion of FSIS Shiga Toxin-Producing Escherichia coli (STEC) Testing to Additional Raw Beef Products. Federal Register, 34397–34402, 85 FR 34397; Docket No. FSIS-2010-0023. 2020. Available online: https://www.federalregister.gov/documents/2020/06/04/2020-12073/expansion-of-fsis-shiga-toxin-producing-escherichia-coli-stec-testing-to-additional-raw-beef (accessed on 13 January 2025).
- Delannoy, S.; Chaves, B.D.; Ison, S.A.; Webb, H.E.; Beutin, L.; Delaval, J.; Billet, I.; Fach, P. Revisiting the STEC Testing Approach: Using espK and espV to Make Enterohemorrhagic Escherichia coli (EHEC) Detection More Reliable in Beef. Front. Microbiol. 2016, 7, 1. [Google Scholar] [CrossRef] [PubMed]
- USDA; FSIS. Microbiology Laboratory Guidebook. 2023. Available online: https://www.fsis.usda.gov/news-events/publications/microbiology-laboratory-guidebook (accessed on 1 November 2024).
- Sugiyama, K.; Inoue, K.; Sakazaki, R. Mitomycin-supplemented washed blood agar for the isolation of Shiga toxin-producing Escherichia coli other than O157:H7. Lett. Appl. Microbiol. 2001, 33, 193–195. [Google Scholar] [CrossRef]
- Paton, A.W.; Paton, J.C. Detection and characterization of Shiga toxigenic Escherichia coli by using multiplex PCR assays for stx1, stx2, eaeA, enterohemorrhagic E. coli hlyA, rfbO111, and rfbO157. J. Clin. Microbiol. 1998, 36, 598–602. [Google Scholar] [CrossRef]
- Bosilevac, J.M.; Katz, T.S.; Arthur, T.M.; Kalchayanand, N.; Wheeler, T.L. Proportions and Serogroups of Enterohemorrhagic Shiga Toxin-producing Escherichia coli in Feces of Fed and Cull Beef and Cull Dairy Cattle at Harvest. J. Food Prot. 2024, 87, 100273. [Google Scholar] [CrossRef] [PubMed]
- DeLong, E.R.; DeLong, D.M.; Clarke-Pearson, D.L. Comparing the areas under two or more correlated receiver operating characteristic curves: A nonparametric approach. Biometrics 1988, 44, 837–845. [Google Scholar] [CrossRef]
- Fawcett, T. An introduction to ROC analysis. Pattern Recognit. Lett. 2006, 27, 861–874. [Google Scholar] [CrossRef]
- Dunn, O.J. Multiple Comparisons among Means. J. Am. Stat. Assoc. 1961, 56, 52–64. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2022; Available online: https://www.R-project.org/ (accessed on 9 December 2024).
- Robin, X.; Turck, N.; Hainard, A.; Tiberti, N.; Lisacek, F.; Sanchez, J.C.; Müller, M. pROC: An open-source package for R and S+ to analyze and compare ROC curves. BMC Bioinform. 2011, 12, 77. [Google Scholar] [CrossRef]
- Kuhn, M. Building Predictive Models in R Using the caret Package. J. Stat. Softw. 2008, 28, 1–26. [Google Scholar] [CrossRef]
- Bosilevac, J.M.; Bugarel, M.; Machado, M.; Carrico, J.A.; Chablain, P.; Briese, D.; Murray, J.; Bailey, J.; Dutta, V. Use of new markers for precise detection of pathogenic Shiga toxin-producing Escherichia coli. J. Food Prot. 2021, 84 (Suppl. A), 113–114. [Google Scholar]
- Hoyle, D.V.; Wee, B.A.; Macleod, K.; Chase-Topping, M.E.; Bease, A.G.; Tongue, S.C.; Gally, D.L.; Delannoy, S.; Fach, P.; Pearce, M.C.; et al. Phylogenetic relationship and virulence composition of Escherichia coli O26:H11 cattle and human strain collections in Scotland; 2002–2020. Front. Microbiol. 2023, 14, 1260422. [Google Scholar] [CrossRef]
- Trabulsi, L.R.; Keller, R.; Gomes, T.A. Typical and Atypical Enteropathogenic Escherichia coli. Emerg. Infect. Dis. 2002, 8, 508–513. [Google Scholar] [CrossRef]
- Bugarel, M.; Beutin, L.; Scheutz, F.; Loukiadis, E.; Fach, P. Identification of genetic markers for differentiation of Shiga toxin-producing, enteropathogenic, and avirulent strains of Escherichia coli O26. Appl. Environ. Microbiol. 2011, 77, 2275–2281. [Google Scholar] [CrossRef] [PubMed]
- Bosilevac, J.M.; Koohmaraie, M. Predicting the presence of non-O157 Shiga toxin-producing Escherichia coli in ground beef by using molecular tests for Shiga toxins, intimin, and O serogroups. Appl. Environ. Microbiol. 2012, 78, 7152–7155. [Google Scholar] [CrossRef] [PubMed]
- Arthur, T.M.; Reno, F.J.; Wheeler, T.L. Validation of a New Method of Sampling Beef Manufacturing Trimmings for Pathogen Testing Using a Manual Sampling Mitt Approach. J. Food Prot. 2024, 87, 100233. [Google Scholar] [CrossRef] [PubMed]
- Suomalainen, L.R.; Reunanen, H.; Ijäs, R.; Valtonen, E.T.; Tiirola, M. Freezing induces biased results in the molecular detection of Flavobacterium columnare. Appl. Environ. Microbiol. 2006, 72, 1702–1704. [Google Scholar] [CrossRef] [PubMed]
- Phalakornkule, C.; Nuchdang, S.; Khemkhao, M.; Mhuantong, W.; Wongwilaiwalin, S.; Tangphatsornruang, S.; Champreda, V.; Kitsuwan, J.; Vatanyoopaisarn, S. Effect of freeze-thaw process on physical properties, microbial activities and population structures of anaerobic sludge. J. Biosci. Bioeng. 2017, 123, 474–481. [Google Scholar] [CrossRef]
- Miguela-Villoldo, P.; Moreno, M.A.; Hernández, M.; Rodríguez-Lázaro, D.; Gallardo, A.; Borge, C.; Quesada, A.; Domínguez, L.; Ugarte-Ruiz, M. Complementarity of Selective Culture and qPCR for Colistin Resistance Screening in Fresh and Frozen Pig Cecum Samples. Front. Microbiol. 2020, 11, 572712. [Google Scholar] [CrossRef]
- Dorsaz, S.; Charretier, Y.; Girard, M.; Gaïa, N.; Leo, S.; Schrenzel, J.; Harbarth, S.; Huttner, B.; Lazarevic, V. Changes in Microbiota Profiles After Prolonged Frozen Storage of Stool Suspensions. Front. Cell. Infect. Microbiol. 2020, 10, 77. [Google Scholar] [CrossRef]
- Conrad, C.C.; Stanford, K.; McAllister, T.A.; Thomas, J.; Reuter, T. Competition during enrichment of pathogenic Escherichia coli may result in culture bias. Facets 2017, 1, 114–126. [Google Scholar] [CrossRef]
- Cooley, M.B.; Jay-Russell, M.; Atwill, E.R.; Carychao, D.; Nguyen, K.; Quiñones, B.; Patel, R.; Walker, S.; Swimley, M.; Pierre-Jerome, E.; et al. Development of a robust method for isolation of shiga toxin-positive Escherichia coli (STEC) from fecal, plant, soil and water samples from a leafy greens production region in California. PLoS ONE 2013, 8, e65716. [Google Scholar] [CrossRef]
- Stromberg, Z.R.; Lewis, G.L.; Marx, D.B.; Moxley, R.A. Comparison of Enrichment Broths for Supporting Growth of Shiga Toxin-Producing Escherichia coli. Curr. Microbiol. 2015, 71, 214–219. [Google Scholar] [CrossRef]
- Bording-Jorgensen, M.; Tyrrell, H.; Lloyd, C.; Chui, L. Comparison of Common Enrichment Broths Used in Diagnostic Laboratories for Shiga Toxin-Producing Escherichia coli. Microorganisms 2021, 9, 503. [Google Scholar] [CrossRef] [PubMed]
- Stromberg, Z.R.; Lewis, G.L.; Moxley, R.A. Comparison of Agar Media for Detection and Quantification of Shiga Toxin-Producing Escherichia coli in Cattle Feces. J. Food Prot. 2016, 79, 939–949. [Google Scholar] [CrossRef]
- Verhaegen, B.; De Reu, K.; Heyndrickx, M.; De Zutter, L. Comparison of Six Chromogenic Agar Media for the Isolation of a Broad Variety of Non-O157 Shigatoxin-Producing Escherichia coli (STEC) Serogroups. Int. J. Environ. Res. Public Health 2015, 12, 6965–6978. [Google Scholar] [CrossRef]
- Delannoy, S.; Tran, M.L.; Fach, P. Insights into the assessment of highly pathogenic Shiga toxin-producing Escherichia coli in raw milk and raw milk cheeses by High Throughput Real-time PCR. Int. J. Food Microbiol. 2022, 366, 109564. [Google Scholar] [CrossRef] [PubMed]
- Food and Agriculture Organization of the United Nations and World Health Organization (FAO and WHO). Shiga Toxin-Producing Escherichia coli (STEC) and Food: Attribution, Characterization, and Monitoring; Microbiological Risk Assessment Series No. 31; FAO: Rome, Italy, 2018; 153p, ISBN 978-92-5-130682-6. [Google Scholar]
- askFSIS. askFSIS Case Number 01051154, STEC Analyses GENE-UP Screen. ÄÉ. 2022. Available online: https://ask.usda.gov/s/article/Questions-posted-on-askFSIS (accessed on 19 July 2022).
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bosilevac, J.M.; Katz, T.S.; Manis, L.E.; Rozier, L.; Day, M. Using Pathogenic Escherichia coli Type III Secreted Effectors espK and espV as Markers to Reduce the Risk of Potentially Enterohemorrhagic Shiga Toxin-Producing Escherichia coli in Beef. Foods 2025, 14, 382. https://doi.org/10.3390/foods14030382
Bosilevac JM, Katz TS, Manis LE, Rozier L, Day M. Using Pathogenic Escherichia coli Type III Secreted Effectors espK and espV as Markers to Reduce the Risk of Potentially Enterohemorrhagic Shiga Toxin-Producing Escherichia coli in Beef. Foods. 2025; 14(3):382. https://doi.org/10.3390/foods14030382
Chicago/Turabian StyleBosilevac, Joseph M., Tatum S. Katz, Leslie E. Manis, Lorenza Rozier, and Michael Day. 2025. "Using Pathogenic Escherichia coli Type III Secreted Effectors espK and espV as Markers to Reduce the Risk of Potentially Enterohemorrhagic Shiga Toxin-Producing Escherichia coli in Beef" Foods 14, no. 3: 382. https://doi.org/10.3390/foods14030382
APA StyleBosilevac, J. M., Katz, T. S., Manis, L. E., Rozier, L., & Day, M. (2025). Using Pathogenic Escherichia coli Type III Secreted Effectors espK and espV as Markers to Reduce the Risk of Potentially Enterohemorrhagic Shiga Toxin-Producing Escherichia coli in Beef. Foods, 14(3), 382. https://doi.org/10.3390/foods14030382





