Biogenic Amines in White Brined Cheeses
Abstract
1. Introduction
2. Materials and Methods
2.1. White Brined Cheese Samples
2.2. Microbiological Analysis
2.3. Identification of Isolates with MALDI-TOF MS Biotyping
2.4. Preparation of Samples and Precolumn Derivatization with Dansyl Chloride
2.4.1. Preparation of Carbonate Buffer
2.4.2. Preparation of Dansyl Chloride at a Concentration of 5 g/L
2.5. Determination of Biogenic Amine Content
2.6. Statistical Analysis
2.7. AI Tools
3. Results and Discussion
3.1. Occurrence of Microorganisms
3.2. Biogenic Amines in White Brined Cheeses
3.3. Microorganisms Isolated from White Brined Cheeses with Biogenic Amine Production Potential
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Garbowska, M.; Pluta, A.; Berthold-Pluta, A. Contents of Functionally Bioactive Peptides, Free Amino Acids, and Biogenic Amines in Dutch-Type Cheese Models Produced with Different Lactobacilli. Molecules 2020, 25, 5465. [Google Scholar] [CrossRef] [PubMed]
- Campos-Góngora, E.; González-Martínez, M.T.; López-Hernández, A.A.; Arredondo-Mendoza, G.I.; Ortega-Villarreal, A.S.; González-Martínez, B.E. Histamine and Tyramine in Chihuahua Cheeses during Shelf Life: Association with the Presence of Tdc and Hdc Genes. Molecules 2023, 28, 3007. [Google Scholar] [CrossRef] [PubMed]
- Linares, D.M.; Del Río, B.; Ladero, V.; Martínez, N.; Fernández, M.; Martín, M.C.; Álvarez, M.A. Factors Influencing Biogenic Amines Accumulation in Dairy Products. Front. Microbiol. 2012, 3, 180. [Google Scholar] [CrossRef] [PubMed]
- Tsouggou, N.; Slavko, A.; Tsipidou, O.; Georgoulis, A.; Dimov, S.G.; Yin, J.; Vorgias, C.E.; Kapolos, J.; Papadelli, M.; Papadimitriou, K. Investigation of the Microbiome of Industrial PDO Sfela Cheese and Its Artisanal Variants Using 16S rDNA Amplicon Sequencing and Shotgun Metagenomics. Foods 2024, 13, 1023. [Google Scholar] [CrossRef]
- Al-Nabulsi, A.A.; Osaili, T.M.; Oqdeh, S.B.; Olaimat, A.N.; Jaradat, Z.W.; Ayyash, M.; Holley, R.A. Antagonistic Effects of Lactobacillus Reuteri against Escherichia Coli O157:H7 in White-Brined Cheese under Different Storage Conditions. J. Dairy Sci. 2021, 104, 2719–2734. [Google Scholar] [CrossRef] [PubMed]
- Geronikou, A.; Larsen, N.; Lillevang, S.K.; Jespersen, L. Occurrence and Identification of Yeasts in Production of White-Brined Cheese. Microorganisms 2022, 10, 1079. [Google Scholar] [CrossRef] [PubMed]
- Terpou, A.; Bosnea, L.; Kanellaki, M.; Plessas, S.; Bekatorou, A.; Bezirtzoglou, E.; Koutinas, A.A. Growth Capacity of a Novel Potential Probiotic Lactobacillus Paracasei K5 Strain Incorporated in Industrial White Brined Cheese as an Adjunct Culture. J. Food Sci. 2018, 83, 723–731. [Google Scholar] [CrossRef]
- Hussein, N.D.; Hassan, J.W.; Osman, M.; El-Omari, K.; Kharroubi, S.A.; Toufeili, I.; Kassem, I.I. Assessment of the Microbiological Acceptability of White Cheese (Akkawi) in Lebanon and the Antimicrobial Resistance Profiles of Associated Escherichia Coli. Antibiotics 2023, 12, 610. [Google Scholar] [CrossRef] [PubMed]
- Mantzourani, I.; Terpou, A.; Alexopoulos, A.; Chondrou, P.; Galanis, A.; Bekatorou, A.; Bezirtzoglou, E.; Koutinas, A.A.; Plessas, S. Application of A Novel Potential Probiotic Lactobacillus Paracasei Strain Isolated from Kefir Grains in the Production of Feta-Type Cheese. Microorganisms 2018, 6, 121. [Google Scholar] [CrossRef] [PubMed]
- Katsouri, E.; Magriplis, E.; Zampelas, A.; Nychas, G.-J.; Drosinos, H.E. Nutritional Characteristics of Prepacked Feta PDO Cheese Products in Greece: Assessment of Dietary Intakes and Nutritional Profiles. Foods 2020, 9, 253. [Google Scholar] [CrossRef] [PubMed]
- Marino, M.; Innocente, N.; Maifreni, M.; Mounier, J.; Cobo-Díaz, J.F.; Coton, E.; Carraro, L.; Cardazzo, B. Diversity within Italian Cheesemaking Brine-Associated Bacterial Communities Evidenced by Massive Parallel 16S rRNA Gene Tag Sequencing. Front. Microbiol. 2017, 8, 2119. [Google Scholar] [CrossRef] [PubMed]
- Broel, N.; Sowa, M.A.; Manhard, J.; Siegl, A.; Weichhard, E.; Zorn, H.; Li, B.; Gand, M. Altering the Chain Length Specificity of a Lipase from Pleurotus Citrinopileatus for the Application in Cheese Making. Foods 2022, 11, 2608. [Google Scholar] [CrossRef] [PubMed]
- Estrada, O.; Ariño, A.; Juan, T. Salt Distribution in Raw Sheep Milk Cheese during Ripening and the Effect on Proteolysis and Lipolysis. Foods 2019, 8, 100. [Google Scholar] [CrossRef]
- Murtaza, M.A.; Anees-Ur-Rehman, M.; Hafiz, I.; Ameer, K.; Celik, O.F. Effects of Probiotic Adjuncts on Physicochemical Properties, Organic Acids Content, and Proteolysis in Cheese Prepared from Buffalo Milk. J. Food Process. Preserv. 2022, 46, e16385. [Google Scholar] [CrossRef]
- Innosa, D.; Ianni, A.; Faccia, M.; Martino, C.; Grotta, L.; Saletti, M.A.; Pomilio, F.; Martino, G. Physical, Nutritional, and Sensory Properties of Cheese Obtained from Goats Fed a Dietary Supplementation with Olive Leaves. Animals 2020, 10, 2238. [Google Scholar] [CrossRef]
- Edigin, E.; Lingamaneni, P.; Sarmiento, J.; Shaka, H.; Patel, S.A.; Edigin, E.; Lingamaneni, P.; Sarmiento, J.D.; Shaka, H.; Patel, S.A. Tilapia Consumption and Scombroid Poisoning. Cureus 2019, 11, e5976. [Google Scholar] [CrossRef]
- Scientific Opinion on Risk Based Control of Biogenic Amine Formation in Fermented Foods. EFSA J. 2011, 9, 2393–2486. [CrossRef]
- Comas-Basté, O.; Latorre-Moratalla, M.L.; Sánchez-Pérez, S.; Veciana-Nogués, M.T.; Vidal-Carou, M.d.C.; Comas-Basté, O.; Latorre-Moratalla, M.L.; Sánchez-Pérez, S.; Veciana-Nogués, M.T.; Vidal-Carou, M.d.C. Histamine and Other Biogenic Amines in Food. From Scombroid Poisoning to Histamine Intolerance. In Biogenic Amines; IntechOpen: London, UK, 2019; ISBN 978-1-78984-134-3. [Google Scholar]
- Domingos-Lopes, M.F.P.; Stanton, C.; Ross, R.P.; Silva, C.C.G. Histamine and Cholesterol Lowering Abilities of Lactic Acid Bacteria Isolated from Artisanal Pico Cheese. J. Appl. Microbiol. 2020, 129, 1428–1440. [Google Scholar] [CrossRef]
- Özkan, E.R.; Demirci, T.; Öztürk, H.İ.; Akın, N. Screening Lactobacillus Strains from Artisanal Turkish Goatskin Casing Tulum Cheeses Produced by Nomads via Molecular and In Vitro Probiotic Characteristics. J. Sci. Food Agric. 2021, 101, 2799–2808. [Google Scholar] [CrossRef] [PubMed]
- Dabadé, D.S.; Jacxsens, L.; Miclotte, L.; Abatih, E.; Devlieghere, F.; De Meulenaer, B. Survey of Multiple Biogenic Amines and Correlation to Microbiological Quality and Free Amino Acids in Foods. Food Control 2021, 120, 107497. [Google Scholar] [CrossRef]
- Kavas, N.; Kavas, G.; Kınık, Ö.; Ateş, M.; Şatır, G.; Kaplan, M. Effect of Probiotic and Symbiotic Microencapsulation Supplementation on the Physico-Chemical Characteristics and Organic Acid Content of Goat Cheese. J. Food Process. Preserv. 2022, 46, e16815. [Google Scholar] [CrossRef]
- Garbowska, M.; Berthold-Pluta, A.; Stasiak-Różańska, L.; Pluta, A. The Impact of the Adjunct Heat-Treated Starter Culture and Lb. Helveticus LH-B01 on the Proteolysis and ACE Inhibitory Activity in Dutch-Type Cheese Model during Ripening. Animals 2021, 11, 2699. [Google Scholar] [CrossRef] [PubMed]
- Garofalo, G.; Ponte, M.; Greco, C.; Barbera, M.; Mammano, M.M.; Fascella, G.; Greco, G.; Salsi, G.; Orlando, S.; Alfonzo, A.; et al. Improvement of Fresh Ovine “Tuma” Cheese Quality Characteristics by Application of Oregano Essential Oils. Antioxidants 2023, 12, 1293. [Google Scholar] [CrossRef] [PubMed]
- Busetta, G.; Ponte, M.; Barbera, M.; Alfonzo, A.; Ioppolo, A.; Maniaci, G.; Guarcello, R.; Francesca, N.; Palazzolo, E.; Bonanno, A.; et al. Influence of Citrus Essential Oils on the Microbiological, Physicochemical and Antioxidant Properties of Primosale Cheese. Antioxidants 2022, 11, 2004. [Google Scholar] [CrossRef] [PubMed]
- Ponte, M.; Maniaci, G.; Di Grigoli, A.; Gannuscio, R.; Ashkezary, M.R.; Addis, M.; Pipi, M.; Alabiso, M.; Todaro, M.; Bonanno, A. Feeding Dairy Ewes with Fresh or Dehydrated Sulla (Sulla Coronarium L.) Forage. 2. Effects on Cheese Enrichment in Bioactive Molecules. Animals 2022, 12, 2462. [Google Scholar] [CrossRef] [PubMed]
- Xian, S.; Li, Y.; Liu, X.; Shen, G.; Zhou, M.; Li, M.; Hou, X.; Li, S.; Luo, Q.; Zhang, Z.; et al. Impact of Microorganisms on Key Processes of Organic Acid Metabolism during the Occurrence and Disappearance of Paocai Pellicle. J. Food Sci. 2024, 89, 5047–5064. [Google Scholar] [CrossRef] [PubMed]
- Li, K.J.; Brouwer-Brolsma, E.M.; Burton-Pimentel, K.J.; Vergères, G.; Feskens, E.J.M. A Systematic Review to Identify Biomarkers of Intake for Fermented Food Products. Genes. Nutr. 2021, 16, 5. [Google Scholar] [CrossRef] [PubMed]
- Münger, L.H.; Garcia-Aloy, M.; Vázquez-Fresno, R.; Gille, D.; Rosana, A.R.R.; Passerini, A.; Soria-Florido, M.-T.; Pimentel, G.; Sajed, T.; Wishart, D.S.; et al. Biomarker of Food Intake for Assessing the Consumption of Dairy and Egg Products. Genes Nutr. 2018, 13, 26. [Google Scholar] [CrossRef]
- Tarrah, A.; Castilhos, J.d.; Rossi, R.C.; Duarte, V.d.S.; Ziegler, D.R.; Corich, V.; Giacomini, A. In Vitro Probiotic Potential and Anti-Cancer Activity of Newly Isolated Folate-Producing Streptococcus thermophilus Strains. Front. Microbiol. 2018, 9, 2214. [Google Scholar] [CrossRef]
- Mah, J.-H.; Park, Y.; Jin, Y.; Lee, J.-H.; Hwang, H.-J. Bacterial Production and Control of Biogenic Amines in Asian Fermented Soybean Foods. Foods 2019, 8, 85. [Google Scholar] [CrossRef]
- Dias, I.; Laranjo, M.; Potes, M.E.; Agulheiro-Santos, A.C.; Ricardo-Rodrigues, S.; Fialho, A.R.; Véstia, J.; Fraqueza, M.J.; Oliveira, M.; Elias, M. Autochthonous Starter Cultures Are Able to Reduce Biogenic Amines in a Traditional Portuguese Smoked Fermented Sausage. Microorganisms 2020, 8, 686. [Google Scholar] [CrossRef]
- Yu, Y.; Li, L.; Xu, Y.; An, K.; Shi, Q.; Yu, Y.; Xu, Z. Evaluation of the Relationship among Biogenic Amines, Nitrite and Microbial Diversity in Fermented Mustard. Molecules 2021, 26, 6173. [Google Scholar] [CrossRef] [PubMed]
- Bavaro, S.L.; Susca, A.; Frisvad, J.C.; Tufariello, M.; Chytiri, A.; Perrone, G.; Mita, G.; Logrieco, A.F.; Bleve, G. Isolation, Characterization, and Selection of Molds Associated to Fermented Black Table Olives. Front. Microbiol. 2017, 8, 1356. [Google Scholar] [CrossRef] [PubMed]
- Al-Holy, M.A.; Al-Nabulsi, A.; Osaili, T.M.; Ayyash, M.M.; Shaker, R.R. Inactivation of Listeria Innocua in Brined White Cheese by a Combination of Nisin and Heat. Food Control 2012, 23, 48–53. [Google Scholar] [CrossRef]
- De Mey, E.; Drabik-Markiewicz, G.; De Maere, H.; Peeters, M.-C.; Derdelinckx, G.; Paelinck, H.; Kowalska, T. Dabsyl Derivatisation as an Alternative for Dansylation in the Detection of Biogenic Amines in Fermented Meat Products by Reversed Phase High Performance Liquid Chromatography. Food Chem. 2012, 130, 1017–1023. [Google Scholar] [CrossRef]
- Sánchez-Pérez, S.; Comas-Basté, O.; Costa-Catala, J.; Iduriaga-Platero, I.; Veciana-Nogués, M.T.; Vidal-Carou, M.C.; Latorre-Moratalla, M.L. The Rate of Histamine Degradation by Diamine Oxidase Is Compromised by Other Biogenic Amines. Front. Nutr. 2022, 9, 897028. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Pérez, S.; Comas-Basté, O.; Veciana-Nogués, M.T.; Latorre-Moratalla, M.L.; Vidal-Carou, M.C. Low-Histamine Diets: Is the Exclusion of Foods Justified by Their Histamine Content? Nutrients 2021, 13, 1395. [Google Scholar] [CrossRef]
- ISO 4833-1:2013; Microbiology of the Food Chain—Horizontal Method for the Enumeration of Microorganisms. Part 1: Colony Count at 30 °C by the Pour Plate Technique. ISO: Geneva, Switzerland, 2013.
- ISO 15214:1998; Microbiology of Food and Animal Feeding Stuffs—Horizontal Method for the Enumeration of Mesophilic Lactic Acid Bacteria—Colony-Count Technique at 30 °C. ISO: Geneva, Switzerland, 1998.
- ISO 4832:2006; Microbiology of Food and Animal Feeding Stuffs—Horizontal Method for the Enumeration of Coliforms—Colony-Count Technique. ISO: Geneva, Switzerland, 2006.
- ISO 6887-1:2017; Microbiology of the Food Chain—Preparation of Test Samples, Initial Suspension and Decimal Dilutions for Microbiological Examination. Part 1: General Rules for the Preparation of the Initial Suspension and Decimal Dilutions. ISO: Geneva, Switzerland, 2017.
- DeMarco, M.L.; Burnham, C.-A.D. Diafiltration MALDI-TOF Mass Spectrometry Method for Culture-Independent Detection and Identification of Pathogens Directly From Urine Specimens. Am. J. Clin. Pathol. 2014, 141, 204–212. [Google Scholar] [CrossRef] [PubMed]
- Combarros-Fuertes, P.; Fernández, D.; Arenas, R.; Diezhandino, I.; Tornadijo, M.E.; Fresno, J.M. Biogenic Amines in Zamorano Cheese: Factors Involved in Their Accumulation. J. Sci. Food Agric. 2016, 96, 295–305. [Google Scholar] [CrossRef]
- Schirone, M.; Tofalo, R.; Perpetuini, G.; Manetta, A.C.; Di Gianvito, P.; Tittarelli, F.; Battistelli, N.; Corsetti, A.; Suzzi, G.; Martino, G. Influence of Iodine Feeding on Microbiological and Physico-Chemical Characteristics and Biogenic Amines Content in a Raw Ewes’ Milk Cheese. Foods 2018, 7, 108. [Google Scholar] [CrossRef]
- Houicher, A.; Kuley, E.; Bensid, A.; Yazgan, H.; Özogul, F. In Vitro Study of Biogenic Amine Production and Gastrointestinal Stress Tolerance by Some Enterococci Strains. J. Sci. Food Agric. 2024, 104, 500–507. [Google Scholar] [CrossRef]
- Tsanasidou, C.; Asimakoula, S.; Sameli, N.; Fanitsios, C.; Vandera, E.; Bosnea, L.; Koukkou, A.-I.; Samelis, J. Safety Evaluation, Biogenic Amine Formation, and Enzymatic Activity Profiles of Autochthonous Enterocin-Producing Greek Cheese Isolates of the Enterococcus Faecium/Durans Group. Microorganisms 2021, 9, 777. [Google Scholar] [CrossRef]
- Pawul-Gruba, M.; Kiljanek, T.; Madejska, A.; Osek, J. Development of a High Performance Liquid Chromatography with Diode Array Detector (HPLC-DAD) Method for Determination of Biogenic Amines in Ripened Cheeses. Molecules 2022, 27, 8194. [Google Scholar] [CrossRef] [PubMed]
- del Valle, L.J.; Ginovart, M.; Gordún, E.; Carbó, R. Histidine Decarboxylase-Positive Lactic Acid Bacteria Strains and the Formation of Histamine in Ripened Cheeses. J. Food Process. Preserv. 2018, 42, e13708. [Google Scholar] [CrossRef]
- Barbieri, F.; Montanari, C.; Gardini, F.; Tabanelli, G. Biogenic Amine Production by Lactic Acid Bacteria: A Review. Foods 2019, 8, 17. [Google Scholar] [CrossRef] [PubMed]
- Spekker, E.; Nagy-Grócz, G. All Roads Lead to the Gut: The Importance of the Microbiota and Diet in Migraine. Neurol. Int. 2023, 15, 1174–1190. [Google Scholar] [CrossRef] [PubMed]
- Benkerroum, N. Biogenic Amines in Dairy Products: Origin, Incidence, and Control Means. Compr. Rev. Food Sci. Food Saf. 2016, 15, 801–826. [Google Scholar] [CrossRef]
- Kandasamy, S.; Yoo, J.; Yun, J.; Kang, H.B.; Seol, K.-H.; Ham, J.-S. Quantitative Analysis of Biogenic Amines in Different Cheese Varieties Obtained from the Korean Domestic and Retail Markets. Metabolites 2021, 11, 31. [Google Scholar] [CrossRef] [PubMed]
- Omer, A.K.; Mohammed, R.R.; Ameen, P.S.M.; Abas, Z.A.; Ekici, K. Presence of Biogenic Amines in Food and Their Public Health Implications: A Review. J. Food Prot. 2021, 84, 1539–1548. [Google Scholar] [CrossRef] [PubMed]
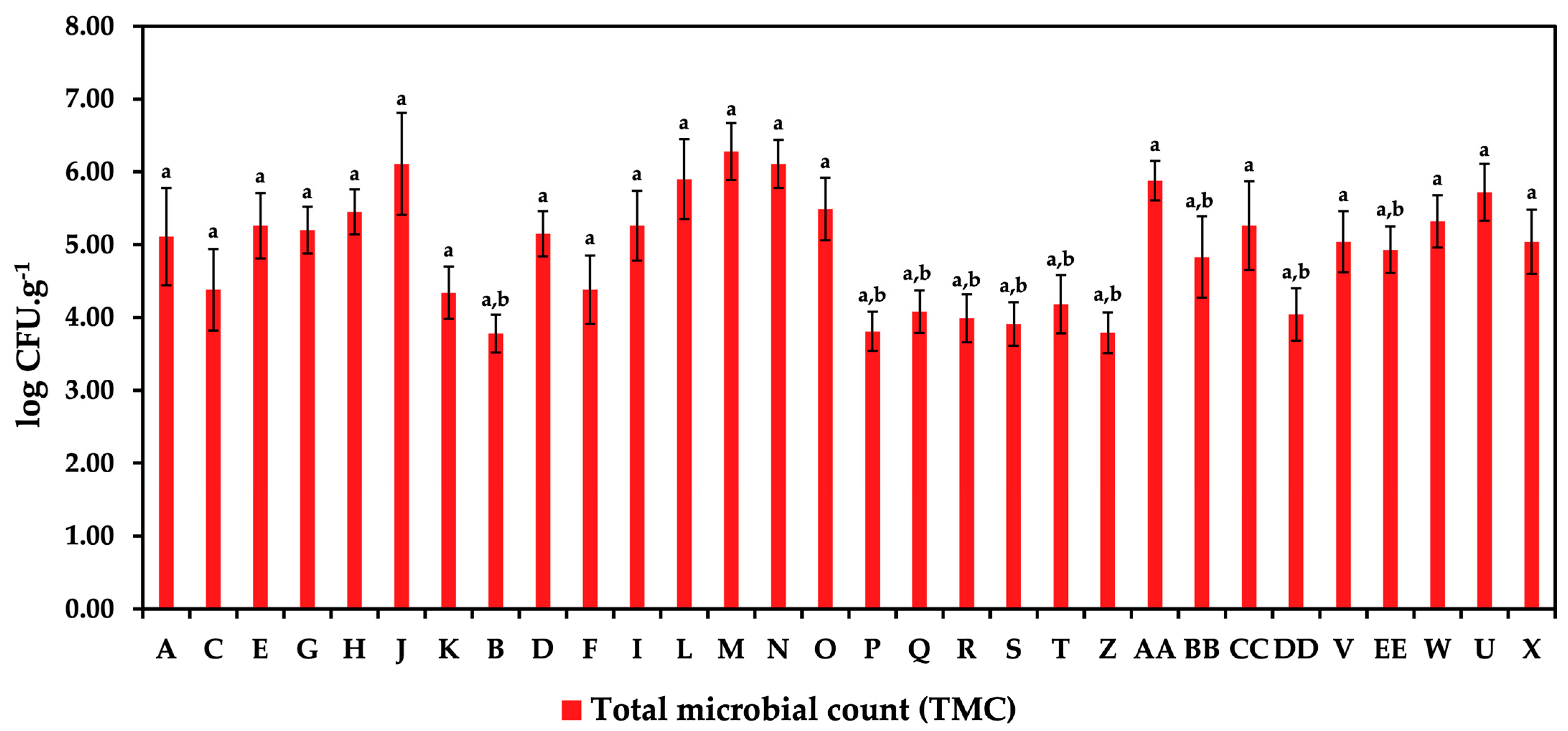
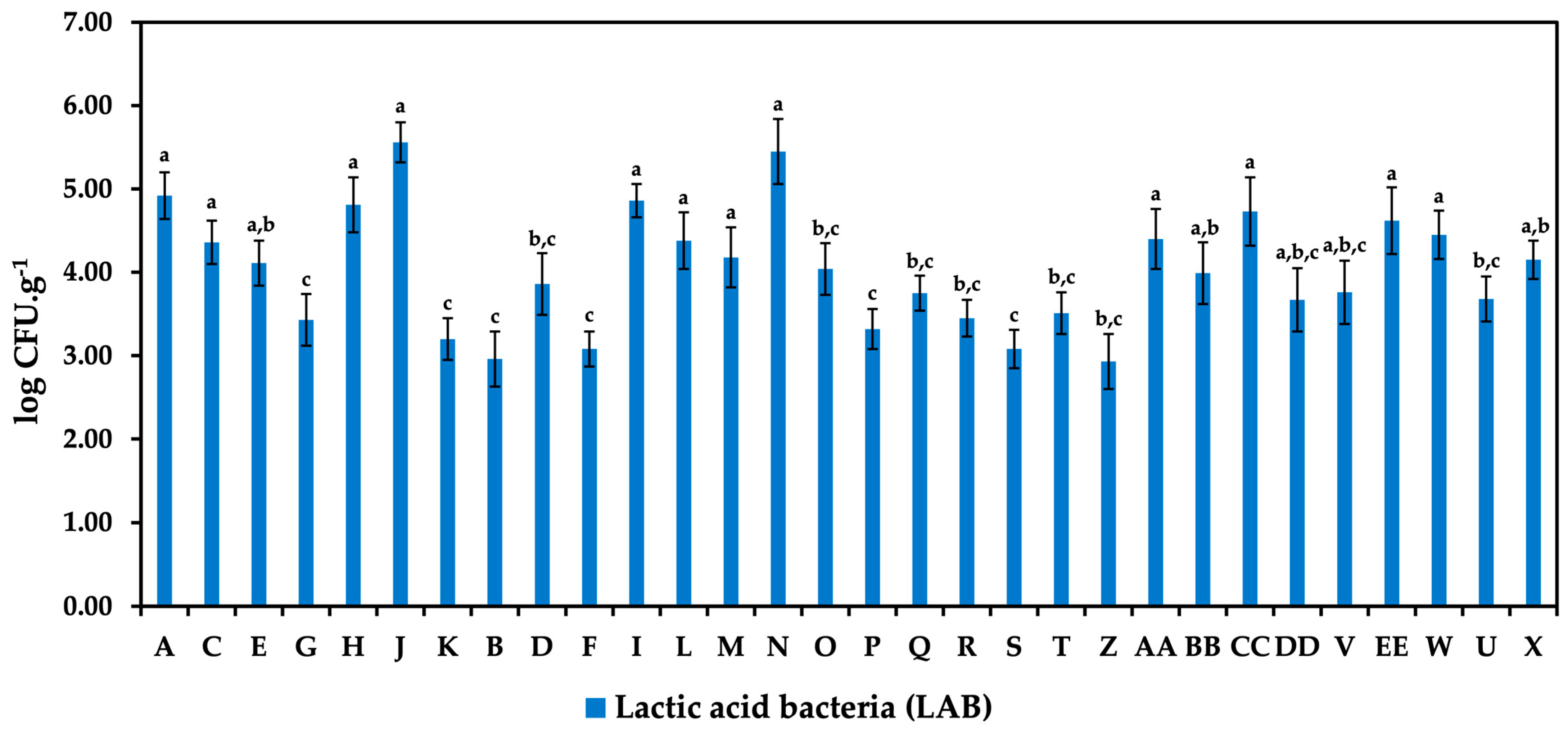
| Sample ID | HIS | TYR | PUT | CAD | SPE | Total BAs |
|---|---|---|---|---|---|---|
| A | ND | 21.08 ± 0.87 | ND | ND | 40.39 ± 2.63 | 61.47 ± 2.77 |
| C | ND | 20.54 ± 1.76 | ND | ND | 57.58 ± 2.50 | 78.12 ± 3.06 |
| E | ND | 15.70 ± 0.89 | ND | ND | 44.24 ± 2.11 | 59.94 ± 2.29 |
| J | ND | 3.99 ± 0.24 | ND | ND | 12.72 ± 0.83 | 16.71 ± 0.86 |
| K | ND | 3.84 ± 0.33 | ND | ND | 12.86 ± 0.73 | 16.70 ± 0.80 |
| B | ND | 7.39 ± 0.16 | ND | ND | 19.39 ± 1.91 | 26.78 ± 1.92 |
| D | ND | 2.38 ± 0.25 | ND | ND | 13.86 ± 0.87 | 16.24 ± 0.91 |
| F | ND | 3.23 ± 0.23 | ND | ND | 16.63 ± 0.96 | 19.86 ± 0.99 |
| L | ND | ND | ND | ND | 11.26 ± 0.56 | 11.26 ± 0.56 |
| M | 26.76 ± 2.11 | 380.43 ± 21.90 | 308.50 ± 19.29 | 31.01 ± 1.66 | 73.41 ± 1.77 | 820.11 ± 29.36 |
| N | ND | 122.71 ± 9.57 | 349.99 ± 0.91 | ND | 12.96 ± 1.84 | 485.66 ± 9.79 |
| O | ND | 133.25 ± 10.99 | 12.27 ± 0.67 | ND | 42.33 ± 2.05 | 187.85 ± 11.20 |
| P | ND | 7.71 ± 0.28 | ND | ND | 11.90 ± 0.67 | 19.61 ± 0.73 |
| Q | ND | 9.09 ± 0.60 | ND | ND | 18.71 ± 0.85 | 27.80 ± 1.04 |
| R | ND | 11.43 ± 0.86 | ND | 11.65 ± 0.65 | 20.97 ± 1.13 | 44.05 ± 1.56 |
| T | ND | 27.57 ± 1.88 | ND | 3.22 ± 0.09 | 5.04 ± 0.47 | 33.83 ± 1.94 |
| V | ND | 3.84 ± 0.33 | ND | ND | 12.86 ± 0.73 | 16.70 ± 0.80 |
| EE | ND | 41.98 ± 2.79 | ND | 3.45 ± 0.17 | 10.90 ± 0.65 | 56.33 ± 2.87 |
| W | ND | ND | ND | ND | 11.26 ± 0.56 | 11.26 ± 0.56 |
| U | ND | ND | ND | ND | 58.32 ± 3.40 | 104.98 ± 3.40 |
| Cheese Sample | Isolate ID | Identified Microorganism | HIS | TYR | TRY | PUT | CAD | SPE | Total BAs |
|---|---|---|---|---|---|---|---|---|---|
| Balkan cheese—consumer packaging | A1 | Lactococcus lactis | 0.60 ± 0.04 | 2.80 ± 0.17 | 3.70 ± 0.19 | 0.80 ± 0.06 | 2.30 ± 0.12 | 2.50 ± 0.15 | 12.70 ± 0.32 |
| A2 | Lacticaseibacillus casei | 2.70 ± 0.16 | 81.70 ± 3.27 | 4.60 ± 0.32 | 10.70 ± 0.75 | 4.90 ± 0.25 | 32.70 ± 0.98 | 137.30 ± 3.52 | |
| A5 | Staphylococcus equorum | 3.90 ± 0.16 | 47.53 ± 1.43 | ND | 5.60 ± 0.34 | 29.10 ± 0.87 | 43.57 ± 3.05 | 129.70 ± 3.50 | |
| C1 | Lacticaseibacillus casei | 0.30 ± 0.01 | 41.20 ± 2.47 | 3.40 ± 0.20 | 1.40 ± 0.04 | 1.40 ± 0.04 | 0.10 ± 0.01 | 47.80 ± 2.48 | |
| C3 | Acinetobacter lwoffii | ND | 38.82 ± 1.16 | ND | 2.70 ± 0.19 | 124.93 ± 8.75 | 37.55 ± 3.00 | 204.00 ± 9.32 | |
| E1 | Lacticaseibacillus casei | 0.10 ± 0.01 | 21.40 ± 1.93 | 2.10 ± 0.13 | 1.60 ± 0.11 | 2.00 ± 0.16 | 30.50 ± 2.44 | 57.70 ± 3.12 | |
| E2 | Serratia marcescens | 0.60 ± 0.05 | 13.50 ± 0.95 | 4.90 ± 0.34 | 1.30 ± 0.07 | 2.00 ± 0.08 | 33.10 ± 2.32 | 55.40 ± 2.53 | |
| E3 | Lacticaseibacillus casei | 3.00 ± 0.18 | 1.80 ± 0.09 | 5.10 ± 0.26 | 35.50 ± 2.49 | 10.80 ± 0.32 | 32.00 ± 1.28 | 88.20 ± 2.83 | |
| E5 | Staphylococcus warneri | 2.00 ± 0.08 | 1.40 ± 0.13 | 11.20 ± 0.45 | 4.30 ± 0.39 | 2.30 ± 0.09 | 0.40 ± 0.01 | 21.60 ± 0.62 | |
| E6 | Staphylococcus equorum | 1.70 ± 0.15 | 2.10 ± 0.06 | 10.30 ± 0.72 | 4.50 ± 0.41 | 2.50 ± 0.23 | ND | 21.10 ± 0.87 | |
| E8 | Kocuria varians | ND | 18.82 ± 0.94 | ND | ND | ND | 25.10 ± 2.01 | 43.92 ± 2.22 | |
| G2 * | Kocuria sp. | 0.40 ± 0.03 | 0.90 ± 0.07 | 2.90 ± 0.26 | 0.80 ± 0.05 | 0.90 ± 0.05 | 0.10 ± 0.01 | 6.00 ± 0.28 | |
| H7 | Serratia marcescens | ND | 5.50 ± 0.50 | ND | ND | ND | 20.44 ± 1.02 | 25.94 ± 1.14 | |
| J3 | Kocuria sp. | 0.40 ± 0.03 | 4.70 ± 0.19 | 2.90 ± 0.26 | 3.80 ± 0.11 | 1.50 ± 0.05 | 0.30 ± 0.02 | 13.60 ± 0.35 | |
| J4 | Bacillus cereus | ND | 4.30 ± 0.13 | ND | ND | ND | 18.03 ± 0.90 | 22.33 ± 0.91 | |
| J5 | Enterococcus durans | ND | 4.43 ± 0.35 | ND | ND | 8.99 ± 0.72 | 24.43 ± 1.71 | 37.85 ± 1.89 | |
| J8 | Enterococcus faecium | ND | 8.06 ± 0.40 | ND | ND | ND | 23.23 ± 0.70 | 31.29 ± 0.81 | |
| J9 | Enterococcus durans | ND | 2.33 ± 0.19 | ND | ND | 6.47 ± 0.19 | 16.53 ± 1.16 | 25.33 ± 1.19 | |
| K1 | Lactococcus lactis | ND | 5.24 ± 0.26 | ND | ND | ND | 22.21 ± 1.78 | 27.45 ± 1.80 | |
| K2 | Lacticaseibacillus paracasei | ND | 1.48 ± 0.07 | ND | ND | ND | 20.22 ± 1.82 | 21.70 ± 1.82 | |
| Balkan cheese—counter-sold | B3 | Lactiplantibacillus plantarum | 0.30 ± 0.03 | 1.50 ± 0.12 | 3.50 ± 0.28 | 0.60 ± 0.02 | 1.50 ± 0.14 | 20.80 ± 1.66 | 28.20 ± 1.70 |
| B4 | Lactococcus cremoris | 0.20 ± 0.01 | 5.10 ± 0.46 | 1.30 ± 0.09 | 1.50 ± 0.14 | 1.30 ± 0.04 | 30.20 ± 2.11 | 39.60 ± 2.17 | |
| D4 | Acinetobacter calcoaceticus | 1.60 ± 0.11 | 1.80 ± 0.13 | 14.10 ± 0.42 | 6.80 ± 0.54 | 2.50 ± 0.20 | 10.40 ± 0.73 | 37.20 ± 1.04 | |
| D5 | Lactococcus lactis | ND | 20.14 ± 1.81 | ND | ND | ND | 17.35 ± 1.39 | 37.49 ± 2.28 | |
| F1 | Enterococcus faecium | 0.30 ± 0.02 | 1.40 ± 0.10 | 2.30 ± 0.14 | 1.20 ± 0.08 | 1.00 ± 0.03 | 5.10 ± 0.26 | 11.30 ± 0.32 | |
| F3 | Bacillus amyloliquefacinns | 0.50 ± 0.04 | 1.60 ± 0.05 | 1.80 ± 0.14 | 0.80 ± 0.07 | 1.40 ± 0.10 | 10.50 ± 0.32 | 16.60 ± 0.37 | |
| F4 | Staphylococcus hominis | 0.40 ± 0.02 | 5.20 ± 0.16 | 2.60 ± 0.18 | 0.80 ± 0.07 | 1.80 ± 0.05 | 8.40 ± 0.59 | 19.20 ± 0.64 | |
| F5 | Bacillus licheniformis | 0.60 ± 0.03 | 2.90 ± 0.20 | 4.50 ± 0.27 | 2.00 ± 0.16 | 1.60 ± 0.13 | 10.30 ± 0.82 | 21.90 ± 0.91 | |
| I1 | Lysinibacillus fusiformis | 0.70 ± 0.06 | 2.10 ± 0.15 | 3.70 ± 0.26 | 1.00 ± 0.05 | 2.40 ± 0.17 | ND | 9.90 ± 0.35 | |
| I2 | Bacillus horneckiae | 0.60 ± 0.04 | 1.80 ± 0.09 | 4.40 ± 0.35 | 1.10 ± 0.04 | 2.40 ± 0.19 | ND | 10.30 ± 0.41 | |
| L1 | Lactiplantibacillus plantarum | ND | 4.30 ± 0.39 | ND | ND | ND | 15.95 ± 1.12 | 20.25 ± 1.18 | |
| L5 | Lacticaseibacillus casei | ND | 1.30 ± 0.12 | ND | ND | ND | 19.30 ± 1.54 | 20.60 ± 1.55 | |
| Feta—counter-sold | M1 | Lactiplantibacillus plantarum | 30.30 ± 1.21 | 152.30 ± 13.71 | 3.40 ± 0.10 | 0.90 ± 0.08 | 1.70 ± 0.14 | 2.40 ± 0.22 | 191.00 ± 13.76 |
| M2 | Levilactobacillus brevis | 0.30 ± 0.02 | 201.90 ± 12.11 | 4.00 ± 0.32 | 0.70 ± 0.04 | 2.20 ± 0.15 | 62.50 ± 2.50 | 271.60 ± 12.37 | |
| M4 | Enterococcus durans | 6.20 ± 0.50 | 10.50 ± 0.53 | 8.10 ± 0.49 | 1282.10 ± 89.75 | 30.30 ± 0.91 | 2.90 ± 0.17 | 1340.10 ± 89.76 | |
| M7 | Enterococcus faecium | 6.00 ± 0.18 | 2.00 ± 0.14 | 12.10 ± 0.48 | 395.80 ± 19.79 | 20.30 ± 1.83 | 70.40 ± 2.11 | 506.60 ± 19.99 | |
| N1 | Klebsiella aerogenes | 0.30 ± 0.01 | 1.70 ± 0.07 | 2.40 ± 0.10 | 199.80 ± 15.98 | 1.60 ± 0.10 | 0.40 ± 0.02 | 206.20 ± 15.98 | |
| N2 | Staphylococcus warneri | 0.40 ± 0.02 | 2.10 ± 0.17 | 2.60 ± 0.08 | 1.90 ± 0.13 | 1.20 ± 0.07 | 1.00 ± 0.09 | 9.20 ± 0.26 | |
| N4 | Serratia marcescens | ND | 37.25 ± 1.12 | ND | 201.60 ± 6.05 | 9.80 ± 0.88 | 34.43 ± 1.03 | 283.08 ± 6.30 | |
| N6 | Lacticaseibacillus casei | 1.60 ± 0.05 | 395.10 ± 23.71 | 9.80 ± 0.49 | 6.40 ± 0.38 | 2.70 ± 0.19 | 2.70 ± 0.24 | 418.30 ± 23.72 | |
| O1 | Staphylococcus warneri | 0.40 ± 0.03 | 4.70 ± 0.42 | 2.90 ± 0.26 | 1.90 ± 0.13 | 1.90 ± 0.17 | 2.40 ± 0.12 | 14.20 ± 0.56 | |
| O3 | Kocuria sp. | 0.40 ± 0.01 | 1.20 ± 0.11 | 3.20 ± 0.16 | 1.70 ± 0.15 | 1.60 ± 0.14 | 30.20 ± 1.51 | 38.30 ± 1.54 | |
| O4 | Psychrobacillus psychrodurans | 0.30 ± 0.01 | 81.20 ± 5.68 | 3.40 ± 0.24 | 1.40 ± 0.08 | 1.40 ± 0.04 | 8.10 ± 0.73 | 95.80 ± 5.74 | |
| O5 | Lacticaseibacillus casei | 0.30 ± 0.02 | 2.00 ± 0.16 | 1.70 ± 0.07 | 1.70 ± 0.07 | 1.40 ± 0.11 | 7.80 ± 0.39 | 14.90 ± 0.45 | |
| O7 | Enterococcus durans | 2.00 ± 0.16 | 113.40 ± 10.21 | ND | 113.40 ± 7.94 | ND | 40.70 ± 2.44 | 269.50 ± 13.16 | |
| O8 | Lactococcus cremoris | 0.30 ± 0.02 | 2.30 ± 0.16 | 1.80 ± 0.09 | 1.50 ± 0.14 | 1.60 ± 0.06 | 0.60 ± 0.02 | 8.10 ± 0.24 | |
| O9 | Enterococcus faecium | 1.60 ± 0.05 | 86.40 ± 3.46 | ND | ND | ND | 4.30 ± 0.34 | 92.30 ± 3.47 | |
| Jadel—consumer packaging | P3 | Lactococcus lactis | 0.40 ± 0.04 | 15.60 ± 0.47 | 2.60 ± 0.16 | 1.00 ± 0.04 | 1.50 ± 0.09 | 20.20 ± 0.81 | 41.30 ± 0.95 |
| Q2 | Klebsiella aerogenes | 0.30 ± 0.02 | 5.00 ± 0.20 | 2.80 ± 0.14 | 1.00 ± 0.09 | 1.90 ± 0.11 | 10.10 ± 0.30 | 21.10 ± 0.42 | |
| Q3 | Staphylococcus equorum | 0.50 ± 0.04 | 6.30 ± 0.57 | 3.10 ± 0.22 | 3.70 ± 0.19 | 2.00 ± 0.06 | 8.10 ± 0.41 | 23.70 ± 0.76 | |
| Q5 | Kocuria varians | 3.10 ± 0.12 | 0.20 ± 0.01 | 3.10 ± 0.22 | 1.70 ± 0.07 | 1.20 ± 0.08 | 4.20 ± 0.21 | 13.50 ± 0.34 | |
| R4 | Bacillus licheniformis | 0.40 ± 0.02 | 1.70 ± 0.10 | 3.00 ± 0.27 | 1.30 ± 0.10 | 1.80 ± 0.09 | 20.80 ± 0.62 | 29.00 ± 0.70 | |
| R6 | Lactiplantibacillus plantarum | ND | 23.61 ± 1.18 | ND | ND | ND | 18.90 ± 1.51 | 42.51 ± 1.92 | |
| T1 ** | Lactococcus lactis | ND | 17.98 ± 0.72 | ND | ND | 8.70 ± 0.78 | 23.38 ± 1.17 | 50.06 ± 1.58 | |
| Akawi—unflavored, stored for 1 year at 20–25 °C | Z1 | Bacillus licheniformis | ND | 15.51 ± 1.40 | ND | 8.51 ± 0.60 | 1.76 ± 0.12 | 10.69 ± 0.32 | 36.47 ± 1.56 |
| Z3 | Lactococcus lactis | ND | 39.61 ± 1.19 | ND | 4.28 ± 0.30 | 1.92 ± 0.15 | 26.98 ± 1.62 | 72.79 ± 2.04 | |
| AA4 | Enterococcus durans | ND | 9.07 ± 0.27 | ND | 13.07 ± 1.05 | 5.41 ± 0.27 | 14.58 ± 0.73 | 42.13 ± 1.33 | |
| AA5 | Enterococcus faecalis | ND | 12.38 ± 0.87 | ND | ND | ND | 28.19 ± 1.13 | 40.57 ± 1.42 | |
| BB1 | Lactiplantibacillus plantarum | ND | 16.38 ± 1.15 | ND | ND | ND | 44.37 ± 3.55 | 60.75 ± 3.73 | |
| BB6 | Lacticaseibacillus racasei | ND | 21.51 ± 1.29 | ND | ND | ND | 39.36 ± 1.18 | 60.87 ± 1.75 | |
| CC1 | Enterococcus durans | ND | 17.08 ± 1.54 | ND | 14.68 ± 0.73 | 7.16 ± 0.50 | 27.72 ± 2.22 | 66.64 ± 2.84 | |
| CC3 | Enterococcus faecium | ND | 15.00 ± 0.60 | ND | 11.07 ± 0.33 | 5.41 ± 0.32 | 22.26 ± 2.00 | 53.74 ± 2.14 | |
| CC6 | Enterococcus durans | ND | 18.53 ± 1.11 | ND | 12.10 ± 1.09 | 5.77 ± 0.23 | 28.93 ± 1.45 | 65.33 ± 2.14 | |
| CC7 | Lacticaseibacillus casei | ND | 15.50 ± 0.93 | ND | 11.80 ± 0.59 | 6.06 ± 0.24 | 25.77 ± 1.55 | 59.13 ± 1.91 | |
| DD2 | Lacticaseibacillus casei | ND | 13.20 ± 1.06 | ND | 14.62 ± 1.32 | 6.77 ± 0.54 | 22.56 ± 1.13 | 57.15 ± 2.10 | |
| DD4 | Lactiplantibacillus paraplantarum | ND | 18.41 ± 1.29 | ND | ND | ND | 34.14 ± 2.05 | 52.55 ± 2.42 | |
| Akawi—gyros flavor | V1 | Klebsiella oxytoca | 0.50 ± 0.04 | 12.40 ± 0.87 | 4.50 ± 0.32 | 2.30 ± 0.18 | 2.00 ± 0.18 | 2.40 ± 0.22 | 24.10 ± 0.98 |
| V3 | Bacillus licheniformis | 4.70 ± 0.42 | 2.10 ± 0.17 | 4.60 ± 0.41 | 3.50 ± 0.21 | 5.80 ± 0.23 | 3.20 ± 0.29 | 23.90 ± 0.75 | |
| V4 | Staphylococcus hominis | 0.70 ± 0.06 | 11.10 ± 1.00 | 3.90 ± 0.31 | 2.90 ± 0.23 | 1.90 ± 0.15 | 2.80 ± 0.11 | 23.30 ± 1.09 | |
| V5 | Kocuria varians | 0.50 ± 0.05 | 3.30 ± 0.23 | 4.10 ± 0.33 | 0.50 ± 0.03 | 1.80 ± 0.14 | 2.80 ± 0.14 | 13.00 ± 0.45 | |
| EE1 | Lactiplantibacillus plantarum | ND | 21.14 ± 0.63 | 10.90 ± 0.33 | 6.30 ± 0.19 | 2.90 ± 0.09 | 37.26 ± 1.49 | 78.50 ± 1.67 | |
| EE2 | Lentilactobacillus hilgardii | ND | 19.61 ± 0.59 | ND | ND | ND | 43.96 ± 3.96 | 63.57 ± 4.00 | |
| EE5 | Enterococcus durans | ND | 16.31 ± 1.14 | ND | 90.41 ± 8.14 | 5.44 ± 0.49 | 26.54 ± 1.33 | 138.70 ± 8.34 | |
| EE6 | Bacillus cereus | ND | 18.59 ± 0.56 | ND | ND | ND | 39.74 ± 3.18 | 58.33 ± 3.23 | |
| EE7 | Staphylococcus warneri | ND | 27.01 ± 1.89 | ND | ND | ND | 50.40 ± 1.51 | 77.41 ± 2.42 | |
| EE8 | Enterococcus faecium | ND | 31.20 ± 1.56 | ND | ND | ND | 32.81 ± 2.62 | 64.01 ± 3.05 | |
| EE9 | Enterococcus durans | ND | 24.77 ± 1.24 | ND | ND | ND | 41.34 ± 2.89 | 66.11 ± 3.15 | |
| Akawi—garlic flavor | W1 | Bacillus cereus | 0.30 ± 0.02 | 1.10 ± 0.07 | 2.80 ± 0.08 | 0.60 ± 0.04 | 1.10 ± 0.09 | 12.10 ± 0.73 | 18.00 ± 0.74 |
| W2 | Staphylococcus warneri | 0.30 ± 0.01 | 1.50 ± 0.06 | 3.10 ± 0.25 | 1.60 ± 0.14 | 1.20 ± 0.10 | 9.70 ± 0.87 | 17.40 ± 0.93 | |
| W3 | Enterococcus faecium | 0.30 ± 0.01 | 1.10 ± 0.04 | 1.40 ± 0.08 | 1.70 ± 0.05 | 1.20 ± 0.06 | 12.70 ± 0.76 | 18.40 ± 0.77 | |
| W4 | Enterococcus faecalis | 0.20 ± 0.01 | 2.00 ± 0.16 | 1.50 ± 0.05 | 4.20 ± 0.38 | 1.00 ± 0.06 | 4.30 ± 0.22 | 13.20 ± 0.47 | |
| Akawi—tzatziki flavor | U1 | Enterococcus durans | ND | 16.71 ± 1.50 | ND | ND | ND | 34.11 ± 1.36 | 50.82 ± 2.03 |
| U2 | Lacticaseibacillus paracasei | ND | 40.02 ± 3.20 | ND | 6.04 ± 0.18 | 5.64 ± 0.45 | 26.33 ± 1.05 | 78.03 ± 3.41 | |
| U3 | Lactococcus lactis | ND | 17.25 ± 1.38 | ND | ND | ND | 34.47 ± 2.07 | 51.72 ± 2.49 | |
| U4 | Bacillus safensis | ND | 43.94 ± 1.76 | 7.02 ± 0.42 | 4.51 ± 0.32 | 9.57 ± 0.38 | 36.77 ± 1.84 | 101.81 ± 2.63 | |
| U5 | Enterococcus durans | ND | 16.83 ± 1.35 | ND | ND | ND | 42.18 ± 2.53 | 59.01 ± 2.87 | |
| U8 | Enterococcus faecium | ND | 14.05 ± 0.42 | ND | ND | ND | 29.99 ± 1.20 | 44.04 ± 1.27 | |
| U9 | Bacillus licheniformis | 1.70 ± 0.14 | 1.80 ± 0.11 | 4.70 ± 0.42 | 490.80 ± 44.17 | 5.10 ± 0.36 | 3.60 ± 0.29 | 507.70 ± 44.18 | |
| X1 | Lactococcus lactis | 0.60 ± 0.04 | 1.80 ± 0.14 | 4.40 ± 0.22 | 1.10 ± 0.03 | 2.20 ± 0.09 | ND | 10.10 ± 0.28 | |
| X2 | Lactococcus cremoris | 0.70 ± 0.03 | 1.70 ± 0.10 | 4.00 ± 0.16 | 1.70 ± 0.12 | 1.90 ± 0.11 | ND | 10.00 ± 0.25 | |
| X3 | Staphylococcus epidermidis | 0.70 ± 0.04 | 1.50 ± 0.06 | 3.30 ± 0.10 | 1.30 ± 0.12 | 1.60 ± 0.14 | ND | 8.40 ± 0.22 | |
| X4 | Lacticaseibacillus paracasei | 0.70 ± 0.04 | 1.40 ± 0.11 | 3.70 ± 0.22 | 1.30 ± 0.12 | 1.80 ± 0.16 | 1.60 ± 0.14 | 10.50 ± 0.35 | |
| X5 | Lactiplantibacillus paraplantarum | 0.80 ± 0.07 | 1.20 ± 0.11 | 4.40 ± 0.31 | 1.40 ± 0.11 | 2.10 ± 0.13 | 2.50 ± 0.23 | 12.40 ± 0.44 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Buňková, L.; Riemel, J.; Purevdorj, K.; Vinter, Š.; Míšková, Z.; Jančová, P. Biogenic Amines in White Brined Cheeses. Foods 2025, 14, 369. https://doi.org/10.3390/foods14030369
Buňková L, Riemel J, Purevdorj K, Vinter Š, Míšková Z, Jančová P. Biogenic Amines in White Brined Cheeses. Foods. 2025; 14(3):369. https://doi.org/10.3390/foods14030369
Chicago/Turabian StyleBuňková, Leona, Jakub Riemel, Khatantuul Purevdorj, Štěpán Vinter, Zuzana Míšková, and Petra Jančová. 2025. "Biogenic Amines in White Brined Cheeses" Foods 14, no. 3: 369. https://doi.org/10.3390/foods14030369
APA StyleBuňková, L., Riemel, J., Purevdorj, K., Vinter, Š., Míšková, Z., & Jančová, P. (2025). Biogenic Amines in White Brined Cheeses. Foods, 14(3), 369. https://doi.org/10.3390/foods14030369





