Abstract
The nutritional content of tiger nut (Cyperus esculentus L.) is abundant, rich in oil, protein, and starch. Conventional methods for assessing the nutrient composition of tiger nuts (TNs) are time-consuming and labor-intensive. Near-infrared spectroscopy (NIR) combined with chemometrics has been widely applied in rapidly predicting the nutritional content of various crops, but its application to TNs is rare. In order to enhance the practicality of the method, this study employed a portable NIR in conjunction with chemometrics to rapidly predict the contents of crude oil (CO), crude protein (CP), and total starch (TS) from TNs. In the period from 2022 to 2023, we collected a total of 75 TN tuber samples of 28 varieties from Xinjiang Uyghur Autonomous Region and Henan Province. The three main components were measured using common chemical analysis methods. Partial least squares regression (PLSR) was utilized to establish prediction models between NIR and chemical indicators. In addition, to further enhance the prediction performance of the models, various preprocessing and variable selection algorithms were utilized to optimize the prediction models. The optimal models for CO, CP, and TS exhibited coefficient of determination (R2) values of 0.8946, 0.8525, and 0.8778, with root mean square error of prediction (RMSEP) values of 1.1764, 0.7470, and 1.4601, respectively. The absolute errors between the predicted and actual values for the three-indicator spectral measurements were 0.80, 0.59, and 0.99. The results demonstrated that the portable NIR combined with chemometrics could be effectively utilized for the rapid analysis of quality-related components in TNs. With further refinements, this approach could revolutionize TN quality assessment and be used to determine optimal harvest times, as well as facilitate the graded marketing of TNs.
1. Introduction
The tuber of tiger nuts (Cyperus esculentus L.) is rich in nutrients, earning it the title of the “King of Oil Crops”. Recent investigations have shown that tiger nuts (TNs) are high in oil content (17–35.43%), and rich in monounsaturated fatty acids, polyphenols, tocopherols, and phytosterols, as well as high-value compounds such as proteins (5–9.7%), carbohydrates (16.2–47%), vitamins (Vitamin A, C, D, and E), minerals, and bioactive compounds [1]. The TN can be used for oil extraction, brewing, and other economically valuable applications, and is well-suited for growth in sandy soils, significantly enhancing the utilization of marginal lands. The oil extracted from TNs shares similarities with olive oil, both of which contain over 70% monounsaturated fatty acids and are rich in polyphenols, tocopherols, and phytosterols [1,2]. The starch from TNs exhibits good resistance to digestion, which is beneficial for lowering blood sugar levels [3]. The proteins in TNs are rich in essential amino acids, particularly lysine, threonine, and leucine [4]. They exhibit high digestibility and favorable nutritional properties, making TN an excellent source of plant-based protein [5]. Since these indicators are crucial for assessing the quality and nutritional value of TNs, providing scientific evidence and guidance for its cultivation, processing, and utilization, there is an urgent need for an efficient detection method to measure these indicators.
Researchers often use conventional chemical analysis methods in the laboratory to detect the chemical components in TNs. For example, protein content is commonly determined using the Kjeldahl method [6] or the Bicinchoninic Acid (BCA) assay [5]. Oil content is measured using the Soxhlet extraction method [6], gas chromatography [7], or liquid chromatography [8], and total starch content is analyzed using a dual-wavelength colorimetric method [3,9]. However, compared to the seeds of crops such as wheat and rapeseed, TNs are larger, and the small sample sizes used in chemical analysis methods may not be representative, failing to accurately reflect the distribution of the chemical components in the sample population. Additionally, chemical analysis is time-consuming and labor-intensive, making it unsuitable for large-scale and rapid quality analysis. Moreover, destructive analysis methods are not convenient for subsequent breeding and variety improvement. Therefore, to meet the systematic evaluation needs of TNs from different regions, cultivation conditions, and varieties, it is necessary to establish a non-destructive and rapid detection system.
There are an increasing number of technologies that involve near-infrared (NIR) light [10]. NIR spectroscopy offers the advantages of speed, non-destructiveness, and multi-component analysis, making it widely used in agriculture [11], food [12], pharmaceuticals [13], petrochemicals [14], and other fields. NIRS utilizes multiple wavelengths of NIR light to retrieve the material information of an object, especially its chemical components [10]. NIR measures the absorption intensity of samples at different wavelengths of near-infrared light to obtain the near-infrared spectrum. NIR primarily records the overtone and combined absorption of vibrations from hydrogen-containing groups X–H (where X = C, N, O), making it applicable to agricultural products for quality assessment, variety identification, and other analytical purposes [15]. NIR technology enables the rapid and non-destructive determination of the typical quality characteristics of food categories [16]. Hence, NIRS is superior for mobile material sensing tasks, such as identifying food compositions, water quality analysis, and crop disease detection [10]. For example, Tarandeep Singh et al. used a convolutional neural network (CNN) to determine the protein content in barley samples, achieving a coefficient of determination (R2) of 0.9962 and a root mean square error prediction (RMSEP) of 0.0823, allowing for the accurate prediction of the protein content in barley [17]. Özcan Çataltas et al. utilized a one-dimensional convolutional autoencoder combined with NIR to detect protein, starch, oil, and moisture content in corn kernels. The R2 values for these were 0.9012, 0.9359, 0.9632, and 0.9716, respectively, with corresponding RMSEP values of 0.1535, 0.2093, 0.0388, and 0.0628 [18]. Edenio Olivares et al. employed logistic regression, support vector machine (SVM), and partial least squares discriminant analysis (PLSDA) models to predict rice protein and amylose content, achieving a high accuracy of 94%, an F1-score of 90%, an average precision of 94%, and a low classification error rate of 4%. This enabled the accurate and non-destructive classification of rice quality [19]. NIR has also made significant contributions to the quantitative analysis of soybean proteins, oils, and fatty acids [20,21,22]. However, there are no relevant articles on near-infrared analytical models for TNs. Therefore, this paper selected NIR spectroscopy, combined with chemometric methods, to establish predictive models for nutritional indicators in TNs. In cereal composition analysis, the rapid detection of oil, protein, and starch using NIR is well established. Consequently, the application of NIR for the analysis of TN components holds potential and is feasible.
Commonly used modeling algorithms, such as partial least squares regression (PLSR) [23] and principal component regression (PCR) [24], typically perform full-spectrum analysis. However, in NIR, the number of variables frequently far exceeds the number of samples. Consequently, collinearity and redundancy are common phenomena in the data matrix, and irrelevant information such as background, noise, and overlapping bands can affect model accuracy and robustness [25]. To fully exploit informative spectra, scientists have developed various variable selection algorithms, including interval partial least squares regression (iPLS) [26], backward interval partial least squares regression (biPLS) [27], moving-window partial least squares regression (MWPLS) [28], uninformative variable elimination (UVE) [29], successive projections algorithm (SPA) [30], and interval combination optimization (ICO) [31]. For instance, NIR combined with ICO was used to establish models for the four major components (total sugars, reducing sugars, total nitrogen, and nicotine) of tobacco, and exhibited a higher prediction accuracy compared to full-spectrum models [32]. Additionally, there is a growing approach toward developing handheld near-infrared spectroscopy devices to be easily applied for in situ determinations. NIR instruments are becoming increasingly smaller in size, less costly, more robust, and operable without specialized training for the operators. Portable NIR spectrometers are poised to broaden the application scope of NIR analytical techniques, thereby contributing significantly to grain inspection, food safety, and other related fields [33]. A portable NIR was used to collect rice spectra within the range of 800–1100 nm and MWPLS was applied to construct an analysis model for amylose content. The results demonstrated good accuracy and stability, with an R2 of 0.96 and an RMSEP of 0.4–0.5%. Furthermore, this model has been piloted in a cereal milling plant [34]. These variable selection algorithms each have unique mechanisms, advantages, and disadvantages in variable selection. In practical applications, the appropriate algorithm or combination of algorithms should be selected based on the characteristics of the spectral data and the analysis goals.
In this study, to enhance the universality and reliability of the near-infrared analysis model, 75 samples of TNs from multiple varieties and regions were collected, and portable NIR was utilized to improve the practicality. Additionally, in order to boost the predictive performance of the model, various preprocessing techniques and variable selection algorithms were employed. Subsequently, quantitative prediction models for crude oil (CO), crude protein (CP), and total starch (TS) were established. Ultimately, the prediction accuracy of the models was verified using unknown samples.
2. Materials and Methods
2.1. Sample Preparation and Near-Infrared Spectrum Collection
In the period from 2022 to 2023, we collected a total of 75 TN tuber samples of 28 varieties (Table S1). After washing, the fresh samples were air-dried outdoors for a week to maintain a moisture content of approximately 10%, then crushed and sieved through a 30-mesh screen. The processed samples were stored in a refrigerator at 4 °C.
The near-infrared spectra of the 75 TN samples were collected using a handheld near-infrared spectrometer (IAS8120, Intelligent Analysis Service Co., Ltd., Wuxi, China). Please see Figure S1 for an image of the instrument. Prior to acquiring the spectral data, the near-infrared spectrometer recorded a white reference spectrum and re-recorded it every 30 min. The 15.0 g TN powder samples were placed in a dedicated sample cup positioned at the light source of the spectrometer. The instrument collected spectra via diffuse reflection with a scanning wavelength range of 900–1700 nm, a resolution of 12 nm, and 20 scans per sample. Each sample was measured three times, and the original spectra were averaged to obtain a mean spectrum for subsequent modeling and analysis. The instrument automatically converts reflectivity to absorbance using the formula A = −log(R), where A represents absorbance and R represents reflectivity.
2.2. The Analyses of Crude Oil, Crude Protein, and Total Starch
Drawing upon the research of Duyi with minor modifications, the CO content of TNs was analyzed using the Soxhlet extraction method [6], with each sample being measured in triplicate for accuracy. A powder sample of 0.5 g was placed in an oven at 80 °C for 2 h to determine its dry weight. Subsequently, the samples were placed in fat-free filter paper thimbles and extracted with petroleum ether (boiling range: 30–60 °C) for 6 h. During the extraction, the petroleum ether was refluxed once every 10–15 min until it became clear and transparent. After extraction, the samples were dried for 30 min, and their dry weight was measured to calculate the crude fat content.
Drawing upon the research of Duyi with minor modifications, the Kjeldahl method was employed to measure the CP content of TNs [6], with each sample being measured in duplicate for accuracy. A 0.5 g sample was weighed into a digestion tube, and 10 milliliters of concentrated sulfuric acid was slowly added. The samples were then digested in a digestion furnace at 250 °C for 30 min, followed by an increase to 350 °C for another 30 min, and finally to 400 °C for 4 h. After cooling, the digest was transferred to a 250 milliliter volumetric flask and diluted to the mark. The nitrogen content was then determined using an automatic Kjeldahl nitrogen analyzer. The crude protein content was calculated by multiplying the nitrogen content by a conversion factor of 6.25.
The TS content was measured using a Total Starch Content Assay Kit (AKSU015, Boxbio, Beijing, China), with each sample being measured in triplicate for accuracy. The detection principle employed is based on the anthrone colorimetric assay [35]. Briefly, 50 mg of sample was weighed into a centrifuge tube and mixed thoroughly with 1 mL of elution buffer. The mixture was then incubated in a sealed water bath at 80 °C for 30 min to prevent water loss. Following incubation, the mixture was centrifuged at 8000 g for 10 min at room temperature. The supernatant was discarded, and the precipitate was retained. The precipitate was thoroughly mixed with 500 μL of distilled water and gelatinized in a water bath at 95 °C for 15 min. After cooling to room temperature, 1 mL of extraction buffer was added and thoroughly mixed. The mixture was then extracted at room temperature for 15 min, with shaking occurring 3–5 times during this period. Following extraction, the mixture was centrifuged at 8000 g for 10 min at room temperature, and the supernatant was collected for measurement. The collected supernatant was appropriately diluted, and the color development reagent was then added. The mixture was incubated at 95 °C for 10 min, cooled to room temperature, and the absorbance at 620 nm was measured to calculate the starch content.
2.3. Algorithms
During the spectral collection process, the influence of instrument noise or light scattering is encountered. Therefore, three preprocessing methods were specifically selected in this research. Savitzky–Golay smoothing (S–G smoothing) [36] is a digital filtering technique utilized for data smoothing and derivative calculation. It can reduce noise while preserving the characteristics of the signal as much as possible. In this study, the size of the smoothing window was set to 5 data points. Standard Normal Variate (SNV) [37] and Multiplicative Scatter Correction (MSC) [38] are primarily used to correct scattering effects in spectral data and are particularly applicable to mitigating the interference of light scattering caused by powder samples.
In addition, in order to remove irrelevant or redundant variables and improve the model performance, four kinds of variable selection algorithms were employed in this study: moving-window partial least squares regression (MWPLS) [28], interval partial least squares regression (iPLS) [26], interval combination optimization (ICO) [31], and uninformative variable elimination combined with the successive projections algorithm (UVE-SPA) [29,39]. Among them, MWPLS and iPLS are interval variable selection algorithms designed to identify specific intervals that are most correlated with model performance. In this study, for iPLS, we divided the entire spectral region into 10 equal parts to determine the optimal spectral region for the model. For MWPLS, the size of the moving window was set to 1/10 of the total number of variables in each iteration to identify the optimal spectral region. ICO is also an interval variable selection algorithm, but unlike the aforementioned two algorithms, it considers the relationship between combinations of intervals and the predicted values, seeking the most relevant combination of intervals. The procedure of ICO is outlined as follows: firstly, the spectra were generated into 40 equal-width intervals and 4000 random combinations via weighted bootstrap sampling. Secondly, the weight of each interval was calculated by the frequency of appearance. Thirdly, the PLS algorithm and five-fold cross-validation were used to extract the ratio of optimal intervals with lower RMSECVs. Finally, the intervals with lower RMSECVs were selected as the optimal intervals. The UVE-SPA is a variable selection method that combines the UVE algorithm and SPA. The main goal of the UVE algorithm is to reduce the number of variables included in the final model, thereby decreasing model complexity and improving model performance. The SPA selects wavelengths through projection analysis, enabling the selection of variable combinations with minimal redundant information and collinearity. Thus, the UVE-SPA can effectively reduce model complexity and enhance predictive accuracy. In the UVE algorithm section of this study, PLSR models were established using the spectral data and chemical values from the training set, with five-fold cross-validation employed. The maximum number of latent variables was set to 10, and information from all 10 components was utilized to eliminate variables that did not contribute any relevant information to the chemical values. For the SPA section, the default minimum number of selected variables was set to 1, and the maximum number was set to one-third of the number of variables selected by the UVE algorithm.
2.4. Software and Datasets
Quantitative analysis models were established for the near-infrared spectral data of TNs, using CO, CP, and TS as the analytical indicators. Each sample was measured three times using NIR, and the average of the three spectra was taken as the representative spectrum for that sample. Additionally, for each indicator, the values were ranked. Subsequently, systematic sampling (with a distance of 3) was used to divide the dataset into a calibration set (50 samples) and a validation set (25 samples). The model performance was optimized using 10-fold cross-validation. Data preprocessing, data modeling, and model plotting were all conducted using MATLAB software (Version R2024b, MathWorks, United States).
2.5. Evaluation Metrics
The model evaluation metrics utilized were the R2, RMSE of cross-validation (RMSECV), and RMSEP. A value of R2 closer to 1 and a smaller RMSEP indicate a stronger predictive performance of the model.
where represents the actual value of the i observation, represents the predicted value of the i observation, represents the mean of the observed values, and n represents the total number of observations.
3. Results
3.1. Sample Composition Content
To ensure that the samples are representative, TNs of different varieties were collected from various provinces, implying a certain degree of variability in their chemical values. This variability ensures the generalization ability and robustness of the model. In this research, for the 75 collected samples, the values for CO, CP, and TS ranged from 8.45 to 26.83, 4.31 to 12.13, and 20.85 to 40.40, respectively. The sampling method employed was systematic sampling after ranking, which ensured that both the maximum and minimum values were included in the calibration set, thereby guaranteeing the representativeness of the modeling samples. The distribution of various indicators in each dataset of TN samples is displayed in Table 1.

Table 1.
The distribution of various indicators in each dataset of TN samples.
3.2. Near-Infrared Spectroscopy and Preprocessing
The sample spectra, which were extracted from TN samples within the wavelength range of 900–1700 nm, are shown in Figure 1A. The spectral curves of the different samples exhibited a consistent trend with nearly identical positions of the absorption peaks, indicating a high degree of similarity in the internal chemical composition of the samples. The differences in absorbance magnitudes could be attributed to variations in the content of individual compounds, as well as to physical effects such as scattering. The spectra primarily contained three characteristic peaks: the second overtone vibrational absorption of C-H at 1200 nm, the first overtone vibrational absorption of O-H at 1460 nm, and the first overtone vibrational absorption of N-H at 1580 nm. Besides containing the chemical information of the sample itself, the spectra also encompassed other irrelevant information, such as electrical noise, sample background, and stray light. In this study, three preprocessing methods (S–G smoothing, SNV, and MSC) were employed to optimize the spectra, as displayed in Figure 1B–D. After the preprocessing was completed, it could be seen solely from the spectrogram that both SNV and MSC had significantly reduced the spectral differences caused by light scattering.
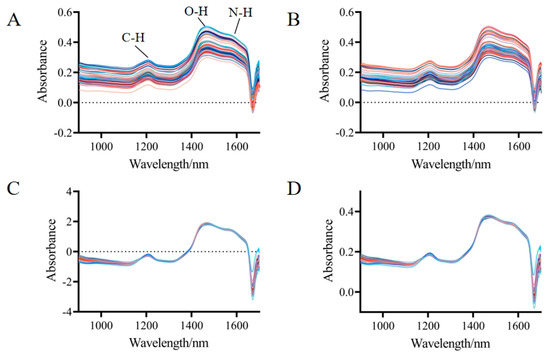
Figure 1.
Near-infrared spectra of TNs. (A) Raw spectra. (B) Savitzky–Golay smoothing (S–G smoothing). (C) Standard Normal Variate (SNV). (D) Multiplicative Scatter Correction (MSC).
3.3. Full-Spectrum Model
Near-infrared models for crude oil (CO), crude protein (CP), and total starch (TS) were established using PLSR. The maximum number of latent variables (LVs) was limited to ten, and the optimal number of LVs was determined by the five-fold cross-validation technique. The models based on raw spectra and those processed with three different preprocessing methods are presented in Table 2 and Figure 2. For the raw spectra models, the RCV2 and RPre2 values for CO, CP, and TS were 0.8025 and 0.8124; 0.8962 and 0.3276; and 0.6408 and 0.8415, respectively. Although the model for CO achieved a certain degree of prediction accuracy, there were issues of overfitting and underfitting in the model performance for CP and TS. After applying the three preprocessing methods, smoothing and SNV did not show significant improvement for the three indicators. However, the data preprocessed with MSC showed a substantial enhancement for TS, with RCV2 and RPre2 values of 0.7167 and 0.8132, respectively, and maintained the model performance for CO, with RCV2 and RPre2 values of 0.8141 and 0.8062, respectively. The results from the aforementioned preprocessing indicated that, although preprocessing could somewhat improve the predictive performance of a model for a certain indicator, the overall improvement was not obvious. In light of this, we adopted four different variable selection algorithms, aiming to explore and construct models with better performances.

Table 2.
Results of full-spectrum analysis models for three components.
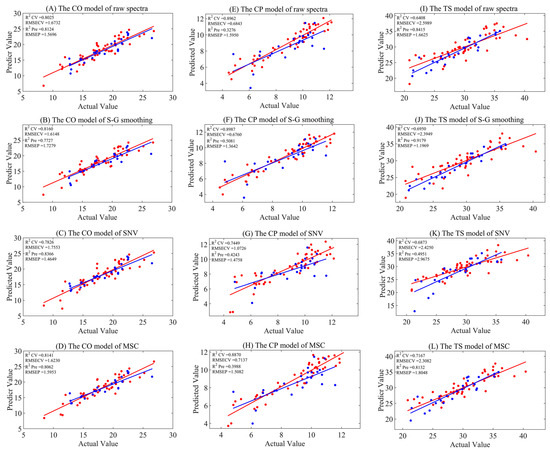
Figure 2.
The model results of full-spectrum analysis combined with three preprocessing methods, where (A–D) represent CO models for raw, S–G smoothing, SNV, and MSC, respectively. (E–H) are CP models for raw, S–G smoothing, SNV, and MSC, respectively. (I–L) are TS models for raw, S–G smoothing, SNV, and MSC, respectively. Red represents the training set, and blue represents the test set.
3.4. Variable Selection Algorithm Model
The results of full-spectrum modeling in this study failed to meet the requirements of practical applications, as too many irrelevant variables caused a decrease in model accuracy. To address this issue, we next employed four variable selection algorithms to screen the spectral ranges suitable for quantitative near-infrared spectroscopy models.
3.4.1. Variable Selection Model for CO
Compared to the full-spectrum model for CO, the combination of S–G smoothing preprocessing with ICO, along with UVE-SPA variable selection models, demonstrated superior model accuracy, with Rpre2 values of 0.8852 and 0.8946, and RMSEP values of 1.2279 and 1.1764. The detailed performances of variable selection algorithms are shown in Table 3 and Figure 3. However, the MWPLS and iPLS models did not contribute to optimization in this context. Interestingly, after SNV and MSC preprocessing, the ICO calibration set models generally exhibited better accuracy, but the performance of the validation set models declined, with Rpre2 values of 0.8177 and 0.8473, and RMSEP values of 1.5471 and 1.5297, respectively. This might be attributed to the fact that SNV and MSC preprocessing highlighted more characteristic variables, leading to the overfitting of the models, which in turn resulted in poorer performance in the validation set.

Table 3.
Results of variable selection analysis models for CO.
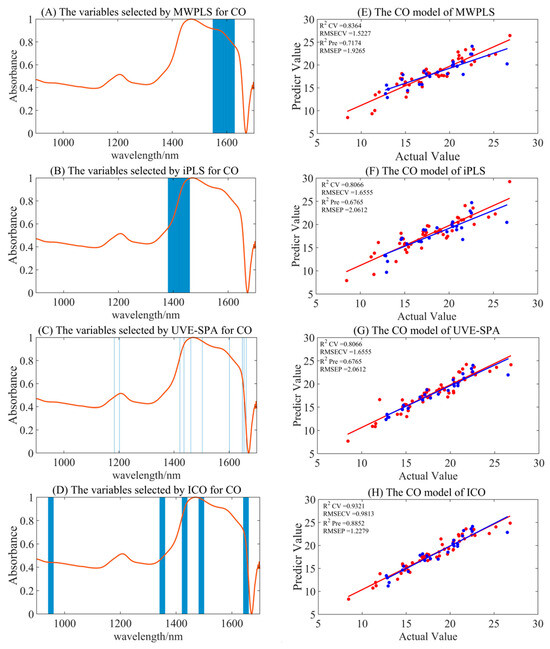
Figure 3.
The summary of the optimal model results for CO under different variable selection algorithms. (A–D) are the selected regions of MWPLS, iPLS, UVE-SPA, and ICO, respectively. (E–H) represent the model results by utilizing the corresponding selected regions. Red represents the training set, and blue represents the test set.
The optimal preprocessing-screened feature variables from the two variable selection methods overlapped. In Figure 2, UVE-SPA variable selection yielded the following wavelengths: 1183, 1202, 1421, 1436, 1461, 1503, 1601, 1648, 1654, and 1662 nm. Meanwhile, ICO variable selection identified the following spectral ranges: 941–960, 1341–1360, 1421–1440, 1481–1500, and 1641–1660 nm. These selected spectral bands primarily reflect the characteristic absorption peaks of C-H bond overtones and combination bands, which correspond to the vibrational absorption of numerous -CH2 and -CH3 groups in CO. The chosen spectral bands possessed certain chemical interpretability and provided the necessary feature information for the model.
3.4.2. Variable Selection Model for CP
The full-spectrum model for CP exhibited significant overfitting, rendering the results impractical. After preprocessing and combining with four variable selection algorithms for modeling (displayed in Table 4 and Figure 4), only the combination of S–G smoothing with the UVE-SPA, and MSC preprocessing with MWPLS yielded reliable modeling results, with Rpre2 values of 0.8525 and 0.7800, and RMSEP values of 0.7470 and 0.9124. The S–G smoothing combined with the UVE-SPA emerged as the optimal model, significantly enhancing model accuracy and stability. The ICO calibration set models generally demonstrated better accuracy, but the performance of the validation set models declined, indicating overall overfitting.

Table 4.
Results of variable selection analysis models for CP.
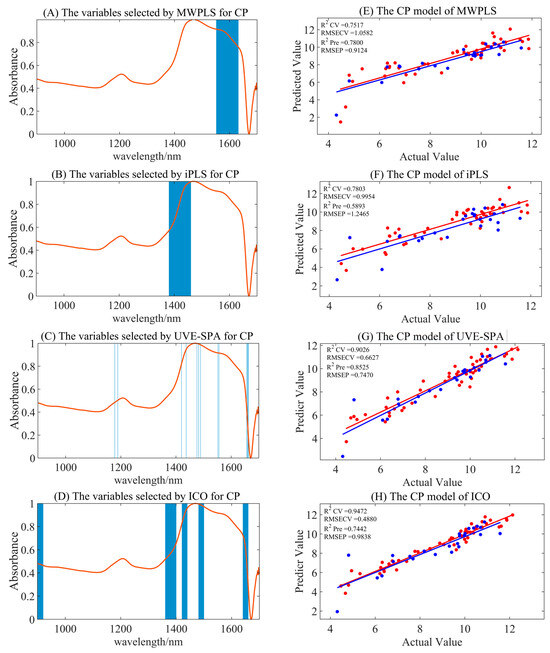
Figure 4.
The summary of optimal model results for CP under different variable selection algorithms. (A–D) are the selected regions of MWPLS, iPLS, UVE-SPA, and ICO, respectively. (E–H) represent the model results by utilizing the corresponding selected regions. Red represents the training set, and blue represents the test set.
In Figure 4, UVE-SPA variable selection identified characteristic wavelengths at 1178, 1189, 1420, 1437, 1476, 1482, 1489, 1552, 1556, 1656, 1659, 1661, and 1662 nm, while the MWPLS variable selection yielded the range of 1552–1632 nm. These selections correspond to the absorption peaks of N-H, C-H, and O-H bonds, and to the abundant NH3, C-H, and -COOH groups in proteins. The selected variable region had a certain degree of chemical interpretability, which might be one of the reasons for the improved model performance after variable selection.
3.4.3. Variable Selection Model of TS
Compared to the full-spectrum model for TS, the MWPLS, iPLS, and UVE-SPA models significantly reduced the degree of overfitting, while the ICO model still exhibited overfitting, as presented in Table 5 and Figure 5. The optimal preprocessing methods for the MWPLS, iPLS, and UVE-SPA models were S–G smoothing, MSC, and MSC, respectively. The corresponding Rpre2 values were 0.8126, 0.7426, and 0.8778, while the RMSEP values were 1.8079, 2.1186, and 1.460, respectively. The UVE-SPA model demonstrated the best accuracy and stability, while also reducing computational load, making it more suitable for portable NIR applications.

Table 5.
Results of the variable selection analysis model for TS.
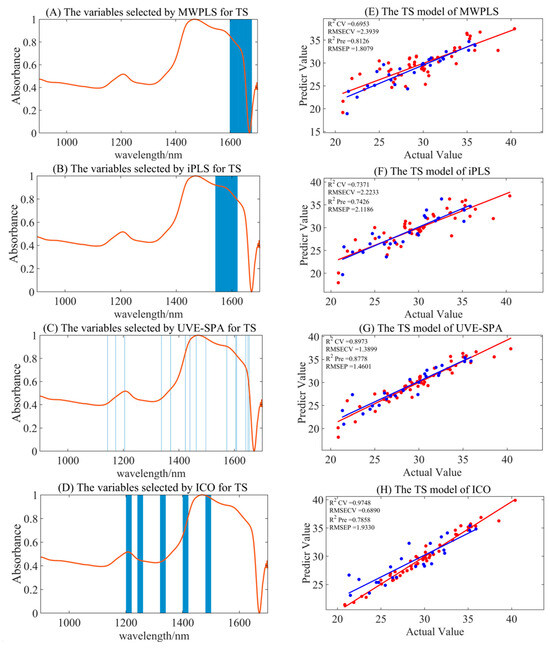
Figure 5.
The summary of optimal model results for TS under different variable selection algorithms. (A–D) are the selected regions of MWPLS, iPLS, UVE-SPA, and ICO, respectively. (E–H) represent the model results by utilizing the corresponding selected regions. Red represents the trainging set, and blue represents the test set.
In Figure 5, the MWPLS variable selection yielded the range of 1599–1679 nm, the iPLS variable selection yielded the range of 1541–1620 nm, and the UVE-SPA identified specific wavelengths at 1143, 1172, 1205, 1337, 1370, 1423, 1440, 1462, 1497, 1572, 1605, 1608, 1641, 1649, and 1653 nm. These selections primarily focused on the characteristic absorption peaks of C-H and O-H bonds, which correspond to the abundant carbon–hydrogen bonds and hydroxyl groups on the glucose carbon chain. This demonstrated that the selected spectral bands had certain chemical interpretability and could significantly improve the stability of the model.
3.5. Comparison of Variable Selection and Full-Spectrum Modeling
The three quality indicators each have their own unique characteristics. As shown in Figure 6, the CO model exhibited the best performance. The full-spectrum model had an average Rpre2 of 0.8366 and an average RMSEP of 1.4649. Among the variable selection algorithms, the UVE-SPA was the most suitable, with the model achieving an average Rpre2 of 0.8946 and an average RMSEP of 1.1764. Setting the interval to one-tenth of the full spectrum for both MWPLS and iPLS did not result in any optimization, which might indicate that the characteristic bands for CO were distributed across multiple regions within the spectrum. Therefore, the UVE-SPA was found to be more suitable.
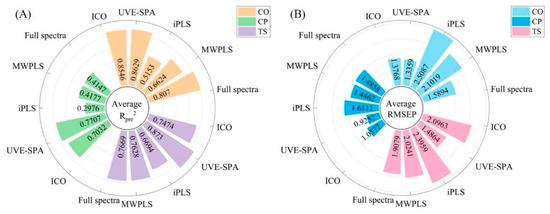
Figure 6.
Comparison of the Rpre2 and RMSEP values by variable selection and full-spectrum PLS. (A) is Rpre2. (B) is RMSEP.
The variable selection effect for CP was the most notable. The full-spectrum model had an average Rpre2 of 0.5081 and an average RMSEP of 1.3642, whereas the most suitable variable selection algorithm was the UVE-SPA, resulting in a model with an average Rpre2 of 0.8525 and an average RMSEP of 0.7470. The UVE-SPA not only significantly improves the accuracy of model predictions but also reduces model overfitting by selecting relevant variables, thus providing a reliable variable screening method for CP modeling.
For TS, the full-spectrum model exhibited an average Rpre2 of 0.8132 and an average RMSEP of 1.8048. Among the variable selection algorithms, the UVE-SPA was the most appropriate, resulting in a model with an average Rpre2 of 0.8778 and an average RMSEP of 1.4601. The UVE-SPA significantly enhanced the stability of the model compared to the full-spectrum model, markedly reducing both overfitting and underfitting, and thus demonstrated greater practicality.
In the full-spectrum modeling, 801 variables were utilized, but the four different variable selection algorithms significantly decreased the number of variables involved in the modeling process. Among them, the UVE-SPA employed the smallest number of variables for modeling and achieved the highest model accuracy and stability. As illustrated in Figure 6, based on the evaluation metric RMSEP, the UVE-SPA clearly outperformed the full-spectrum model. Furthermore, the Rpre2 values of the full-spectrum models for all three indicators were not as satisfactory as those obtained with the UVE-SPA. Evidently, the UVE-SPA was the most suitable variable selection algorithm in this study, particularly for the CP and TS indicators, where the full-spectrum modeling performance was relatively poorer.
4. Discussion
In this study, four variable selection algorithms were utilized to extract the characteristic wavelength bands for the determination of crude oil, crude protein, and total starch in TNs. The performance of these algorithms was compared with that of a PLS full-spectrum model, revealing that UVE-SPA variable selection significantly optimized the model’s effectiveness. The optimal models for CO, CP, and TS exhibited R2 values of 0.8946, 0.8525, and 0.8778, with RMSEP values of 1.1764, 0.7470, and 1.4601, respectively. The absolute errors between the predicted and actual values for the three-indicator spectral measurements were 0.80, 0.59, and 0.99 (Table S2). The overall results indicated that variable selection holds considerable potential for enhancing the performance of PLS full-spectrum models.
Furthermore, there exist notable differences in the results obtained from different variable selection models. NIR combined with ICO was used to establish models for the four major components (total sugars, reducing sugars, total nitrogen, and nicotine) of tobacco, resulting in R2 values of 0.985, 0.9408, 0.937, 0.9648, respectively, and RMSEP values of 0.8672, 1.4464, 0.0432, 0.1078, respectively [29]. Additionally, MWPLS was applied to construct an analysis model for amylose content. The results demonstrated good accuracy and stability, with an R2 of 0.96 and an RMSEP of 0.4–0.5% [30]. However, in this study, the roles of both methods were limited and did not achieve the expected effects. We believe that the information distribution of the three indicators spans multiple spectral bands, and the selection of a single band limits the model’s ability to acquire characteristic information. A prediction model for soluble solids in Hami melons was established using MC-UVE-SPA, with correlation coefficients ranging from 0.81 to 0.93 and RMSEP values between 0.95 and 0.99 [40]. While UVE-SPA variable selection significantly optimized the prediction model for TNs, there is still room for improvement compared to near-infrared prediction models for major crops such as rice. Deep learning demonstrates stronger feature selection capabilities compared to machine learning. Zhangchu et al. utilized convolutional neural networks for feature selection and established near-infrared prediction models for total anthocyanin content, total flavonoids, and total phenolics in dried black goji berries [41]. Deep learning is well suited for large datasets, and as the number of samples accumulates, the enormous potential of deep learning as a method for modeling and feature extraction in regression problems can be anticipated.
Previous studies have reported promising results in predicting crude fat, crude protein, and total starch using NIRS in different materials. Yakubu A. B. et al. established NIRS prediction models for soybean oil and protein using PLS, achieving R2 values of 0.992 and 0.995, respectively, with corresponding RMSEP values of 0.208 and 0.280 [19]. Özcan Çataltas et al. utilized a one-dimensional convolutional autoencoder combined with NIRS to detect protein, starch, and oil content in corn kernels, obtaining R2 values of 0.9012, 0.9359, and 0.9632, respectively, with corresponding RMSEP values of 0.1535, 0.2093, and 0.0388. However, in another dataset reported in the same study, the R2 values for the protein, starch, and oil models were 0.8995, 0.7988, and 0.8199, respectively, with corresponding RMSEP values of 0.1548, 0.3709, and 0.0857 [15]. These R2 values are comparable to those of our model, but the RMSEP values are smaller. RMSEP is an indicator that measures the deviation between the predicted and actual values of a model. Given the similar R2 values, we hypothesize that the differences in errors between the traditional detection methods used for the two crops may be the cause. The detection system for soybeans is more developed. To obtain a more accurate NIRS model for TNs, we must continue to optimize the traditional detection methods for this crop to stabilize the reference values.
5. Conclusions
In this study, to achieve the rapid detection of the three major nutritional components in TNs, a portable near-infrared spectrometer, combined with chemometrics, was used to establish rapid prediction models for these nutrients. Among these models, the one using full-spectrum data combined with three preprocessing methods exhibited poor performance. To enhance the performance of the model, four variable selection algorithms were employed to construct near-infrared analysis models for CO, CP, and TS in TNs. The results demonstrated that the models built using the UVE-SPA for all three indicators surpassed the full-spectrum models, making the UVE-SPA the most suitable variable selection algorithm for TN analysis. Especially for CP and TS, the characteristic wavelengths selected by the UVE-SPA directly reflected the correspondence between sample composition and spectral information. The UVE-SPA eliminated non-informative variables, thereby enhancing the predictive capability of the model and significantly reducing its computational load.
This study developed near-infrared non-destructive analysis models for three conventional evaluation components of TNs and improved the predictive and generalization abilities of the models through the use of variable selection algorithms. After ensuring the predictive performance of the NIR model, it demonstrated significant advantages, such as rapidness (approximately 30 s per sample), simultaneous multi-component analysis, and environmental friendliness (generating no exhaust gases or liquid waste), and provided new technologies and methods for TN quality assessment, determination of the optimal harvest time, and graded sales. In addition, in our study, we modeled the data by grinding TNs into powder. In the future, to further accelerate the rapid detection of the nutritional components in TNs, we could consider directly detecting the intact oil shale and establishing a mathematical prediction model.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/foods14030366/s1, Figure S1. IAS8120 handheld near-infrared spectrometer; Table S1. information of TN samples; Table S2. Comparison between the predicted values of crude oil and the true values from analytical methods; Table S3. Comparison between the predicted values of crude protein and the true values from analytical methods; Table S4. Comparison between the predicted values of total starch and the true values from analytical methods.
Author Contributions
Conceptualization, J.X. and L.X.; methodology, X.J. and D.G.; software, X.J. and J.X.; validation, X.Z.; formal analysis, X.J.; investigation, Y.S., R.M., L.C., K.T., J.S., T.S. and X.A.; resources, X.Z.; data curation, D.G.; writing—original draft preparation, X.J.; writing—review and editing, J.X. and L.X.; supervision, J.X.; project administration, L.X.; funding acquisition, L.X. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Key Research Development Project of Xinjiang Autonomous Region (2022B02040-3) and Xinjiang Autonomous Region Major Special Project (2024A02001).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding authors.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Rebezov, M.; Khan, M.U.; Bouyahya, A.; Imran, M.; Tufail, T.; Loretts, O.; Neverova, O.; Artyukhova, S.; Kuznetsova, E.; Ermolaev, V.; et al. Nutritional and technical aspect of Tiger Nut and its micro-constituents: An overview. Food Rev. Int. 2021, 39, 3262–3282. [Google Scholar] [CrossRef]
- Sánchez-Zapata, E.; Fernández-López, J.; Pérez-Alvarez, J.A. Tiger Nut (Cyperus esculentus) Commercialization: Health Aspects, Composition, Properties, and Food Applications. Compr. Rev. Food Sci. Food Saf. 2012, 11, 366–377. [Google Scholar] [CrossRef]
- Zhang, R.-Y.; Liu, C.; Chen, P.-X.; Jiang, M.-M.; Zhu, W.-X.; Liu, H.-M. Sequential extraction of oligosaccharide and polysaccharides from defatted tiger nut (Cyperus esculentus) meal for its comprehensive utilization. J. Food Meas. Charact. 2023, 17, 4357–4370. [Google Scholar] [CrossRef]
- Yu, Y.; Jiang, X.; Lu, X.; Zhang, T.; Cai, R.; Zheng, H.; Gao, F. Nutritional, structural and functional properties of protein fractions from tiger nut (Cyperus esculentus L.) seed meal. J. Food Meas. Charact. 2024, 18, 9867–9878. [Google Scholar] [CrossRef]
- Ijarotimi, O.S.; Yinusa, M.A.; Adegbembo, P.A.; Adeniyi, M.D. Chemical compositions, functional properties, antioxidative activities, and glycaemic indices of raw and fermented tigernut tubers (Cyperus esculentus Lativum) flour. J. Food Biochem. 2018, 42, e12591. [Google Scholar] [CrossRef]
- Du, Y.; Zhang, Y.; Chai, X.; Li, X.; Ullah, A.; Islam, W.; Zhang, Z.; Zeng, F. Effects of different tillage systems and mowing time on nutrient accumulation and forage nutritive value of Cyperus esculentus. Front. Plant Sci. 2023, 14, 1162572. [Google Scholar] [CrossRef]
- Zou, Z.; Zheng, Y.; Zhang, Z.; Xiao, Y.; Xie, Z.; Chang, L.; Zhang, L.; Zhao, Y. Molecular characterization of oleosin genes in Cyperus esculentus, a Cyperaceae plant producing oil in underground tubers. Plant Cell Rep. 2023, 42, 1791–1808. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Wang, Y.; Wei, Z.; Zhang, X.; Jiao, B.; Tian, Y.; Yan, F.; Li, J.; Liu, Y.; Yang, X.; et al. Analysis of oil synthesis pathway in Cyperus esculentus tuber and identification of oleosin and caleosin genes. J. Plant Physiol. 2023, 284, 153961. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Jiang, Y.; Tong, Y.; Leng, J.; Zhou, T.; Gao, Z.; Liu, H.; Zhu, C.; Zhang, W.; Yang, R. An Innovative Strategy of Comprehensive Utilization of Tiger Nuts (Cyperus esculentus L.): Simultaneous Extraction of Oil and Glucose Syrup by Amylolysis-Assisted Aqueous Extraction Process. Food Bioprocess Technol. 2025, 18, 1283–1295. [Google Scholar] [CrossRef]
- Jiang, W.; Goncalves, J.; Kostakos, V. Mobile near-infrared sensing—A systematic review on devices, data, modeling, and applications. J. ACM Comput. Surv. 2024, 56, 1–36. [Google Scholar] [CrossRef]
- Duan, J.; Huang, Y.; Li, Z.; Zheng, B.; Li, Q.; Xiong, Y.; Wu, L.; Min, S. Determination of 27 chemical constituents in Chinese southwest tobacco by FT-NIR spectroscopy. Ind. Crops Prod. 2012, 40, 21–26. [Google Scholar] [CrossRef]
- Ritota, M.; Manzi, P. Melamine Detection in Milk and Dairy Products: Traditional Analytical Methods and Recent Developments. Food Anal. Methods 2018, 11, 128–147. [Google Scholar] [CrossRef]
- Zontov, Y.V.; Balyklova, K.S.; Titova, A.V.; Rodionova, O.Y.; Pomerantsev, A.L. Chemometric aided NIR portable instrument for rapid assessment of medicine quality. J. Pharm. Biomed. Anal. 2016, 131, 87–93. [Google Scholar] [CrossRef] [PubMed]
- Syunyaev, R.Z.; Balabin, R.M.; Akhatov, I.S.; Safieva, J.O. Adsorption of Petroleum Asphaltenes onto Reservoir Rock Sands Studied by Near-Infrared (NIR) Spectroscopy. Energy Fuels 2009, 23, 1230–1236. [Google Scholar] [CrossRef]
- Chao, T.; Xin, Q.; Li, M. An ensemble method based on a self-organizing map for near-infrared spectral calibration of complex beverage samples. Anal. Bioanal. Chem. 2008, 392, 515–521. [Google Scholar]
- Fodor, M.; Matkovits, A.; Benes, E.L.; Jókai, Z. The role of near-infrared spectroscopy in food quality assurance: A review of the past two decades. Foods 2024, 13, 3501. [Google Scholar] [CrossRef]
- Singh, T.; Garg, N.M.; Iyengar, S.R.S.; Singh, V. Near-infrared hyperspectral imaging for determination of protein content in barley samples using convolutional neural network. J. Food Meas. Charact. 2023, 17, 3548–3560. [Google Scholar] [CrossRef]
- Cataltas, O.; Tutuncu, K. Detection of protein, starch, oil, and moisture content of corn kernels using one-dimensional convolutional autoencoder and near-infrared spectroscopy. PeerJ Comput. Sci. 2023, 9, e1266. [Google Scholar] [CrossRef]
- Díaz, E.O.; Iino, H.; Koyama, K.; Kawamura, S.; Koseki, S.; Lyu, S. Non-destructive quality classification of rice taste properties based on near-infrared spectroscopy and machine learning algorithms. Food Chem. 2023, 429, 136907. [Google Scholar] [CrossRef]
- Zhang, G.; Li, P.; Zhang, W.; Zhao, J. Analysis of multiple soybean phytonutrients by near-infrared reflectance spectroscopy. Anal. Bioanal. Chem. 2017, 409, 3515–3525. [Google Scholar] [CrossRef]
- Mourya, V.; Kumar, V.; Rani, A.; Jain, M.; Husain, S.M. Near-Infrared Reflectance Spectroscopy for Protein Content in Soybean Flour and Screening of Germplasm Across Different Countries. Agric. Res. 2016, 5, 29–34. [Google Scholar] [CrossRef]
- Yakubu, A.B.; Shaibu, A.S.; Mohammed, S.G.; Ibrahim, H.; Mohammed, I.B. NIR-Based Prediction for Protein, Oil, and Fatty Acids in Soybean (Glycine max (L.) Merrill) Seeds. Food Anal. Methods 2024, 17, 1592–1600. [Google Scholar]
- Höskuldsson, A. PLS regression methods. J. Chemom. 1988, 2, 211–228. [Google Scholar] [CrossRef]
- Mevik, B.H.; Cederkvist, H.R. Mean squared error of prediction (MSEP) estimates for principal component regression (PCR) and partial least squares regression (PLSR). J. Chemom. 2004, 18, 422–429. [Google Scholar] [CrossRef]
- Zhang, X.; Li, W.; Yin, B.; Chen, W.; Kelly, D.P.; Wang, X.; Zheng, K.; Du, Y. Improvement of near infrared spectroscopic (NIR) analysis of caffeine in roasted Arabica coffee by variable selection method of stability competitive adaptive reweighted sampling (SCARS). Spectrochim. Acta. A Mol. Biomol. Spectrosc. 2013, 114, 350–356. [Google Scholar] [CrossRef]
- Pereira, A.F.C.; Pontes, M.J.C.; Neto, F.F.G.; Santos, S.R.B.; Galvão, R.K.H.; Araújo, M.C.U. NIR spectrometric determination of quality parameters in vegetable oils using iPLS and variable selection. Food Res. Int. 2008, 41, 341–348. [Google Scholar] [CrossRef]
- Leardi, R.; Nørgaard, L. Sequential application of backward interval partial least squares and genetic algorithms for the selection of relevant spectral regions. J. Chemom. 2004, 18, 486–497. [Google Scholar] [CrossRef]
- Fu, H.-Y.; Huan, S.-Y.; Xu, L.; Tang, L.-J.; Jiang, J.-H.; Wu, H.-L.; Shen, G.-L.; Yu, R.-Q. Moving Window Partial Least-Squares Discriminant Analysis for Identification of Different Kinds of Bezoar Samples by near Infrared Spectroscopy and Comparison of Different Pattern Recognition Methods. J. Infrared Spectrosc. 2007, 15, 291–297. [Google Scholar] [CrossRef]
- Ye, S.; Wang, D.; Min, S. Successive projections algorithm combined with uninformative variable elimination for spectral variable selection. Chemom. Intell. Lab. Syst. 2008, 91, 194–199. [Google Scholar] [CrossRef]
- Araújo, M.C.U.; Saldanha, T.C.B.; Galvão, R.K.H.; Yoneyama, T.; Chame, H.C.; Visani, V. The successive projections algorithm for variable selection in spectroscopic multicomponent analysis. Chemom. Intell. Lab. Syst. 2001, 57, 65–73. [Google Scholar] [CrossRef]
- Song, X.; Huang, Y.; Yan, H.; Xiong, Y.; Min, S. A novel algorithm for spectral interval combination optimization. Anal. Chim. Acta 2016, 948, 19–29. [Google Scholar] [CrossRef]
- Li, Q.; Huang, Y.; Song, X.; Zhang, J.; Min, S. Spectral interval combination optimization (ICO) on rapid quality assessment of Solanaceae plant: A validation study. J. Food Sci. Technol. 2019, 56, 2158–2166. [Google Scholar] [CrossRef] [PubMed]
- Iino, H.; Kawamura, S.; Díaz, E.O.; Ishizu, H.; Nagata, T.; Koseki, S. Non-destructive measurement of rice amylose content using near-infrared spectroscopy for application at grain elevators and milling plants. J. Food Meas. Charact. 2024, 18, 8275–8288. [Google Scholar] [CrossRef]
- Gullifa, G.; Barone, L.; Papa, E.; Giuffrida, A.; Materazzi, S.; Risoluti, R. Portable NIR spectroscopy: The route to green analytical chemistry. Front. Chem. 2023, 11, 1214825. [Google Scholar] [CrossRef]
- Morris, D.L. Quantitative determination of carbohydrates with Dreywood’s anthrone reagent. Science 1948, 107, 254–255. [Google Scholar] [CrossRef] [PubMed]
- Luo, J.W.; Ying, K.; Bai, J. Savitzky-Golay smoothing and differentiation filter for even number data. Signal Process. 2005, 85, 1429–1434. [Google Scholar] [CrossRef]
- Bi, Y.; Yuan, K.; Xiao, W.; Wu, J.; Shi, C.; Xia, J.; Chu, G.; Zhang, G.; Zhou, G. A local pre-processing method for near-infrared spectra, combined with spectral segmentation and standard normal variate transformation. Anal. Chim. Acta 2016, 909, 30–40. [Google Scholar] [CrossRef] [PubMed]
- Thennadil, S.N.; Martens, H.; Kohler, A. Physics-based multiplicative scatter correction approaches for improving the performance of calibration models. Appl. Spectrosc. 2006, 60, 315–321. [Google Scholar] [CrossRef]
- Jiang, W.; Lu, C.; Zhang, Y.; Ju, W.; Wang, J.; Hong, F.; Wang, T.; Ou, C. Moving-window-improved Monte Carlo uninformative variable elimination combining successive projections algorithm for near-infrared spectroscopy (NIRS). J. Spectrosc. 2020, 3590301. [Google Scholar] [CrossRef]
- Hu, R.; Zhang, L.; Yu, Z.; Zhai, Z.; Zhang, R. Optimization of soluble solids content prediction models in “Hami” melons by means of Vis-NIR spectroscopy and chemometric tools. Infrared Phys. Technol. 2019, 102, 102999. [Google Scholar] [CrossRef]
- Zhang, C.; Wu, W.; Zhou, L.; Cheng, H.; Ye, X.; He, Y. Developing deep learning based regression approaches for determination of chemical compositions in dry black goji berries (Lycium ruthenicum Murr.) using near-infrared hyperspectral imaging. Food Chem. 2020, 319, 126536. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).