A Comprehensive Study via Proton Nuclear Magnetic Resonance of a Variety of Omega-3 Lipid-Rich Supplements Available in the Spanish Market: Acyl Group Profile, Minor Components, and Oxidative Status
Abstract
1. Introduction
2. Materials and Methods
2.1. Samples of Study
2.2. Analysis by 1H NMR Spectroscopy
2.2.1. Operating Conditions
2.2.2. Identification and Quantification of Some Types of Compounds and Structures Present in the Liquid Matrix of the Samples
2.3. Statistical Analysis
3. Results and Discussion
3.1. Information Obtained via 1H NMR About Supplement Composition in Acyl Groups and the Structures Supporting Them
3.1.1. Analysis of the 1H NMR Spectra of Some ω-3 and ω-6 Lipid Standard Compounds
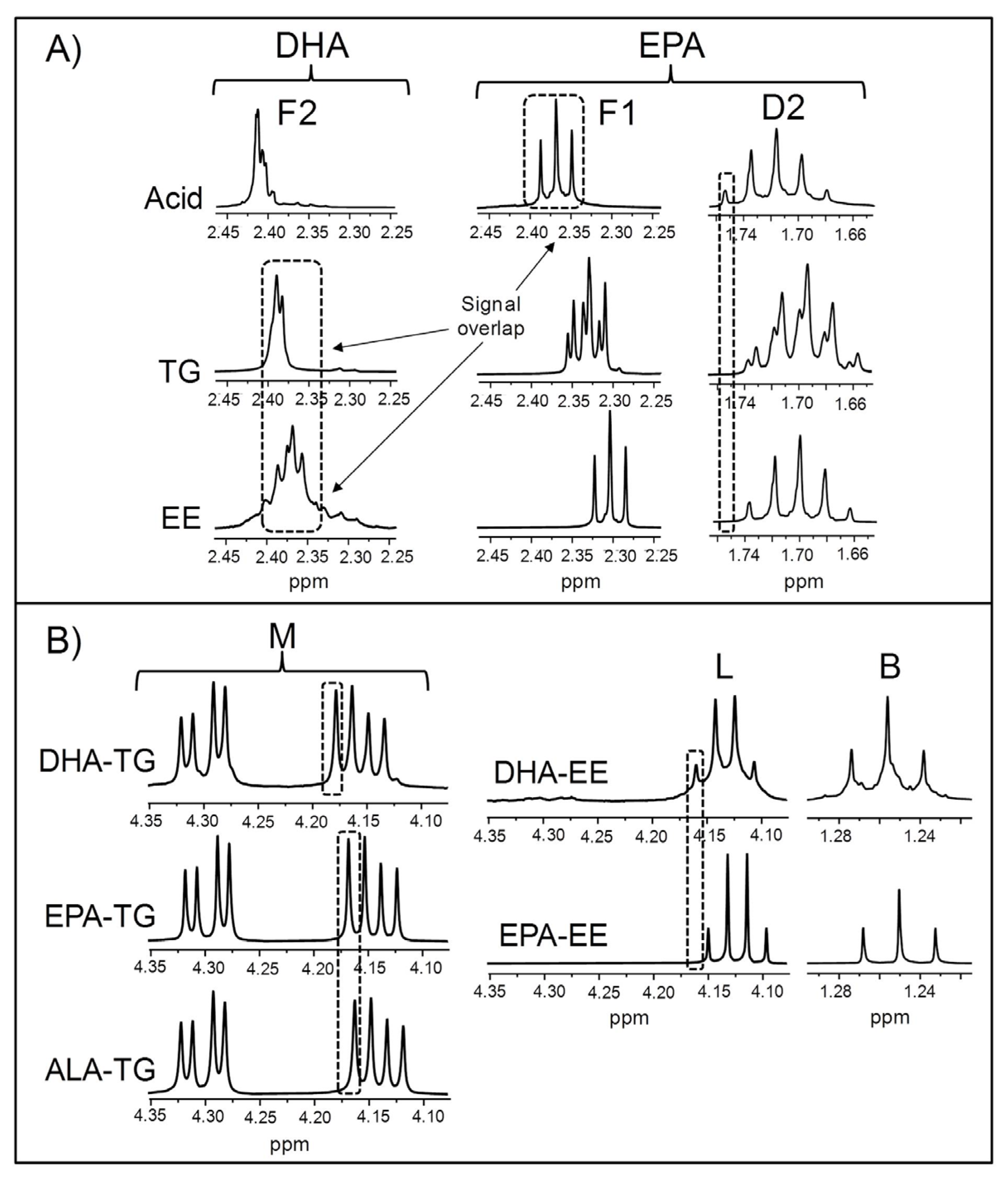
3.1.2. Visual Analysis of the 1H NMR Spectra of the Studied Supplements
3.1.3. Proportions of the Various Types of Acyl Groups in the Samples Studied
Some Considerations Related to the Determination of the Molar Percentages of Certain Kinds of ω-3 Acyl Groups in Some Supplements
Data Obtained
3.2. Information About Supplement Minor Components
3.2.1. Tocopherols
3.2.2. Sterols
3.2.3. Other Types of Minor Components of Varied Nature
3.3. Information About Supplement Oxidation Level
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Punia, S.; Sandhu, K.S.; Siroha, A.K.; Dhull, S.B. Omega 3-metabolism, absorption, bioavailability and health benefits—A review. PharmaNutrition 2019, 10, 100162. [Google Scholar] [CrossRef]
- Zartmann, A.; Völcker, L.; Hammann, S. Quantitative analysis of fatty acids and vitamin E and total lipid profiling of dietary supplements from the German market. Eur. Food Res. Technol. 2023, 249, 1035–1048. [Google Scholar] [CrossRef]
- Hegde, M.V.; Zanwar, A.A.; Adekar, S.P. Omega-3 Fatty Acids; Springer International Publishing: Cham, Switzerland, 2016. [Google Scholar] [CrossRef]
- Shahidi, F.; Ambigaipalan, P. Novel functional food ingredients from marine sources. Curr. Opin. Food Sci. 2015, 2, 123–129. [Google Scholar] [CrossRef]
- Myers, A.; Cumberford, G. Ahiflower Oil—The Rising GLA Alternative to Evening Primrose for Women & Vegans. Integr. Med. A Clin. J. 2021, 20, 30–33. [Google Scholar]
- Whelan, J. Dietary Stearidonic Acid Is a Long Chain (n-3) Polyunsaturated Fatty Acid with Potential Health Benefits. J. Nutr. 2009, 139, 5–10. [Google Scholar] [CrossRef]
- Shahidi, F.; Ambigaipalan, P. Omega-3 Polyunsaturated Fatty Acids and Their Health Benefits. Annu. Rev. Food Sci. Technol. 2018, 9, 345–381. [Google Scholar] [CrossRef]
- Prasad, P.; Savyasachi, S.; Reddy, L.P.A.; Sreedhar, R.V. Physico-Chemical Characterization, Profiling of Total Lipids and Triacylglycerol Molecular Species of Omega-3 Fatty Acid Rich B. arvensis Seed Oil from India. J. Oleo Sci. 2019, 68, 209–223. [Google Scholar] [CrossRef]
- Martín-Olmedo, J.J.; Jurado-Fasoli, L. Impact of technological processing on the bioavailability of omega-3 fatty acids in fish oil: A review. Crit. Rev. Food Sci. Nutr. 2025, 65, 7468–7478. [Google Scholar] [CrossRef]
- Xie, D.; Gong, M.; Wei, W.; Jin, J.; Wang, X.; Wang, X.; Jin, Q. Antarctic Krill (Euphausia superba) Oil: A Comprehensive Review of Chemical Composition, Extraction Technologies, Health Benefits, and Current Applications. Compr. Rev. Food Sci. Food Saf. 2019, 18, 514–534. [Google Scholar] [CrossRef]
- Kutzner, L.; Ostermann, A.I.; Konrad, T.; Riegel, D.; Hellhake, S.; Schuchardt, J.P.; Schebb, N.H. Lipid Class Specific Quantitative Analysis of n-3 Polyunsaturated Fatty Acids in Food Supplements. J. Agric. Food Chem. 2017, 65, 139–147. [Google Scholar] [CrossRef]
- Remy, C.; Danoun, S.; Delample, M.; Morris, C.; Gilard, V.; Balayssac, S. Characterization of fatty acid forms using benchtop NMR in omega-3 oil supplements. Magn. Reson. Chem. 2023, 62, 328–336. [Google Scholar] [CrossRef]
- Li, M.H.; Robinson, E.H.; Tucker, C.S.; Manning, B.B.; Khoo, L. Effects of dried algae Schizochytrium sp., a rich source of docosahexaenoic acid, on growth, fatty acid composition, and sensory quality of channel catfish Ictalurus punctatus. Aquaculture 2009, 292, 232–236. [Google Scholar] [CrossRef]
- Neubronner, J.; Schuchardt, J.P.; Kressel, G.; Merkel, M.; von Schacky, C.; Hahn, A. Enhanced increase of omega-3 index in response to long-term n-3 fatty acid supplementation from triacylglycerides versus ethyl esters. Eur. J. Clin. Nutr. 2011, 65, 247–254. [Google Scholar] [CrossRef]
- Opperman, M.; Benade, S. Analysis of the omega-3 fatty acid content of South African fish oil supplements: A follow-up study: Cardiovascular topics. Cardiovasc. J. Afr. 2013, 24, 297–302. [Google Scholar] [CrossRef] [PubMed]
- Ahonen, E.; Damerau, A.; Suomela, J.P.; Kortesniemi, M.; Linderborg, K.M. Oxidative stability, oxidation pattern and α-tocopherol response of docosahexaenoic acid (DHA, 22:6n–3)-containing triacylglycerols and ethyl esters. Food Chem. 2022, 387, 132882. [Google Scholar] [CrossRef] [PubMed]
- Martín, D.; Terrón, A.; Fornari, T.; Reglero, G.; Torres, C.F. Oxidative stabilization of ultra-high omega-3 concentrates as ethyl esters or triacylglycerols. Food Res. Int. 2012, 45, 336–341. [Google Scholar] [CrossRef]
- Sullivan Ritter, J.C.; Budge, S.M.; Jovica, F.; Reid, A.J.M. Oxidation Rates of Triacylglycerol and Ethyl Ester Fish Oils. J. Am. Oil Chem. Soc. 2015, 92, 561–569. [Google Scholar] [CrossRef]
- Esterbauer, H.; Schaur, R.J.; Zollner, H. Chemistry and biochemistry of 4-hydroxynonenal, malonaldehyde and related aldehydes. Free Rad. Biol. Med. 1991, 11, 81–128. [Google Scholar] [CrossRef]
- Miyashita, K.; Takagi, T. Study on the Oxidative Rate and Prooxidant Activity of Free Fatty Acids. J. Am. Oil Chem. Soc. 1986, 63, 1380–1384. [Google Scholar] [CrossRef]
- Hands, J.M.; Anderson, M.L.; Cooperman, T.; Frame, L.A. A Multi-Year Rancidity Analysis of 72 Marine and Microalgal Oil Omega-3 Supplements. J. Diet. Suppl. 2024, 21, 195–206. [Google Scholar] [CrossRef]
- Jackowski, S.A.; Alvi, A.Z.; Mirajkar, A.; Imani, Z.; Gamalevych, Y.; Shaikh, N.A.; Jackowski, G. Oxidation levels of North American over-the-counter n-3 (omega-3) supplements and the influence of supplement formulation and delivery form on evaluating oxidative safety. J. Nutr. Sci. 2015, 4, e30. [Google Scholar] [CrossRef] [PubMed]
- Abdulhussain, H.; Khonji, A.; Alsaloom, A.; Meshaima, H.; AlKooheji, L.; Aldoseri, M.; Al-Mannai, M.; Freije, A. Determination of eicosapentaenoic acid and docosahexaenoic acid contents and the oxidation level of fish oil supplements from Bahrain market. Arab. J. Basic Appl. Sci. 2023, 30, 472–481. [Google Scholar] [CrossRef]
- Albert, B.B.; Derraik, J.G.; Cameron-Smith, D.; Hofman, P.L.; Tumanov, S.; Villas-Boas, S.G.; Garg, M.L.; Cutfield, W.S. Fish oil supplements in New Zealand are highly oxidised and do not meet label content of n-3 PUFA. Sci. Rep. 2015, 5, 7928. [Google Scholar] [CrossRef] [PubMed]
- Bannenberg, G.; Mallon, C.; Edwards, H.; Yeadon, D.; Yan, K.; Johnson, H.; Ismail, A. Omega-3 Long-Chain Polyunsaturated Fatty Acid Content and Oxidation State of Fish Oil Supplements in New Zealand. Sci. Rep. 2017, 7, 1488. [Google Scholar] [CrossRef]
- Bannenberg, G.; Rice, H.B.; Bernasconi, A.; Ferrari, A.; Mallon, C.; Navarrete, L.; Hughes, G.; Igarashi, J.; Persons, K.; Latynski, L.; et al. Ingredient label claim compliance and oxidative quality of EPA/DHA omega-3 retail products in the US. J. Food Compos. Anal. 2020, 88, 103435. [Google Scholar] [CrossRef]
- Chee, K.M.; Gong, J.X.; Good Rees, D.M.; Meydanl, M.; Ausman, L.; Johnson, J.; Siguel, E.N.; Schaefer, E.J. Fatty Acid Content of Marine Oil Capsules. Lipids 1990, 25, 523–528. [Google Scholar] [CrossRef]
- Damerau, A.; Ahonen, E.; Kortesniemi, M.; Puganen, A.; Tarvainen, M.; Linderborg, K.M. Evaluation of the composition and oxidative status of omega-3 fatty acid supplements on the Finnish market using NMR and SPME-GC–MS in comparison with conventional methods. Food Chem. 2020, 330, 127194. [Google Scholar] [CrossRef]
- De Gouvêa, H.R.; de Paula, D.F.; de Pinho Silva, T.A.; Campos, A.F.C.; Ito, M.K. Fatty Acid Content, Oxidation Markers and Mercury in Fish Oil Supplements Commercialized in Brasília, Brazil. Orbital Electron. J. Chem. 2019, 11, 168–177. [Google Scholar] [CrossRef]
- Fantoni, C.M.; Cuccio, A.P.; Barrera-Arellano, D. Brazilian Encapsulated Fish Oils: Oxidative Stability and Fatty Acid Composition. J. Am. Oil Chem. Soc. 1996, 73, 251–253. [Google Scholar] [CrossRef]
- Galuch, M.B.; Carbonera, F.; Magon, T.F.; Silveira, R.D.; dos Santos, P.D.; Pizzo, J.S.; Santos, O.O.; Visentainer, J.V. Quality Assessment of Omega-3 Supplements Available in the Brazilian Market. J. Braz. Chem. Soc. 2018, 29, 631–638. [Google Scholar] [CrossRef]
- Hamilton, K.; Brooks, P.; Holmes, M.; Cunningham, J.; Russell, F.D. Evaluation of the composition of omega-3 fatty acids in dietary oil supplements. Nutr. Diet. 2010, 67, 182–189. [Google Scholar] [CrossRef]
- Hasanpour, M.; Rezaie, A.; Iranshahy, M.; Yousefi, M.; Saberi, S.; Iranshahi, M. 1H NMR-based metabolomics study of the lipid profile of omega-3 fatty acid supplements and some vegetable oils. J. Pharm. Biomed. Anal. 2024, 238, 115848. [Google Scholar] [CrossRef] [PubMed]
- Heller, M.; Gemming, L.; Tung, C.; Grant, R. Oxidation of fish oil supplements in Australia. Int. J. Food Sci. Nutr. 2019, 70, 540–550. [Google Scholar] [CrossRef] [PubMed]
- Jansson, P.; Kay, B. Aldehydes identified in commercially available ω-3 supplements via 1H NMR spectroscopy. Nutrition 2019, 60, 74–79. [Google Scholar] [CrossRef]
- Karsli, B. Comparative analysis of the fatty acid composition of commercially available fish oil supplements in Turkey: Public health risks and benefits. J. Food Compos. Anal. 2021, 103, 104105. [Google Scholar] [CrossRef]
- Kartal, M.; Kurucu, S.; Aslan, S.; Özbay, Ö.; Ceyhan, T.; Sayar, E.; Cevheroǧlu, Ş. Comparison of ω-3 Fatty Acids by GC-MS in Frequently Consumed Fish and Fish Oil Preparations on the Turkish Market. Fabad J. Pharm. Sci. 2003, 28, 201–205. [Google Scholar]
- Killeen, D.P.; Marshall, S.N.; Burgess, E.J.; Gordon, K.C.; Perry, N.B. Raman Spectroscopy of Fish Oil Capsules: Polyunsaturated Fatty Acid Quantitation Plus Detection of Ethyl Esters and Oxidation. J. Agric. Food Chem. 2017, 65, 3551–3558. [Google Scholar] [CrossRef]
- Kleiner, A.C.; Cladis, D.P.; Santerre, C.R. A comparison of actual versus stated label amounts of EPA and DHA in commercial omega-3 dietary supplements in the United States. J. Sci. Food Agric. 2015, 95, 1260–1267. [Google Scholar] [CrossRef]
- Kolanowski, W. Omega-3 LC PUFA Contents and Oxidative Stability of Encapsulated Fish Oil Dietary Supplements. Int. J. Food Prop. 2010, 13, 498–511. [Google Scholar] [CrossRef]
- Lee, J.B.; Kim, M.K.; Kim, B.K.; Kim, J.Y.; Lee, K.G. Analysis of polychlorinated biphenyls (PCBs), heavy metals and omega-3 fatty acids in commercially available Korean functional fish oil supplements. Int. J. Food Sci. Technol. 2016, 51, 2217–2224. [Google Scholar] [CrossRef]
- Lopes, T.I.B.; Pereira, E.S.; Freitas, D.D.S.; Oliveira, S.L.; Alcantara, G.B. Spectral profiles of commercial omega-3 supplements: An exploratory analysis by ATR-FTIR and 1H NMR. J. Food Sci. Technol. 2020, 57, 1251–1257. [Google Scholar] [CrossRef]
- Lorensia, A.; Budiono, R.; Suryadinata, R.V.; Tiarasari, N. Quantitative determination of EPA and DHA in fish oil capsules for cardiovascular disease therapy in Indonesia by GC-MS. J. Public Health Res. 2021, 10, 2159. [Google Scholar] [CrossRef] [PubMed]
- Nevigato, T.; Masci, M.; Caproni, R. Quality of Fish-Oil-Based Dietary Supplements Available on the Italian Market: A Preliminary Study. Molecules 2021, 26, 5015. [Google Scholar] [CrossRef] [PubMed]
- Nichols, P.D.; Dogan, L.; Sinclair, A. Australian and New Zealand Fish Oil Products in 2016 Meet Label Omega-3 Claims and Are Not Oxidized. Nutrients 2016, 8, 703. [Google Scholar] [CrossRef] [PubMed]
- Ozyurt, G.; Ekmen, D.; Durmuş, M.; Ucar, Y. Assessment of the safety of dietary fish oil supplements in terms of content and quality. Environ. Sci. Pollut. Res. Int. 2022, 29, 25006–25019. [Google Scholar] [CrossRef]
- Ozyurt, G.; Şimşek, A.; Etyemez, M.; Polat, A. Fatty Acid Composition and Oxidative Stability of Fish Oil Products in Turkish Retail Market. J. Aquat. Food Prod. Technol. 2013, 22, 322–329. [Google Scholar] [CrossRef]
- Pasini, F.; Gómez-Caravaca, A.M.; Blasco, T.; Cvejić, J.; Caboni, M.F.; Verardo, V. Assessment of Lipid Quality in Commercial Omega-3 Supplements Sold in the French Market. Biomolecules 2022, 12, 1361. [Google Scholar] [CrossRef]
- Sprague, M.; Cooper, S.; Tocher, D.R.; Betancor, M.B. Encapsulated Fish Oil Products Available in the UK Meet Regulatory Guidelines With Respect to EPA + DHA Contents and Oxidative Status. Eur. J. Lipid Sci. Technol. 2018, 120, 1800105. [Google Scholar] [CrossRef]
- Srigley, C.T.; Rader, J.I. Content and Composition of Fatty Acids in Marine Oil Omega-3 Supplements. J. Agric. Food Chem. 2014, 62, 7268–7278. [Google Scholar] [CrossRef]
- Ritter, J.C.S.; Budge, S.M.; Jovica, F. Quality analysis of commercial fish oil preparations. J. Sci. Food Agric. 2013, 93, 1935–1939. [Google Scholar] [CrossRef]
- Yi, T.; Li, S.M.; Fan, J.Y.; Fan, L.L.; Zhang, Z.F.; Luo, P.; Zhang, X.J.; Wang, J.G.; Zhu, L.; Zhao, Z.Z.; et al. Comparative analysis of EPA and DHA in fish oil nutritional capsules by GC-MS. Lipids Health Dis. 2014, 13, 190. [Google Scholar] [CrossRef]
- Plans, M.; Wenstrup, M.J.; Rodriguez-Saona, L.E. Application of Infrared Spectroscopy for Characterization of Dietary Omega-3 Oil Supplements. J. Am. Oil Chem. Soc. 2015, 92, 957–966. [Google Scholar] [CrossRef]
- Dais, P.; Misiak, M.; Hatzakis, E. Analysis of marine dietary supplements using NMR spectroscopy. Anal. Methods 2015, 7, 5226–5238. [Google Scholar] [CrossRef]
- Lv, J.; Wang, C.; Zhang, X.; Lv, Z.; Yu, M. 1H NMR Quantification of DHA and EPA in Fish Oil. J. Ocean Univ. China 2020, 19, 1193–1197. [Google Scholar] [CrossRef]
- Amorim, T.L.; Granato, Á.S.; de Oliveira Mendes, T.; de Oliveira, M.A.L.; Amarante, G.W.; de la Fuente, M.A.; Gomez-Cortes, P. Lipid classification of fish oil omega-3 supplements by 1H NMR and multivariate analysis. J. Food Compos. Anal. 2021, 102, 104060. [Google Scholar] [CrossRef]
- Jairoun, A.A.; Shahwan, M.; Zyoud, S.E.H. Fish oil supplements, oxidative status, and compliance behaviour: Regulatory challenges and opportunities. PLoS ONE 2020, 15, e0244688. [Google Scholar] [CrossRef]
- Guillén, M.D.; Goicoechea, E. Toxic Oxygenated α,β-Unsaturated Aldehydes and their Study in Foods: A Review. Crit. Rev. Food Sci. Nutr. 2008, 48, 119–136. [Google Scholar] [CrossRef]
- Halvorsen, B.L.; Blomhoff, R. Determination of lipid oxidation products in vegetable oils and marine omega-3 supplements. Food Nutr. Res. 2011, 55, 5792. [Google Scholar] [CrossRef]
- Emami, S.; Zhang, Z.; Taha, A.Y. Quantitation of Oxylipins in Fish and Algae Oil Supplements Using Optimized Hydrolysis Procedures and Ultra-High Performance Liquid Chromatography Coupled to Tandem Mass-Spectrometry. J. Agric. Food Chem. 2020, 68, 9329–9344. [Google Scholar] [CrossRef]
- Koch, E.; Kampschulte, N.; Schebb, N.H. Comprehensive Analysis of Fatty Acid and Oxylipin Patterns in n3-PUFA Supplements. J. Agric. Food Chem. 2022, 70, 3979–3988. [Google Scholar] [CrossRef]
- Spiteller, G. Furan Fatty Acids: Occurrence, Synthesis, and Reactions. Are Furan Fatty Acids Responsible for the Cardioprotective Effects of a Fish Diet? Lipids 2005, 40, 755–771. [Google Scholar] [CrossRef] [PubMed]
- Gottstein, V.; Müller, M.; Günther, J.; Kuballa, T.; Vetter, W. Direct 1H NMR Quantitation of Valuable Furan Fatty Acids in Fish Oils and Fish Oil Fractions. J. Agric. Food Chem. 2019, 67, 11788–11795. [Google Scholar] [CrossRef] [PubMed]
- Jensen, S.; Ragnarsdottir, O.; Johannsson, R. Marine Sources of Furan Fatty Acids. J. Aquat. Food Prod. Technol. 2019, 28, 74–83. [Google Scholar] [CrossRef]
- Vetter, W.; Laure, S.; Wendlinger, C.; Mattes, A.; Smith, A.W.T.; Knight, D.W. Determination of Furan Fatty Acids in Food Samples. J. Am. Oil Chem. Soc. 2012, 89, 1501–1508. [Google Scholar] [CrossRef]
- Weinbinder, D.D.; Manzanos, M.J.; Ibargoitia, M.L.; Sopelana, P. Methylated furan fatty acids in ω-3 lipid-rich dietary supplements: A complementary study using proton nuclear magnetic resonance and direct immersion solid-phase microextraction followed by gas chromatography–mass spectrometry. LWT-Food Sci. Technol. 2025, 228, 118025. [Google Scholar] [CrossRef]
- Guillén, M.D.; Ruiz, A. Edible oils: Discrimination by 1H nuclear magnetic resonance. J. Sci. Food Agric. 2003, 83, 338–346. [Google Scholar] [CrossRef]
- Guillén, M.D.; Ruiz, A. Rapid simultaneous determination by proton NMR of unsaturation and composition of acyl groups in vegetable oils. Eur. J. Lipid Sci. Technol. 2003, 105, 688–696. [Google Scholar] [CrossRef]
- Guillén, M.D.; Ruiz, A. Study of the oxidative stability of salted and unsalted salmon fillets by 1H nuclear magnetic resonance. Food Chem. 2004, 86, 297–304. [Google Scholar] [CrossRef]
- Martínez-Yusta, A.; Goicoechea, E.; Guillén, M.D. A Review of Thermo-Oxidative Degradation of Food Lipids Studied by 1H NMR Spectroscopy: Influence of Degradative Conditions and Food Lipid Nature. Compr. Rev. Food Sci. Food Saf. 2014, 13, 838–859. [Google Scholar] [CrossRef]
- Vidal, N.P.; Manzanos, M.J.; Goicoechea, E.; Guillén, M.D. Quality of farmed and wild sea bass lipids studied by 1H NMR: Usefulness of this technique for differentiation on a qualitative and a quantitative basis. Food Chem. 2012, 135, 1583–1591. [Google Scholar] [CrossRef]
- Williamson, K.; Hatzakis, E. NMR Spectroscopy as a Robust Tool for the Rapid Evaluation of the Lipid Profile of Fish Oil Supplements. J. Vis. Exp. 2017, 123, e55547. [Google Scholar] [PubMed]
- Martin-Rubio, A.S.; Sopelana, P.; Ibargoitia, M.L.; Guillén, M.D. Prooxidant effect of α-tocopherol on soybean oil. Global monitoring of its oxidation process under accelerated storage conditions by 1H nuclear magnetic resonance. Food Chem. 2018, 245, 312–323. [Google Scholar] [CrossRef] [PubMed]
- Guillén, M.D.; Ruiz, A.; Cabo, N.; Chirinos, R.; Pascual, G. Characterization of Sacha Inchi (Plukenetia volubilis L.) Oil by FTIR Spectroscopy and 1H NMR. Comparison with Linseed Oil. J. Am. Oil Chem. Soc. 2003, 80, 755–762. [Google Scholar] [CrossRef]
- Nieva-Echevarría, B.; Goicoechea, E.; Manzanos, M.J.; Guillén, M.D. A method based on 1H NMR spectral data useful to evaluate the hydrolysis level in complex lipid mixtures. Food Res. Int. 2014, 66, 379–387. [Google Scholar] [CrossRef]
- Guillén, M.D.; Uriarte, P.S. Study by 1H NMR spectroscopy of the evolution of extra virgin olive oil composition submitted to frying temperature in an industrial fryer for a prolonged period of time. Food Chem. 2012, 134, 162–172. [Google Scholar] [CrossRef]
- Baker, J.K.; Myers, C.W. One-Dimensional and Two-Dimensional 1H-and 13C-Nuclear Magnetic Resonance (NMR) Analysis of Vitamin E Raw Materials or Analytical Reference Standards. Pharm. Res. 1991, 8, 763–770. [Google Scholar] [CrossRef]
- Ruiz-Aracama, A.; Goicoechea, E.; Guillén, M.D. Direct study of minor extra-virgin olive oil components without any sample modification. 1H NMR multisupression experiment: A powerful tool. Food Chem. 2017, 228, 301–314. [Google Scholar] [CrossRef]
- Bouvier-Navé, P.; Husselstein, T.; Benveniste, P. Two families of sterol methyltransferases are involved in the first and the second methylation steps of plant sterol biosynthesis. Eur. J. Biochem. 1998, 256, 88–96. [Google Scholar] [CrossRef]
- Zhang, X.; Cambrai, A.; Miesch, M.; Roussi, S.; Raul, F.; Aoude-Werner, D.; Marchioni, E. Separation of Δ5- and Δ7-Phytosterols by Adsorption Chromatography and Semipreparative Reversed Phase High-Performance Liquid Chromatography for Quantitative Analysis of Phytosterols in Foods. J. Agric. Food Chem. 2006, 54, 1196–1202. [Google Scholar] [CrossRef]
- Guillén, M.D.; Carton, I.; Goicoechea, E.; Uriarte, P.S. Characterization of Cod Liver Oil by Spectroscopic Techniques. New Approaches for the Determination of Compositional Parameters, Acyl Groups, and Cholesterol from 1H Nuclear Magnetic Resonance and Fourier Transform Infrared Spectral Data. J. Agric. Food Chem. 2008, 56, 9072–9079. [Google Scholar] [CrossRef]
- Forgo, P.; Kövér, K.E. Gradient enhanced selective experiments in the 1H NMR chemical shift assignment of the skeleton and side-chain resonances of stigmasterol, a phytosterol derivative. Steroids 2004, 69, 43–50. [Google Scholar] [CrossRef]
- Santos, J.S.; Escher, G.B.; da Silva Pereira, J.M.; Marinho, M.T.; do Prado-Silva, L.; Sant’Ana, A.S.; Dutra, L.D.; Barison, A.; Granato, D. 1H NMR combined with chemometrics tools for rapid characterization of edible oils and their biological properties. Ind. Crops Prod. 2018, 116, 191–200. [Google Scholar] [CrossRef]
- Atta-ur-Rahman, A.; Yaqoob, M.; Farooq, A.; Anjum, S.; Asif, F.; Choudhary, M.I. Fungal Transformation of (1R,2S,5R)-(−)-Menthol by Cephalosporium aphidicola. J. Nat. Prod. 1998, 61, 1340–1342. [Google Scholar] [CrossRef] [PubMed]
- Salvino, R.A.; Aroulanda, C.; De Filpo, G.; Celebre, G.; De Luca, G. Metabolic composition and authenticity evaluation of bergamot essential oil assessed by nuclear magnetic resonance spectroscopy. Anal. Bioanal. Chem. 2022, 414, 2297–2313. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.H.; Kim, H.K.; Wilson, E.G.; Erkelens, C.; Trijzelaar, B.; Verpoorte, R. Quantitative analysis of retinol and retinol palmitate in vitamin tablets using 1H-nuclear magnetic resonance spectroscopy. Anal. Chim. Acta 2004, 512, 141–147. [Google Scholar] [CrossRef]
- Gottlieb, H.E.; Kotlyar, V.; Nudelman, A. NMR Chemical Shifts of Common Laboratory Solvents as Trace Impurities. J. Org. Chem. 1997, 62, 7512–7515. [Google Scholar] [CrossRef]
- Nieva-Echevarría, B.; Goicoechea, E.; Guillén, M.D. Polyunsaturated lipids and vitamin A oxidation during cod liver oil in vitro gastrointestinal digestion. Antioxidant effect of added BHT. Food Chem. 2017, 232, 733–743. [Google Scholar] [CrossRef]
- Manini, P.; Camera, E.; Picardo, M.; Napolitano, A.; d’Ischia, M. Free radical oxidation of coriolic acid (13-(S)-hydroxy-9Z,11E-octadecadienoic Acid). Chem. Phys. Lipids 2005, 134, 161–171. [Google Scholar] [CrossRef]
- Gardner, H.; Weisleder, D. Hydroperoxides from Oxidation of Linoleic and Linolenic Acids by Soybean Lipoxygenase: Proof of the trans-11 Double Bond. Lipids 1972, 7, 191–193. [Google Scholar] [CrossRef]
- Kikuchi, M.; Yaoita, Y.; Kikuchi, M. Monohydroxy-Substituted Polyunsaturated Fatty Acids from Swertia japonica. Helv. Chim. Acta 2008, 91, 1857–1862. [Google Scholar] [CrossRef]
- Goicoechea, E.; Guillén, M.D. Analysis of Hydroperoxides, Aldehydes and Epoxides by 1H Nuclear Magnetic Resonance in Sunflower Oil Oxidized at 70 and 100 °C. J. Agric. Food Chem. 2010, 58, 6234–6245. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, R.; Varras, P.C.; Siskos, M.G.; Siddiqui, H.; Choudhary, M.I.; Gerothanassis, I.P. NMR and Computational Studies as Analytical and High-Resolution Structural Tool for Complex Hydroperoxides and Diastereomeric Endo-Hydroperoxides of Fatty Acids in Solution-Exemplified by Methyl Linolenate. Molecules 2020, 25, 4902. [Google Scholar] [CrossRef] [PubMed]
- Guillén, M.D.; Ruiz, A. Formation of hydroperoxy- and hydroxyalkenals during thermal oxidative degradation of sesame oil monitored by proton NMR. Eur. J. Lipid Sci. Technol. 2004, 106, 680–687. [Google Scholar] [CrossRef]
- Guillén, M.D.; Ruiz, A. Monitoring the oxidation of unsaturated oils and formation of oxygenated aldehydes by proton NMR. Eur. J. Lipid Sci. Technol. 2005, 107, 36–47. [Google Scholar] [CrossRef]
- Guillén, M.D.; Uriarte, P.S. Contribution to Further Understanding of the Evolution of Sunflower Oil Submitted to Frying Temperature in a Domestic Fryer: Study by 1H Nuclear Magnetic Resonance. J. Agric. Food Chem. 2009, 57, 7790–7799. [Google Scholar] [CrossRef]
- Martin-Rubio, A.S.; Sopelana, P.; Guillén, M.D. Influence of minor components on lipid bioaccessibility and oxidation during in vitro digestion of soybean oil. J. Sci. Food Agric. 2019, 99, 4793–4800. [Google Scholar] [CrossRef]
- Nieva-Echevarría, B.; Goicoechea, E.; Manzanos, M.J.; Guillén, M.D. 1H NMR and SPME-GC/MS study of hydrolysis, oxidation and other reactions occurring during in vitro digestion of non-oxidized and oxidized sunflower oil. Formation of hydroxy-octadecadienoates. Food Res. Int. 2017, 91, 171–182. [Google Scholar] [CrossRef]
- Falch, E.; Anthonsen, H.W.; Axelson, D.E.; Aursand, M. Correlation Between 1H NMR and Traditional Methods for Determining Lipid Oxidation of Ethyl Docosahexaenoate. J. Am. Oil Chem. Soc. 2004, 81, 1105–1110. [Google Scholar] [CrossRef]
- Jiang, Y.; Fan, K.W.; Tsz-Yeung Wong, R.; Chen, F. Fatty Acid Composition and Squalene Content of the Marine Microalga Schizochytrium mangrovei. J. Agric. Food Chem. 2004, 52, 1196–1200. [Google Scholar] [CrossRef]
- Bayır, A.; Haliloǧlu, H.İ.; Sirkecioǧlu, A.N.; Aras, N.M. Fatty acid composition in some selected marine fish species living in Turkish waters. J. Sci. Food Agric. 2006, 86, 163–168. [Google Scholar] [CrossRef]
- Orban, E.; Nevigato, T.; Lena, G.D.; Casini, I.; Marzetti, A. Differentiation in the Lipid Quality of Wild and Farmed Seabass (Dicentrarchus labrax) and Gilthead Sea Bream (Sparus aurata). J. Food Sci. 2003, 68, 128–132. [Google Scholar] [CrossRef]
- Carlini, G.C.G.; Roschel, G.G.; Ferrari, R.A.; Alencar, S.M.; Ota, H.C.; da Silveira, T.F.F.; Castro, I.A. Chemical characterization of Echium plantagineum seed oil obtained by three methods of extraction. J. Food Sci. 2021, 86, 5307–5317. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez-Luna, K.; Ansorena, D.; Astiasarán, I. Fatty acid profile, sterols, and squalene content comparison between two conventional (olive oil and linseed oil) and three non-conventional vegetable oils (echium oil, hempseed oil, and moringa oil). J. Food Sci. 2022, 87, 1489–1499. [Google Scholar] [CrossRef] [PubMed]
- Prasad, P.; Anjali, P.; Sreedhar, R.V. Plant-based stearidonic acid as sustainable source of omega-3 fatty acid with functional outcomes on human health. Crit. Rev. Food Technol. 2020, 61, 1725–1737. [Google Scholar] [CrossRef]
- Sullivan, J.C.; Budge, S.M.; St-Onge, M. Determining Ethyl esters in Fish Oil with Solid Phase Microextraction and GC–MS. J. Am. Oil Chem. Soc. 2009, 86, 743–748. [Google Scholar] [CrossRef]
- Turan, S.; Karabulut, I.; Vural, H. Influence of sn-1, 3-lipase-catalysed interesterification on the oxidative stability of soybean oil-based structured lipids. J. Sci. Food Agric. 2007, 87, 90–97. [Google Scholar] [CrossRef]
- Tyl, C.E.; Brecker, L.; Wagner, K.H. 1H NMR spectroscopy as tool to follow changes in the fatty acids of fish oils. Eur. J. Lipid Sci. Technol. 2008, 110, 141–148. [Google Scholar] [CrossRef]
- Gibson, R.A. Australian fish-An excellent source of both arachidonic acid and ω-3 polyunsaturated fatty acids. Lipids 1983, 18, 743–752. [Google Scholar] [CrossRef]
- De Leonardis, A.; Macciola, V. Behaviour of cod liver oil during the autoxidation process. Eur. J. Lipid Sci. Technol. 2006, 108, 871–876. [Google Scholar] [CrossRef]
- Guil-Guerrero, J.L.; Belarbi, E.H. Purification Process for Cod Liver Oil Polyunsaturated Fatty Acids. J. Am. Oil Chem. Soc. 2001, 78, 477–484. [Google Scholar] [CrossRef]
- Peng, S.; Chen, C.; Shi, Z.; Wang, L. Amino Acid and Fatty Acid Composition of the Muscle Tissue of Yellowfin Tuna (Thunnus albacares) and Bigeye Tuna (Thunnus obesus). J. Food Nutr. Res. 2013, 1, 42–45. [Google Scholar]
- Shim, S.M.; Dorworth, L.E.; Lasrado, J.A.; Santerre, C.R. Mercury and Fatty Acids in Canned Tuna, Salmon, and Mackerel. J. Food Sci. 2004, 69, C681–C684. [Google Scholar] [CrossRef]
- Byelashov, O.A.; Sinclair, A.J.; Kaur, G. Dietary sources, current intakes, and nutritional role of omega-3 docosapentaenoic acid. Lipid Technol. 2015, 27, 79–82. [Google Scholar] [CrossRef] [PubMed]
- Fiori, L.; Solana, M.; Tosi, P.; Manfrini, M.; Strim, C.; Guella, G. Lipid profiles of oil from trout (Oncorhynchus mykiss) heads, spines and viscera: Trout by-products as a possible source of omega-3 lipids? Food Chem. 2012, 134, 1088–1095. [Google Scholar] [CrossRef]
- Hosomi, R.; Tanizaki, T.; Ikawa, S.; Tsushima, T.; Misawa, Y.; Baba, N.; Yoshida, M.; Fukunaga, K. Effect of 6, 9, 12, 15-Hexadecatetraenoic Acid (C16:4n-1)-Ethyl Ester on Lipid Content and Fatty Acid Composition in the Blood and Organs of Mice. J. Oleo Sci. 2021, 70, 703–712. [Google Scholar] [CrossRef]
- Shahidi, F.; Wanasundara, U.N. Omega-3 fatty acid concentrates: Nutritional aspects and production technologies. Trends Food Sci. Technol. 1998, 9, 230–240. [Google Scholar] [CrossRef]
- Bratu, A.; Mihalache, M.; Hanganu, A.; Chira, N.A.; Todaşcă, M.C.; Roşca, S. Quantitative determination of fatty acids from fish oils using GC-MS method and 1H-NMR spectroscopy. UPB Sci. Bull. B 2013, 75, 23–32. [Google Scholar]
- Knothe, G.; Kenar, J.A. Determination of the fatty acid profile by 1H-NMR spectroscopy. Eur. J. Lipid Sci. Technol. 2004, 106, 88–96. [Google Scholar] [CrossRef]
- Caño-Ochoa, S.D.; Ruiz-Aracama, A.; Guillén, M.D. Alpha-Tocopherol, a Powerful Molecule, Leads to the Formation of Oxylipins in Polyunsaturated Oils Differently to the Temperature Increase: A Detailed Study by Proton Nuclear Magnetic Resonance of Walnut Oil Oxidation. Antioxidants 2022, 11, 604. [Google Scholar] [CrossRef]
- Nestel, P.J.; Mori, T.A. Dietary patterns, dietary nutrients and cardiovascular disease. Rev. Cardiovasc. Med. 2022, 23, 17. [Google Scholar] [CrossRef]
- Nattagh-Eshtivani, E.; Barghchi, H.; Pahlavani, N.; Barati, M.; Amiri, Y.; Fadel, A.; Khosravi, M.; Talebi, S.; Arzhang, P.; Ziaei, R.; et al. Biological and pharmacological effects and nutritional impact of phytosterols: A comprehensive review. Phytother. Res. 2022, 36, 299–322. [Google Scholar] [CrossRef] [PubMed]
- Lv, J.; Yang, X.; Ma, H.; Hu, X.; Wei, Y.; Zhou, W.; Li, L. The oxidative stability of microalgae oil (Schizochytrium aggregatum) and its antioxidant activity after simulated gastrointestinal digestion: Relationship with constituents. Eur. J. Lipid Sci. Technol. 2015, 117, 1928–1939. [Google Scholar] [CrossRef]
- Chen, J.; Jiao, R.; Jiang, Y.; Bi, Y.; Chen, Z.Y. Algal Sterols are as Effective as β-Sitosterol in Reducing Plasma Cholesterol Concentration. J. Agric. Food Chem. 2014, 62, 675–681. [Google Scholar] [CrossRef] [PubMed]
- Alberdi-Cedeño, J.; Ibargoitia, M.L.; Cristillo, G.; Sopelana, P.; Guillén, M.D. A new methodology capable of characterizing most volatile and less volatile minor edible oils components in a single chromatographic run without solvents or reagents. Detection of new components. Food Chem. 2017, 221, 1135–1144. [Google Scholar] [CrossRef]
- Schwartz, H.; Ollilainen, V.; Piironen, V.; Lampi, A.M. Tocopherol, tocotrienol and plant sterol contents of vegetable oils and industrial fats. J. Food Compos. Anal. 2008, 21, 152–161. [Google Scholar] [CrossRef]
- Wright, J. Essential Oils. In Food Flavourings, 2nd ed.; Ashurst, P.R., Ed.; Blackie and Son Ltd.: New York, NY, USA, 1991; pp. 24–53. [Google Scholar]
- Ibáñez, M.D.; Sanchez-Ballester, N.M.; Blázquez, M.A. Encapsulated Limonene: A Pleasant Lemon-Like Aroma with Promising Application in the Agri-Food Industry. A Review. Molecules 2020, 25, 2598. [Google Scholar] [CrossRef]
- Mołdoch, J.; Agacka-Mołdoch, M.; Jóźwiak, G.; Wojtunik-Kulesza, K. Biological Activity of Monoterpene-Based Scaffolds: A Natural Toolbox for Drug Discovery. Molecules 2025, 30, 1480. [Google Scholar] [CrossRef]
- Babu, B.; Wu, J.T. Production of natural butylated hydroxytoluene as an antioxidant by freshwater phytoplankton. J. Phycol. 2008, 44, 1447–1454. [Google Scholar] [CrossRef]
- Standal, I.B.; Carvajal, A.K.; Mozuraityte, R.; Storrø, I.; Størseth, T.; Abbasi, E.; Aursand, M. High-Resolution NMR as Tool to Study Enzyme-Catalyzed Production of Fatty Acid Ethyl Esters from Marine Oils. In Modern Magnetic Resonance; Webb, G.A., Ed.; Springer International Publishing: Cham, Switzerland, 2017; pp. 1853–1866. [Google Scholar] [CrossRef]








| Sample | Oil Nature | Some Composition Data According to the Label |
|---|---|---|
| S1 (c) | Cod liver oil | (*), vitamin A (from cod liver oil and retinyl palmitate) |
| S2 (ne) | Fish oil | In 1.14 g oil (1.25 mL): 184 mg EPA, 115 mg DHA 50% mixed tocopherols, natural lemon aroma |
| S3 (ne) | Tuna oil | In 4489 mg oil (5 mL): 1212 mg ω-3 lipids, 852.91 mg DHA, 197.51 mg EPA natural lemon aroma, α-tocopherol |
| S4 (c) | Cold-pressed linseed (Linum usitatissimum) oil | (*), α-tocopherol |
| S5 (c) | Refined ahiflower (Buglossoides arvensis) oil | In 1000 mg oil (1 cap): 395 mg ALA, 159 mg SDA, 83 mg LA, 41 mg GLA |
| S6 (c) | Echium (Echium plantegineum) seed oil | In 1 g oil (2 caps): 469 mg ω-3 lipids, 325 mg ALA, 144 mg SDA, 146 mg LA, 112 mg GLA |
| S7 (ne) | Schizochytrium sp. microalgae oil | 50% DHA ascorbyl palmitate, natural blend of tocopherols, natural lemon aroma |
| S8 (c) | Schizochytrium sp. microalgae oil | In 1000 mg oil (2 caps): 550 mg ω-3 lipids, 300 mg DHA, 150 mg EPA D-α-tocopherol (vitamin E) |
| S9 (c) | Fish oil, cold-pressed borage (Borago officinalis L.) oil | In 400 mg fish oil (1 cap): 72 mg EPA, 48 mg DHA In 100 mg borage oil (1 cap): 22.5 mg GLA D-α-tocopherol |
| S10 (c) | Cold-pressed linseed oil, Schizochytrium sp. microalgae oil | In 600 mg lipids (1 cap): 300 mg ω-3 lipids, ≥200 mg ALA, ≥75 mg DHA natural mint essential oil, natural vitamin E |
| S11 (c) | Fish oil | In 2000 mg oil (2 caps): 700 mg EPA, 500 mg DHA vitamin E (D-α-tocoferol) |
| S12 (ne) | Concentrated fish oil (standardised 75% omega-3) | 3 g ω-3 lipids (5 mL): 1.6 g EPA, 0.8 g DHA, 0.6 g other ω-3 aroma, tocopherol-rich extract |
| S13 (c) | Purified fish oil (50% DHA, 20% EPA) | In 500 mg oil (1 cap): Minimum 225 mg DHA (in its natural TG form), Minimum 90 mg EPA tocopherol mixture |
| S14 (c) | Concentrated fish oil | In 1 g fish oil (2 caps): 0.6 g ω-3 lipids (guaranteed), 0.5 g DHA, 0.1 g EPA α-tocopherol acetate (vitamin E) |
| S15 (c) | Purified fish oil | In 547.75 mg oil (1 cap): 465 mg ω-3 lipids, 450 mg EPA (in TG form) vitamin E (natural tocopherol blend) |
| S16 (c) | Purified deodorised fish oil | In 527 mg oil (1 cap): Minimum 421.6 mg ω-3 lipids, Minimum 400.5 mg DHA, Maximum 26.4 mg EPA (in TG form) blend of natural tocopherols |
| S17 (ne) | Fish oil (80% DHA) | In 1.02 g oil (1 mL): 800 mg DHA (in TG form), 40 mg EPA δ-tocopherol, lemon, and mint aromas |
| Signal | Chemical Shift (ppm) | Multiplicity | Type of Protons a | Compound or Type of Compounds |
|---|---|---|---|---|
| A1 | 0.88 b,c | t | -CH3 | Saturated, monounsaturated ω-9 and/or ω-7 acyl groups and fatty acids |
| 0.89 b,c | t | -CH3 | Polyunsaturated ω-6 acyl groups and fatty acids | |
| A2 | 0.97 b,c | t | -CH3 | Polyunsaturated ω-3 acyl groups and fatty acids |
| B | 1.25 d | t | ROCO-CH2-CH3 | EE |
| C | 1.19–1.44 b,c,e,f | m * | -(CH2)n- | Acyl groups and fatty acids |
| D1 | 1.61 b,g | m | -OCO-CH2-CH2- | Acyl groups in TG, except for DHA, DPA ω-6, EPA, ARA, SDA, and GLA groups |
| 1.62 g | m | -OCO-CH2-CH2- | Acyl groups in 1,2-DG, except for DHA, DPA ω-6, EPA and ARA groups | |
| 1.63 g | m | -OCO-CH2-CH2-, COOH-CH2-CH2- | Acyl groups in 1-MG, 1,3-DG and fatty acids, except for DHA, DPA ω-6, EPA and ARA groups | |
| 1.64 g | m | -OCO-CH2-CH2- | Acyl groups in 2-MG, except for DHA, DPA ω-6, EPA and ARA groups | |
| 1.65 | m | -OCO-CH2-CH2- | SDA ** and GLA ** acyl groups in methyl ester form | |
| D2 | 1.70 c,g | m | -OCO-CH2-CH2- | EPA and ARA acyl groups in TG and EE ** |
| 1.71 | m | -OCO-CH2-CH2- | ARA acyl groups in methyl ester form ** | |
| 1.72 c,g | m | COOH-CH2-CH2- | EPA and ARA (acids) | |
| E | 1.92–2.17 c,e,g | m *** | -CH2-CH=CH- | Acyl groups and fatty acids except for -CH2- of DHA and DPA ω-6 groups in β-position in relation to the carbonyl/carboxyl group |
| F1 | 2.25–2.36 c,g | dt (TG)/t (EE) | -OCO-CH2- | Acyl groups in TG and EE **, except for DHA and DPA ω-6 groups |
| 2.33 g | m | -OCO-CH2- | Acyl groups in 1,2-DG, except for DHA and DPA ω-6 groups | |
| 2.35 g | t | -OCO-CH2-, COOH-CH2- | Acyl groups in 1-MG, 1,3-DG, and fatty acids, except for DHA and DPA ω-6 groups and EPA (acid) | |
| 2.37/2.35 § | t | COOH-CH2- | EPA (acid) ** | |
| 2.38 g | t | -OCO-CH2- | Acyl groups in 2-MG, except for DHA and DPA ω-6 groups | |
| F2 | 2.34–2.42 | m | -OCO-CH2-CH2- | DHA acyl groups in EE form ** |
| 2.36–2.42 c,g | m | -OCO-CH2-CH2- | DHA acyl groups in TG and DPA ω-6 acyl groups in methyl ester form ** | |
| 2.38–2.44 g | m | COOH-CH2-CH2- | DHA and DPA ω-6 ** (acids) | |
| G1 | 2.77 b | t | =HC-CH2-CH= | Diunsaturated ω-6 acyl groups and fatty acids |
| G2 | 2.77–2.90 b,c | m | =HC-CH2-CH= | Other polyunsaturated ω-6 and ω-3 acyl groups and fatty acids |
| G3 | 2.79–2.89 | m | =HC-CH2-CH= | SDA acyl groups in methyl ester form ** |
| H1 | 3.65 g,h | ddd | ROCH2-CHOH-CH2OH | Glyceryl group in 1-MG |
| I1 | 3.73 g,h | d/t §§ | ROCH2-CH(OR′)-CH2OH | Glyceryl group in 1,2-DG |
| J1 | 3.84 g,h | d/t §§ | HOCH2-CH(OR)-CH2OH | Glyceryl group in 2-MG |
| H2 | 3.94 g,h | m | ROCH2-CHOH-CH2OH | Glyceryl group in 1-MG |
| K | 4.05–4.21 g,h | m | ROCH2-CHOH-CH2OR’ | Glyceryl group in 1,3-DG |
| L | 4.12 d | q | ROCO-CH2-CH3 | EE |
| H3 | 4.18 g,h | ddd | ROCH2-CHOH-CH2OH | Glyceryl group in 1-MG |
| M | 4.22 b | ddd | ROCH2-CH(OR′)-CH2OR″ | Glyceryl group in TG |
| I2 | 4.28 g,h | ddd | ROCH2-CH(OR′)-CH2OH | Glyceryl group in 1,2-DG |
| J2 | 4.93 g,h | m | HOCH2-CH(OR)-CH2OH | Glyceryl group in 2-MG |
| I3 | 5.08 g,h | m | ROCH2-CH(OR′)-CH2OH | Glyceryl group in 1,2-DG |
| N1 | 4.95–5.07 c | dq,dq | -CH=CH2 | Unsaturated ω-1 acyl groups |
| O | 5.20–5.26 b | m | ROCH2-CH(OR’)-CH2OR″ | Glyceryl group in TG |
| P | 5.28–5.46 g | m | -CH=CH- | Acyl groups and fatty acids |
| N2 | 5.75–5.86 c | m | -CH=CH2 | Unsaturated ω-1 acyl groups |
| DHA + DPA ω-6 | EPA + ARA | Linolenic | SDA | Total ω-3 | Other ω-3 * | Linoleic ** | ω-1 | Total Unsat | |
|---|---|---|---|---|---|---|---|---|---|
| Fish oils | |||||||||
| S1 | 9.30 ± 0.10 b | 10.50 ± 0.22 d | - | - | 25.03 ± 0.43 a | 5.23 ± 0.32 d | 3.65 ± 0.26 ab | 0.95 ± 0.06 d | 78.23 ± 0.46 c |
| S2 | 9.63 ± 0.08 b | 18.13 ± 0.58 g | - | - | 32.95 ± 0.72 b | 5.01 ± 0.23 d | 4.36 ± 0.02 b | 2.37 ± 0.02 g | 69.51 ± 0.19 a |
| S3 | 24.60 ± 0.07 f | 7.49 ± 0.15 c | - | - | 33.84 ± 0.13 b | 1.75 ± 0.08 bc | 3.27 ± 0.03 ab | 0.08 ± 0.00 a | 69.46 ± 0.27 a |
| Vegetable oils | |||||||||
| S4 | - | - | 53.49 ± 0.33 d | - | *** | - | 14.36 ± 0.09 e | - | 88.26 ± 0.18 de |
| S5 | - | - | 41.09 ± 0.86 c | 19.50 ± 0.93 b | 60.59 ± 0.07 f | nd | 12.00 ± 0.17 d | - | 91.86 ± 0.45 efg |
| S6 | - | - | 30.03 ± 1.02 a | 13.93 ± 0.48 a | 43.96 ± 0.55 c | nd | 14.43 ± 0.13 e | - | 86.17 ± 0.39 d |
| Microalgae oils | |||||||||
| S7 | 65.98 ± 0.01 j | 0.59 ± 0.01 a | - | - | 56.99 ± 0.25 e | nd | - | - | 76.22 ± 0.00 bc |
| S8 | 33.37 ± 0.03 g | 21.40 ± 0.03 h | - | - | 57.64 ± 0.08 e | 2.87 ± 0.07 c | 2.91 ± 0.02 a | - | 74.24 ± 0.60 b |
| Mixtures of vegetable and marine oils | |||||||||
| S9A | 7.83 ± 0.25 a | 13.41 ± 0.23 de | - | - | 26.32 ± 0.28 a | 5.08 ± 0.76 d | 11.11 ± 1.31 d | 1.71 ± 0.13 e | 74.91 ± 1.36 b |
| S9B | 8.18 ± 0.04 a | 14.59 ± 0.06 f | - | - | 25.58 ± 0.00 a | 2.81 ± 0.10 c | 11.09 ± 0.04 d | 1.82 ± 0.00 f | 73.30 ± 0.47 b |
| S10 | 15.42 ± 0.11 c | 1.06 ± 0.03 a | 34.43 ± 0.26 b | - | 50.91 ± 0.18 d | nd | 9.61 ± 0.46 c | - | 85.72 ± 0.34 d |
| Fish oil concentrates | |||||||||
| Acyl groups in EE form | |||||||||
| S11 | 22.34 ± 0.63 e | 35.83 ± 0.79 i | - | - | 66.72 ± 0.52 g | 8.55 ± 0.68 e | 3.84 ± 0.27 ab | 0.35 ± 0.05 c | 90.20 ± 0.01 ef |
| S12A | 19.03 ± 0.69 d | 50.67 ± 0.83 l | - | - | 75.27 ± 0.26 i | 5.58 ± 0.12 d | 2.75 ± 0.24 a | 0.26 ± 0.00 bc | 95.07 ± 4.06 g |
| S12B | 19.10 ± 0.04 d | 46.60 ± 0.09 k | - | - | 71.75 ± 0.37 h | 6.05 ± 0.32 d | 2.77 ± 0.08 a | 0.32 ± 0.00 c | 91.03 ± 0.13 ef |
| Acyl groups mainly in TG form | |||||||||
| S13 | 40.87 ± 0.02 h,**** | 24.17 ± 0.04 i | - | - | 68.07 ± 0.05 g | 3.03 ± 0.02 c | 3.09 ± 0.12 a | 0.28 ± 0.00 bc | 88.83 ± 0.00 de |
| S14 | 44.69 ± 0.03 i,**** | 13.78 ± 0.35 ef | - | - | 72.95 ± 1.40 h | 14.49 ± 1.02 f | - | 0.30 ± 0.01 c | 91.06 ± 0.50 ef |
| S15 | - | 88.02 ± 0.14 m | - | - | 88.52 ± 0.61 j | 0.49 ± 0.47 ab | - | 0.17 ± 0.00 ab | 92.59 ± 0.09 fg |
| S16 | 80.46 ± 0.02 l | 6.51 ± 0.01 b | - | - | 92.67 ± 0.15 l | 5.71 ± 0.19 d | - | 0.07 ± 0.00 a | 98.15 ± 0.06 h |
| S17A | 82.42 ± 0.19 m | 12.51 ± 0.09 d | - | - | 96.65 ± 0.85 m | 1.71 ± 0.75 bc | - | - | 99.35 ± 0.47 h |
| S17B | 78.87 ± 0.17 k | 13.36 ± 0.25 de | - | - | 91.81 ± 0.81 k | - | - | - | 96.97 ± 0.29 h |
| DHA | EPA | Linolenic | SDA | Total ω-3 Groups | |
|---|---|---|---|---|---|
| Fish oils | |||||
| S2 | 10.09 (0.5↓) a | 16.14 (2.0↑) | - | - | - |
| S3 | 19.00 (5.6↑) | 4.40 (3.1↑) | - | - | 27.00 (6.8↑) |
| Vegetable oils | |||||
| S5 | - | - | 39.50 (1.6↑) | 15.90 (3.6↑) | - |
| S6 | - | - | 32.50 (2.5↓) | 14.40 (0.5↓) | 46.90 (2.9↓) |
| Microalgae oils | |||||
| S7 | 50.00 b | - | - | - | - |
| S8 | 30.00 (3.4↑) | 15.00 (6.4↑) | - | - | 55.00 (2.6↑) |
| Mixtures of vegetable and marine oils | |||||
| S9 | 9.60 | 14.40 | - | - | - |
| A: (1.8↓)-/B: (1.4↓) | A: (1.0↓)/B: (0.2↑) | ||||
| S10 | Minimum: 12.50 | - | Minimum: 33.33 | - | 50.00 (0.9↑) |
| (2.9↑) | (1.1↑) | ||||
| Fish oil concentrates | |||||
| S11 | 25.00 (2.7↓) | 35.00 (0.8↑) | - | - | - |
| S12 | 26.67 c | 53.33 c | - | - | 75.00 |
| A: 25.28 d (1.4↓) | A: 67.32 d (14.0↑) | A: (0.3↑) | |||
| B: 26.62 d (<0.1↓) | B: 64.95 d (11.6↑) | B: (3.3↓) | |||
| S13 | Minimum: 45.00 | Minimum: 18.00 | - | - | - |
| (4.1↓) | (6.2↑) | ||||
| S14 | Guaranteed: 50.00 | Guaranteed: 10.00 | - | - | Guaranteed: 60.00 |
| (5.3↓) | (3.8↑) | (13.0↑) | |||
| S15 | - | 82.15 (5.9↑) | - | - | 84.89 (3.6↑) |
| S16 | Minimum: 76.00 | Maximum: 5.00 | - | - | Minimum: 80.00 |
| (4.5↑) | (1.5↑) | (12.7↑) | |||
| S17 | 78.43 | 3.92 | - | - | - |
| A: (4.0↑)/B: (0.4↑) | A: (8.6↑)/B: (9.4↑) | ||||
| Tocopherols | Sterols | ||||||
|---|---|---|---|---|---|---|---|
| α-T | γ-T | δ-T | Signal at 0.68 ppm * | DiMe-St | Stigm | ∆7-Aven | |
| Fish oils | |||||||
| S1 | - | - | - | 3.97 ± 0.14 g | - | - | - |
| S2 | - | nq | nq | 5.73 ± 0.14 j | - | - | - |
| S3 | 1.81 ± 0.03 b,** | 1.89 ± 0.03 g | 0.79 ± 0.09 c | 1.95 ± 0.01 e | - | - | - |
| Vegetable oils | |||||||
| S4 | 7.95 ± 0.30 f | 0.39 ± 0.00 a | - | 1.83 ± 0.01 de | 1.26 ± 0.01 a | nq | 0.12 ± 0.00 a |
| S5 | - | 0.38 ± 0.03 a | - | 1.58 ± 0.00 c | - | - | 0.19 ± 0.02 b |
| S6 | - | 0.72 ± 0.01 b | - | 2.89 ± 0.04 f | - | - | 0.50 ± 0.00 c |
| Microalgae oils | |||||||
| S7 | - | 2.73 ± 0.02 h | 0.58 ± 0.00 b | 2.90 ± 0.00 f | - | - | - |
| S8 | 4.16 ± 0.04 e | 0.90 ± 0.01 c | 0.21 ± 0.04 a | 0.81 ± 0.00 a | - | 2.87 ± 0.00 | - |
| Mixtures of vegetable and marine oils | |||||||
| S9A | 3.62 ± 0.16 d | - | - | 5.20 ± 0.04 i | - | - | - |
| S9B | - | 3.09 ± 0.04 f | 1.67 ± 0.02 d | 4.74 ± 0.03 h | - | - | - |
| S10 | - | 0.67 ± 0.08 b | - | 1.72 ± 0.01 cd | 1.41 ± 0.11 b | nq | - |
| Fish oil concentrates | |||||||
| Acyl groups in EE form | |||||||
| S11 | 3.02 ± 0.17 c | - | - | 1.36 ± 0.08 b | - | - | - |
| S12A | - | 1.22 ± 0.13 e | 0.62 ± 0.16 b | - | - | - | - |
| S12B | - | 1.89 ± 0.02 g | nq | - | - | - | - |
| Acyl groups mainly in TG form | |||||||
| S13 | - | 0.90 ± 0.01 c | 0.51 ± 0.01 b | 6.20 ± 0.06 k | - | - | - |
| S14 | 4.37 ± 0.05 e | 1.06 ± 0.02 d | 0.47 ± 0.02 b | 1.88 ± 0.04 de | - | - | - |
| S15 | 0.76 ± 0.01 a | 0.45 ± 0.03 a | nq | - | - | - | - |
| S16 | 1.68 ± 0.01 b | 3.87 ± 0.03 i | 1.83 ± 0.06 e | - | - | - | - |
| S17A | - | - | - | - | - | - | - |
| S17B | - | - | - | - | - | - | - |
| Flavour Components * | Others | |||||
|---|---|---|---|---|---|---|
| Limonene | Geranial | Neral | β-Pinene | Menthol | ||
| S1 | - | - | - | - | - | Retinyl esters: 1.01 ± 0.00 |
| S2 | 22.16 ± 0.92 c | 1.21 ± 0.04 c | 0.75 ± 0.12 b | 2.77 ± 0.02 d | - | - |
| S3 | 0.50 ± 0.01 a | 0.38 ± 0.00 a | 0.24 ± 0.01 a | - | - | Ethanol: 56.12 ± 0.61 |
| S7 | 0.56 ± 0.01 a | - | - | - | - | BHT: 0.18 ± 0.00 |
| S10 | - | - | - | - | 3.46 ± 0.24 a | - |
| S12A | 3.32 ± 0.27 b | 0.33 ± 0.04 a | 0.31 ± 0.08 a | 1.06 ± 0.22 b | - | - |
| S12B | 3.91 ± 0.03 b | 0.34 ± 0.01 a | 0.22 ± 0.01 a | 0.71 ± 0.02 a | - | - |
| S17A | 35.81 ± 0.56 d | 0.87 ± 0.03 b | 0.70 ± 0.01 b | 1.66 ± 0.05 c | 14.07 ± 0.26 b | - |
| S17B | 94.15 ± 1.25 e | 3.03 ± 0.00 d | 1.85± 0.03 c | 4.55 ± 0.11 e | 37.62 ± 0.22 c | - |
| HPO-c-Z,E- dEs | HO-c-Z,E- dEs | Aldehydes | |||||
|---|---|---|---|---|---|---|---|
| n-alk | 2E-alkn | 2E,4E- alkdn | 2Z,4E- alkdn | Non- Identified * | |||
| S1 | - | - | - | - | - | - | - |
| S2 | 0.57 ± 0.09 ab | - | 0.14 ± 0.01 b | - | - | - | - |
| S3 | 0.47 ± 0.04 ab | - | - | - | - | - | - |
| S4 | 0.42 ± 0.09 a | 1.61 ± 0.10 b | 0.10 ± 0.01 a | - | - | - | - |
| S5 | 1.61 ± 0.03 e | - | - | - | - | - | - |
| S6 | 2.57 ± 0.11 f | - | - | 0.07 ± 0.01 | 0.09 ± 0.01 | 0.07 ± 0.01 a | - |
| S7 | 1.27 ± 0.15 cd | - | - | - | - | - | 0.32 ± 0.09 a |
| S8 | 0.36 ± 0.06 a | - | - | - | - | - | - |
| S9A | 1.14 ± 0.02 b | - | - | - | - | - | - |
| S9B | 0.75 ± 0.06 cd | - | - | - | - | - | - |
| S10 | - | 0.72 ± 0.01 a | - | - | - | - | - |
| S11 | 2.58 ± 0.19 f | - | - | - | - | - | - |
| S12A | - | - | - | - | - | - | - |
| S12B | - | - | - | - | - | - | - |
| S13 | 0.62 ± 0.02 ab | - | - | - | - | - | - |
| S14 | 1.05 ± 0.15 c | - | - | - | - | 0.11 ± 0.00 b | - |
| S15 | 1.34 ± 0.02 d | - | - | - | - | - | - |
| S16 | 2.59 ± 0.03 f | - | - | - | - | - | 0.31 ± 0.08 a |
| S17A | 3.87 ± 0.07 g | - | 0.10 ± 0.01 a | - | - | - | 0.57 ± 0.02 c |
| S17B | 4.18 ± 0.08 h | - | 0.36 ± 0.04 c | - | - | - | 0.46 ± 0.01 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Weinbinder, D.D.; Manzanos, M.J.; Sopelana, P. A Comprehensive Study via Proton Nuclear Magnetic Resonance of a Variety of Omega-3 Lipid-Rich Supplements Available in the Spanish Market: Acyl Group Profile, Minor Components, and Oxidative Status. Foods 2025, 14, 4217. https://doi.org/10.3390/foods14244217
Weinbinder DD, Manzanos MJ, Sopelana P. A Comprehensive Study via Proton Nuclear Magnetic Resonance of a Variety of Omega-3 Lipid-Rich Supplements Available in the Spanish Market: Acyl Group Profile, Minor Components, and Oxidative Status. Foods. 2025; 14(24):4217. https://doi.org/10.3390/foods14244217
Chicago/Turabian StyleWeinbinder, Dafne Denise, María J. Manzanos, and Patricia Sopelana. 2025. "A Comprehensive Study via Proton Nuclear Magnetic Resonance of a Variety of Omega-3 Lipid-Rich Supplements Available in the Spanish Market: Acyl Group Profile, Minor Components, and Oxidative Status" Foods 14, no. 24: 4217. https://doi.org/10.3390/foods14244217
APA StyleWeinbinder, D. D., Manzanos, M. J., & Sopelana, P. (2025). A Comprehensive Study via Proton Nuclear Magnetic Resonance of a Variety of Omega-3 Lipid-Rich Supplements Available in the Spanish Market: Acyl Group Profile, Minor Components, and Oxidative Status. Foods, 14(24), 4217. https://doi.org/10.3390/foods14244217






