Impact of Frying Olive Oil Type on the Physicochemical and Sensory Quality of Commercial Chicken Nuggets
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Experimental Design and Pan-Frying of the Nuggets
2.3. Physicochemical Characterisation of Nuggets
2.3.1. Moisture and Fat Composition
2.3.2. Cooking Performance and Instrumental Colour
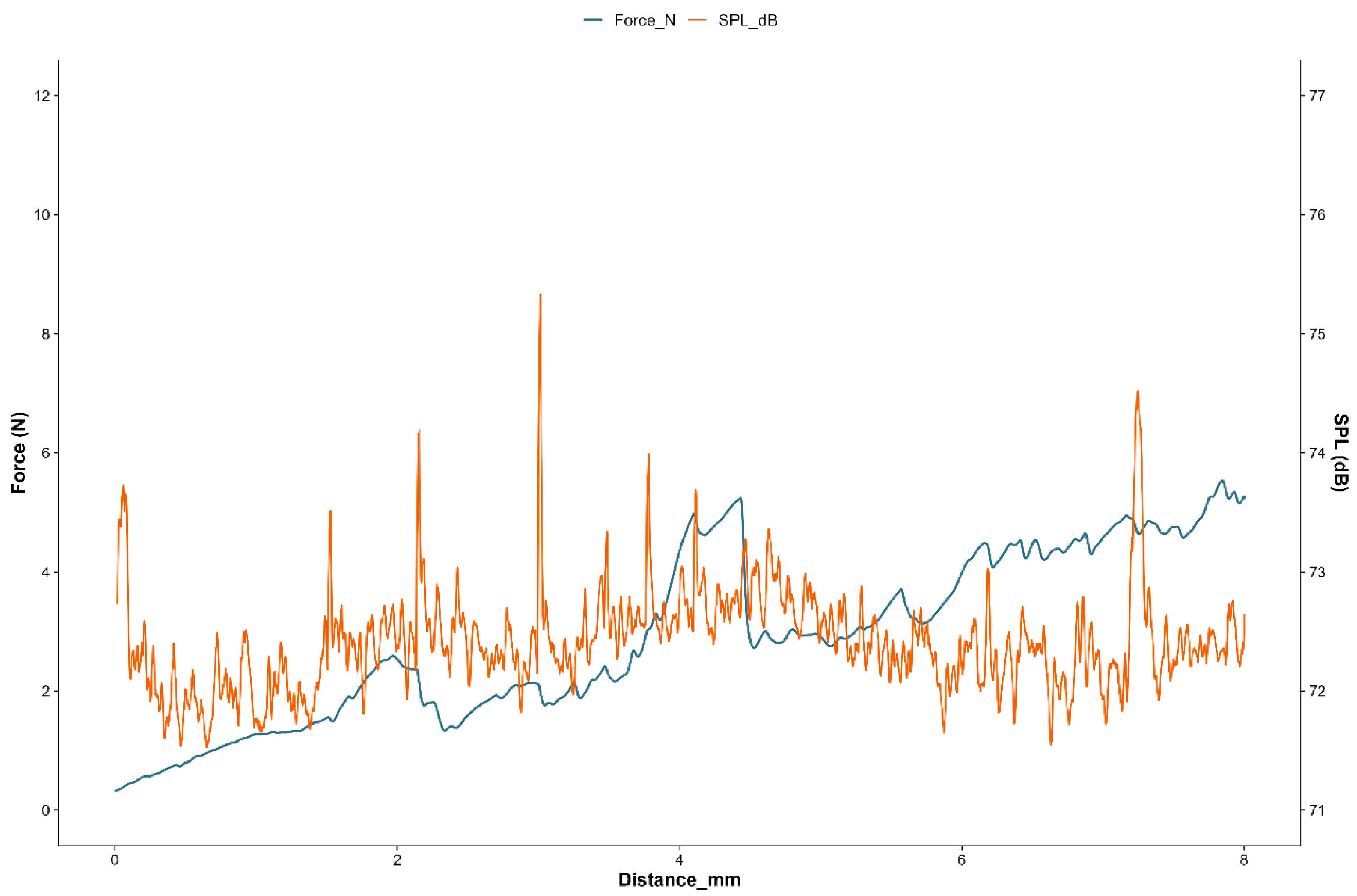
2.3.3. Instrumental Texture and Sound Emission Analysis
2.3.4. Volatile Determination
2.4. Sensorial Analysis
2.5. Statistical Analysis
3. Results and Discussion
3.1. Moisture and Fat Composition of Fried Nuggets
3.2. Cooking Performance and Instrumental Colour Determination
3.3. Mechanical and Acoustic Properties
3.4. Volatile Compounds
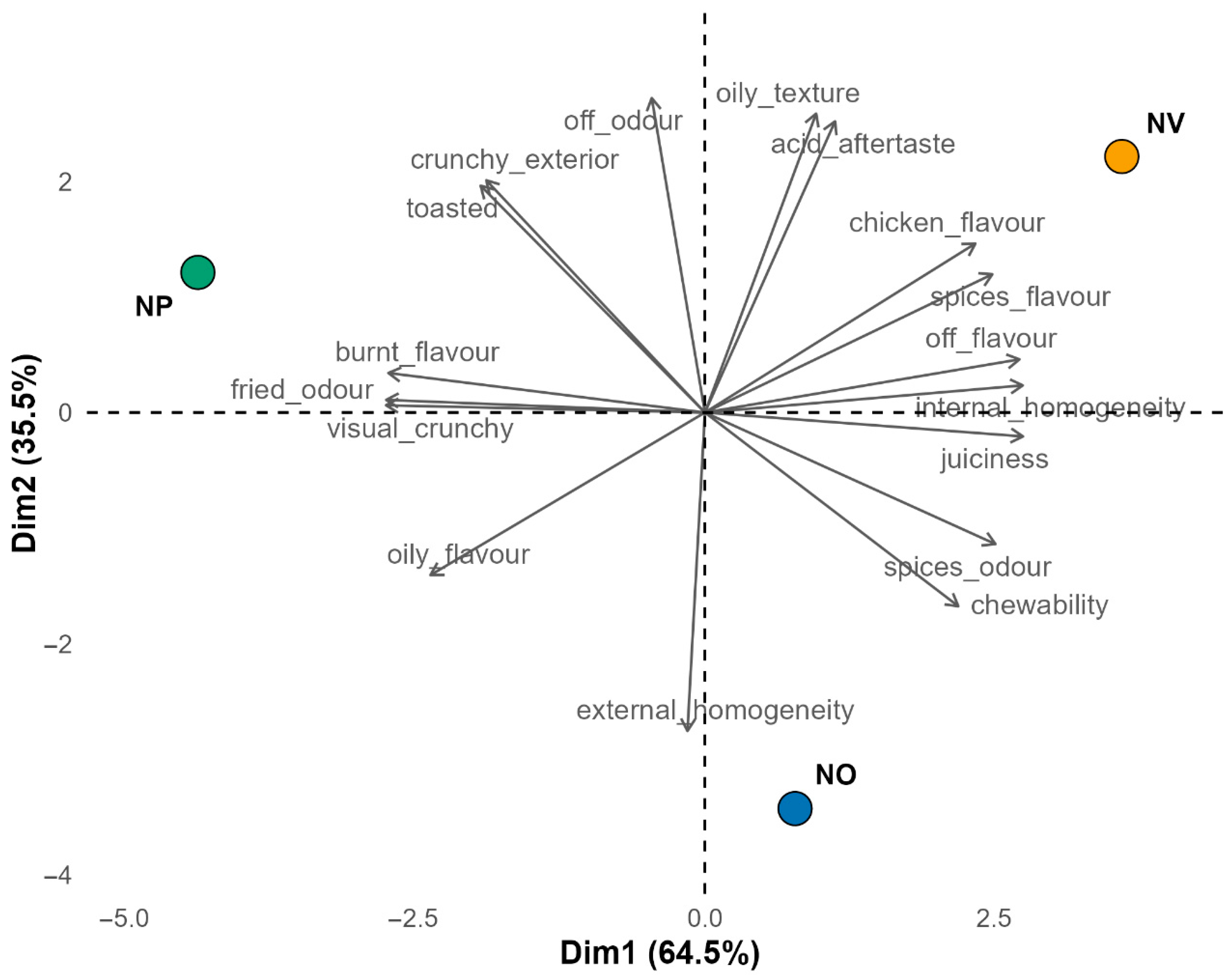
3.5. Sensory Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- The Commission of the European Communities. Commission Regulation (Ec) No 1019/2002 of 13 June 2002 on Marketing Standards for Olive Oil; The Commission of the European Communities: Brussels, Belgium, 2002; pp. 27–31. [Google Scholar]
- Casal, S.; Malheiro, R.; Sendas, A.; Oliveira, B.P.P.; Pereira, J.A. Olive Oil Stability under Deep-Frying Conditions. Food Chem. Toxicol. 2010, 48, 2972–2979. [Google Scholar] [CrossRef]
- Gonçalves, M.; Vale, N.; Silva, P. Neuroprotective Effects of Olive Oil: A Comprehensive Review of Antioxidant Properties. Antioxidants 2024, 13, 762. [Google Scholar] [CrossRef]
- Mateos, R.; Sarria, B.; Bravo, L. Nutritional and Other Health Properties of Olive Pomace Oil. Crit. Rev. Food Sci. Nutr. 2020, 60, 3506–3521. [Google Scholar] [CrossRef]
- Holgado, F.; Ruiz-Méndez, M.V.; Velasco, J.; Márquez-Ruiz, G. Performance of Olive-Pomace Oils in Discontinuous and Continuous Frying. Comp. Behav. Sunflower Oils High-Oleic Sunflower Oils. Foods 2021, 10, 3081. [Google Scholar] [CrossRef]
- Chiou, A.; Kalogeropoulos, N. Virgin Olive Oil as Frying Oil. Compr. Rev. Food Sci. Food Saf. 2017, 16, 632–646. [Google Scholar] [CrossRef] [PubMed]
- Demiray, I.D.; Ergezer, H.; Demiray, E.; Süfer, Ö. Deep-Fat Frying of Chicken Nuggets: Impacts on Mass Transfer and Some Quality Indices. Food Sci. Nutr. 2025, 13, e70451. [Google Scholar] [CrossRef]
- Kochhar, S.P. 6-The Composition of Frying Oils. In Frying; Rossell, J.B., Ed.; Woodhead Publishing Series in Food Science, Technology and Nutrition; Woodhead Publishing: Cambridge, UK, 2001; pp. 87–114. ISBN 978-1-85573-556-9. [Google Scholar]
- European Commission Olive Oil and Table Olives: Imports, Exports and Prices of Olives and Different Qualities of Oil; European Commission: Brussels, Belgium, 2025.
- MAPA. Evolución de Los Precios En Aceite de Oliva; Ministerio de Agricultura, Pesca y Alimentación: Madrid, España, 2025.
- Official Methods of Analysis. Official Methods of Analysis, 17th ed.; Method 950.46; Aoac International: Gaithersburg, MD, USA, 2000. [Google Scholar]
- Pintado, T.; Herrero, A.M.; Jiménez-Colmenero, F.; Pasqualin Cavalheiro, C.; Ruiz-Capillas, C. Chia and Oat Emulsion Gels as New Animal Fat Replacers and Healthy Bioactive Sources in Fresh Sausage Formulation. Meat Sci. 2018, 135, 6–13. [Google Scholar] [CrossRef]
- Weihrauch, J.L.; Posati, L.P.; Anderson, B.A.; Exler, J. Lipid Conversion Factors for Calculating Fatty Acid Contents of Foods. J. Am. Oil. Chem. Soc. 1977, 54, 36–40. [Google Scholar] [CrossRef]
- Bradski, G. The OpenCV Library. Dobb’s J. Softw. Tools 2000, 120, 122–125. [Google Scholar]
- Harris, C.R.; Millman, K.J.; van der Walt, S.J.; Gommers, R.; Virtanen, P.; Cournapeau, D.; Wieser, E.; Taylor, J.; Berg, S.; Smith, N.J.; et al. Array Programming with NumPy. Nature 2020, 585, 357–362. [Google Scholar] [CrossRef] [PubMed]
- The Pandas Development Team Pandas-Dev/Pandas: Pandas 2020. Available online: https://zenodo.org/records/16918803 (accessed on 20 September 2025).
- van der Walt, S.; Schönberger, J.L.; Nunez-Iglesias, J.; Boulogne, F.; Warner, J.D.; Yager, N.; Gouillart, E.; Yu, T. Contributors, the scikit-image Scikit-Image: Image Processing in Python. PeerJ 2014, 2, e453. [Google Scholar] [CrossRef]
- Alvarez, M.D.; Velarde, C.; Barrios, L.; Herranz, B. Understanding the Crispy–Crunchy Texture of Raw Red Pepper and Its Change with Storage Time. J. Texture Stud. 2020, 51, 120–133. [Google Scholar] [CrossRef]
- Pérez-Palacios, T.; Ruiz-Carrascal, J.; Jiménez-Martín, E.; Solomando, J.C.; Antequera, T. Improving the Lipid Profile of Ready-to-Cook Meat Products by Addition of Omega-3 Microcapsules: Effect on Oxidation and Sensory Analysis. J. Sci. Food Agric. 2018, 98, 5302–5312. [Google Scholar] [CrossRef]
- Tzompa-Sosa, D.A.; Provijn, P.; Gellynck, X.; Schouteten, J.J. Frying Dough with Yellow Mealworm Oil: Aroma Profile and Consumer Perception at a Central Location Test and at Home. J. Food Sci. 2023, 88, A130–A146. [Google Scholar] [CrossRef] [PubMed]
- R Core Team R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2024.
- Posit Team RStudio: Integrated Development Environment for R; R Posit Team: Boston, MA, USA, 2024.
- Meyners, M.; Jaeger, S.R.; Ares, G. On the Analysis of Rate-All-That-Apply (RATA) Data. Food Qual. Prefer. 2016, 49, 1–10. [Google Scholar] [CrossRef]
- Wickham, H.; Averick, M.; Bryan, J.; Chang, W.; McGowan, L.D.; François, R.; Grolemund, G.; Hayes, A.; Henry, L.; Hester, J.; et al. Welcome to the Tidyverse. J. Open Source Softw. 2019, 4, 1686. [Google Scholar] [CrossRef]
- Kassambara, A. Rstatix: Pipe-Friendly Framework for Basic Statistical Tests; The R Foundation: Vienna, Austria, 2023. [Google Scholar]
- Lê, S.; Josse, J.; Husson, F. FactoMineR: An R Package for Multivariate Analysis. J. Stat. Softw. 2008, 25, 1–18. [Google Scholar] [CrossRef]
- Kassambara, A.; Mundt, F. Factoextra: Extract and Visualize the Results of Multivariate Data Analyses; The R Foundation: Vienna, Austria, 2020. [Google Scholar]
- Ruiz-Méndez, M.-V.; Velasco, J.; Lastrucci, A.S.; Márquez-Ruiz, G. Lipid Quality Changes in French Fries, Chicken Croquettes, and Chicken Nuggets Fried with High-Linoleic and High-Oleic Sunflower Oils in Domestic Deep Fryers. Foods 2024, 13, 2419. [Google Scholar] [CrossRef] [PubMed]
- Milićević, D.; Vranić, D.; Mašić, Z.; Parunović, N.; Trbović, D.; Nedeljković-Trailović, J.; Petrović, Z. The Role of Total Fats, Saturated/Unsaturated Fatty Acids and Cholesterol Content in Chicken Meat as Cardiovascular Risk Factors. Lipids Health Dis. 2014, 13, 42. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Ngadi, M.O.; Adedeji, A.A. Shrinkage of Chicken Nuggets During Deep-Fat Frying. Int. J. Food Prop. 2010, 13, 404–410. [Google Scholar] [CrossRef]
- Krokida, M.K.; Oreopoulou, V.; Maroulis, Z.B.; Marinos-Kouris, D. Colour Changes during Deep Fat Frying. J. Food Eng. 2001, 48, 219–225. [Google Scholar] [CrossRef]
- Aliberti, G.; Alamprese, C.; Torri, L.; Casiraghi, E.; Giovanelli, G. Effects of Air Convection Cooking on Chicken Nugget Quality. LWT 2025, 218, 117514. [Google Scholar] [CrossRef]
- Castro-López, R.; Mba, O.I.; Gómez-Salazar, J.A.; Cerón-García, A.; Ngadi, M.O.; Sosa-Morales, M.E. Evaluation of Chicken Nuggets during Air Frying and Deep-Fat Frying at Different Temperatures. Int. J. Gastron. Food Sci. 2023, 31, 100631. [Google Scholar] [CrossRef]
- Varela, P.; Salvador, A.; Fiszman, S.M. Methodological Developments in Crispness Assessment: Effects of Cooking Method on the Crispness of Crusted Foods. LWT-Food Sci. Technol. 2008, 41, 1252–1259. [Google Scholar] [CrossRef]
- Albert, Á.; Salvador, A.; Hough, G.; Fiszman, S. Influence of Outer Layer Formulation on the Sensory Properties of Microwaved Breaded Nuggets. Int. J. Food Prop. 2014, 17, 829–841. [Google Scholar] [CrossRef]
- Şişik Oğraş, Ş.; Kaplan, H. The Effects of Frying Method and Oil Type on the Quality Attributes of Turkey Nuggets and Frying Oils. Eur. J. Lipid Sci. Technol. 2022, 124, 2100023. [Google Scholar] [CrossRef]
- Cao, Y.; Wu, G.; Zhang, F.; Xu, L.; Jin, Q.; Huang, J.; Wang, X. A Comparative Study of Physicochemical and Flavor Characteristics of Chicken Nuggets during Air Frying and Deep Frying. J. Americ. Oil. Chem. Soc. 2020, 97, 901–913. [Google Scholar] [CrossRef]
- Giuffrè, A.M.; Capocasale, M.; Macrì, R.; Caracciolo, M.; Zappia, C.; Poiana, M. Volatile Profiles of Extra Virgin Olive Oil, Olive Pomace Oil, Soybean Oil and Palm Oil in Different Heating Conditions. LWT 2020, 117, 108631. [Google Scholar] [CrossRef]
- Flavour Development, Analysis and Perception in Food and Beverages; Parker, J.K., Elmore, S., Methven, L., Eds.; Elsevier: Amsterdam, The Netherlands, 2014; ISBN 978-1-78242-111-5. [Google Scholar]
- Pérez-Córdoba, R.; Quesada-Granados, J.J.; Ramírez-Anaya, J.d.P.; Peña-Díaz, J.; Blanca-Herrera, R.; Samaniego-Sánchez, C. Bioactive Compounds in Spanish Extra Virgin Olive Oils: Migration and Stability According to the Culinary Technique Used. Food Res. Int. 2023, 172, 113191. [Google Scholar] [CrossRef]
- Suloi, A.N.F.; Suwanto, A.; Anwar, M.; Suryani, R.; Djalal, M.; Bastian, F.; Dirpan, A.; Bahmid, N.A. Volatile Composition and Antimicrobial Properties of Ground Spices: Investigating the Correlation Using Principal Component Analysis. J. Agric. Food Res. 2025, 21, 101849. [Google Scholar] [CrossRef]
- Shahidi, F. Headspace Volatile Aldehydes as Indicators of Lipid Oxidation in Foods. In Headspace Analysis of Foods and Flavors: Theory and Practice; Rouseff, R.L., Cadwallader, K.R., Eds.; Springer: Boston, MA, USA, 2001; pp. 113–123. ISBN 978-1-4615-1247-9. [Google Scholar]
- Maarse, H. Volatile Compounds in Foods and Beverages; Routledge: New York, NY, USA, 2017; ISBN 978-0-203-73428-5. [Google Scholar]
- Domínguez, R.; Pateiro, M.; Gagaoua, M.; Barba, F.J.; Zhang, W.; Lorenzo, J.M. A Comprehensive Review on Lipid Oxidation in Meat and Meat Products. Antioxidants 2019, 8, 429. [Google Scholar] [CrossRef]
- Shahidi, F.; Hossain, A. Role of Lipids in Food Flavor Generation. Molecules 2022, 27, 5014. [Google Scholar] [CrossRef]
- McGorrin, R.J. The Significance of Volatile Sulfur Compounds in Food Flavors. In Volatile Sulfur Compounds in Food; ACS Symposium Series; American Chemical Society: Washington, DC, USA, 2011; Volume 1068, pp. 3–31. ISBN 978-0-8412-2616-6. [Google Scholar]
- Mlostoń, G.; Romański, J.; Weigand, W.; Heimgartner, H. Organic and Coordination Chemistry of 1,2,4-Trithiolanes. Eur. J. Org. Chem. 2019, 2019, 1867–1875. [Google Scholar] [CrossRef]
- Izawa, K.; Amino, Y.; Kohmura, M.; Ueda, Y.; Kuroda, M. 4.16-Human–Environment Interactions–Taste. In Comprehensive Natural Products II; Liu, H.-W., Mander, L., Eds.; Elsevier: Oxford, UK, 2010; pp. 631–671. ISBN 978-0-08-045382-8. [Google Scholar]
- Sabikun, N.; Bakhsh, A.; Rahman, M.S.; Hwang, Y.-H.; Joo, S.-T. Volatile and Nonvolatile Taste Compounds and Their Correlation with Umami and Flavor Characteristics of Chicken Nuggets Added with Milkfat and Potato Mash. Food Chem. 2021, 343, 128499. [Google Scholar] [CrossRef]
- Wang, F.; Shen, H.; Liu, T.; Yang, X.; Yang, Y.; Guo, Y. Formation of Pyrazines in Maillard Model Systems: Effects of Structures of Lysine-Containing Dipeptides/Tripeptides. Foods 2021, 10, 273. [Google Scholar] [CrossRef]
- Flament, I.; Willhalm, B.; Ohloff, G. New Developments in Meat Aroma Research. In Flavor in Foods and Beverages; Charalambous, G., Inglett, I., Eds.; Academic: New York, NY, USA, 1978; pp. 15–32. [Google Scholar]
- Wang, D.; Liu, Z.; Chen, W.; Lan, X.; Zhan, S.; Sun, Y.; Su, W.; Lin, C.-C.; Ni, L. Comparative Study of the Volatile Fingerprints of Roasted and Unroasted Oolong Tea by Sensory Profiling and HS-SPME-GC-MS. Curr. Res. Food Sci. 2023, 6, 100442. [Google Scholar] [CrossRef]
- Jiménez-Martín, E.; Pérez-Palacios, T.; Carrascal, J.R.; Rojas, T.A. Enrichment of Chicken Nuggets with Microencapsulated Omega-3 Fish Oil: Effect of Frozen Storage Time on Oxidative Stability and Sensory Quality. Food Bioprocess Technol. 2016, 9, 285–297. [Google Scholar] [CrossRef]
- Yan, J.; Alewijn, M.; van Ruth, S.M. From Extra Virgin Olive Oil to Refined Products: Intensity and Balance Shifts of the Volatile Compounds versus Odor. Molecules 2020, 25, 2469. [Google Scholar] [CrossRef]
- Lasekan, O.; Teoh, L.S. Contribution of Aroma Compounds to the Antioxidant Properties of Roasted White Yam (Dioscorea rotundata). BMC Chem. 2019, 13, 133. [Google Scholar] [CrossRef] [PubMed]
- Ansorena, D.; Gimeno, O.; Astiasarán, I.; Bello, J. Analysis of Volatile Compounds by GC–MS of a Dry Fermented Sausage: Chorizo de Pamplona. Food Res. Int. 2001, 34, 67–75. [Google Scholar] [CrossRef]
- Thierry, A.; Maillard, M.-B.; Bonnarme, P.; Roussel, E. The Addition of Propionibacterium Freudenreichii to Raclette Cheese Induces Biochemical Changes and Enhances Flavor Development. J. Agric. Food Chem. 2005, 53, 4157–4165. [Google Scholar] [CrossRef]
- Kalua, C.M.; Allen, M.S.; Bedgood, D.R.; Bishop, A.G.; Prenzler, P.D.; Robards, K. Olive Oil Volatile Compounds, Flavour Development and Quality: A Critical Review. Food Chem. 2007, 100, 273–286. [Google Scholar] [CrossRef]
- MAPA. Informe Anual Del Consumo Alimentario En España 2024; Ministerio de Agricultura, Pesca y Alimentación: Madrid, España, 2024.
- Ares, G.; Bruzzone, F.; Vidal, L.; Cadena, R.S.; Giménez, A.; Pineau, B.; Hunter, D.C.; Paisley, A.G.; Jaeger, S.R. Evaluation of a Rating-Based Variant of Check-All-That-Apply Questions: Rate-All-That-Apply (RATA). Food Qual. Prefer. 2014, 36, 87–95. [Google Scholar] [CrossRef]
- Delarue, J.; Lawlor, J.B. Rapid SENSORY Profiling Techniques: Applications in New Product Development and Consumer Research; Woodhead Publishing: Cambridge, UK, 2022; ISBN 978-0-12-821937-9. [Google Scholar]
- Taylor, A.J. Release and Transport of Flavors In Vivo: Physicochemical, Physiological, and Perceptual Considerations. Compr. Rev. Food Sci. Food Saf. 2002, 1, 45–57. [Google Scholar] [CrossRef] [PubMed]
| Nutritional Profile | |
|---|---|
| Total energy (Kcal) | 241 |
| Total fat (g) | 11 |
| Saturated fat (g) | 1.3 |
| Total carbohydrate (g) | 26 |
| Total sugars (g) | 4.4 |
| Dietary fibre (g) | 1.4 |
| Proteins (g) | 8.8 |
| Salt (g) | 1.2 |
| NO | NP | NV | |
|---|---|---|---|
| Moisture | 39.60 (1.21) a | 39.85 (2.23) a | 39.84 (1.02) a |
| Fat | 18.00 (0.33) a | 18.17 (0.44) a | 18.58 (0.37) a |
| NO | NP | NV | |
|---|---|---|---|
| Palmitic acid (C16:00) | 2.49 (0.04) b | 2.27 (0.04) a | 2.42 (0.05) b |
| Stearic acid (C18:00) | 0.56 (0.01) b | 0.54 (0.01) a | 0.58 (0.01) b |
| ΣSFA | 3.25 (0.05) b | 3.04 (0.06) a | 3.20 (0.07) b |
| Palmitoleic acid (C16:1n-7) | 0.27 (0.00) b | 0.23 (0.00) a | 0.26 (0.01) b |
| cis-Vaccenic acid (C18:1n7c) | 0.41 (0.01) b | 0.38 (0.01) a | 0.43 (0.01) c |
| Oleic acid (C18:1n9c) | 10.53 (0.22) a | 10.80 (0.30) a | 11.18 (0.19) b |
| ΣMUFA | 11.26 (0.23) a | 11.47 (0.31) a | 11.91 (0.20) b |
| Linoleic acid (C18:2n6c) | 2.42 (0.04) b | 2.58 (0.05) b | 2.37 (0.07) a |
| Linolenic acid (C18:3n3) | 0.16 (0.00) b | 0.17 (0.00) b | 0.16 (0.01) a |
| ΣPUFA | 2.60 (0.04) b | 2.76 (0.05) b | 2.54 (0.07) a |
| Σn3 | 0.16 (0.00) a | 0.17 (0.00) b | 0.16 (0.01) a |
| Σn6 | 2.44 (0.04) a | 2.59 (0.05) b | 2.38 (0.07) a |
| Sample | Area | Perimeter | Weight |
|---|---|---|---|
| NO | 5.18 (0.84) a | 4.47 (0.68) a | 12.94 (0.82) a |
| NP | 4.76 (0.82) a | 4.81 (0.66) a | 12.26 (0.78) a |
| NV | 5.20 (0.90) a | 3.27 (1.02) a | 13.33 (1.27) a |
| Sample | R | G | B |
|---|---|---|---|
| NO | 43.18 (2.35) | 64.82 (2.61) | 48.21 (3.57) |
| NP | 41.27 (5.39) | 62.49 (6.18) | 48.99 (3.62) |
| NV | 40.07 (9.83) | 61.48 (5.57) | 43.93 (1.00) |
| Stats | F = 0.947, p = 0.439 | F = 0.626, p = 0.566 | F = 3.041, p = 0.122 |
| Instrumental Mechanical Parameters | Instrumental AED Parameters | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Sample | Max F (N) | Work FD (mJ) | Peaks F * (-) | Drop off F (N) | Max Peak SPLmax (dB) | Peaks AED ** (-) | Mean AED Peaks (dB) | Area AED (dB mm) | Drop off AED (dB) |
| NO | 6.86 (0.52) a | 31.70 (1.84) a,b | 19.00(1.30) a | 0.37(0.09) a | 76.75(0.44) a | 6.00(1.72) a | 75.35(0.42) a | 581.11(0.25) a | 3.53(0.41) a |
| NP | 6.78 (0.48) a | 27.30 (2.22) a | 20.00 (1.56) a | 0.37 (0.06) a | 77.21 (0.28) a,b | 5.00 (1.46) a | 74.96 (0.10) a | 566.74 (21.23) a | 3.34 (0.18) a |
| NV | 7.74 (0.53) a | 34.29 (1.38) b | 20.00 (0.68) a | 0.38 (0.01) a | 77.98 (0.16) b | 8.00 (0.36) a | 75.03 (0.24) a | 570.35 (5.53) a | 3.44 (0.05) a |
| Compound | LRI 1 | ID 2 | NO | NP | NV | F-Value (p-Value) 3 |
|---|---|---|---|---|---|---|
| Alcohol | ||||||
| 1-Octen-3-ol | 1444 | A | 1.57 (1.38) | 2.50 (0.63) | 2.84 (0.61) | |
| Alyphatic | ||||||
| Total alyphatic | 59.83 (52.48) | 40.51 (45.74) | 224.54 (270.82) | |||
| Pentane | B | 10.61 (9.38) | 8.39 (7.63) | 11.70 (11.72) | ||
| n-Hexane | B | 29.47 (36.21) | 15.41 (26.68) | 253.37 (288.16) | ||
| Heptane | B | 5.30 (4.88) | 3.52 (6.09) | 10.94 (3.38) | ||
| 4,5-Dimethyl-octane | B | 1.52 (1.37) | nd 4 | 2.82 (2.63) | ||
| 5-Methyl-nonane | B | 1.25 (1.20) | 2.66 (2.68) | 3.42 (1.54) | ||
| Octane | 804 | B | 3.28 (2.93) | 3.93 (1.44) | 5.42 (1.19) | |
| (1-Methylethyl)-cyclopropane | 1107 | B | 1.54 (1.34) | nd | nd | |
| 2-Methyl-azetidine | 1252 | B | 6.85 (2.65) | 6.61 (1.28) | 7.82 (1.06) | |
| Nitrosamine | ||||||
| Nitroso-methane | 870 | B | 14.52 (2.60) a | 60.41 (6.05) b | 15.51 (0.94) a | 139.82 (<0.001) |
| Aldehydes | ||||||
| Total aldehydes | 190.57 (110.66) | 242.27 (96.13) | 259.37 (45.61) | |||
| 2-Methyl-propanal | 807 | A | 17.63 (18.52) | 30.41 (24.24) | 17.26 (3.15) | |
| Propanal | 816 | B | 6.84 (2.30) | 7.78 (1.79) | 10.05 (1.55) | |
| 2-Methyl-butanal | 858 | B | 32.76 (20.87) | 51.62 (23.24) | 45.38 (9.60) | |
| 3-Methyl-butanal | 860 | B | 59.57 (36.92) | 85.07 (38.07) | 75.28 (14.96) | |
| Pentanal | 863 | B | 6.79 (5.88) | nd | 10.47 (1.68) | |
| Hexanal | 1072 | A | 58.03 (25.12) | 57.25 (8.03) | 86.77 (14.49) | |
| Nonanal | 1392 | A | 8.96 (2.64) | 7.46 (0.94) | 10.18 (0.73) | |
| 2,4-Decadienal | 1800 | A | nd | 2.67 (0.08) a | 3.98 (0.30) b | 53.15 (0.002) |
| Ketones | ||||||
| Total ketones | 15.22 (3.40) a | 26.62 (2.77) ab | 40.07 (8.23) b | 16.08 (0.004) | ||
| 1-(3-Cyclopenten-1-yl)-2-pentanone | 811 | B | 0.49 (0.43) | nd | nd | |
| Acetone | 823 | A | nd | nd | 18.17 (3.39) | |
| 4-Methyl-3-oxolanone | 853 | B | 2.42 (0.82) | nd | 3.71 (0.82) | |
| 2,3-Pentanedione | 1050 | A | 3.62 (1.36) a | 7.47 (1.68) b | 5.44 (0.65) ab | 6.56 (0.031) |
| Dihydroxyacetophenone | 1249 | B | nd | nd | 1.83 (1.59) | |
| 1-(2-Furanyl)-ethanone | 1484 | A | 4.22 (0.50) a | 12.01 (1.42) c | 8.56 (0.81) b | 46.67 (<0.001) |
| 2,3-Dioxo-4-phenyl butyrolactone | 1498 | B | 2.38 (0.28) a | 3.45 (0.06) b | nd | 41.69 (0.003) |
| Maltol | 1953 | A | 1.43 (0.11) a | 3.69 (0.33) b | 1.37 (0.21) a | 96.91 (<0.001) |
| Terpenes | ||||||
| Total terpenes | 218.60 (76.21) a | 172.75 (27.93) a | 344.81 (13.69) b | 10.55 (0.011) | ||
| α-Pinene | 1015 | A | 26.91 (14.35) | 21.89 (6.27) | 41.06 (1.30) | |
| α-Thujene | 1020 | A | 19.90 (8.58) | 16.06 (4.00) | 29.01 (1.98) | |
| β-Pinene | 1091 | A | 33.12 (14.43) ab | 25.75 (5.99) a | 51.00 (3.15) b | 5.97 (0.037) |
| β-Sabinene | 1106 | A | 35.26 (12.50) ab | 24.13 (5.50) a | 50.71 (5.24) b | 7.49 (0.023) |
| α-Terpinene | 1130 | A | 2.34 (2.14) | nd | nd | |
| 3-Carene | 1139 | A | 22.45 (6.77) a | 24.27 (3.10) a | 40.23 (6.82) b | 8.44 (0.018) |
| α-Sabinene | 1157 | B | nd | nd | 11.69 (0.04) | |
| Isoterpinolene | 1172 | B | nd | nd | 4.80 (0.16) | |
| D-Limonene | 1192 | A | 47.56 (11.08) a | 38.52 (3.66) a | 75.83 (3.96) b | 22.44 (0.002) |
| α-Phellandrene | 1199 | A | 9.17 (2.74) ab | 5.81 (0.45) a | 12.27 (2.11) b | 7.74 (0.022) |
| β-Terpinene | 1243 | A | 4.78 (2.47) ab | 3.63 (0.90) a | 8.20 (0.36) b | 7.19 (0.026) |
| Caryophyllene | 1600 | A | 17.12 (2.33) ab | 12.68 (0.52) a | 20.02 (2.41) b | 10.67 (0.011) |
| Sulphur compounds | ||||||
| Total sulphur compounds | 181.84 (64.75) | 119.11 (41.06) | 214.07 (35.35) | |||
| Hydrogen sulphide | B | 1.82 (0.89) | nd | 1.64 (0.34) | ||
| Methanethiol | B | 10.71 (5.89) | 4.90 (1.66) | 6.83 (0.86) | ||
| Dimethyl sulphide | 807 | A | 0.98 (0.62) | 1.22 (0.31) | 1.26 (0.05) | |
| 1-Propanethiol | 830 | A | 5.23 (3.64) | 4.02 (2.01) | 7.67 (2.40) | |
| Methyl-thiirane | 847 | A | 128.63 (51.83) | 63.13 (29.25) | 147.59 (26.86) | |
| 3-(Methylthio)-1-propene | 877 | A | 2.84 (1.16) | 3.70 (0.46) | 3.39 (0.63) | |
| Dimethyl disulphide | 1061 | A | 5.83 (2.86) | 10.96 (3.51) | 9.52 (1.33) | |
| Methyl 2-propenyl disulphide | 1277 | A | 10.17 (3.53) | 15.22 (1.51) | 15.50 (2.02) | |
| Dimethyl trisulphide | 1375 | A | 2.29 (0.94) | 4.46 (1.16) | 3.24 (0.12) | |
| Diallyl disulphide | 1467 | A | 8.52 (3.27) | 6.11 (0.74) | 10.68 (1.80) | |
| C6 H10 S2 | 1472 | B | 1.19 (0.38) | 1.20 (0.35) | 1.73 (0.38) | |
| Methyl 2-propenyl trisulphide | 1585 | A | 3.06 (1.08) | 3.82 (0.56) | 4.27 (0.51) | |
| Allyl trisulphide | 1784 | A | 0.57 (0.24) | 0.36 (0.09) | 0.74 (0.12) | |
| Furan | ||||||
| Total furan | 23.99 (5.72) a | 55.62 (4.93) b | 43.99 (5.51) b | 26.38 (0.001) | ||
| 3-Methyl-furan | 842 | A | 5.69 (2.98) | 6.19 (1.13) | 3.47 (0.89) | |
| 2,3-Dihydro-4-methyl-furan | 1082 | B | nd | 4.03 (0.23) | 4.99 (0.92) | |
| 2-Pentyl-furan | 1227 | A | 6.55 (2.00) | 7.39 (1.27) | 7.26 (1.05) | |
| Furfural | 1448 | A | 11.75 (0.89) a | 28.14 (3.18) | 22.14 (2.51) b | 35.96 (<0.001) |
| 2-Furanmethanol | 1659 | A | nd | 9.87 (0.61) b | 6.13 (0.44) a | 72.79 (0.001) |
| Pyrazines | ||||||
| Total pyrazines | 33.52 (3.74) a | 124.76 (14.12) c | 80.11 (6.01) b | 75.04 (<0.001) | ||
| Pyrazine | 1202 | A | nd | 5.92 (0.56) b | 4.78 (0.26) a | 10.16 (0.033) |
| Methyl-pyrazine | 1264 | A | 28.70 (2.62) a | 70.69 (8.83) c | 46.31 (3.85) b | 40.15 (<0.001) |
| 2,5-Dimethyl-pyrazine | 1322 | A | nd | 8.06 (0.88) | nd | |
| 2,6-Dimethyl-pyrazine | 1329 | A | 4.82 (1.13) a | 12.41 (0.78) c | 7.26 (0.56) b | 61.27 (<0.001) |
| Ethyl-pyrazine | 1334 | A | nd | 12.39 (1.13) b | 8.01 (0.99) a | 25.53 (0.007) |
| 2,3-Dimethyl-pyrazine | 1347 | A | nd | 5.30 (0.56) b | 2.85 (0.33) a | 42.46 (0.003) |
| 2-Ethyl-6-methyl-pyrazine | 1384 | A | nd | 4.37 (0.61) b | 2.63 (0.55) a | 13.44 (0.021) |
| 2-Ethyl-3-methyl-pyrazine | 1401 | A | nd | 5.61 (0.92) b | 3.26 (0.40) a | 16.70 (0.015) |
| Pyrimidine | ||||||
| 4,6-Dimethyl-pyrimidine | 1322 | B | nd | nd | 5.01 (0.36) | |
| Benzene | ||||||
| 1-Ethyl-2,3-dimethyl-benzene | 1268 | B | 21.30 (6.83) | 18.26 (1.40) | 28.36 (1.40) | |
| Esters | ||||||
| Total esters | 4.78 (1.17) a | 26.31 (5.79) b | 22.02 (1.65) b | 31.11 (0.001) | ||
| Ethyl isobutyrate | 882 | B | 2.08 (0.05) | 2.54 (1.17) | 1.58 (0.36) | |
| 2-Propynyl propionate | 1104 | B | nd | 17.68 (4.05) | 15.21 (1.04) | |
| Methyl acetate | 1294 | B | 3.39 (0.04) a | 6.08 (0.85) b | 3.88 (0.17) a | 24.91 (0.001) |
| Octyl formate | 1549 | A | nd | nd | 1.35 (0.13) | |
| Acids | ||||||
| Total acids | 13.92 (4.68) a | 27.56 (2.14) b | 21.94 (2.73) b | 12.47 (0.007) | ||
| Propiolic acid | B | nd | nd | 10.30 (1.78) | ||
| 2-Acetoxysuccinic acid | 886 | B | nd | 12.99 (3.14) | nd | |
| Acetic acid | 1439 | A | 5.98 (1.20) a | 9.03 (1.03) b | 5.06 (0.71) a | 12.86 (0.007) |
| Hexanoic acid | 1827 | A | 7.94 (4.16) | 5.55 (1.97) | 6.57 (2.36) |
| Attribute | NO | NP | NV | F-Value (p-Value) |
|---|---|---|---|---|
| Toasted appearance | 1.77 (0.81) a | 2.23 (0.71) b | 1.96 (0.71) ab | 6.21 (0.003) |
| Visual crunchy | 2.61 (0.53) | 2.68 (0.51) | 2.57 (0.50) | 0.78 (0.462) |
| External homogeneity | 2.23 (0.76) | 2.05 (0.77) | 2.00 (0.74) | 1.96 (0.147) |
| Internal homogeneity | 2.16 (0.68) | 2.09 (0.72) | 2.21 (0.68) | 0.60 (0.540) |
| Spice odour | 1.21 (0.97) | 0.98 (0.88) | 1.20 (1.02) | 1.32 (0.272) |
| Off odour | 0.11 (0.31) | 0.14 (0.40) | 0.14 (0.44) | 0.22 (0.780) |
| Fried odour | 1.77 (0.79) ab | 2.04 (0.99) b | 1.64 (0.82) a | 3.99 (0.022) |
| Crunchy exterior | 2.54 (0.63) | 2.70 (0.54) | 2.61 (0.59) | 1.23 (0.297) |
| Juiciness | 2.11 (0.76) | 1.93 (0.76) | 2.18 (0.79) | 2.01 (0.143) |
| Chewability | 2.55 (0.63) | 2.38 (0.62) | 2.50 (0.50) | 1.82 (0.169) |
| Oily texture | 1.14 (0.92) | 1.16 (0.89) | 1.18 (0.79) | 0.04 (0.956) |
| Chicken flavour | 1.62 (0.82) | 1.61 (0.80) | 1.73 (0.82) | 0.63 (0.512) |
| Oily flavour | 1.09 (0.77) | 1.11 (0.87) | 1.00 (0.83) | 0.50 (0.610) |
| Spice flavour | 1.30 (0.99) | 1.23 (0.83) | 1.52 (0.91) | 1.89 (0.157) |
| Burnt flavour | 0.62 (0.89) ab | 0.96 (1.03) b | 0.52 (0.76) a | 4.70 (0.013) |
| Off flavour | 0.30 (0.60) | 0.25 (0.51) | 0.36 (0.77) | 0.50 (0.605) |
| Acid aftertaste | 0.16 (0.46) | 0.23 (0.54) | 0.32 (0.61) | 1.88 (0.158) |
| Overall Liking | 6.71 (1.42) | 6.41 (1.53) | 6.82 (1.62) | 1.23 (0.298) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pintado, T.; Álvarez, M.D.; Herranz, B.; Delgado-Pando, G. Impact of Frying Olive Oil Type on the Physicochemical and Sensory Quality of Commercial Chicken Nuggets. Foods 2025, 14, 3315. https://doi.org/10.3390/foods14193315
Pintado T, Álvarez MD, Herranz B, Delgado-Pando G. Impact of Frying Olive Oil Type on the Physicochemical and Sensory Quality of Commercial Chicken Nuggets. Foods. 2025; 14(19):3315. https://doi.org/10.3390/foods14193315
Chicago/Turabian StylePintado, Tatiana, María Dolores Álvarez, Beatriz Herranz, and Gonzalo Delgado-Pando. 2025. "Impact of Frying Olive Oil Type on the Physicochemical and Sensory Quality of Commercial Chicken Nuggets" Foods 14, no. 19: 3315. https://doi.org/10.3390/foods14193315
APA StylePintado, T., Álvarez, M. D., Herranz, B., & Delgado-Pando, G. (2025). Impact of Frying Olive Oil Type on the Physicochemical and Sensory Quality of Commercial Chicken Nuggets. Foods, 14(19), 3315. https://doi.org/10.3390/foods14193315






