Honeysuckle Extracts (Lonicera japonica Thunb.): Understanding Insights into the Antioxidant Effect on Preserving Qualities of Rabbit Meat During Refrigerated Storage
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of HE
2.2. Total Phenolic (TPC), Flavonoid (TFC), and Chlorogenic Acid (CGA) Contents Measurement
2.3. DPPH and ABTS Assay
2.4. Preparation of Rabbit Meat
2.5. Determination of Moisture Content, Water Activity (Aw)
2.6. Color and Texture Profile Analysis (TPA)
2.7. Thiobarbituric Acid Reactive Substance (TBARS) Assay
2.8. Total Volatile Basic-Nitrogen (TVB-N) Assay
2.9. Microbiological Analysis
2.10. Statistical Analysis
3. Results
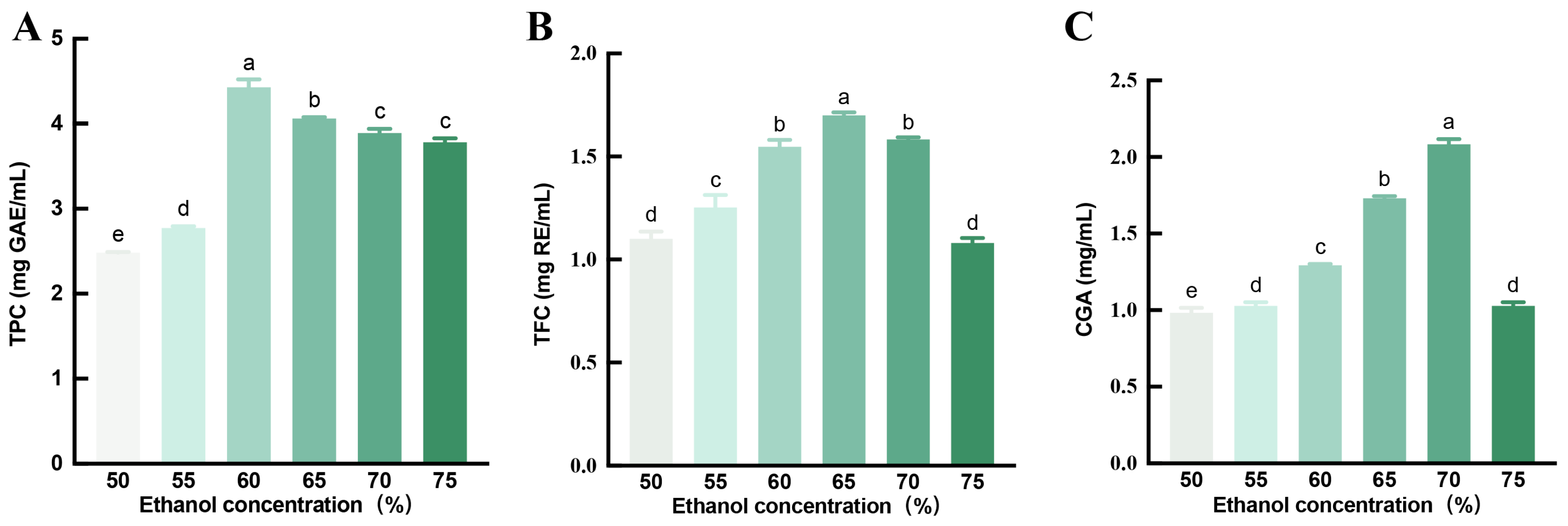
3.1. Total Phenolic, Flavonoids, and Chlorogenic Acid Content of the HE
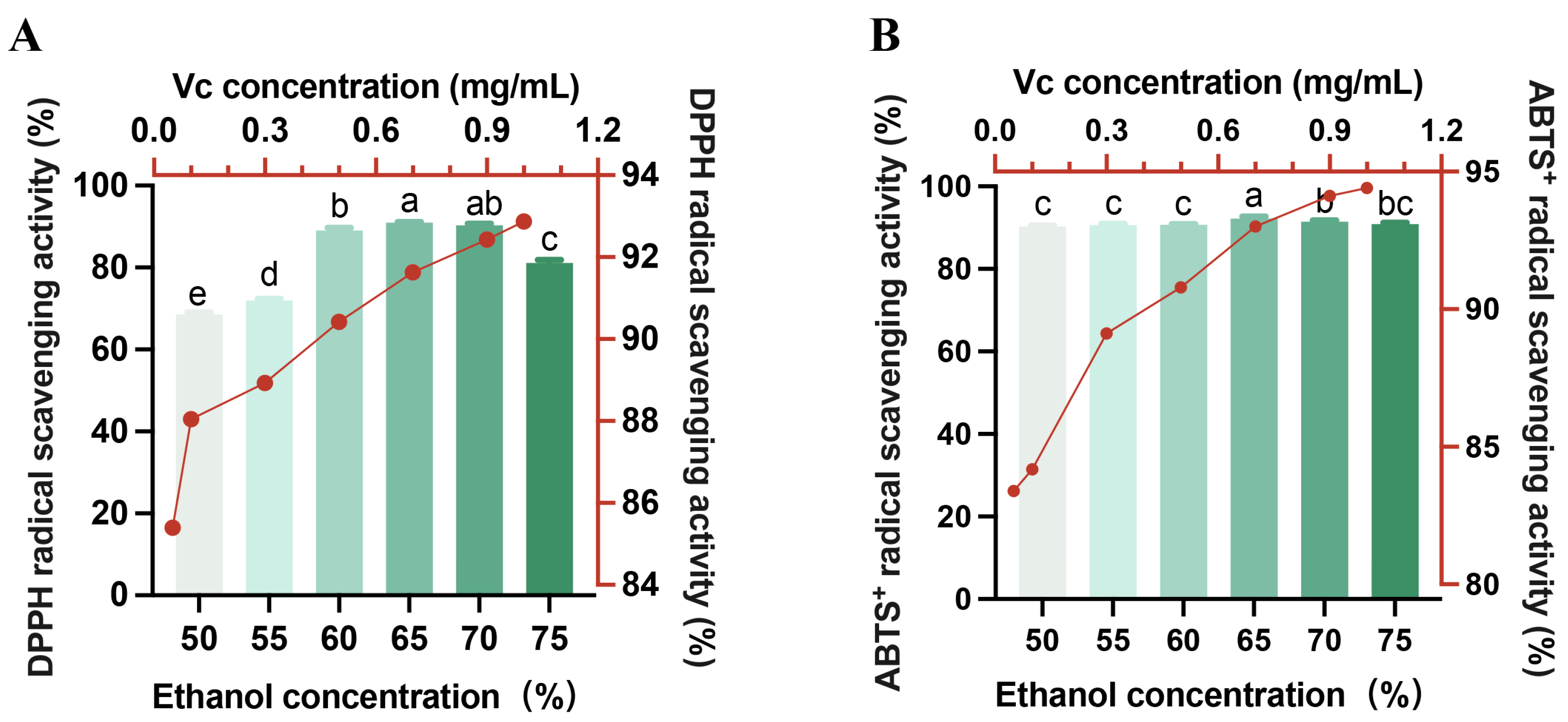
3.2. Antioxidant Activity of the HE
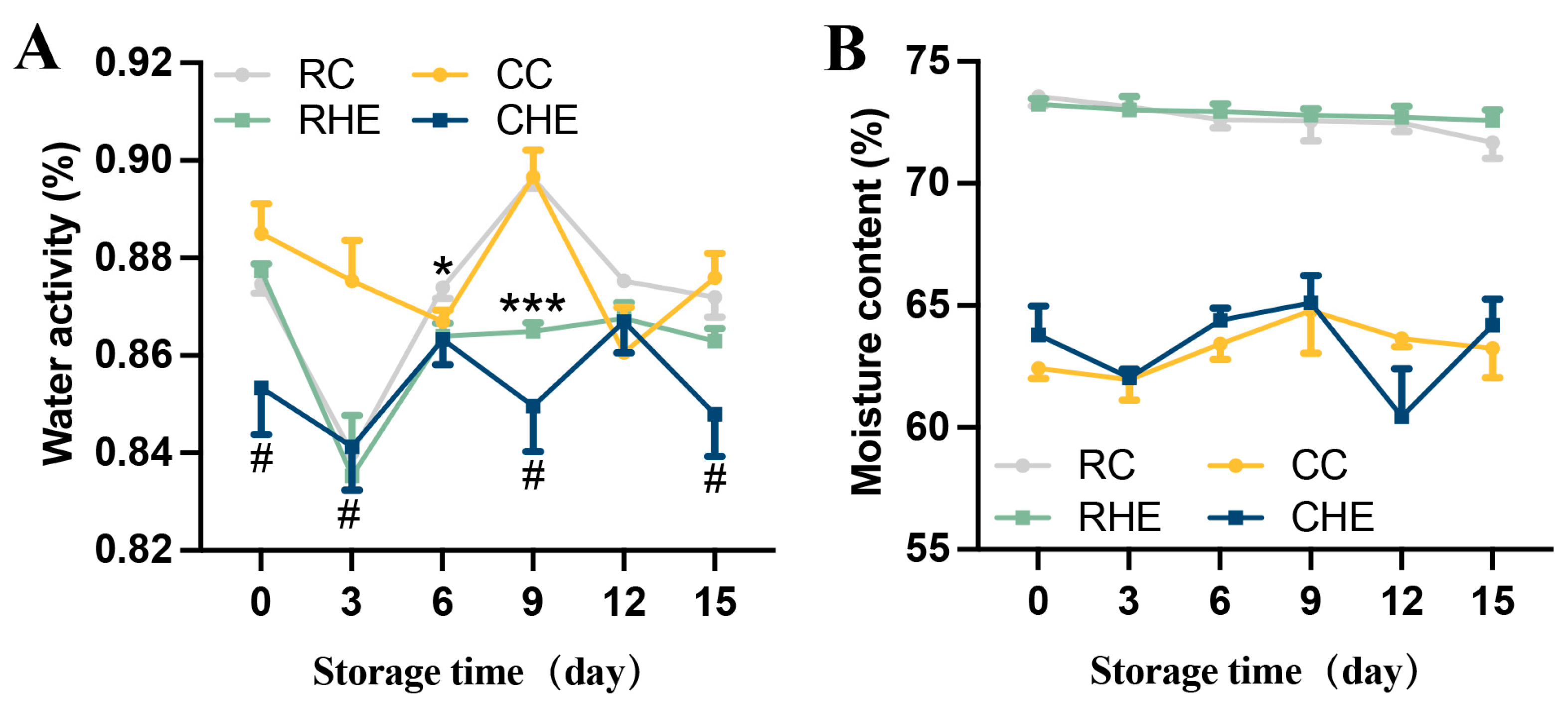
3.3. Effect of HE on the Aw and Moisture Content of Pre-Treated Rabbit Meat During Refrigerated Storage
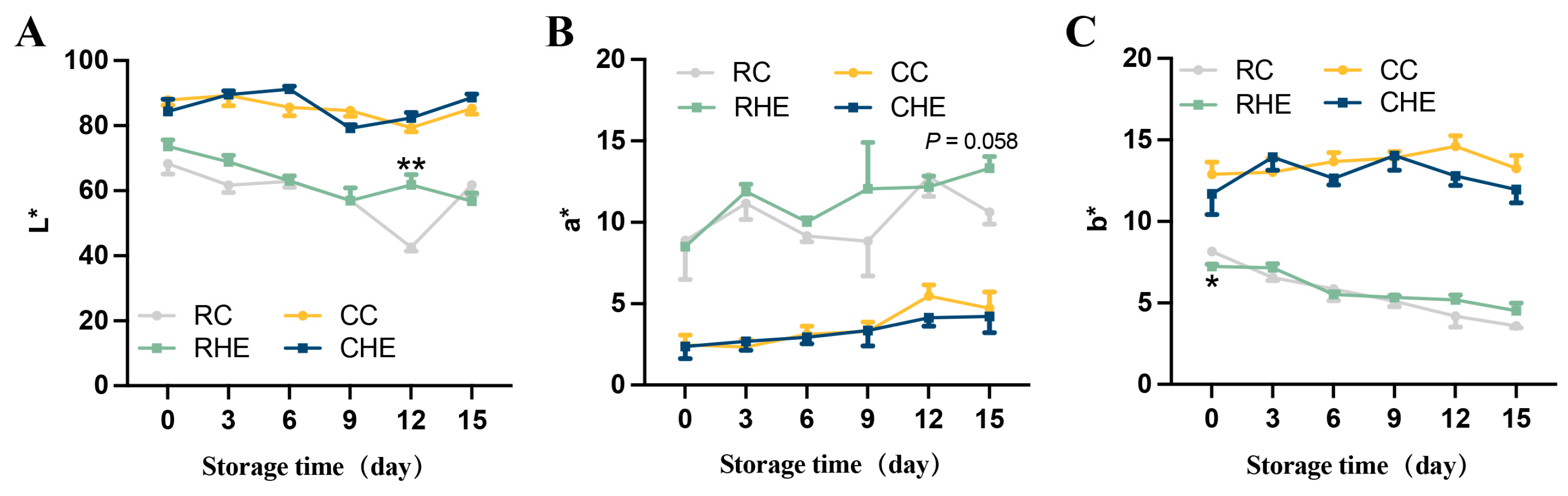
3.4. Effect of HE on Color of Pre-Treated Rabbit Meat During Refrigerated Storage
3.5. Effect of HE on Texture Profile of Pre-Treated Rabbit During Refrigerated Storage
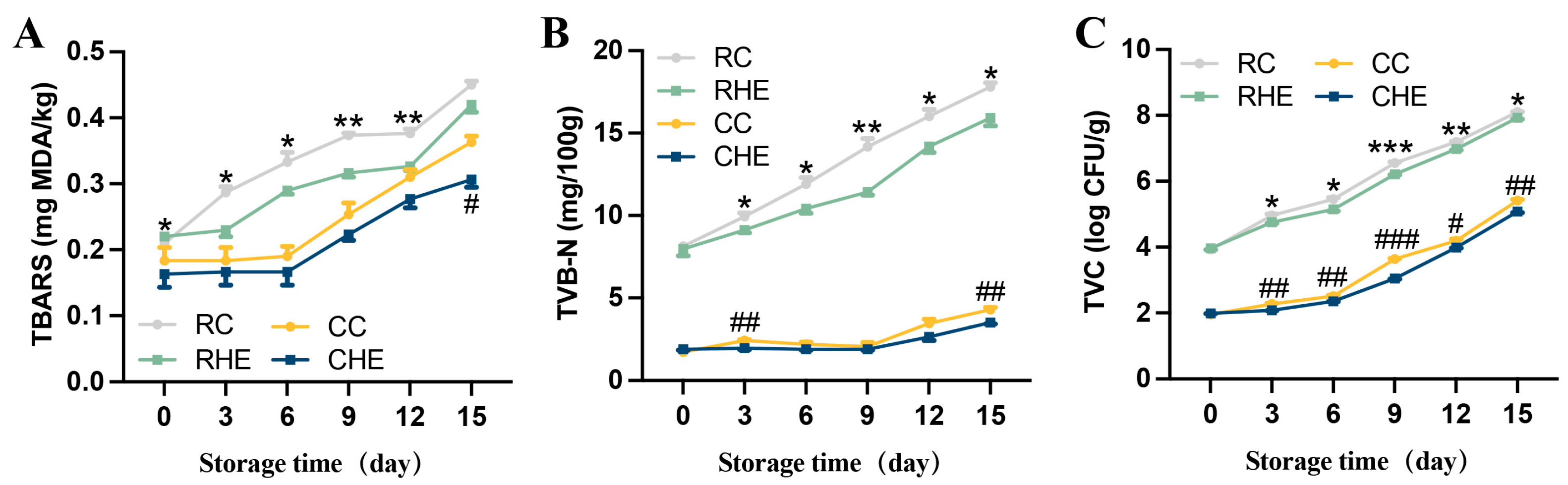
3.6. Effect of HE on Lipid Oxidation of Pre-Treated Rabbit During Refrigerated Storage
3.7. Effect of HE on Antimicrobial Activity of Pre-Treated Rabbit During Refrigerated Storage
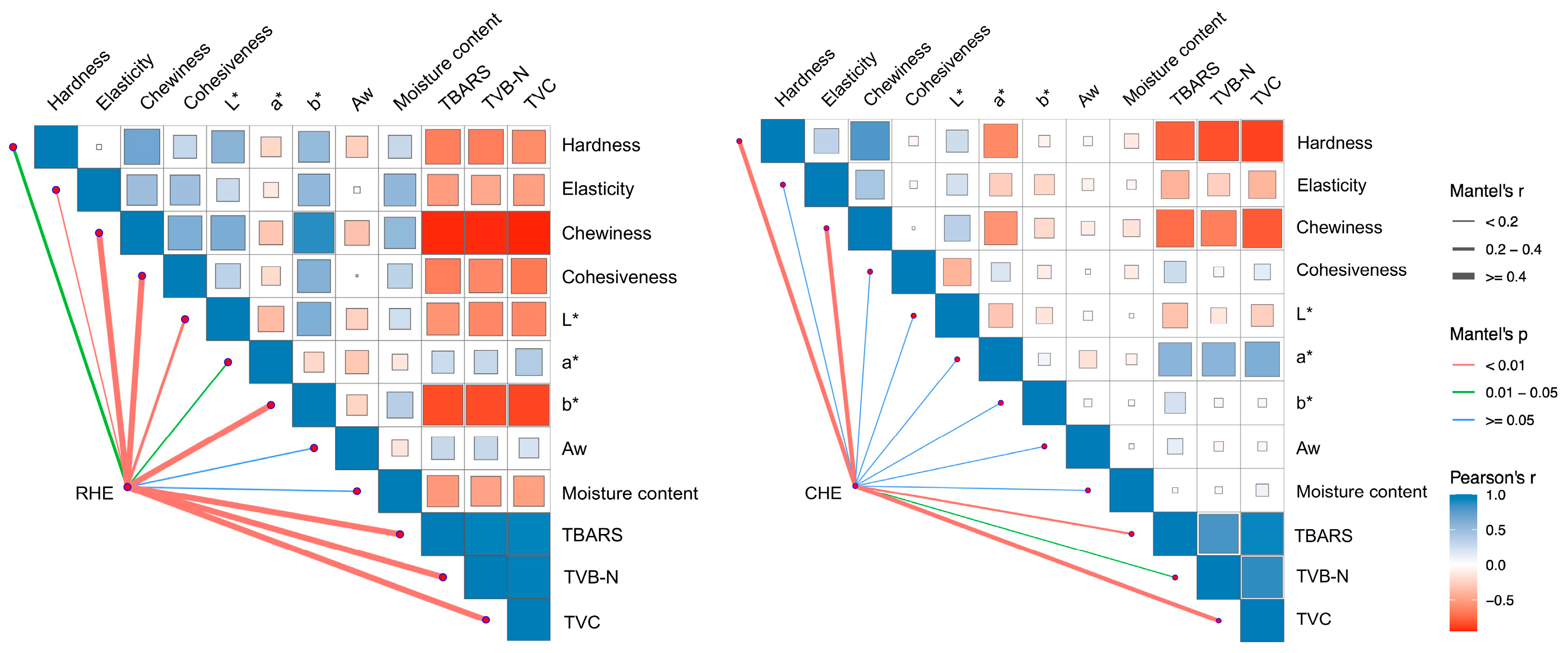
3.8. Correlation Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kantono, K.; Hamid, N.; Ma, Q.; Chadha, D.; Oey, I. Consumers’ perception and purchase behaviour of meat in China. Meat Sci. 2021, 179, 108548. [Google Scholar] [CrossRef]
- Klurfeld, D.M. Research gaps in evaluating the relationship of meat and health. Meat Sci. 2015, 109, 86–95. [Google Scholar] [CrossRef]
- Dalle Zotte, A.; Szendro, Z. The role of rabbit meat as functional food. Meat Sci. 2011, 88, 319–331. [Google Scholar] [CrossRef]
- Cao, R.; Wang, B.; Bai, T.; Zhu, Y.; Cheng, J.; Zhang, J. Structural and functional impacts of glycosylation-induced modifications in rabbit myofibrillar proteins. Int. J. Biol. Macromol. 2024, 283, 137583. [Google Scholar] [CrossRef]
- Siddiqui, S.A.; Bhowmik, S.; Afreen, M.; Ucak, I.; Ikram, A.; Gerini, F.; Mehdizadeh, M.; Ayivi, R.D.; Castro-Munoz, R. Bodybuilders and high-level meat consumers’ behavior towards rabbit, beef, chicken, turkey, and lamb meat: A comparative review. Nutrition 2024, 119, 112305. [Google Scholar] [CrossRef]
- Escriba-Perez, C.; Baviera-Puig, A.; Buitrago-Vera, J.; Montero-Vicente, L. Consumer profile analysis for different types of meat in Spain. Meat Sci. 2017, 129, 120–126. [Google Scholar] [CrossRef]
- Wang, Z.; He, Z.; Gan, X.; Li, H. Interrelationship among ferrous myoglobin, lipid and protein oxidations in rabbit meat during refrigerated and superchilled storage. Meat Sci. 2018, 146, 131–139. [Google Scholar] [CrossRef]
- Lan, Y.; Shang, Y.; Song, Y.; Dong, Q. Changes in the quality of superchilled rabbit meat stored at different temperatures. Meat Sci. 2016, 117, 173–181. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Liu, A.; Hu, S.; Ares, I.; Martinez-Larranaga, M.R.; Wang, X.; Martinez, M.; Anadon, A.; Martinez, M.A. Synthetic phenolic antioxidants: Metabolism, hazards and mechanism of action. Food Chem. 2021, 353, 129488. [Google Scholar] [CrossRef] [PubMed]
- Carocho, M.; Morales, P.; Ferreira, I.C.F.R. Antioxidants: Reviewing the chemistry, food applications, legislation and role as preservatives. Trends Food Sci. Technol. 2018, 71, 107–120. [Google Scholar] [CrossRef]
- Inguglia, E.S.; Song, Z.; Kerry, J.P.; O’Sullivan, M.G.; Hamill, R.M. Addressing Clean Label Trends in Commercial Meat Processing: Strategies, Challenges and Insights from Consumer Perspectives. Foods 2023, 12, 2062. [Google Scholar] [CrossRef]
- Vicario, A.L.; García, M.G.; Ochoa, N.A.; Quiroga, E. Assessing the impact of chanar and green tea extracts on pectin active packaging for extended food preservation. Food Hydrocoll. 2024, 153, 110009. [Google Scholar] [CrossRef]
- de Farias Marques, A.D.J.; de Lima Tavares, J.; de Carvalho, L.M.; Leite Abreu, T.; Alves Pereira, D.; Moreira Fernandes Santos, M.; Suely Madruga, M.; de Medeiros, L.L.; Kenia Alencar Bezerra, T. Oxidative stability of chicken burgers using organic coffee husk extract. Food Chem. 2022, 393, 133451. [Google Scholar] [CrossRef]
- de Figueiredo, M.J.; Grisi, C.V.B.; Santiago, A.M.; Vieira, E.A.; Cordeiro, A.; Vilela, A.F.; Viana, A.D.; de Sousa, S.; Conrado, L.S. Characterization and application of Croton blanchetianus Baill extract for lamb ribs preservation. Food Chem. 2022, 373, 131404. [Google Scholar] [CrossRef]
- Bai, T.; Wang, X.; Du, W.; Cheng, J.; Zhang, J.; Zhang, Y.; Klinjapo, R.; Asavasanti, S.; Yasurin, P. Recent Advances, Challenges, and Functional Applications of Natural Phenolic Compounds in the Meat Products Industry. Antioxidants 2025, 14, 138. [Google Scholar] [CrossRef]
- Al Jumayi, H.A.; Allam, A.Y.; El-Beltagy, A.E.; Algarni, E.H.; Mahmoud, S.F.; El Halim Kandil, A.A. Bioactive Compound, Antioxidant, and Radical Scavenging Activity of Some Plant Aqueous Extracts for Enhancing Shelf Life of Cold-Stored Rabbit Meat. Antioxidants 2022, 11, 1056. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; He, Z.; Zhang, D.; Li, H. Antioxidant activity of purslane extract and its inhibitory effect on the lipid and protein oxidation of rabbit meat patties during chilled storage. J. Sci. Food Agric. 2021, 101, 1953–1962. [Google Scholar] [CrossRef]
- Shah, M.A.; Bosco, S.J.; Mir, S.A. Plant extracts as natural antioxidants in meat and meat products. Meat Sci. 2014, 98, 21–33. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Ding, J.; Chen, S.; Chen, J.; Wang, C.; Li, J.; Shi, H.; Yin, X.; Wang, J.; Liu, J.; et al. Alleviation of colitis by honeysuckle MIR2911 via direct regulation of gut microbiota. J. Control. Release 2024, 376, 123–137. [Google Scholar] [CrossRef]
- Gao, S.; Shan, Y.; Wang, Y.; Wang, W.; Li, J.; Tan, H. Polysaccharides from Lonicera japonica Thunb.: Extraction, purification, structural features and biological activities—A review. Int. J. Biol. Macromol. 2024, 281, 136472. [Google Scholar] [CrossRef] [PubMed]
- Ran, J.; Tang, Y.; Mao, W.; Meng, X.; Jiao, L.; Li, Y.; Zhao, R.; Zhou, H. Optimization of the fermentation process and antioxidant activity of mixed lactic acid bacteria for honeysuckle beverage. Front. Microbiol. 2024, 15, 1364448. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Lin, L.; Zhao, M. A new perspective to explore the bioactive ingredients of honeysuckle tea infusion by structure, function and stability characterization of self-assembled nano/microaggregates. Food Res. Int. 2025, 204, 115923. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wang, Z.; Xue, Q.; Zhen, L.; Wang, Y.; Cao, J.; Liu, Y.; Khan, A.; Zhao, T.; Cheng, G. Effect of ultra-high pressure pretreatment on the phenolic profiles, antioxidative activity and cytoprotective capacity of different phenolic fractions from Que Zui tea. Food Chem. 2023, 409, 135271. [Google Scholar] [CrossRef] [PubMed]
- Opris, O.; Lung, I.; Soran, M.L.; Stegarescu, A.; Cesco, T.; Ghendov-Mosanu, A.; Podea, P. Sturza, R. Efficient Extraction of Total Polyphenols from Apple and Investigation of Its SPF Properties. Molecules 2022, 27, 1679. [Google Scholar] [CrossRef]
- GB 5009.238-2016; Chinese National Food Safety Standard—Determination of Water Activity of Food. National Health and Family Planning Commission: Beijing, China, 2016.
- GB 5009.181-2016; Chinese National Food Safety Standard—Determination of Malondialdehyde in Foods. National Health and Family Planning Commission: Beijing, China, 2016.
- GB 5009.228-2016; Chinese National Food Safety Standard—Determination of Total Volatile Basic Nitrogen in Foods. National Health and Family Planning Commission: Beijing, China, 2016.
- GB 4789.2-2022; Chinese National Food Safety Standard—Food Microbiological Examination: Aerobic Plate Count. National Health Commission: Beijing, China, 2022.
- Lima, L.; Pereira, A.I.; Vaz, C.B.; Ferreira, O.; Dias, M.I.; Heleno, S.A.; Calhelha, R.C.; Barros, L.; Carocho, M. Optimization of heat and ultrasound-assisted extraction of Eucalyptus globulus leaves reveals strong antioxidant and antimicrobial properties. Food Chem. 2025, 479, 143755. [Google Scholar] [CrossRef]
- Chang, Y.W.; Chen, Y.L.; Park, S.H.; Yap, E.E.S.; Sung, W.C. Characterization of Functional Ingredients Extracted with Ethanol Solvents from Ponkan (Citrus reticulata) By-Products Using the Microwave Vacuum Drying Method Combined with Ultrasound-Assisted Extraction. Foods 2024, 13, 2129. [Google Scholar] [CrossRef]
- Suman, S.P.; Nair, M.N.; Joseph, P.; Hunt, M.C. Factors influencing internal color of cooked meats. Meat Sci. 2016, 120, 133–144. [Google Scholar] [CrossRef]
- Yang, X.; Ma, Y.; Huang, T.; Huang, J.; Huang, M. Effect of modified starch-chitosan coating incorporated with Gongju extract on the shelf life of salted duck. Int. J. Biol. Macromol. 2025, 311, 143870. [Google Scholar] [CrossRef]
- Gul, P.; Khan, J.; Li, Q.; Liu, K. Moringa oleifera in a modern time: A comprehensive review of its nutritional and bioactive composition as a natural solution for managing diabetes mellitus by reducing oxidative stress and inflammation. Food Res. Int. 2025, 201, 115671. [Google Scholar] [CrossRef]
- Das, A.A.; Waldeck-Weiermair, M.; Yadav, S.; Spyropoulos, F.; Pandey, A.; Dutta, T.; Covington, T.A.; Michel, T. Differential aortic aneurysm formation provoked by chemogenetic oxidative stress. J. Clin. Investig. 2025, 135, e188743. [Google Scholar] [CrossRef]
- Tang, X.; Liu, X.; Zhong, J.; Fang, R. Potential Application of Lonicera japonica Extracts in Animal Production: From the Perspective of Intestinal Health. Front. Microbiol. 2021, 12, 719877. [Google Scholar] [CrossRef]
- Somwongin, S.; Sirilun, S.; Chantawannakul, P.; Anuchapreeda, S.; Yawootti, A.; Chaiyana, W. Ultrasound-assisted green extraction methods: An approach for cosmeceutical compounds isolation from Macadamia integrifolia pericarp. Ultrason. Sonochem. 2023, 92, 106266. [Google Scholar] [CrossRef]
- Gao, S.H.; Zhao, T.R.; Liu, Y.P.; Wang, Y.F.; Cheng, G.G.; Cao, J.X. Phenolic constituents, antioxidant activity and neuroprotective effects of ethanol extracts of fruits, leaves and flower buds from Vaccinium dunalianum Wight. Food Chem. 2022, 374, 131752. [Google Scholar] [CrossRef]
- Rice-Evans, C.A.; Miller, N.J. Antioxidant activities of flavonoids as bioactive components of food. Biochem. Soc. Trans. 1996, 24, 790–795. [Google Scholar] [CrossRef]
- Seo, O.N.; Kim, G.-S.; Park, S.; Lee, J.H.; Kim, Y.-H.; Lee, W.S.; Lee, S.J.; Kim, C.Y.; Jin, J.S.; Choi, S.K.; et al. Determination of polyphenol components of Lonicera japonica Thunb. using liquid chromatography–tandem mass spectrometry: Contribution to the overall antioxidant activity. Food Chem. 2012, 134, 572–577. [Google Scholar] [CrossRef]
- Saa, R.W.; Fombang, E.N.; Ndjantou, E.B.; Njintang, N.Y. Treatments and uses of Moringa oleifera seeds in human nutrition: A review. Food Sci. Nutr. 2019, 7, 1911–1919. [Google Scholar] [CrossRef] [PubMed]
- Silchenko, S.; Nessah, N.; Li, J.; Li, L.B.; Huang, Y.; Owen, A.J.; Hidalgo, I.J. In vitro dissolution absorption system (IDAS2): Use for the prediction of food viscosity effects on drug dissolution and absorption from oral solid dosage forms. Eur. J. Pharm. Sci. 2020, 143, 105164. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, Y.Q.; Zou, Y.H.; Zhang, W.G. Effects of ultrasound-assisted cooking on quality characteristics of spiced beef during cold storage. Lwt Food Sci. Technol. 2021, 136, 110359. [Google Scholar] [CrossRef]
- Liang, X.; Huang, X.; Li, C.; Kong, B.; Cao, C.; Sun, F.; Zhang, H.; Liu, Q.; Shen, L. Effect of different natural antioxidants on the quality promotion of pork chip snacks during storage as revealed by lipid profiles and volatile flavor compounds. Food Chem. 2025, 478, 143716. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Xu, M.; He, W.; Li, X.; Qiu, C.; Zhang, J. Unraveling the Physicochemical Properties and Bacterial Communities in Rabbit Meat during Chilled Storage. Foods 2024, 13, 623. [Google Scholar] [CrossRef]
- Khan, S.; Hashim, S.B.H.; Arslan, M.; Zhang, K.; Siman, L.; Mukhtar, A.; Zhihua, L.; Tahir, H.E.; Zhai, X.; Shishir, M.R.I.; et al. Development of an active biogenic silver nanoparticles composite film based on berry wax and chitosan for rabbit meat preservation. Int. J. Biol. Macromol. 2024, 275, 133128. [Google Scholar] [CrossRef] [PubMed]
- Cheng, J.; Tang, D.; Yang, H.; Wang, X.; Lin, Y.; Liu, X. The effects of mulberry polyphenols on the digestibility and absorption properties of pork myofibrillar protein in vitro. Meat Sci. 2023, 202, 109205. [Google Scholar] [CrossRef] [PubMed]
- Zheng, O.; Chen, H.; Jia, X.; Liu, Y.; Sun, Q.; Wang, Z.; Liu, S. Inhibitory Effect of Honeysuckle (Lonicera japonica Thunb.) Extract on the Melanosis and Quality Deterioration of Pacific White Shrimp (Litopenaeus vannamei) During Cold Storage. Foods 2025, 14, 2928. [Google Scholar] [CrossRef] [PubMed]
- Cherrak, S.A.; Mokhtari-Soulimane, N.; Berroukeche, F.; Bensenane, B.; Cherbonnel, A.; Merzouk, H.; Elhabiri, M. In Vitro Antioxidant versus Metal Ion Chelating Properties of Flavonoids: A Structure-Activity Investigation. PLoS ONE 2016, 11, e0165575. [Google Scholar] [CrossRef]
- Zheng, S.L.; Wang, Y.M.; Chi, C.F.; Wang, B. Chemical Characterization of Honeysuckle Polyphenols and Their Alleviating Function on Ultraviolet B-Damaged HaCaT Cells by Modulating the Nrf2/NF-kappaB Signaling Pathways. Antioxidants 2024, 13, 294. [Google Scholar] [CrossRef]
- Cai, J.R.; Chen, Q.S.; Wan, X.M.; Zhao, J.W. Determination of total volatile basic nitrogen (TVB-N) content and Warner-Bratzler shear force (WBSF) in pork using Fourier transform near infrared (FT-NIR) spectroscopy. Food Chem. 2011, 126, 1354–1360. [Google Scholar] [CrossRef]
- GB2707—2016; Chinese National Food Safety Standard—Fresh (Frozen) Livestock and Poultry Products. National Health and Family Planning Commission, China Food and Drug Administration, Ministry of Agriculture: Beijing, China, 2016.
- Yim, D.G.; Seo, J.K.; Yum, H.W.; Zahid, M.A.; Park, J.Y.; Parvin, R.; Go, J.; Jin, S.K.; Koo, O.K.; Yang, H.S. Effects of Caesalpinia sappan L. extract on the color stability, antioxidant and antimicrobial activity in cooked pork sausages during cold storage. Lwt Food Sci. Technol. 2019, 112, 108235. [Google Scholar] [CrossRef]
| Storage Time (Day) | Hardness (g) | Elasticity (ratio) | Chewiness (g) | Cohesiveness (ratio) | |
|---|---|---|---|---|---|
| RC | 0 | 1625.84 ± 75.88 a | 0.72 ± 0.04 a | 949.51 ± 15.61 a | 0.65 ± 0.02 a |
| 3 | 1346.31 ± 183.29 a | 0.65 ± 0.04 a | 791.14 ± 20.73 b* | 0.55 ± 0.04 a | |
| 6 | 1213.90 ± 133.92 a | 0.65 ± 0.04 a | 659.50 ± 18.61 c* | 0.51 ± 0.04 a | |
| 9 | 1183.48 ± 180.99 a | 0.65 ± 0.05 a | 479.96 ± 46.64 d* | 0.50 ± 0.05 a | |
| 12 | 1085.69 ± 207.64 a | 0.63 ± 0.04 a | 504.32 ± 16.63 d | 0.51 ± 0.05 a | |
| 15 | 1079.29 ± 101.34 a | 0.60 ± 0.03 a | 410.16 ± 33.49 d | 0.49 ± 0.02 a | |
| RHE | 0 | 1762.13 ± 193.96 a | 0.69 ± 0.03 a | 932.34 ± 33.10 a | 0.65 ± 0.03 a |
| 3 | 1717.08 ± 33.08 a | 0.69 ± 0.03 a | 877.88 ± 6.86 a | 0.58 ± 0.07 ab | |
| 6 | 1582.60 ± 37.92 a | 0.64 ± 0.04 a | 760.61 ± 35.40 b | 0.54 ± 0.02 ab | |
| 9 | 1571.21 ± 56.96 a | 0.62 ± 0.04 a | 632.41 ± 32.77 c | 0.46 ± 0.03 b | |
| 12 | 1459.47 ± 199.71 a | 0.61 ± 0.01 a | 579.93 ± 18.87 c | 0.43 ± 0.01 b | |
| 15 | 1233.67 ± 79.02 a | 0.60 ± 0.03 a | 441.76 ± 14.74 d | 0.44 ± 0.01 b | |
| CC | 0 | 1939.41 ± 33.76 A | 0.66 ± 0.03 A | 969.36 ± 36.73 A | 0.62 ± 0.04 A |
| 3 | 1776.18 ± 59.89 A | 0.67 ± 0.09 A | 827.40 ± 34.67 AB* | 0.55 ± 0.01 A | |
| 6 | 1630.84 ± 80.05 AB | 0.58 ± 0.02 A | 558.91 ± 19.10 BC* | 0.53 ± 0.02 A | |
| 9 | 1548.04 ± 128.52 ABC | 0.56 ± 0.02 A | 596.64 ± 50.33 BC | 0.53 ± 0.08 A | |
| 12 | 1290.29 ± 73.72 BC | 0.54 ± 0.01 A | 486.59 ± 30.62 C | 0.64 ± 0.01 A | |
| 15 | 1185.29 ± 44.55 C | 0.56 ± 0.01 A | 389.40 ± 7.25 C | 0.59 ± 0.04 A | |
| CHE | 0 | 1936.80 ± 87.47 A | 0.61 ± 0.01 A | 1097.23 ± 30.74 A | 0.68 ± 0.01 A |
| 3 | 1887.09 ± 116.62 A | 0.60 ± 0.02 A | 1097.59 ± 132.71 A | 0.53 ± 0.02 B | |
| 6 | 1789.55 ± 128.22 AB | 0.61 ± 0.04 A | 838.76 ± 176.49 AB | 0.57 ± 0.01 AB | |
| 9 | 1659.84 ± 105.22 AB | 0.59 ± 0.005 A | 546.80 ± 13.25 BC | 0.62 ± 0.01 AB | |
| 12 | 1390.89 ± 73.27 BC | 0.58 ± 0.05 A | 483.80 ± 57.14 C | 0.64 ± 0.08 AB | |
| 15 | 1155.29 ± 56.66 C | 0.59 ± 0.03 A | 408.37 ± 26.14 C | 0.62 ± 0.02 AB |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, X.; Chen, W.; Yang, S.; Wang, B.; Hu, X.; Dong, Z.; Zhang, J. Honeysuckle Extracts (Lonicera japonica Thunb.): Understanding Insights into the Antioxidant Effect on Preserving Qualities of Rabbit Meat During Refrigerated Storage. Foods 2025, 14, 4194. https://doi.org/10.3390/foods14244194
Huang X, Chen W, Yang S, Wang B, Hu X, Dong Z, Zhang J. Honeysuckle Extracts (Lonicera japonica Thunb.): Understanding Insights into the Antioxidant Effect on Preserving Qualities of Rabbit Meat During Refrigerated Storage. Foods. 2025; 14(24):4194. https://doi.org/10.3390/foods14244194
Chicago/Turabian StyleHuang, Xiaohua, Wenjiao Chen, Siyi Yang, Bo Wang, Xinyu Hu, Zhilan Dong, and Jiamin Zhang. 2025. "Honeysuckle Extracts (Lonicera japonica Thunb.): Understanding Insights into the Antioxidant Effect on Preserving Qualities of Rabbit Meat During Refrigerated Storage" Foods 14, no. 24: 4194. https://doi.org/10.3390/foods14244194
APA StyleHuang, X., Chen, W., Yang, S., Wang, B., Hu, X., Dong, Z., & Zhang, J. (2025). Honeysuckle Extracts (Lonicera japonica Thunb.): Understanding Insights into the Antioxidant Effect on Preserving Qualities of Rabbit Meat During Refrigerated Storage. Foods, 14(24), 4194. https://doi.org/10.3390/foods14244194






