Identification of Novel Umami Peptides from Yak Bone Collagen and Mechanism Exploration Through In Silico Discovery, Molecular Docking, and Electronic Tongue
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
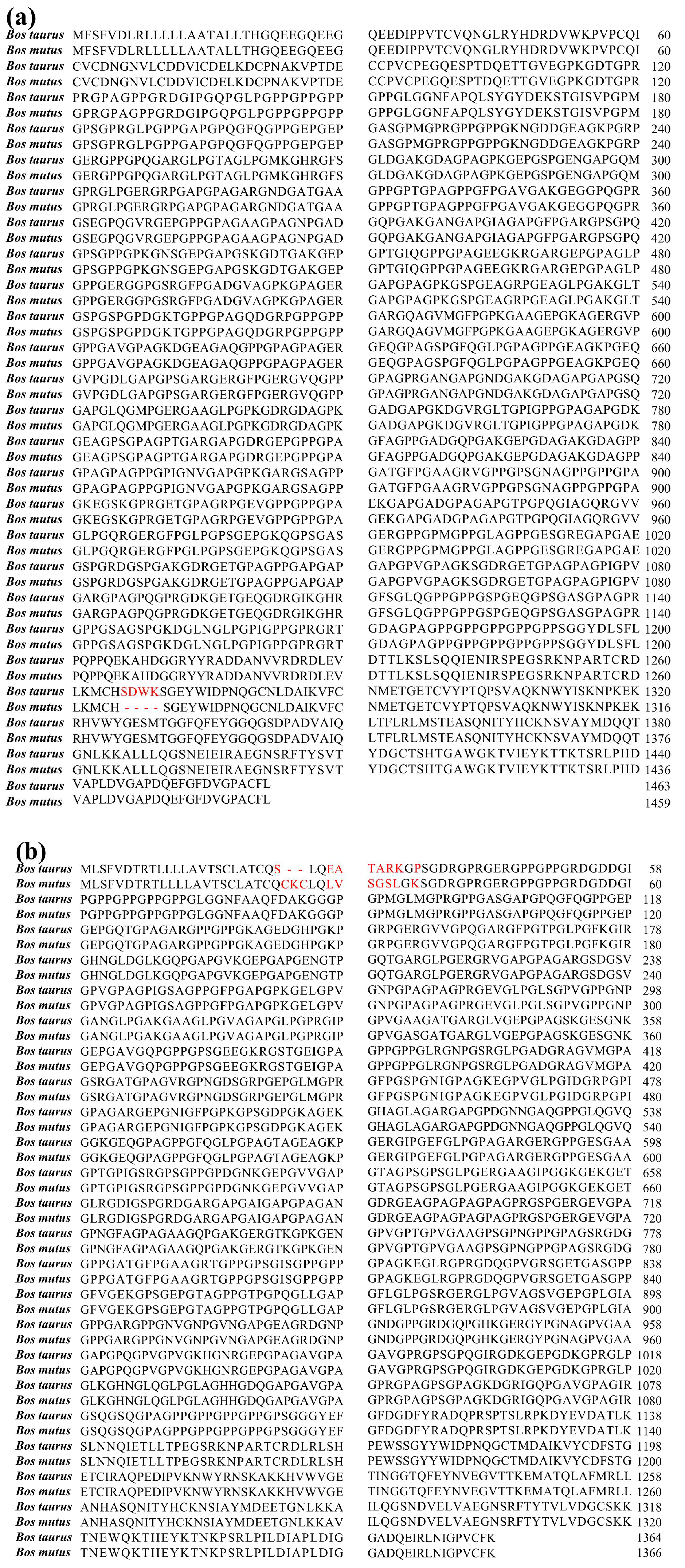
2.2. Comparison of Collagen Sequences from Yak Bone and Bovine Bone
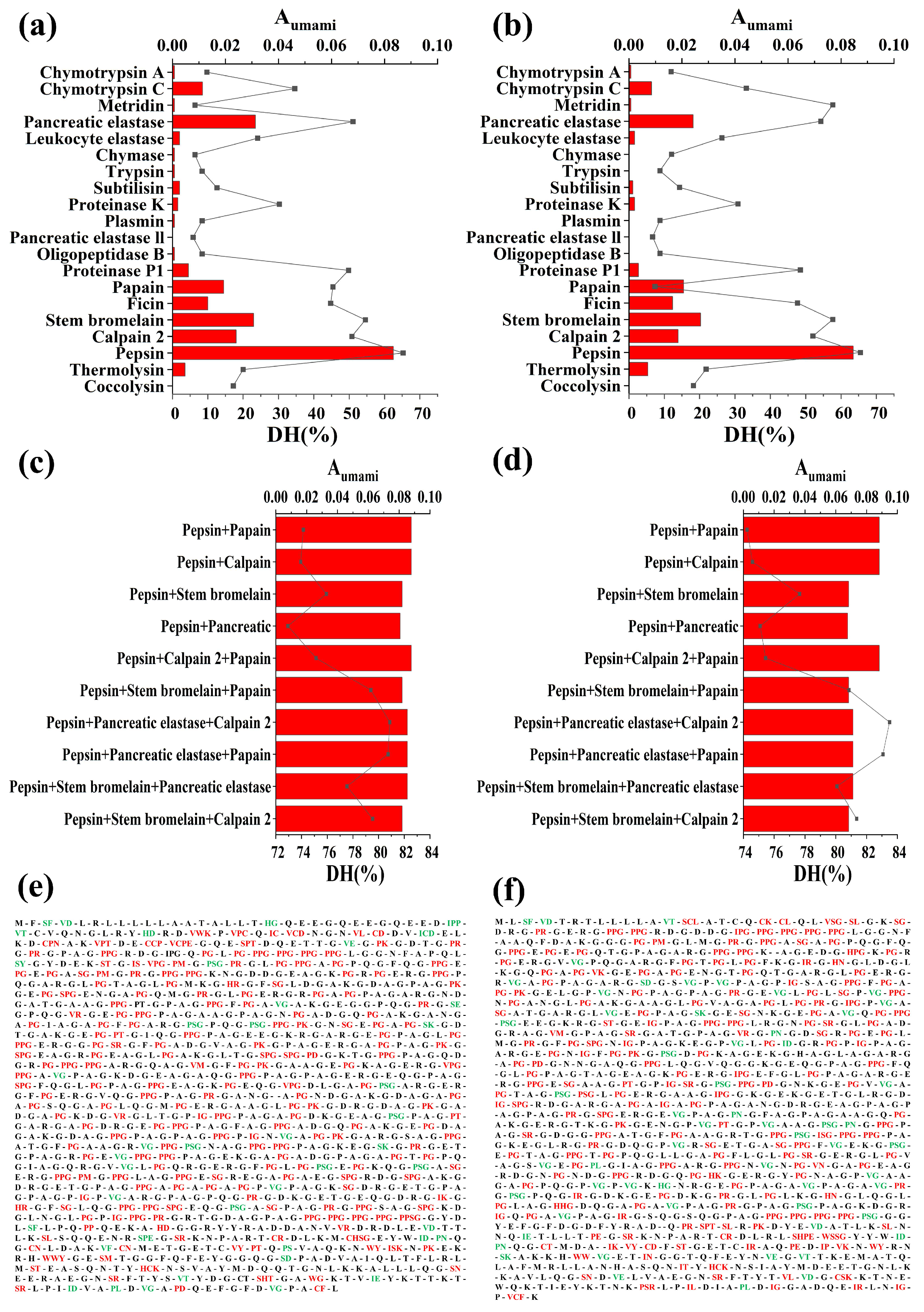
2.3. Simulated Proteolysis and Peptide Screening
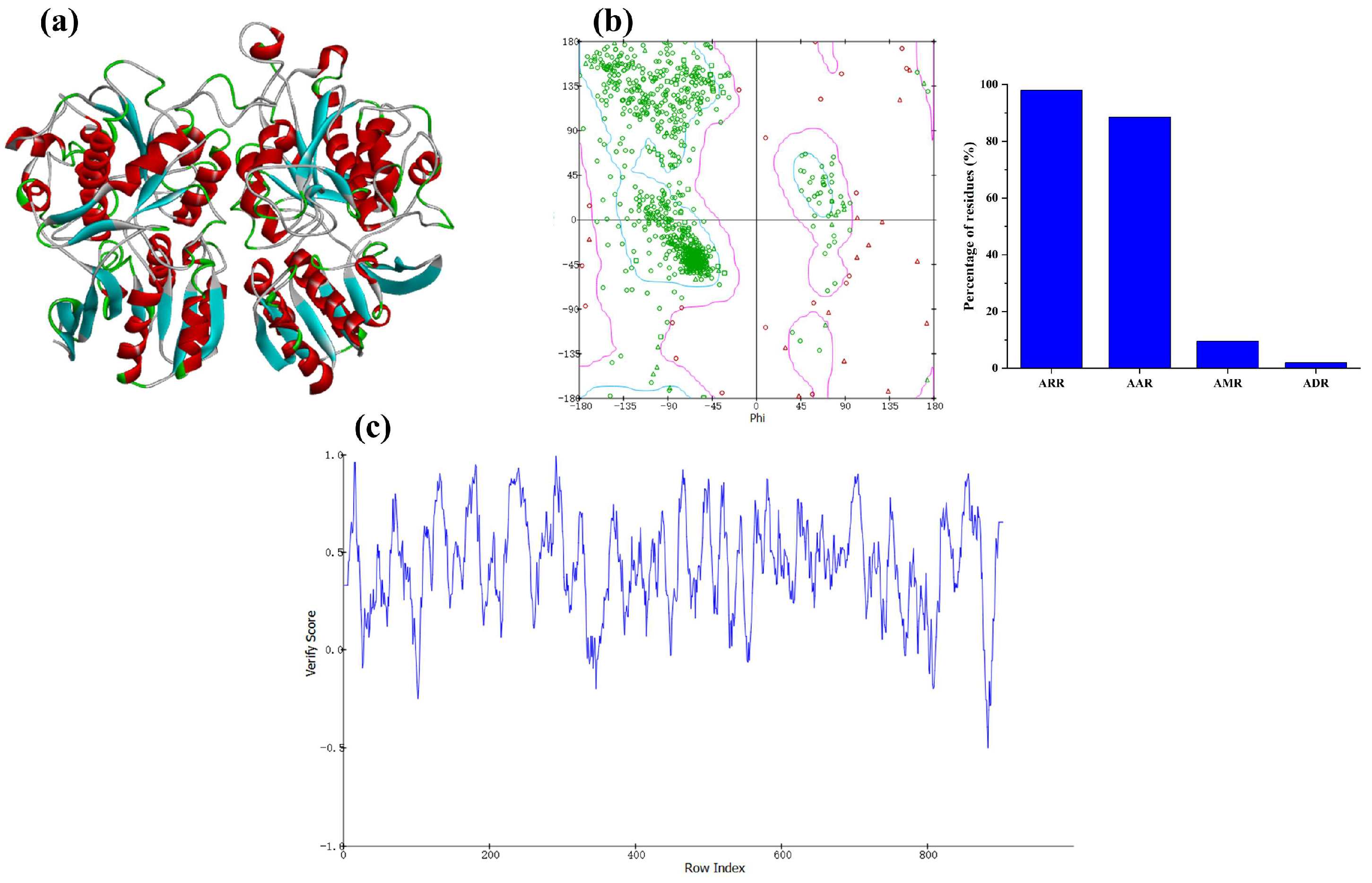
2.4. Homologous Modeling of T1R1/T1R3 Receptor
2.5. Molecular Docking Between Peptides and T1R1/T1R3
2.6. In Silico Analyses of Physicochemical Properties
2.7. Solid-Phase Synthesis of Umami Peptide Candidates
2.8. Identification of Umami Peptides by Sensory Evaluation
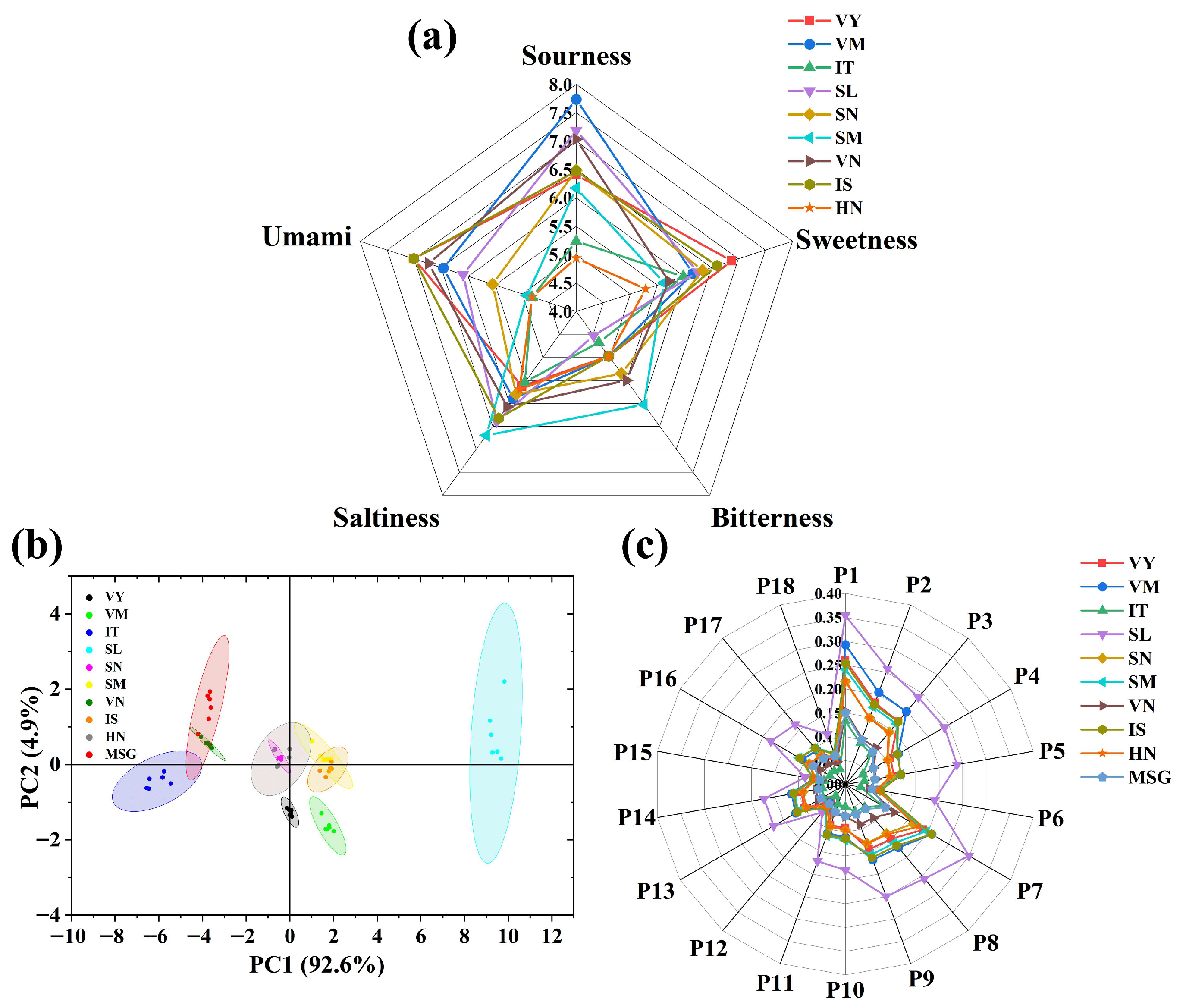
2.9. Electronic Tongue Measurement of Identified Umami Peptides
2.10. Statistical Analysis
3. Results and Discussion
3.1. Sequence Alignment of Bone Collagens from Yak and Bovine
3.2. Simulated Proteolysis of Yak Bone Collagen
3.3. Release Profile and Screening of Peptides
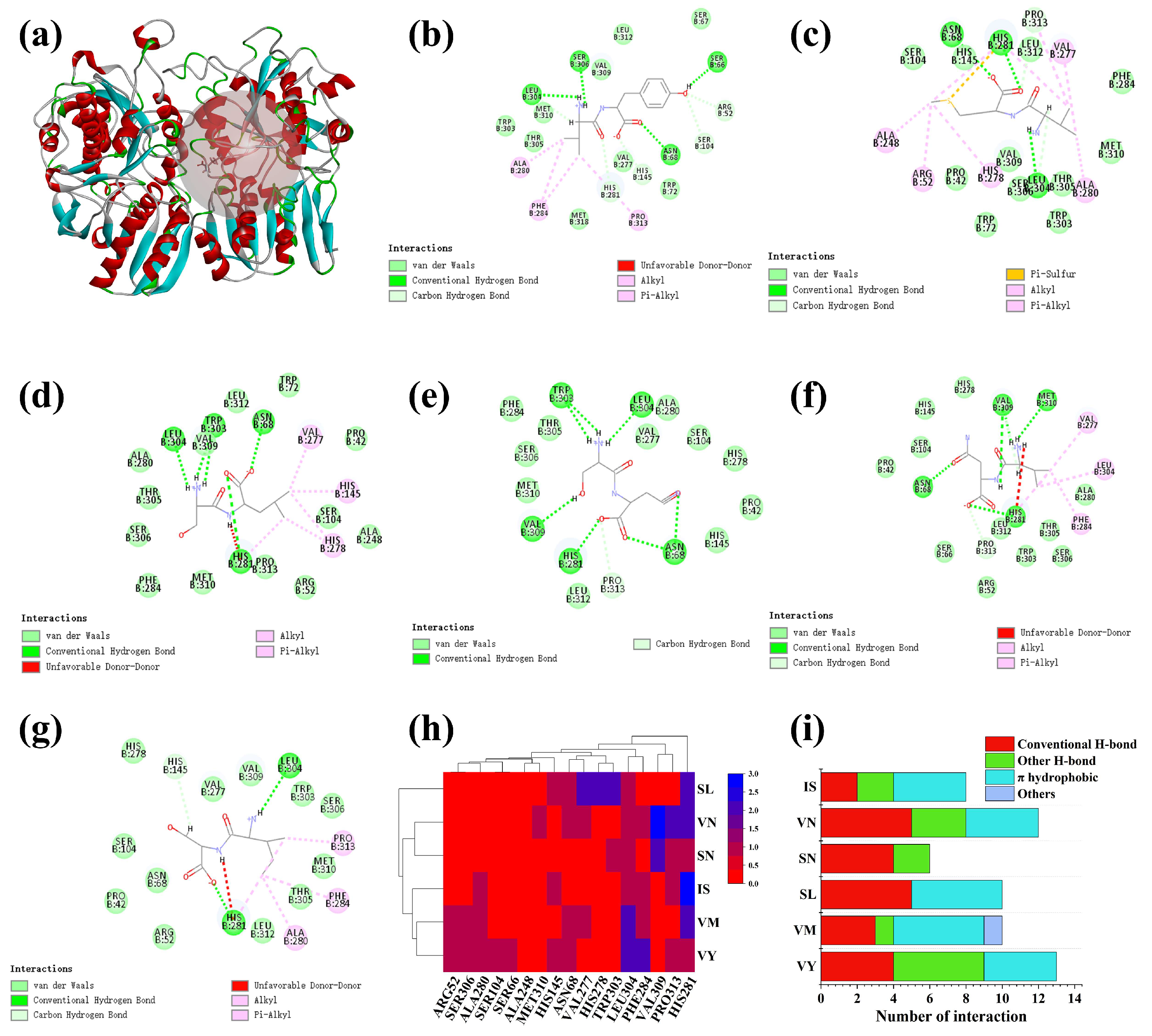
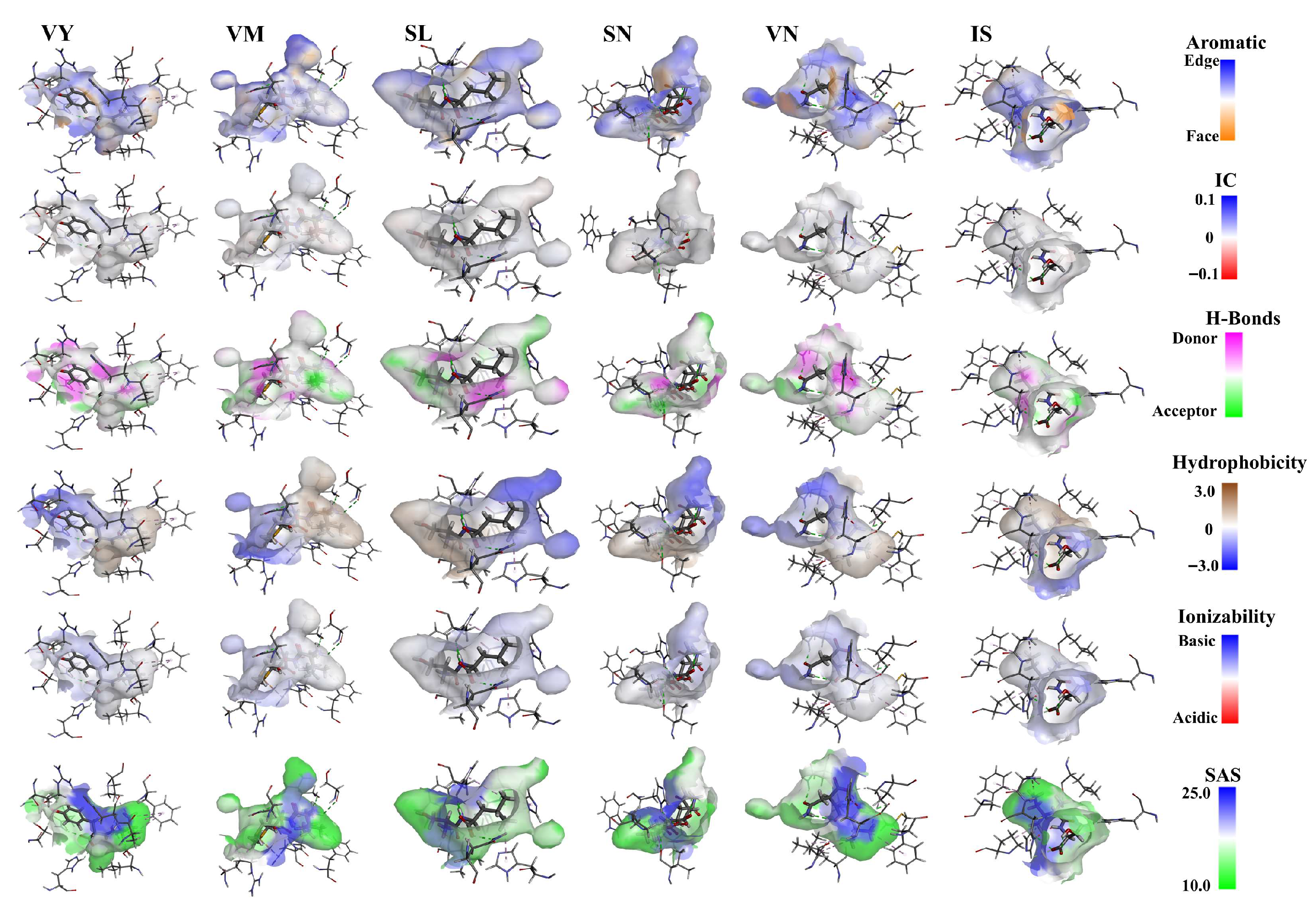
3.4. Molecular Docking of Screened Peptides with T1R1/T1R3 Receptor
3.5. Analysis of Physicochemical Properties for Potential Umami Peptides
3.6. Synthesis and Identification of Umami Peptides by Sensory Evaluation
3.7. Electronic Tongue Analysis of Identified Novel Umami Peptides
3.8. Potential Interactive Mechanism of Identified Peptides with Umami Receptors T1R1/T1R3
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Qin, X.; Shen, Q.; Guo, Y.; Li, X.; Liu, J.; Ye, M.; Wang, H.; Jia, W.; Zhang, C. Physicochemical properties, digestibility and anti-osteoporosis effect of yak bone powder with different particle sizes. Food Res. Int. 2021, 145, 110401. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Li, X.; Zhao, K.; Li, H.; Liu, J.; Da, S.; Ciren, D.; Tang, H. In vitro digestion and fermentation combined with microbiomics and metabolomics reveal the mechanism of superfine yak bone powder regulating lipid metabolism by altering human gut microbiota. Food Chem. 2023, 410, 135441. [Google Scholar] [CrossRef] [PubMed]
- Guo, Z.; Liu, C.; Hu, B.; Zhu, L.; Yang, Y.; Liu, F.; Gu, Z.; Xin, Y.; Zhang, L. Simulated gastrointestinal digestion of yak bone collagen hydrolysates and insights into its effects on gut microbiota composition in mice. Food Biosci. 2021, 44, 101463. [Google Scholar] [CrossRef]
- Gao, S.; Hong, H.; Zhang, C.; Wang, K.; Zhang, B.; Han, Q.-A.; Liu, H.; Luo, Y. Immunomodulatory effects of collagen hydrolysates from yak (Bos grunniens) bone on cyclophosphamide-induced immunosuppression in BALB/c mice. J. Funct. Foods 2019, 60, 103420. [Google Scholar] [CrossRef]
- Guo, Z.; Yi, D.; Hu, B.; Zhu, L.; Zhang, J.; Yang, Y.; Liu, C.; Shi, Y.; Gu, Z.; Xin, Y.; et al. Supplementation with yak (Bos grunniens) bone collagen hydrolysate altered the structure of gut microbiota and elevated short-chain fatty acid production in mice. Food Sci. Hum. Wellness 2023, 12, 1637–1645. [Google Scholar] [CrossRef]
- Zhou, H.; Wei, J.; Wang, Z.; Bai, L.; Wang, Q.; Wei, Y.; Hu, X.; Tian, X.; Zhang, F. Anti-osteoporosis properties and regulatory impact on gut microbiota of yak bone meal in ovariectomized osteoporotic mice. Food Biosci. 2025, 66, 106267. [Google Scholar] [CrossRef]
- Yang, Y.; Zhu, L.; Guo, Z.; Liu, C.; Hu, B.; Li, M.; Gu, Z.; Xin, Y.; Sun, H.; Guan, Y.; et al. Yak bone collagen-derived anti-inflammatory bioactive peptides alleviate lipopolysaccharide-induced inflammatory by inhibiting the NF-κB signaling pathway and nitric oxide production. Food Biosci. 2023, 52, 102423. [Google Scholar] [CrossRef]
- Šefčíková, Z.; Špirková, A.; Kovaříková, V.; Rušinová, L.; Baran, V.; Pisko, J.; Babeľová, J.; Fabian, D.; Čikoš, Š. The consumption of monosodium glutamate during the periconceptional period can impair preimplantation embryo development. Reprod. Toxicol. 2025, 137, 109014. [Google Scholar] [CrossRef]
- Jiang, Y.-H.; Li, Y.-Y.; Huang, P.-M.; Xin, W.-G.; Zhou, Y.-X.; Zheng, Y.; Ibrahim, A.A.; Suo, H.-Y. Decoding of novel umami-enhancing peptides from Hericium Erinaceus and its mechanisms by virtual screening, multisensory techniques, and molecular simulation approaches. Food Chem. X 2025, 31, 103105. [Google Scholar] [CrossRef]
- Wang, W.; Zhou, X.; Liu, Y. Characterization and evaluation of umami taste: A review. TrAC Trends Anal. Chem. 2020, 127, 115876. [Google Scholar] [CrossRef]
- Zhang, R.; Zhou, Z.; Ji, Z.; Ren, Q.; Liu, S.; Xu, Y.; Mao, J. Identification and characterization of novel dual-function antioxidant and umami peptides from protein hydrolysates of Huangjiu lees. Food Qual. Saf. 2024, 8, fyae011. [Google Scholar] [CrossRef]
- Fu, B.; Li, M.; Chang, Z.; Yi, J.; Cheng, S.; Du, M. Identification of novel umami peptides from oyster hydrolysate and the mechanisms underlying their taste characteristics using machine learning. Food Chem. 2025, 473, 142970. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.; Kim, H.-J.; Jo, C. Novel umami-enhancing peptides of beef M. Semimembranosus hydrolysates and interactions with the T1R1/T1R3 taste receptor. Food Chem. 2025, 463, 141368. [Google Scholar] [CrossRef] [PubMed]
- Gu, Y.; Zhang, J.; Niu, Y.; Sun, B.; Liu, Z.; Mao, X.; Zhang, Y. Virtual screening and characteristics of novel umami peptides from porcine type I collagen. Food Chem. 2024, 434, 137386. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Xie, X.; Wang, J.; Xu, Y.; Yi, S.; Zhu, W.; Mi, H.; Li, T.; Li, J. Identification, taste characteristics and molecular docking study of novel umami peptides derived from the aqueous extract of the clam meretrix meretrix Linnaeus. Food Chem. 2020, 312, 126053. [Google Scholar] [CrossRef]
- Yang, D.; Li, C.; Li, L.; Chen, S.; Hu, X.; Xiang, H. Taste mechanism of umami peptides from Chinese traditional fermented fish (Chouguiyu) based on molecular docking using umami receptor T1R1/T1R3. Food Chem. 2022, 389, 133019. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, J.; Liang, L.; Sun, B.; Zhang, Y. Identification and virtual screening of novel umami peptides from chicken soup by molecular docking. Food Chem. 2023, 404, 134414. [Google Scholar] [CrossRef]
- Chen, M.; Gao, X.; Pan, D.; Xu, S.; Zhang, H.; Sun, Y.; He, J.; Dang, Y. Taste characteristics and umami mechanism of novel umami peptides and umami-enhancing peptides isolated from the hydrolysates of Sanhuang Chicken. Eur. Food Res. Technol. 2021, 247, 1633–1644. [Google Scholar] [CrossRef]
- Huang, H.; Chen, Y.; Hong, J.; Yuan, X.; Tian, W.; Zhao, D.; Sun, B.; Sun, J.; Wu, J.; Huang, M.; et al. Exploration of the flavor mechanism of novel umami peptides from lager beer: HPLC-Q-TOF-MS combined with flavor perception, molecular docking and molecular dynamics simulation. J. Food Compos. Anal. 2025, 141, 107338. [Google Scholar] [CrossRef]
- Zhang, J.; Wu, Y.; Tang, H.; Li, H.; Da, S.; Ciren, D.; Peng, X.; Zhao, K. Identification, characterization, and insights into the mechanism of novel dipeptidyl peptidase-IV inhibitory peptides from yak hemoglobin by in silico exploration, molecular docking, and in vitro assessment. Int. J. Biol. Macromol. 2024, 259, 129191. [Google Scholar] [CrossRef]
- Sánchez, A.; Vázquez, A. Bioactive peptides: A review. Food Qual. Saf. 2017, 1, 29–46. [Google Scholar] [CrossRef]
- Lin, K.; Zhang, L.-W.; Han, X.; Xin, L.; Meng, Z.-X.; Gong, P.-M.; Cheng, D.-Y. Yak milk casein as potential precursor of angiotensin I-converting enzyme inhibitory peptides based on in silico proteolysis. Food Chem. 2018, 254, 340–347. [Google Scholar] [CrossRef] [PubMed]
- Minkiewicz, P.; Iwaniak, A.; Darewicz, M. BIOPEP-UWM Database of Bioactive Peptides: Current Opportunities. Int. J. Mol. Sci. 2019, 20, 5978. [Google Scholar] [CrossRef] [PubMed]
- Chang, J.; Li, X.; Liang, Y.; Feng, T.; Sun, M.; Song, S.; Yao, L.; Wang, H.; Hou, F. Novel umami peptide from Hypsizygus marmoreus hydrolysate and molecular docking to the taste receptor T1R1/T1R3. Food Chem. 2023, 401, 134163. [Google Scholar] [CrossRef]
- Zhai, Y.; Peng, W.; Luo, W.; Wu, J.; Liu, Y.; Wang, F.; Li, X.; Yu, J.; Wang, S. Component stabilizing mechanism of membrane-separated hydrolysates on frozen surimi. Food Chem. 2024, 431, 137114. [Google Scholar] [CrossRef]
- Wang, W.; Yang, L.; Ning, M.; Liu, Z.; Liu, Y. A rational tool for the umami evaluation of peptides based on multi-techniques. Food Chem. 2022, 371, 131105. [Google Scholar] [CrossRef]
- Zhao, J.; Liao, S.; Han, J.; Xie, Y.; Tang, J.; Zhao, J.; Shao, W.; Wang, Q.; Lin, H. Revealing the Secret of Umami Taste of Peptides Derived from Fermented Broad Bean Paste. J. Agric. Food Chem. 2023, 71, 4706–4716. [Google Scholar] [CrossRef]
- Wang, H.; Chen, D.; Lu, W.; Dang, Y.; Liu, Z.; Chen, G.; Wang, B.; Zhang, C.; Xiao, C. Novel salty peptides derived from bovine bone: Identification, taste characteristic, and salt-enhancing mechanism. Food Chem. 2024, 447, 139035. [Google Scholar] [CrossRef]
- Xu, X.; Yu, M.; Raza, J.; Song, H.; Gong, L.; Pan, W. Study of the mechanism of flavor compounds formed via taste-active peptides in bovine bone protein extract. LWT 2021, 137, 110371. [Google Scholar] [CrossRef]
- Huang, X.; Liao, J.; Shi, P.; Pei, X.; Wang, C. Virtual screening and directional preparation of xanthine oxidase inhibitory peptides derived from hemp seed protein. Food Sci. Hum. Wellness 2024, 13, 3652–3660. [Google Scholar] [CrossRef]
- Gao, R.; Kong, F.; Mu, G.; Zhao, X.; Cheng, J.; Qian, F. Screening of enzymes for bi-functional whey protein hydrolysates: Virtual enzymolysis, fragmentomics, and molecular docking in silico. Food Res. Int. 2025, 214, 116629. [Google Scholar] [CrossRef]
- Castañeda-Valbuena, D.; Berenguer-Murcia, Á.; Fernandez-Lafuente, R.; Morellon-Sterling, R.; Tacias-Pascacio, V.G. Biological activities of peptides obtained by pepsin hydrolysis of fishery products. Process Biochem. 2022, 120, 53–63. [Google Scholar] [CrossRef]
- Fu, Z.; Akula, S.; Thorpe, M.; Hellman, L. Marked difference in efficiency of the digestive enzymes pepsin, trypsin, chymotrypsin, and pancreatic elastase to cleave tightly folded proteins. Biol. Chem. 2021, 402, 861–867. [Google Scholar] [CrossRef] [PubMed]
- Budseekoad, S.; Yupanqui, C.T.; Sirinupong, N.; Alashi, A.M.; Aluko, R.E.; Youravong, W. Structural and functional characterization of calcium and iron-binding peptides from mung bean protein hydrolysate. J. Funct. Foods 2018, 49, 333–341. [Google Scholar] [CrossRef]
- Tu, M.; Cheng, S.; Lu, W.; Du, M. Advancement and prospects of bioinformatics analysis for studying bioactive peptides from food-derived protein: Sequence, structure, and functions. TrAC Trends Anal. Chem. 2018, 105, 7–17. [Google Scholar] [CrossRef]
- Kong, Y.; Yang, X.; Ding, Q.; Zhang, Y.-Y.; Sun, B.-G.; Chen, H.-T.; Sun, Y. Comparison of non-volatile umami components in chicken soup and chicken enzymatic hydrolysate. Food Res. Int. 2017, 102, 559–566. [Google Scholar] [CrossRef]
- Xiong, Y.; Gao, X.; Pan, D.; Zhang, T.; Qi, L.; Wang, N.; Zhao, Y.; Dang, Y. A strategy for screening novel umami dipeptides based on common feature pharmacophore and molecular docking. Biomaterials 2022, 288, 121697. [Google Scholar] [CrossRef]
- Pa’ee, K.F.; Razali, N.; Sarbini, S.R.; Ramonaran Nair, S.N.; Yong Tau Len, K.; Abd-Talib, N. The production of collagen type I hydrolyzate derived from tilapia (Oreochromis sp.) skin using thermoase PC10F and its in silico analysis. Food Biotechnol. 2021, 35, 1–21. [Google Scholar] [CrossRef]
- Heres, A.; Mora, L.; Toldrá, F. Bioactive and Sensory Di- and Tripeptides Generated during Dry-Curing of Pork Meat. Int. J. Mol. Sci. 2023, 24, 1574. [Google Scholar] [CrossRef]
- Alim, A.; Song, H.; Zou, T. Analysis of meaty aroma and umami taste in thermally treated yeast extract by means of sensory-guided screening. Eur. Food Res. Technol. 2020, 246, 2119–2133. [Google Scholar] [CrossRef]
- Feng, X.; Wang, R.; Lu, J.; Du, Q.; Cai, K.; Zhang, B.; Xu, B. Taste properties and mechanism of umami peptides from fermented goose bones based on molecular docking and molecular dynamics simulation using umami receptor T1R1/T1R3. Food Chem. 2024, 443, 138570. [Google Scholar] [CrossRef]
- Yu, Z.; Kang, L.; Zhao, W.; Wu, S.; Ding, L.; Zheng, F.; Liu, J.; Li, J. Identification of novel umami peptides from myosin via homology modeling and molecular docking. Food Chem. 2021, 344, 128728. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Li, M.; Zhang, G.; Tian, Y.; Kong, F.; Xiong, S.; Zhao, S.; Jia, D.; Manyande, A.; Du, H. Identification of novel antioxidant peptides from snakehead (Channa argus) soup generated during gastrointestinal digestion and insights into the anti-oxidation mechanisms. Food Chem. 2021, 337, 127921. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Guo, Z.; Yang, Y.; Hu, B.; Zhu, L.; Li, M.; Gu, Z.; Xin, Y.; Sun, H.; Guan, Y.; et al. Identification of dipeptidyl peptidase-IV inhibitory peptides from yak bone collagen by in silico and in vitro analysis. Eur. Food Res. Technol. 2022, 248, 3059–3069. [Google Scholar] [CrossRef]
- Rudrapal, M.; Gogoi, N.; Chetia, D.; Khan, J.; Banwas, S.; Alshehri, B.; Alaidarous, M.A.; Laddha, U.D.; Khairnar, S.J.; Walode, S.G. Repurposing of phytomedicine-derived bioactive compounds with promising anti-SARS-CoV-2 potential: Molecular docking, MD simulation and drug-likeness/ADMET studies. Saudi J. Biol. Sci. 2022, 29, 2432–2446. [Google Scholar] [CrossRef]
- Behrens, M.; Meyerhof, W.; Hellfritsch, C.; Hofmann, T. Sweet and Umami Taste: Natural Products, Their Chemosensory Targets, and Beyond. Angew. Chem. Int. Ed. 2011, 50, 2220–2242. [Google Scholar] [CrossRef]
- Hajeb, P.; Jinap, S. Umami Taste Components and Their Sources in Asian Foods. Crit. Rev. Food Sci. Nutr. 2015, 55, 778–791. [Google Scholar] [CrossRef]
- Liu, Z.; Zhu, Y.; Wang, W.; Zhou, X.; Chen, G.; Liu, Y. Seven novel umami peptides from Takifugu rubripes and their taste characteristics. Food Chem. 2020, 330, 127204. [Google Scholar] [CrossRef]
- Zhang, Z.; Chen, W.; Tao, L.; Wei, X.; Gao, L.; Gao, Y.; Suo, J.; Yu, W.; Hu, Y.; Yang, B.; et al. Ethylene treatment promotes umami taste-active amino acids accumulation of Torreya grandis nuts post-harvest by comparative chemical and transcript analyses. Food Chem. 2023, 408, 135214. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, L.; Pérez-Moreno, J.; Bin, L.; Zhang, F.; Yu, F. Novel umami peptides from two Termitomyces mushrooms and molecular docking to the taste receptor T1R1/T1R3. Food Sci. Hum. Wellness 2024, 13, 1055–1064. [Google Scholar] [CrossRef]
- Wei, L.; Shi, C.; Li, D.; Yuan, X.; Yu, X.; Liang, B.; Wu, J.; Zhang, Y.; Dai, Z.; Lu, Y.; et al. Discovery of novel umami peptides and their bitterness masking effects from yellowfin tuna (Thunnus albacares) via peptidomics, multisensory evaluation, and molecular docking approaches. Food Chem. 2025, 489, 145028. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Li, C.; Cui, Z.; Wang, W.; Liu, Y. Novel umami peptides from Takifugu rubripes muscle via virtual hydrolysis and screening strategy combined with sensory evaluation and biosensor. J. Future Foods 2025, 6, 545–553. [Google Scholar] [CrossRef]
- Li, Y.; Sun, Y.; Ren, M.; Zhong, T.; Xu, X.; Sui, W.; Jin, Y.; Zhang, M.; Wu, T. Identification and characterization of novel umami peptides from pea hydrolysate by virtual hydrolysis. Food Biosci. 2025, 63, 105620. [Google Scholar] [CrossRef]
- Chang, L.; Zhang, X.; Zhang, Z.; Cai, F.; Yu, H.; Lin, D.; Liu, H.; Zhao, Q. Isolation and characterization of novel umami peptides from bay scallop (Argopecten irradians) and molecular docking to the T1R1/T1R3 taste receptor. LWT 2024, 207, 116645. [Google Scholar] [CrossRef]
- Jia, R.; He, Y.; Liao, G.; Yang, Z.; Gu, D.; Pu, Y.; Huang, M.; Wang, G. Identification of umami peptides from Wuding chicken by Nano-HPLC-MS/MS and insights into the umami taste mechanisms. Food Res. Int. 2023, 172, 113208. [Google Scholar] [CrossRef]
- Fu, B.; Wu, D.; Cheng, S.; Xu, X.; Zhang, L.; Wang, L.; El-Seedi, H.R.; Liu, H.; Du, M. Three novel umami peptides derived from the alcohol extract of the Pacific oyster (Crassostrea gigas): Identification, characterizations and interactions with T1R1/T1R3 taste receptors. Food Sci. Hum. Wellness 2024, 13, 146–153. [Google Scholar] [CrossRef]
- Wang, S.; Dermiki, M.; Methven, L.; Kennedy, O.B.; Cheng, Q. Interactions of umami with the four other basic tastes in equi-intense aqueous solutions. Food Qual. Prefer. 2022, 98, 104503. [Google Scholar] [CrossRef]
- Yu, Z.; Liu, M.; Xu, J.; Fan, X.; Wang, W.; Sun, Y.; Pan, D. Rapid screening of novel umami peptides in high-umami scored air-dried chicken under low-salt processing based on molecular sensory techniques. Food Biosci. 2024, 62, 105479. [Google Scholar] [CrossRef]
- Dang, Y.; Gao, X.; Xie, A.; Wu, X.; Ma, F. Interaction Between Umami Peptide and Taste Receptor T1R1/T1R3. Cell Biochem. Biophys. 2014, 70, 1841–1848. [Google Scholar] [CrossRef]
- Yang, C.; Ge, X.; Ge, C.; Zhao, P.; Liang, S.; Xiao, Z. Taste characterization and molecular docking study of novel umami flavor peptides in Yanjin black bone Chicken meat. Food Chem. 2025, 464, 141695. [Google Scholar] [CrossRef]

| Peptide | Proportion of Umami Residues (%) | Water Solubility (log S) | Toxicity | Allergen |
|---|---|---|---|---|
| AHD | 100.00 | 1.52 | Non | Allergen |
| CCP | 0.00 | 1.45 | Non | Allergen |
| CSK | 33.33 | 2.28 | Non | Allergen |
| HPG | 66.67 | 1.02 | Non | Allergen |
| IPG | 33.33 | 0.46 | Non | Allergen |
| IPP | 0.00 | −0.30 | Non | Allergen |
| ISK | 33.33 | 1.36 | Non | Allergen |
| NSG | 66.67 | 3.19 | Non | Allergen |
| PPSG | 50.00 | 1.30 | Non | Allergen |
| SCL | 33.33 | 1.05 | Non | Allergen |
| SHPE | 50.00 | 1.83 | Non | Allergen |
| SPG | 66.67 | 2.52 | Non | Allergen |
| VKN | 33.33 | 1.56 | Non | Allergen |
| VPC | 33.33 | 0.75 | Non | Allergen |
| VPT | 66.67 | 1.10 | Non | Allergen |
| VWY | 66.67 | −2.00 | Non | Allergen |
| WSSG | 75.00 | 0.92 | Non | Allergen |
| AG | 100.00 | 1.82 | Non | Non |
| AR | 50.00 | 2.37 | Non | Non |
| CD | 50.00 | 1.80 | Non | Non |
| CF | 50.00 | 0.32 | Non | Non |
| CHSG | 75.00 | 1.93 | Non | Non |
| CK | 0.00 | 1.14 | Non | Non |
| CL | 0.00 | 0.13 | Non | Non |
| CN | 0.00 | 2.22 | Non | Non |
| CPN | 0.00 | 2.35 | Non | Non |
| CR | 0.00 | 1.71 | Non | Non |
| CT | 50.00 | 1.67 | Non | Non |
| DPG | 66.67 | 2.29 | Non | Non |
| EVG | 100.00 | 1.80 | Non | Non |
| HCK | 33.33 | 1.10 | Non | Non |
| HG | 100.00 | 1.44 | Non | Non |
| HHG | 100.00 | 1.18 | Non | Non |
| HK | 50.00 | 1.22 | Non | Non |
| HR | 50.00 | 1.50 | Non | Non |
| IC | 0.00 | 2.22 | Non | Non |
| ICD | 33.33 | 0.99 | Non | Non |
| IG | 50.00 | 0.96 | Non | Non |
| IK | 0.00 | 1.27 | Non | Non |
| IL | 0.00 | 0.25 | Non | Non |
| IN | 0.00 | 2.10 | Non | Non |
| IR | 0.00 | 1.54 | Non | Non |
| IS | 50.00 | 1.38 | Non | Non |
| ISG | 66.67 | 1.63 | Non | Non |
| IT | 50.00 | 1.26 | Non | Non |
| KPG | 33.33 | 1.46 | Non | Non |
| LK | 0.00 | 1.11 | Non | Non |
| LR | 0.00 | 1.37 | Non | Non |
| PD | 50.00 | 2.03 | Non | Non |
| PG | 50.00 | 1.76 | Non | Non |
| PM | 0.00 | 1.04 | Non | Non |
| PRG | 33.33 | 1.50 | Non | Non |
| PS | 50.00 | 2.25 | Non | Non |
| PSR | 33.33 | 2.67 | Non | Non |
| SF | 100.00 | 1.03 | Non | Non |
| SG | 100.00 | 2.45 | Non | Non |
| SHT | 100.00 | 2.01 | Non | Non |
| SK | 50.00 | 1.86 | Non | Non |
| SL | 50.00 | 0.84 | Non | Non |
| SM | 50.00 | 1.22 | Non | Non |
| SN | 50.00 | 2.93 | Non | Non |
| SPGE | 75.00 | 2.59 | Non | Non |
| SPT | 66.67 | 2.51 | Non | Non |
| SR | 50.00 | 2.12 | Non | Non |
| ST | 100.00 | 2.38 | Non | Non |
| VCD | 66.67 | 1.01 | Non | Non |
| VCF | 66.67 | −0.48 | Non | Non |
| VCPE | 50.00 | 1.33 | Non | Non |
| VK | 50.00 | 1.52 | Non | Non |
| VL | 50.00 | 0.50 | Non | Non |
| VM | 50.00 | 0.88 | Non | Non |
| VN | 50.00 | 2.53 | Non | Non |
| VPG | 66.67 | 1.17 | Non | Non |
| VR | 50.00 | 1.78 | Non | Non |
| VSG | 100.00 | 2.27 | Non | Non |
| VW | 50.00 | −0.77 | Non | Non |
| VWK | 33.33 | −0.81 | Non | Non |
| VY | 100.00 | 0.25 | Non | Non |
| WG | 50.00 | −0.11 | Non | Non |
| WY | 50.00 | −1.29 | Non | Non |
| HN | 50.00 | 1.98 | Non | Non |
| PT | 50.00 | 1.89 | Non | Non |
| Peptide | Mass | pI | Net Charge | Hydrophobicity (kcal/mol) | HIA Probability | HOB (%) |
|---|---|---|---|---|---|---|
| IS | 218.1263 | 5.46 | 0 | 7.24 | 1 | 88 |
| IT | 232.1419 | 5.36 | 0 | 7.03 | 1 | 83 |
| SL | 218.1263 | 5.50 | 0 | 7.11 | 1 | 87 |
| SM | 236.0828 | 5.33 | 0 | 7.69 | 1 | 82 |
| SN | 219.0853 | 5.34 | 0 | 9.21 | 1 | 77 |
| VM | 248.1191 | 5.40 | 0 | 6.77 | 1 | 91 |
| VN | 231.1216 | 5.41 | 0 | 8.29 | 1 | 86 |
| VY | 280.1419 | 5.45 | 0 | 6.73 | 1 | 74 |
| HN | 269.1122 | 7.69 | 0 | 11.08 | 1 | 61 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mei, Y.; Wu, X.; Xie, R.; Wu, Y.; Du, H.; Chen, W.; Hu, J.; Zhao, K.; Guo, R.; Zhang, J. Identification of Novel Umami Peptides from Yak Bone Collagen and Mechanism Exploration Through In Silico Discovery, Molecular Docking, and Electronic Tongue. Foods 2025, 14, 4057. https://doi.org/10.3390/foods14234057
Mei Y, Wu X, Xie R, Wu Y, Du H, Chen W, Hu J, Zhao K, Guo R, Zhang J. Identification of Novel Umami Peptides from Yak Bone Collagen and Mechanism Exploration Through In Silico Discovery, Molecular Docking, and Electronic Tongue. Foods. 2025; 14(23):4057. https://doi.org/10.3390/foods14234057
Chicago/Turabian StyleMei, Yimeng, Xiaoli Wu, Ruoyu Xie, Yulong Wu, Hongying Du, Wenxuan Chen, Jun Hu, Ke Zhao, Runfang Guo, and Jin Zhang. 2025. "Identification of Novel Umami Peptides from Yak Bone Collagen and Mechanism Exploration Through In Silico Discovery, Molecular Docking, and Electronic Tongue" Foods 14, no. 23: 4057. https://doi.org/10.3390/foods14234057
APA StyleMei, Y., Wu, X., Xie, R., Wu, Y., Du, H., Chen, W., Hu, J., Zhao, K., Guo, R., & Zhang, J. (2025). Identification of Novel Umami Peptides from Yak Bone Collagen and Mechanism Exploration Through In Silico Discovery, Molecular Docking, and Electronic Tongue. Foods, 14(23), 4057. https://doi.org/10.3390/foods14234057





