Valorization of Brewer’s Spent Grain Liquid Fraction for the Development of a Pasteurized Strawberry-Based Blend Juice
Abstract
1. Introduction
2. Materials and Methods
2.1. BSG Liquid Fraction, Strawberry Pulp, and Blend Juice
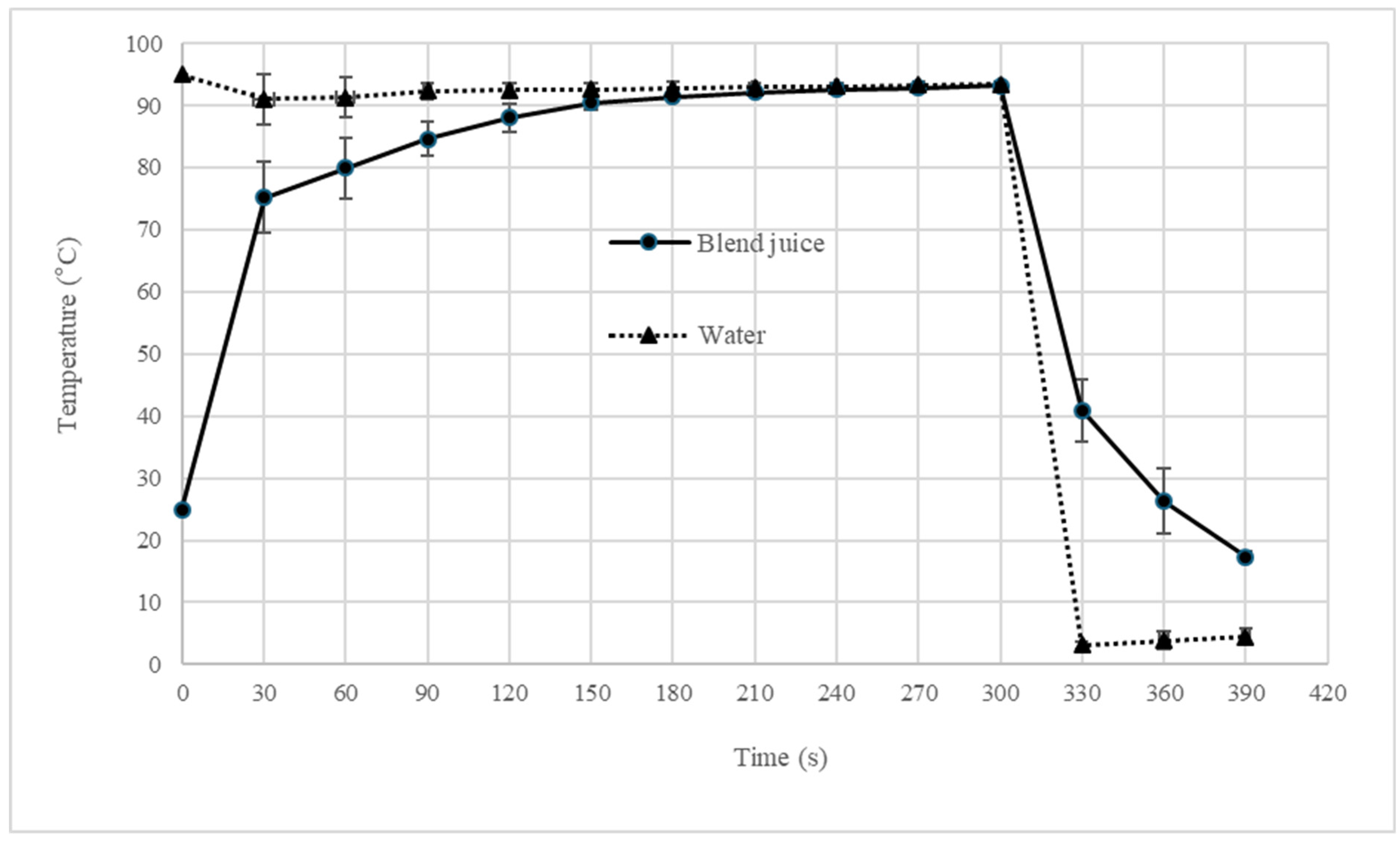
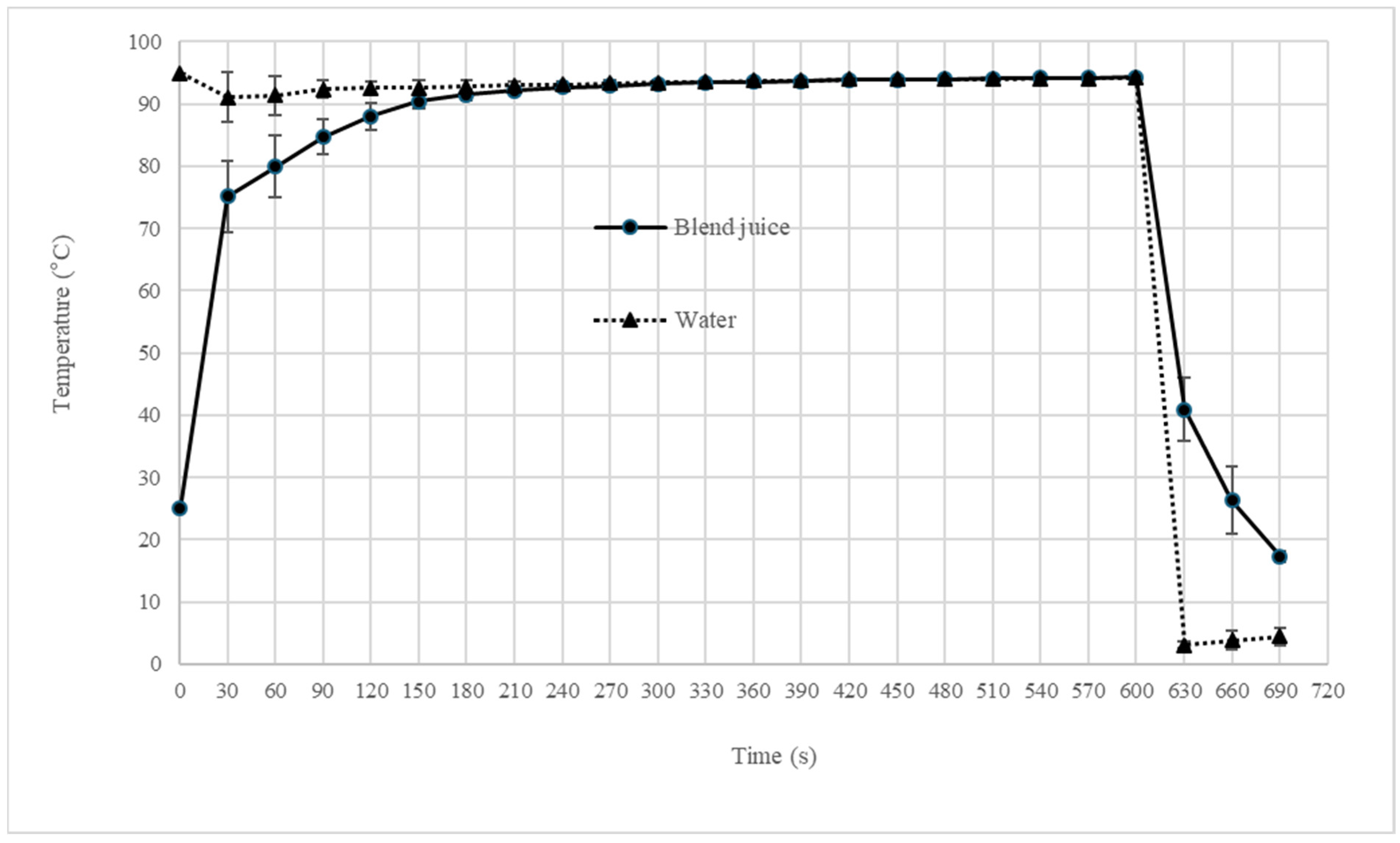
2.2. Blend Juice Pasteurization
2.3. Shelf-Life of Pasteurized Blend Juice
2.4. Analysis
2.4.1. Moisture Content
2.4.2. Instrumental Color
2.4.3. Soluble Solids
2.4.4. Reducing Sugar Content
2.4.5. pH
2.4.6. Titratable Acidity (TA)
2.4.7. Total Polyphenol Content (TPC)
2.4.8. Ascorbic Acid Content
2.4.9. Microbiology Determination
2.5. Sugar Characterization by HPLC
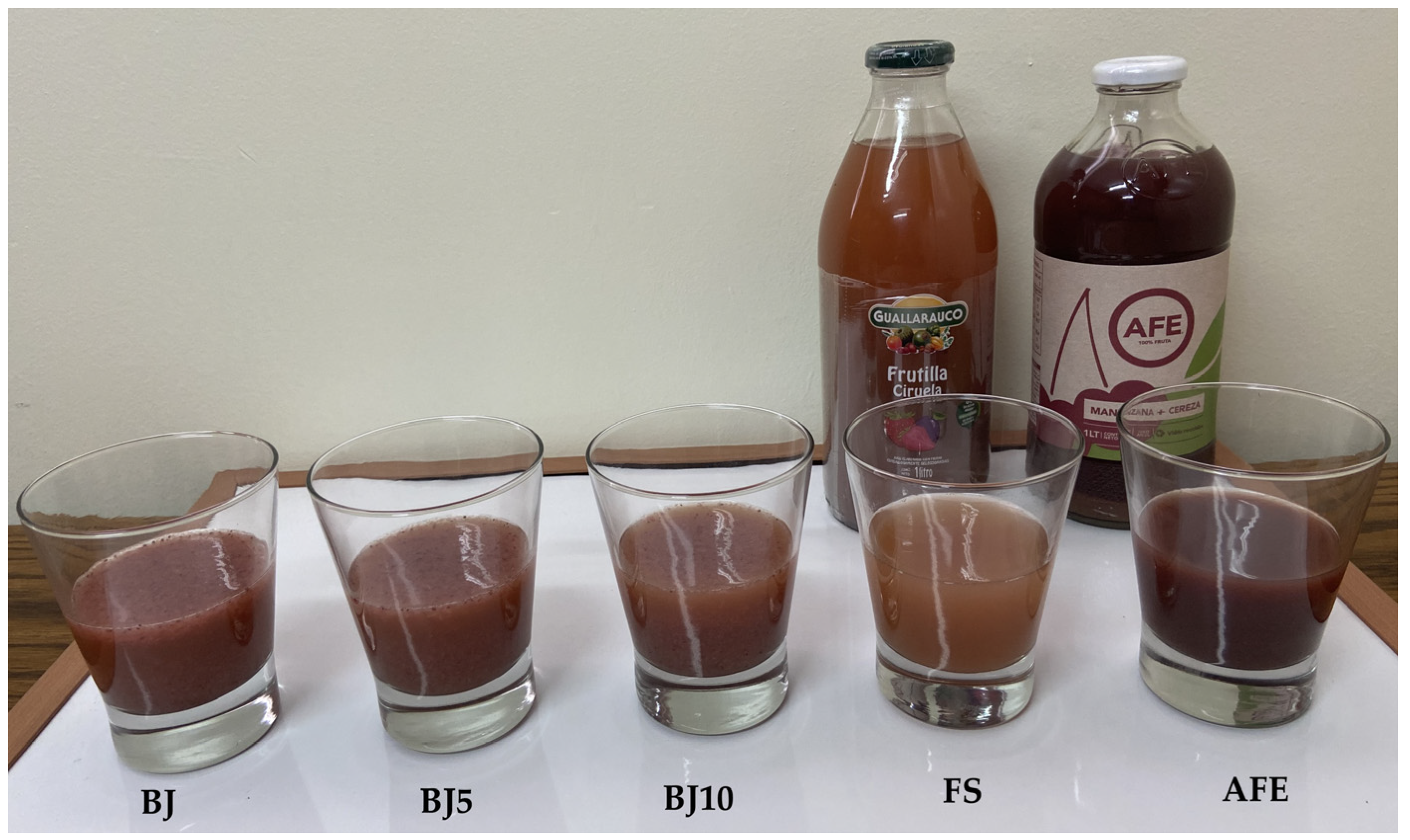
2.6. Sensory Evaluation
2.7. Statistical Analysis
3. Results and Discussion
3.1. Characterization of BSG Liquid Fraction, Strawberry Pulp, and Blend Juice
3.2. Effect of Pasteurization on Blend Juice
3.3. Sensory Evaluation of Blend Juices
3.4. Shelf-Life of Pasteurized Blend Juice
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lynch, K.M.; Steffen, E.J.; Arendt, E.K. Brewers’ spent grain: A review with an emphasis on food and health. J. Inst. Brew. 2016, 122, 553–568. [Google Scholar] [CrossRef]
- Naibaho, J.; Korzeniowska, M.; Sitanggang, A.B.; Lu, Y.; Julianti, E. Brewers’ spent grain as a food ingredient: Techno-processing properties, nutrition, acceptability, and market. Trends Food Sci. Technol. 2024, 152, 104685. [Google Scholar] [CrossRef]
- Ravanal, M.C.; Doussoulin, J.P.; Mougenot, B. Does sustainability matter in the global beer industry? Bibliometrics trends in recycling and the circular economy. Front. Sustain. Food Syst. 2024, 8, 1437910. [Google Scholar] [CrossRef]
- Verni, M.; Pontonio, E.; Krona, A.; Jacob, S.; Pinto, D.; Rinaldi, F.; Verardo, V.; Díaz-de-Cerio, E.; Coda, R.; Rizzello, C.G. Bioprocessing of brewers’ spent grain enhances its antioxidant activity: Characterization of phenolic compounds and bioactive peptides. Front. Microbiol. 2020, 11, 1831. [Google Scholar] [CrossRef]
- Finley, J.W.; Walkera, C.E.; Hautala, E. Utilisation of press water from brewer’s spent grains. J. Sci. Food Agric. 1976, 27, 655–660. [Google Scholar] [CrossRef]
- El-Shafey, E.I.; Gameiro, M.L.F.; Correia, P.F.M.; De Carvalho, J.M.R. Dewatering of brewer’s spent grain using a membrane filter press: A pilot plant study. Sep. Sci. Technol. 2004, 39, 3237–3261. [Google Scholar] [CrossRef]
- Machado, R.M.; Rodrigues, R.A.D.; Henriques, C.M.C.; Gameiro, M.L.F.; Ismael, M.R.C.; Reis, M.T.A.; Freire, J.P.B.; Carvalho, J.M.R. Dewatering of brewer’s spent grain using an integrated membrane filter press with vacuum drying capabilities. Sep. Sci. Technol. 2016, 51, 692–700. [Google Scholar] [CrossRef]
- Ruíz, C.; Schalchli, H.; Melo, P.S.; Moreira, C.d.S.; Sartori, A.d.G.O.; de Alencar, S.M.; Scheuermann, E.S. Effect of physical separation with ultrasound application on brewers’ spent grain to obtain powders for potential application in foodstuffs. Foods 2024, 13, 3000. [Google Scholar] [CrossRef] [PubMed]
- Madsen, S.K.; Priess, C.A.P.; Wätjen, S.; Øzmerih, M.A.; Mohammadifar, C.; Bang-Berthelsen, H. Development of a yoghurt alternative, based on plant-adapted lactic acid bacteria, soy drink and the liquid fraction of brewers’ spent grain. FEMS Microbiol. Lett. 2021, 368, fnab093. [Google Scholar] [CrossRef]
- Akermann, A.; Weiermüller, J.; Christmann, J.; Guirande, L.; Glaser, G.; Knaus, A.; Ulber, R. Brewers’ spent grain liquor as a feedstock for lactate production with Lactobacillus delbrueckii subsp. Lactis. Eng. Life Sci. 2019, 20, 168–180. [Google Scholar] [CrossRef]
- Shetty, R.; Petersen, F.R.; Podduturi, R.; Molina, G.E.S.; Wätjen, A.P.; Madsen, S.K.; Zioga, E.; Øzmerih, S.; Hobley, T.J.; Bang-Berthelsen, C.H. Fermentation of brewer’s spent grain liquids to increase shelf life and give an organic acid enhanced ingredient. LWT–Food Sci. Technol. 2023, 182, 114911. [Google Scholar] [CrossRef]
- Bjerregaard, M.F.; Charalampidis, A.; Frøding, R.; Shetty, R.; Pastell, H.; Jacobsen, C.; Zhuang, S.; Pinelo, M.; Hansen, P.B.; Hobley, T.J. Processing of brewing by-products to give food ingredient streams. Eur. Food Res. Technol. 2018, 245, 545–558. [Google Scholar] [CrossRef]
- Herbst, G.; Hamerski, F.; Errico, M.; Corazza, M.L. Pressurized liquid extraction of brewer’s spent grain: Kinetics and crude extracts characterization. J. Ind. Eng. Chem. 2021, 102, 370–383. [Google Scholar] [CrossRef]
- Lee, Y.C.; Lee, S.H. Flavor quality of concentrated strawberry pulp with aroma recovery. J. Food Qual. 1992, 1992, 15321–15332. [Google Scholar] [CrossRef]
- Gonçalves, G.A.S.; Resende, N.S.; Carvalho, E.E.N.; de Resende, J.V.; Vilas Boas, E.V. de B. Effect of pasteurisation and freezing method on bioactive compounds and antioxidant activity of strawberry pulp. Int. J. Food Sci. Nutr. 2017, 68, 682–694. [Google Scholar] [CrossRef]
- López-Ortiz, A.; Salgado, M.N.; Nair, P.K.; Balbuena, A.O.; Méndez-Lagunas, L.L.; Hernández-Díaz, W.N.; Guerrero, L.L. Improved preservation of the color and bioactive compounds in strawberry pulp dried under UV-Blue blocked solar radiation. Clean. Circ. Bioecon. 2024, 9, 100112. [Google Scholar] [CrossRef]
- Jensen, G.S.; Wu, X.; Patterson, K.M.; Barnes, J.; Carter, S.G. In vitro and in vivo antioxidant and anti-inflammatory capacities of an antioxidant-rich fruit and berry juice blend. Results of a pilot and randomized, double-blinded, placebo-controlled, crossover study. J. Agric. Food Chem. 2008, 56, 8326–8333. [Google Scholar] [CrossRef] [PubMed]
- Jensen, G.S.; Ager, D.M.; Redman, K.A.; Mitzner, M.A.; Benson, K.F. Pain reduction and improvement in range of motion after daily consumption of an açai (Euterpe oleracea Mart.) pulp-fortified polyphenolic-rich fruit and berry juice blend. J. Med. Food 2011, 14, 702–711. [Google Scholar] [CrossRef]
- Atef, A.M.; Nadir, A.S.; Mostafa, T.R. Studies on sheets properties made from juice and puree of pumpkin and some other fruit blends. J. Appl. Sci. Res. 2012, 8, 2632–2639. [Google Scholar]
- Salgado, J.M.; Ferreira, T.R.B.; Biazotto, F.O.; Dias, C.T.S. Increased antioxidant content in juice enriched with dried extract of pomegranate (Punica granatum) peel. Plant Foods Hum. Nutr. 2012, 67, 39–43. [Google Scholar] [CrossRef] [PubMed]
- Feng, X.; Zhou, Z.; Wang, X.; Bi, X.; Ma, Y. Comparison of high hydrostatic pressure, ultrasound, and heat treatments on the quality of strawberry–apple–lemon juice blend. Foods 2020, 9, 218. [Google Scholar] [CrossRef]
- Xue, L.; Zheng, Z.; Wu, Y.; Zhang, L.; Zhang, H.; Yang, N.; Xu, X.; Jin, Y.; Meng, M.; Wang, F. Induced electric field as alternative pasteurization to improve microbiological safety and quality of bayberry juice. Food Chem. 2025, 463, 141137. [Google Scholar] [CrossRef]
- Instituto de Salud Pública de Chile. Manual Métodos de Análisis Físico-Químicos de Alimentos, Aguas y Suelo; Andros Ltd.: London, UK, 1998; pp. 13–14. [Google Scholar]
- Ihl, M.; Aravena, L.; Scheuermann, E.; Uquiche, E.; Bifani, V. Effect of immersion solutions on shelf-life of minimally processed lettuce. LWT-Food Sci. Technol. 2003, 36, 591–599. [Google Scholar] [CrossRef]
- Pathare, P.B.; Opara, U.L.; Al-Said, F.A.J. Colour measurement and analysis in fresh and processed foods: A review. Food Bioprocess Technol. 2013, 6, 36–60. [Google Scholar] [CrossRef]
- Adekunte, A.; Tiwari, B.; Cullen, P.; Scannell, A.; O’Donnell, C. Effect of sonication on colour, ascorbic acid and yeast inactivation in tomato juice. Food Chem. 2010, 122, 500–507. [Google Scholar] [CrossRef]
- Scheuermann, E.; Ihl, M.; Beraud, L.; Quiroz, A.; Salvo, S.; Alfaro, S.; Bustos, R.O.; Seguel, I. Effects of packaging and preservation treatments on the shelf life of murtilla fruit (Ugni molinae Turcz) in cold storage. Packag. Technol. Sci. 2013, 27, 241–248. [Google Scholar] [CrossRef]
- Miller, G.L. Use of dinitrosalicylic acid reagent for determination of reducing sugar. Anal. Chem. 1959, 31, 426–428. [Google Scholar] [CrossRef]
- Pirce, F.; Vieira, T.M.F.S.; Augusto-Obara, T.R.; de Alencar, S.M.; Romero, F.; Scheuermann, E. Effects of convective drying assisted by ultrasound and osmotic solution on polyphenol, antioxidant and microstructure of murtilla (Ugni molinae Turcz) fruit. J. Food Sci. Technol. 2021, 58, 138–146. [Google Scholar] [CrossRef] [PubMed]
- Instituto de Salud Pública de Chile. Manual de Técnicas Microbiológicas Para Alimentos y Aguas; Andros Ltd.: London, UK, 1998; pp. 14–17. [Google Scholar]
- Choo, Y.X.; Teh, L.K.; Tan, C.X. Effects of sonication and thermal pasteurization on the nutritional, antioxidant, and microbial properties of noni juice. Molecules 2023, 28, 313. [Google Scholar] [CrossRef]
- Lepaus, B.M.; Santos, A.K.P.d.O.; Spaviero, A.F.; Daud, P.S.; de São José, J.F.B. Thermosonication of orange-carrot juice blend: Overall quality during refrigerated storage, and sensory acceptance. Molecules 2023, 28, 2196. [Google Scholar] [CrossRef] [PubMed]
- Cendrowski, A.; Przybył, J.L.; Studnicki, M. Physicochemical characteristics, vitamin C, total polyphenols, antioxidant capacity, and sensory preference of mixed juices prepared with rose fruits (Rosa rugosa) and apple or strawberry. Appl. Sci. 2024, 14, 113. [Google Scholar] [CrossRef]
- Carciochi, R.A.; Sologubik, C.A.; Fernández, M.B.; Manrique, G.D.; D’Alessandro, L.G. Extraction of antioxidant phenolic compounds from brewer’s spent grain: Optimization and kinetics modeling. Antioxidants 2018, 7, 45. [Google Scholar] [CrossRef]
- Meneses, N.G.T.; Martins, S.; Teixeira, J.A.; Mussatto, S.I. Influence of extraction solvents on the recovery of antioxidant phenolic compounds from brewer’s spent grains. Sep. Purif. Technol. 2013, 108, 152–158. [Google Scholar] [CrossRef]
- Bonifácio-Lopes, T.; Castro, L.M.G.; Vilas-Boas, A.; Campos, D.; Teixeira, J.A.; Pintado, M. Impact of gastrointestinal digestion simulation on brewer’s spent grain green extracts and their prebiotic activity. Food Res. Int. 2023, 165, 112515. [Google Scholar] [CrossRef]
- Ogundele, O.M.A.; Awolu, O.O.; Badejo, A.A.; Nwachukwu, I.D.; Fagbemi, T.N. Development of functional beverages from blends of Hibiscus sabdariffa extract and selected fruit juices for optimal antioxidant properties. Food Sci. Nutr. 2016, 4, 679–685. [Google Scholar] [CrossRef] [PubMed]
- Prandi, B.; Ferri, M.; Monari, S.; Zurlini, C.; Cigognini, I.; Verstringe, S.; Schaller, D.; Walter, M.; Navarini, L.; Tassoni, A.; et al. Extraction and chemical characterization of functional phenols and proteins from coffee (Coffea arabica) by-products. Biomolecules 2021, 11, 1571. [Google Scholar] [CrossRef] [PubMed]
- Robertson, J.A.; I’Anson, K.J.; Treimo, J.; Faulds, C.B.; Brocklehurst, T.F.; Eijsink, V.G.H.; Waldron, K.W. Profiling brewers’ spent grain for composition and microbial ecology at the site of production. LWT–Food Sci. Technol. 2010, 43, 890–896. [Google Scholar] [CrossRef]
- Basiony, M.; Saleh, A.; Hassabo, R.; AL-Fargah, A. The effect of using pomegranate and strawberry juices with red beet puree on the physicochemical, microbial and sensory properties of yoghurt. J. Food Meas. Charact. 2023, 17, 5024–5033. [Google Scholar] [CrossRef]
- Moutaouakil, S.E.L.; Madhi, Y.E.L.; Inekach, S.; Chauiyakh, O.; SBAI, N.; Benzakour, A.; Ouhssine, M. Evaluation of the physicochemical and microbiological quality of strawberry pulp in a moroccan food industry company. Res. J. Pharm. Technol. 2024, 17, 2505–2509. [Google Scholar] [CrossRef]
- Radziejewska-Kubzdela, E. Effect of ultrasonic, thermal and enzymatic treatment of mash on yield and content of bioactive compounds in strawberry juice. Appl. Sci. 2023, 13, 4268. [Google Scholar] [CrossRef]
- Kanwal, F.; Ren, D.; Kanwal, W.; Ding, M.; Su, J.; Shang, X. The potential role of nondigestible raffinose family oligosaccharides as prebiotics. Glycobiology 2023, 33, 274–288. [Google Scholar] [CrossRef] [PubMed]
- Mandha, J.; Shumoy, H.; Matemu, A.O.; Raes, K. Characterization of fruit juices and effect of pasteurization and storage conditions on their microbial, physicochemical, and nutritional quality. Food Biosci. 2023, 51, 102335. [Google Scholar] [CrossRef]
| Parameter | BSG-LF | SP | BJ |
|---|---|---|---|
| Moisture content (%, w.b.) | 87.0 ± 0.1 a | 69.2 ± 0.1 c | 82.4 ± 0.1 b |
| Color | |||
| L* | 43.7 ± 4.3 a | 30.1 ± 0.6 b | 34.4 ± 0.4 b |
| a* | 0.2 ± 0.3 c | 15.9 ± 0.8 a | 8.9 ± 0.1 b |
| b* | 12.9 ± 0.2 a | 11.4 ± 0.4 b | 10.6 ± 0.5 b |
| ΔE | 13.2 ± 2.9 a | 8.4 ± 0.4 a | - |
| Soluble solid (°Brix) | 13.5 ± 0.1 c | 29.1 ± 0.1 a | 17.2 ± 0.2 b |
| Reducing sugar (g 100 g−1 d.m.) | 65.6 ± 1.3 a | 20.9 ± 0.1 c | 47.4 ± 0.8 b |
| pH | 6.26 ± 0.09 a | 3.64 ± 0.16 c | 4.28 ± 0.03 c |
| Titratable acidity (%) | 0.065 ± 0.007 c | 0.663 ± 0.067 a | 0.243 ± 0.002 b |
| TPC (mg GAE 100 g−1 d.m.) | 169.4 ± 13.2 b | 256.6 ± 10.3 a | 181.2 ± 3.3 b |
| Ascorbic acid (mg 100 g−1 d.m.) | <16.7 | 30.9 ± 3.2 a | 23.2 ± 1.5 b |
| Microbiology | |||
| AMB (log CFU mL−1) | 4.8 ± 0.1 a | Absent | 3.5 ± 0.0 b |
| YM (log CFU mL−1) | Absent | Absent | Absent |
| Sample | Fructose | Glucose | Maltose | Raffinose |
|---|---|---|---|---|
| BSG-LF | n.d. | 12.16 ± 0.16 d | 61.83 ± 0.72 a | 17.14 ± 0.39 a |
| SP | 28.50 ± 0.76 a | 31.05 ± 1.12 a | n.d. | n.d. |
| BJ | 11.22 ± 0.70 b | 23.87 ± 0.54 b | 34.58 ± 1.06 b | 11.84 ± 1.20 b |
| BJ5 | 10.44 ± 0.42 b | 19.26 ± 0.46 c | 31.75 ± 0.96 c | 10.92 ± 0.86 b |
| BJ10 | 11.19 ± 0.64 b | 20.82 ± 1.02 c | 34.50 ± 1.68 b | 11.48 ± 0.61 b |
| Parameter | BJ | BJ5 | BJ10 |
|---|---|---|---|
| Moisture content (%, w.b.) | 82.4 ± 0.1 a | 81.9 ± 0.1 b | 81.7 ± 0.1 b |
| Color | |||
| L* | 34.4 ± 0.4 a | 33.5 ± 0.6 a | 33.9 ± 1.0 a |
| a* | 8.9 ± 0.1 a | 8.7 ± 0.6 a | 8.7 ± 0.7 a |
| b* | 10.6 ± 0.5 a | 9.4 ± 0.8 a | 9.9 ± 0.7 a |
| ΔE | - | 1.7 ± 0.5 a | 1.2 ± 0.3 a |
| Soluble solid (° Brix) | 17.2 ± 0.2 b | 17.7 ± 0.2 a | 18.0 ± 0.0 a |
| Reducing sugar (g 100 g−1 d.m.) | 47.4 ± 0.8 a | 48.9 ± 2.9 a | 49.4 ± 3.4 a |
| pH | 4.28 ± 0.03 a | 4.18 ± 0.02 ab | 4.14 ± 0.07 b |
| Titratable acidity (%) | 0.243 ± 0.002 a | 0.259 ± 0.031 a | 0.260 ± 0.030 a |
| TPC (mg GAE 100 g−1 d.m.) | 181.2 ± 3.3 ab | 172.8 ± 4.4 b | 186.8 ± 1.8 a |
| Ascorbic acid (mg 100 g−1 d.m.) | 23.2 ± 1.5 a | 27.9 ± 2.5 a | 26.7 ± 3.6 a |
| Microbiology | |||
| AMB (log CFU mL−1) | 3.5 ± 0.0 | Absent | Absent |
| YM (log CFU mL−1) | Absent | Absent | Absent |
| Sample | Color | Appearance | Consistency | Aroma | Taste | Overall Acceptance | Purchase Intent |
|---|---|---|---|---|---|---|---|
| BJ | 7.2 ± 1.3 a | 6.5 ± 1.4 a | 6.9 ± 1.1 a | 6.8 ± 1.4 a | 7.2 ± 1.5 a | 6.7 ± 1.5 a | 6.7 ± 1.5 a |
| BJ5 | 7.0 ± 1.5 a | 6.1 ± 1.8 a | 6.5 ± 1.4 a | 6.6 ± 1.6 a | 6.9 ± 1.6 ab | 6.7 ± 1.5 a | 6.5 ± 1.3 a |
| BJ10 | 6.7 ± 1.6 ab | 6.0 ± 2.0 a | 6.7 ± 1.3 a | 6.5 ± 1.4 a | 6.5 ± 1.2 ab | 6.6 ± 1.4 a | 6.2 ± 1.7 a |
| FS | 5.7 ± 1.7 b | 5.8 ± 1.6 a | 6.8 ± 1.6 a | 6.5 ± 1.6 a | 5.8 ± 1.8 b | 6.2 ± 1.8 a | 5.6 ± 2.3 a |
| AFE | 6.4 ± 2.0 ab | 6.2 ± 2.2 a | 6.8 ± 1.5 a | 6.2 ± 1.4 a | 6.3 ± 1.6 ab | 6.2 ± 1.7 a | 5.7 ± 2.1 a |
| Parameter | Immediately After Pasteurized | Storage Time at 25 °C (Week) | ||||
|---|---|---|---|---|---|---|
| 1 | 2 | 4 | 8 | |||
| Moisture content (%, w.b.) | BJ5 | 81.9 ± 0.1 aA | 81.6 ± 0.0 abA | 81.3 ± 0.2 bA | 81.7 ± 0.1 abA | 81.4 ± 0.2 bA |
| BJ10 | 81.7 ± 0.1 aA | 80.7 ± 0.2 cA | 80.9 ± 0.2 bcA | 81.4 ± 0.0 abA | 80.9 ± 0.0 bcA | |
| Color | ||||||
| L* | BJ5 | 33.5 ± 0.6 aA | 36.1 ± 2.6 aA | 34.3 ± 2.0 aA | 34.9 ± 1.7 aA | 34.6 ± 0.4 aA |
| BJ10 | 33.9 ± 1.0 bA | 37.3 ± 2.6 aA | 33.9 ± 0.7 bA | 33.6 ± 1.0 bA | 33.4 ± 0.6 bB | |
| a* | BJ5 | 8.7 ± 0.6 aA | 8.5 ± 0.5 aA | 7.3 ± 0.8 bA | 6.5 ± 0.5 bB | 6.8 ± 0.5 bA |
| BJ10 | 8.7 ± 0.7 abA | 9.0 ± 0.7 aA | 7.6 ± 0.5 bcA | 7.1 ± 0.4 cA | 7.1 ± 0.6 cA | |
| b* | BJ5 | 9.4 ± 0.8 aA | 10.5 ± 2.3 aA | 8.1 ± 0.5 aA | 9.7 ± 2.2 aA | 10.1 ± 1.6 aA |
| BJ10 | 9.9 ± 0.7 aA | 12.1 ± 2.6 aA | 9.3 ± 1.2 aA | 9.8 ± 1.7 aA | 9.8 ± 0.4 aA | |
| ΔE | BJ5 | - | 2.9 ± 2.5 aA | 2.7 ± 1.4 aA | 3.6 ± 2.1 aA | 3.2 ± 0.7 aA |
| BJ10 | - | 4.4 ± 2.6 aA | 1.8 ± 0.9 aA | 2.5 ± 0.7 aA | 2.0 ± 1.0 aA | |
| Soluble solid (°Brix) | BJ5 | 17.7 ± 0.2 bcA | 17.5 ± 0.0 cB | 18.0 ± 0.1 abB | 18.2 ± 0.3 aA | 18.3 ± 0.2 aB |
| BJ10 | 18.0 ± 0.0 dA | 19.1 ± 0.1 aA | 18.9 ± 0.1 bA | 18.5 ± 0.2 cA | 19.0 ± 0.1 abA | |
| Reducing sugar (g 100 g−1 d.m.) | BJ5 | 48.9 ± 2.9 aA | 46.2 ± 0.4 aA | 48.0 ± 5.2 aA | 49.1 ± 0.9 aA | 47.2 ± 0.7 aB |
| BJ10 | 49.4 ± 3.4 aA | 49.3 ± 3.4 aA | 42.4 ± 0.2 bA | 48.6 ± 1.7 aA | 49.4 ± 0.3 aA | |
| pH | BJ5 | 4.18 ± 0.02 bA | 4.07 ± 0.01 cB | 4.19 ± 0.01 bA | 4.17 ± 0.01 bB | 4.27 ± 0.01 aA |
| BJ10 | 4.14 ± 0.07 aA | 4.17 ± 0.01 aA | 4.17 ± 0.01 aA | 4.24 ± 0.01 aA | 4.27 ± 0.01 aA | |
| Titratable acidity (%) | BJ5 | 0.259 ± 0.031 aA | 0.254 ± 0.001 aA | 0.247 ± 0.001 aA | 0.242 ± 0.002 aA | 0.252 ± 0.001 aA |
| BJ10 | 0.260 ± 0.030 aA | 0.280 ± 0.001 aB | 0.246 ± 0.002 aA | 0.250 ± 0.000 aA | 0.251 ± 0.001 aA | |
| TPC (mg GAE 100 g−1 d.m.) | BJ5 | 172.8 ± 4.4 aB | 170.0 ± 2.2 aB | 147.8 ± 0.3 bB | 149.0 ± 7.8 bB | 134.0 ± 3.7 cA |
| BJ10 | 186.8 ± 1.8 abA | 195.7 ± 5.1 aA | 160.1 ± 0.0 cA | 175.9 ± 7.5 bA | 136.2 ± 7.8 dA | |
| Ascorbic acid (mg 100 g−1 d.m.) | BJ5 | 27.9 ± 2.5 aA | 23.5 ± 1.3 abA | 21.1 ± 0.9 bA | 22.9 ± 0.9 abA | 22.0 ± 1.5 abA |
| BJ10 | 26.7 ± 3.6 aA | 22.2 ± 1.2 aA | 23.1 ± 1.5 aA | 23.2 ± 0.6 aA | 21.4 ± 0.3 aA | |
| Microbiology | ||||||
| AMB (log CFU mL−1) | BJ5 | Absent | Absent | Absent | Absent | Absent |
| BJ10 | Absent | Absent | Absent | Absent | Absent | |
| YM (log CFU mL−1) | BJ5 | Absent | Absent | Absent | Absent | Absent |
| BJ10 | Absent | Absent | Absent | Absent | Absent | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Neira Monsalve, V.A.; Devia Valenzuela, B.B.; Peña Arias, A.I.; Schalchli Sáez, H.L.; Melo, P.S.; de Souza Moreira, C.; de Oliveira Sartori, A.G.; Vergara Ojeda, C.A.; de Alencar, S.M.; Scheuermann Salinas, E.S. Valorization of Brewer’s Spent Grain Liquid Fraction for the Development of a Pasteurized Strawberry-Based Blend Juice. Foods 2025, 14, 4053. https://doi.org/10.3390/foods14234053
Neira Monsalve VA, Devia Valenzuela BB, Peña Arias AI, Schalchli Sáez HL, Melo PS, de Souza Moreira C, de Oliveira Sartori AG, Vergara Ojeda CA, de Alencar SM, Scheuermann Salinas ES. Valorization of Brewer’s Spent Grain Liquid Fraction for the Development of a Pasteurized Strawberry-Based Blend Juice. Foods. 2025; 14(23):4053. https://doi.org/10.3390/foods14234053
Chicago/Turabian StyleNeira Monsalve, Valentina Ariela, Bastián Benjamín Devia Valenzuela, Alonso Ignacio Peña Arias, Heidi Laura Schalchli Sáez, Priscilla Siqueira Melo, Carolina de Souza Moreira, Alan Giovanini de Oliveira Sartori, Christian Arnoldo Vergara Ojeda, Severino Matias de Alencar, and Erick Sigisfredo Scheuermann Salinas. 2025. "Valorization of Brewer’s Spent Grain Liquid Fraction for the Development of a Pasteurized Strawberry-Based Blend Juice" Foods 14, no. 23: 4053. https://doi.org/10.3390/foods14234053
APA StyleNeira Monsalve, V. A., Devia Valenzuela, B. B., Peña Arias, A. I., Schalchli Sáez, H. L., Melo, P. S., de Souza Moreira, C., de Oliveira Sartori, A. G., Vergara Ojeda, C. A., de Alencar, S. M., & Scheuermann Salinas, E. S. (2025). Valorization of Brewer’s Spent Grain Liquid Fraction for the Development of a Pasteurized Strawberry-Based Blend Juice. Foods, 14(23), 4053. https://doi.org/10.3390/foods14234053







