Effects of Hot-Air Drying Conditions on Quality Attributes of Meat and Shell of Dried Shrimp
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Hot-Air Drying (HAD)
2.3. Moisture Content Determination
2.4. Drying Rate (DR)
2.5. Color Measurements
2.6. Texture Profile Analysis (TPA)
2.7. Rehydration Ratio
2.8. Sensory Evaluation
2.9. E-Nose Analysis
2.10. Microbiological Testing
2.11. Correlation Analysis
2.12. Partial Least Squares Regression
2.13. Levenberg–Marquardt Algorithm Optimized Artificial Neural Network (LM-ANN)
2.14. Statistical Analysis
3. Results
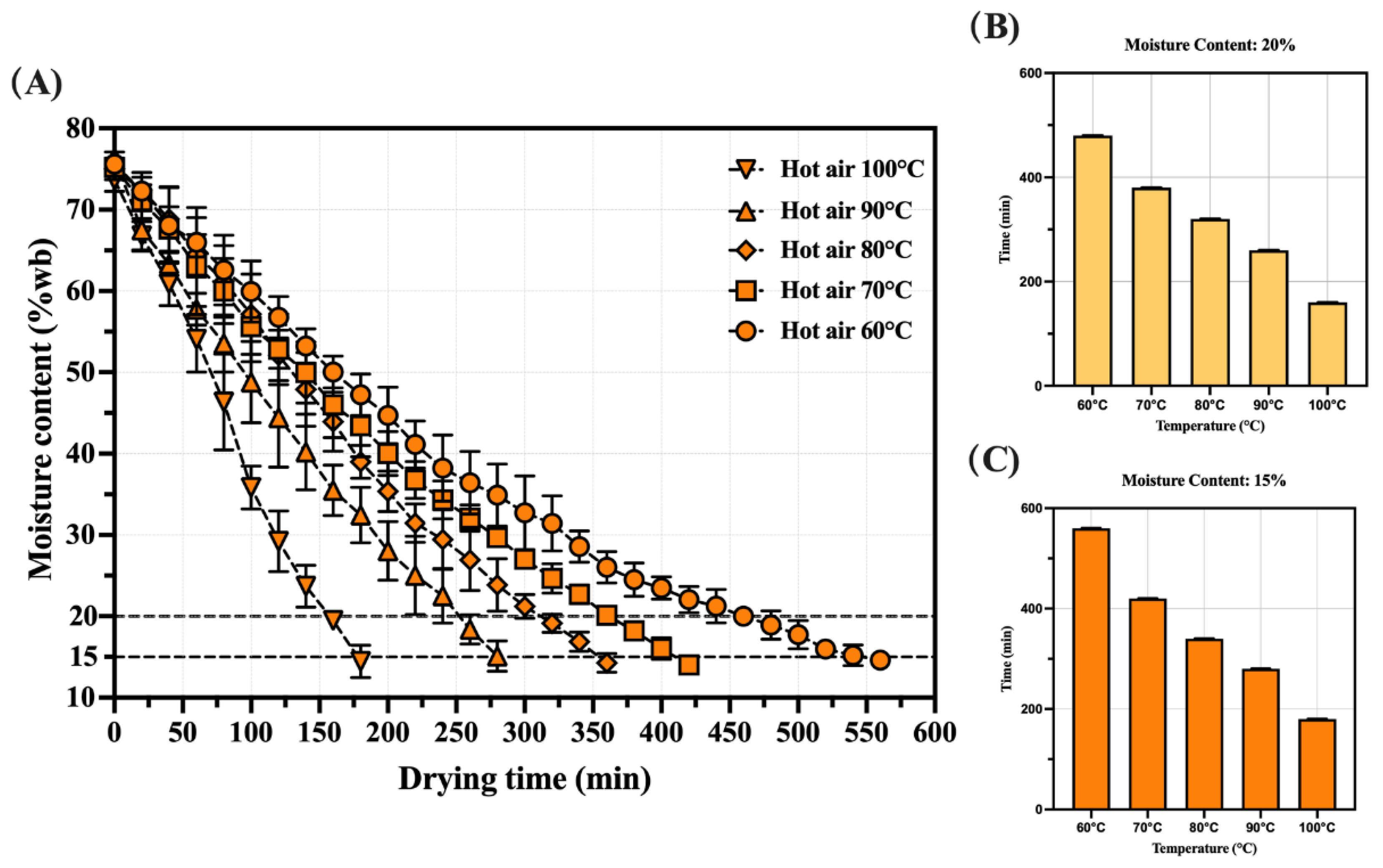
3.1. Characteristics of the Hot-Air Dring Curve for Whole Shrimp
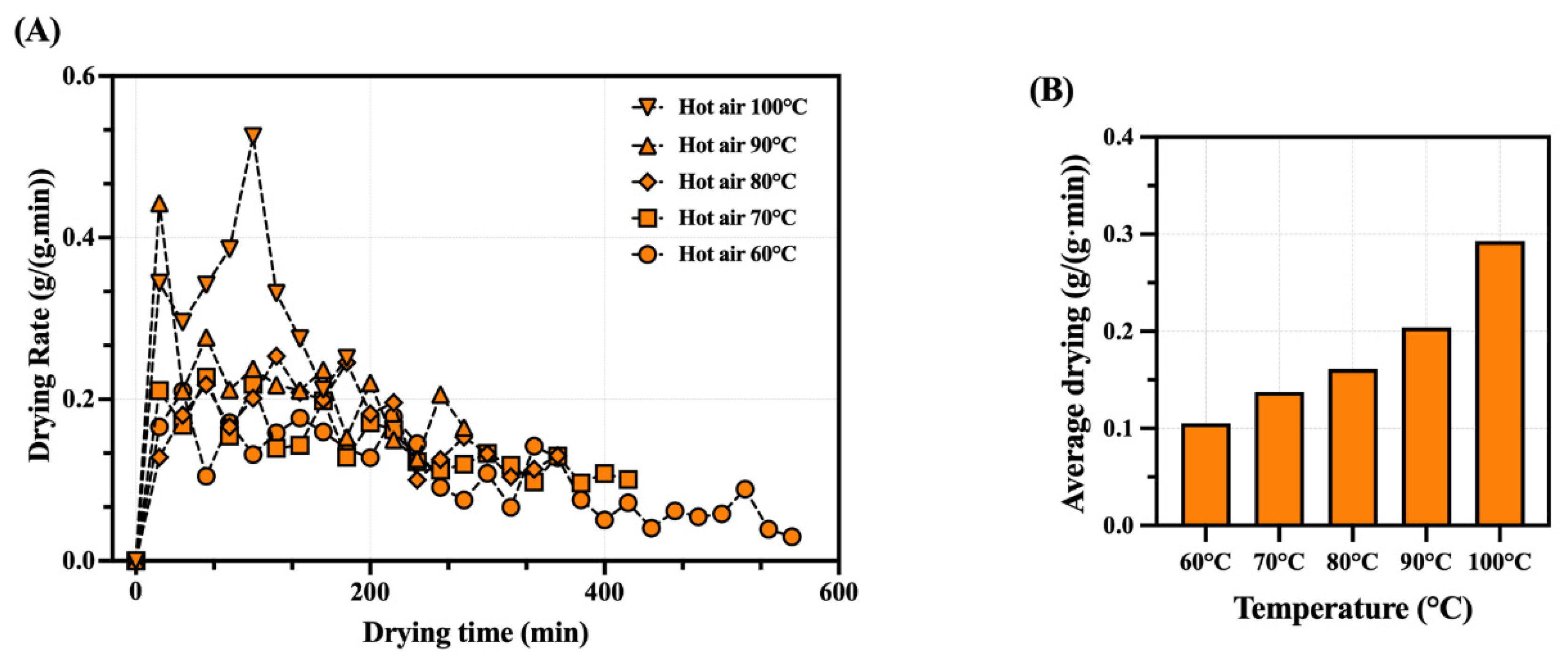
3.2. Drying Rate Characteristics of Whole Dried Shrimp for Hot-Air Drying Treatment
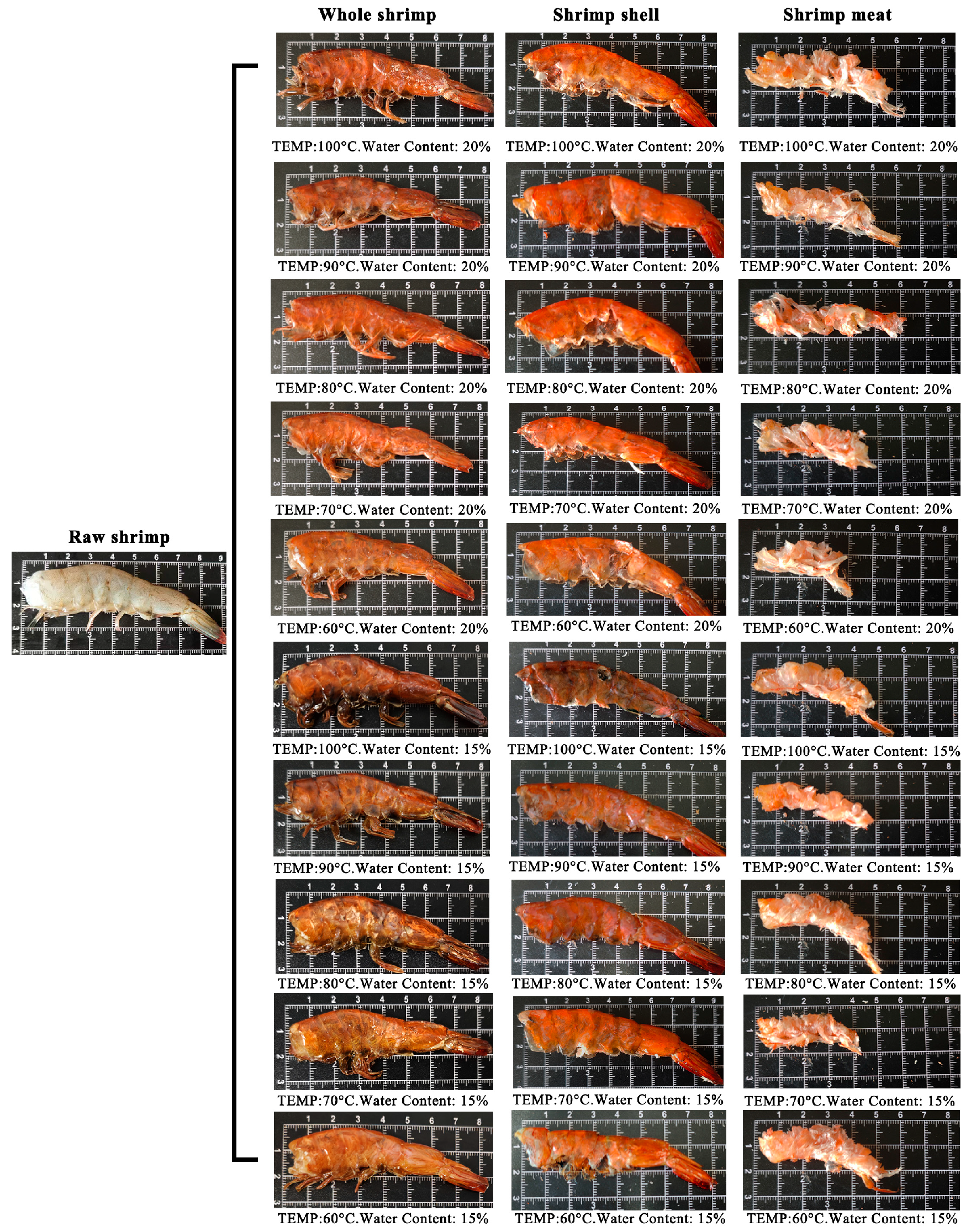
3.3. Morphological Observation of Whole Dried Shrimp and Its Separate Shell/Meat
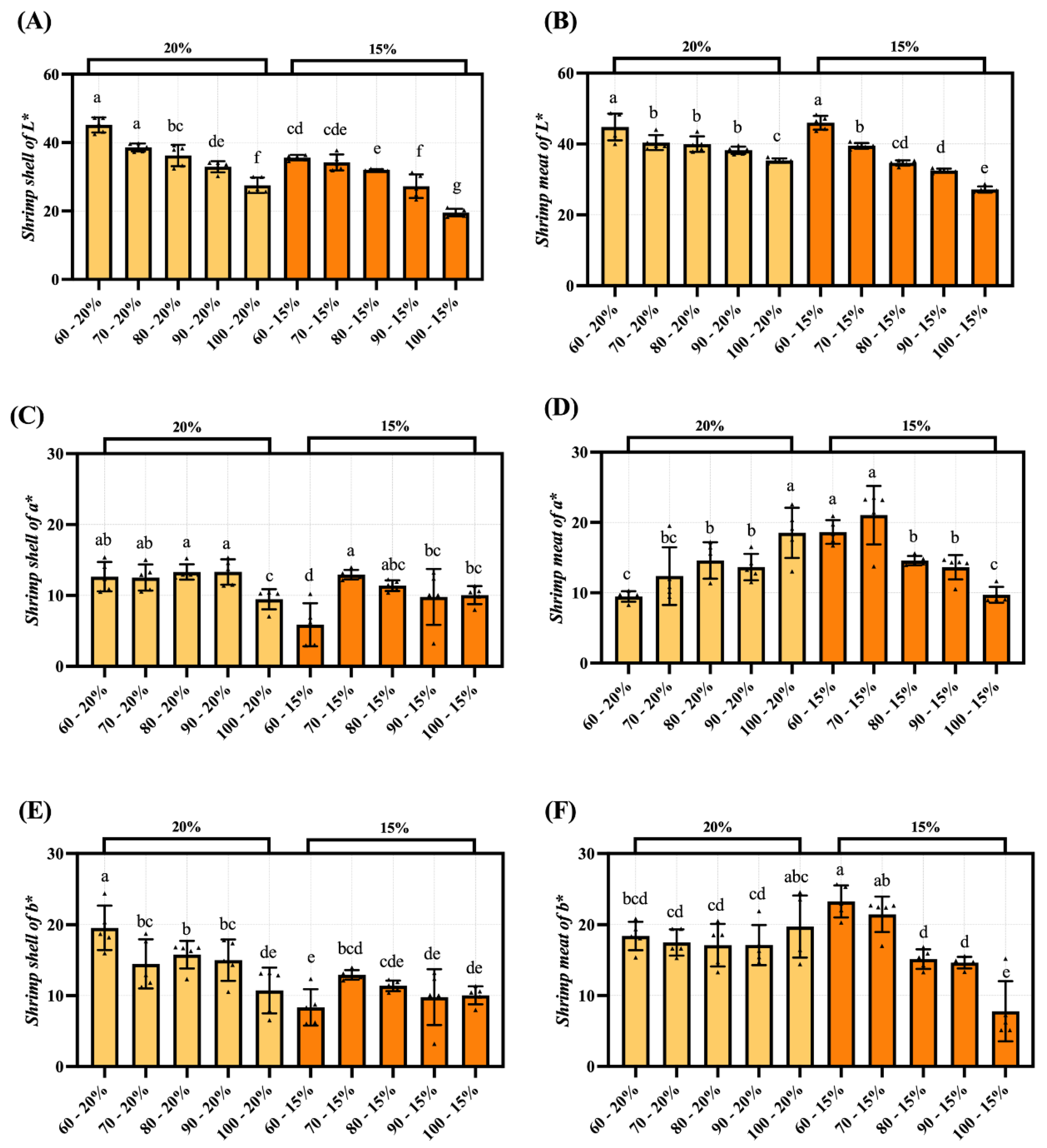
3.4. Color Difference Analysis of Dried Shrimp Shell and Meat
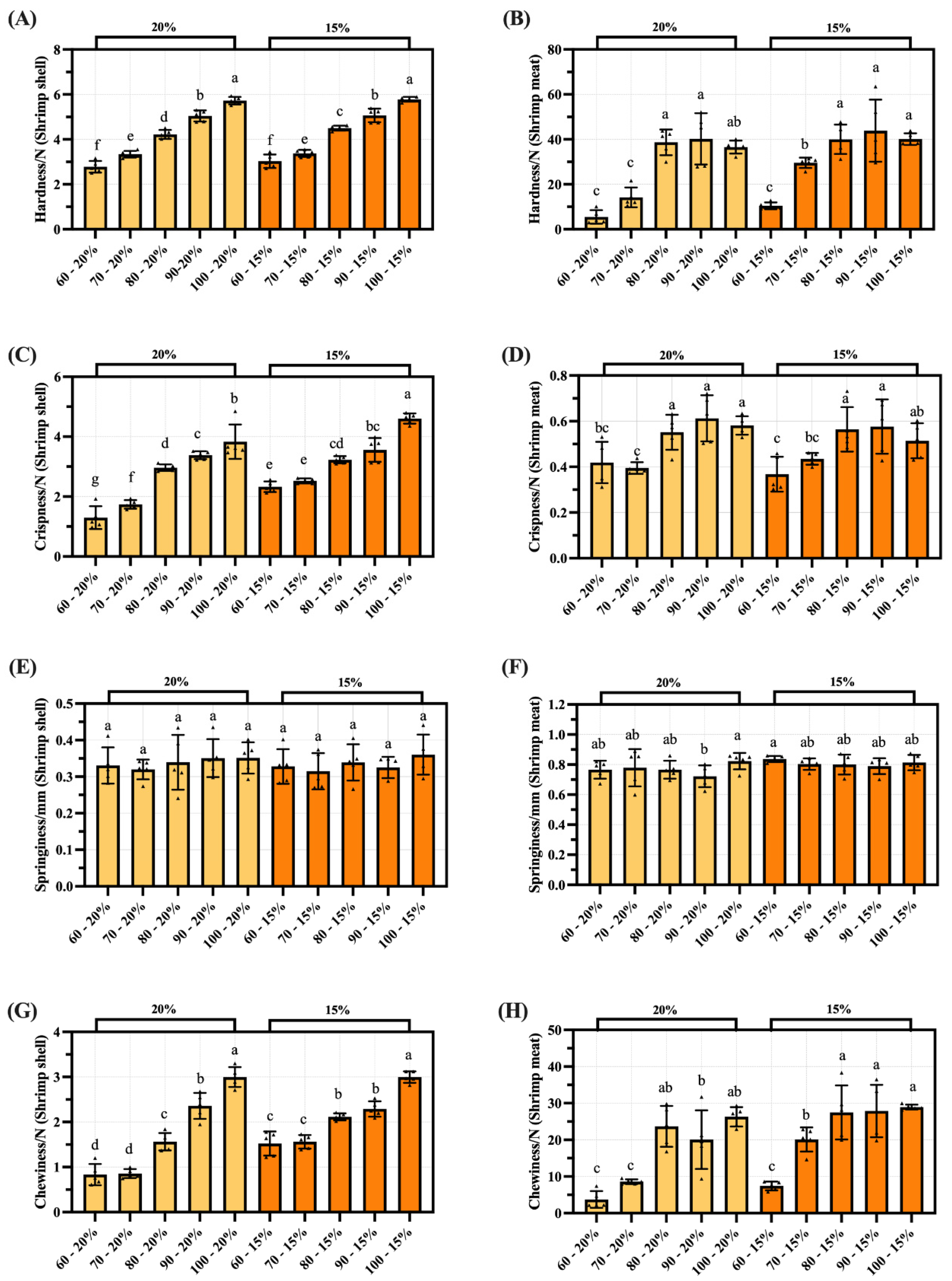
3.5. TPA of Dried Shrimp Shell and Meat
3.6. The Impact of Hot-Air Drying Temperature on the Rehydration Ratio of Dried Shrimp Shell and Meat
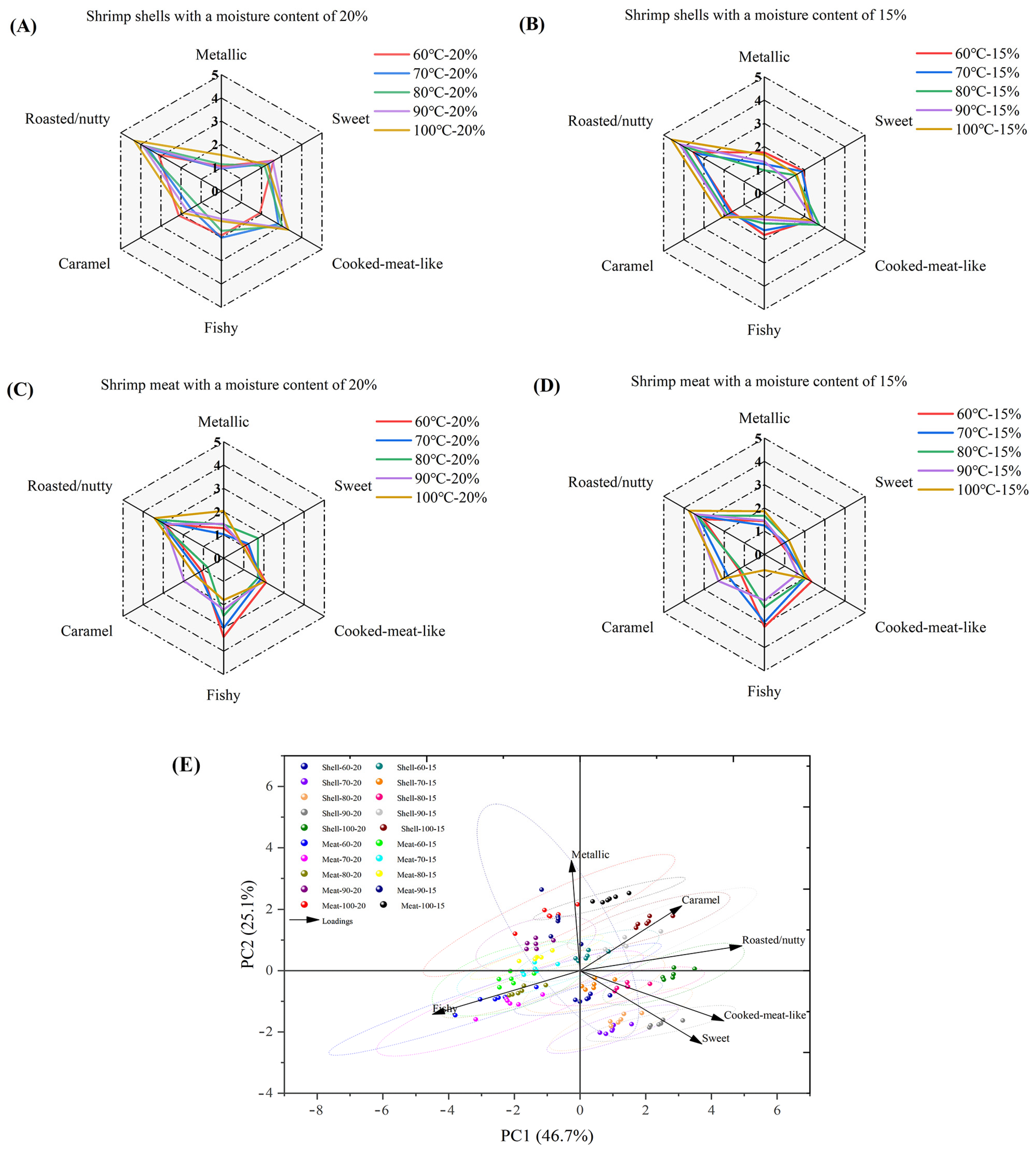
3.7. Sensory Evaluation of Dried Shrimp Shell and Meat
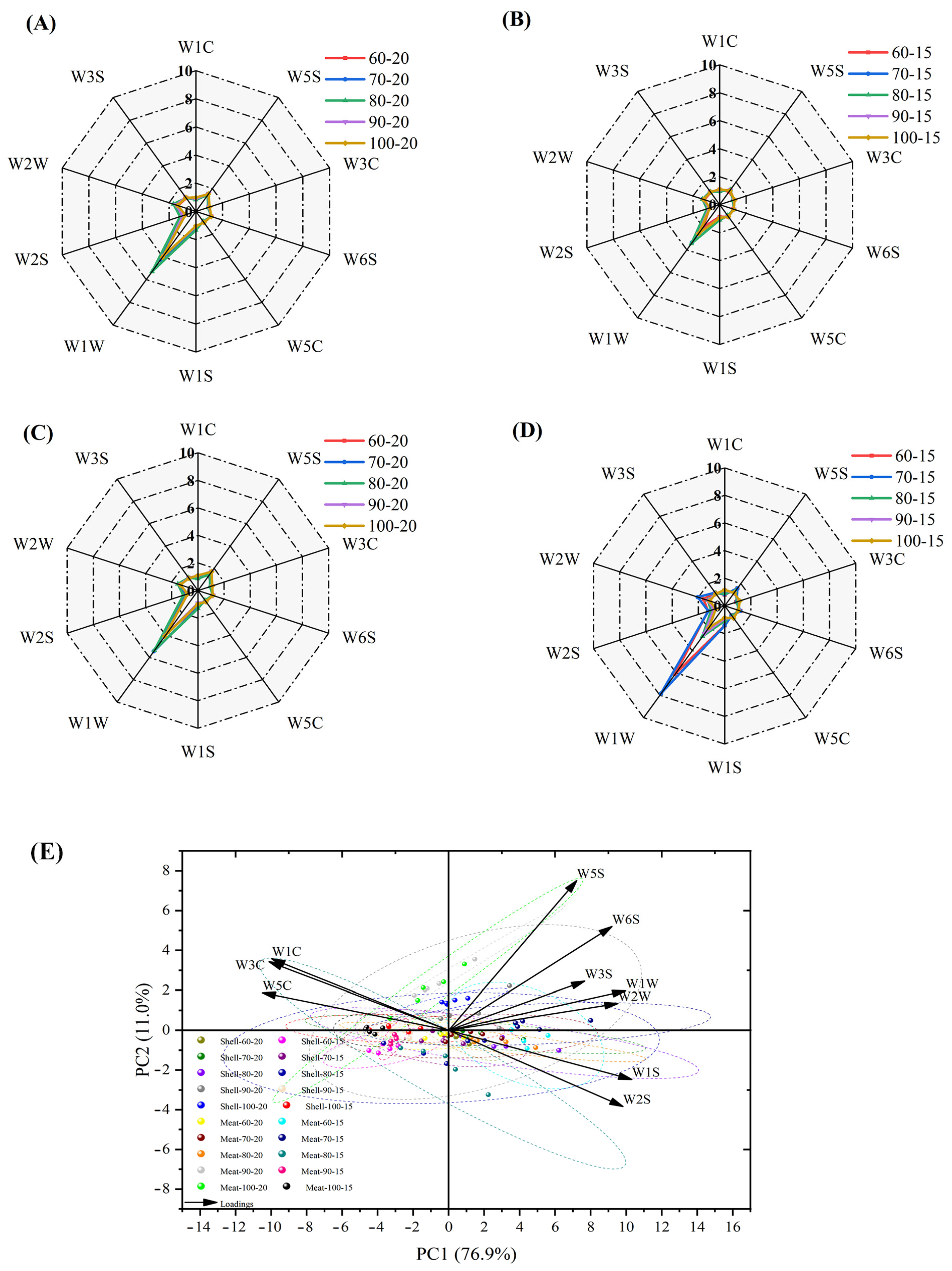
3.8. E-Nose Analysis of Dried Shrimp Shell and Meat
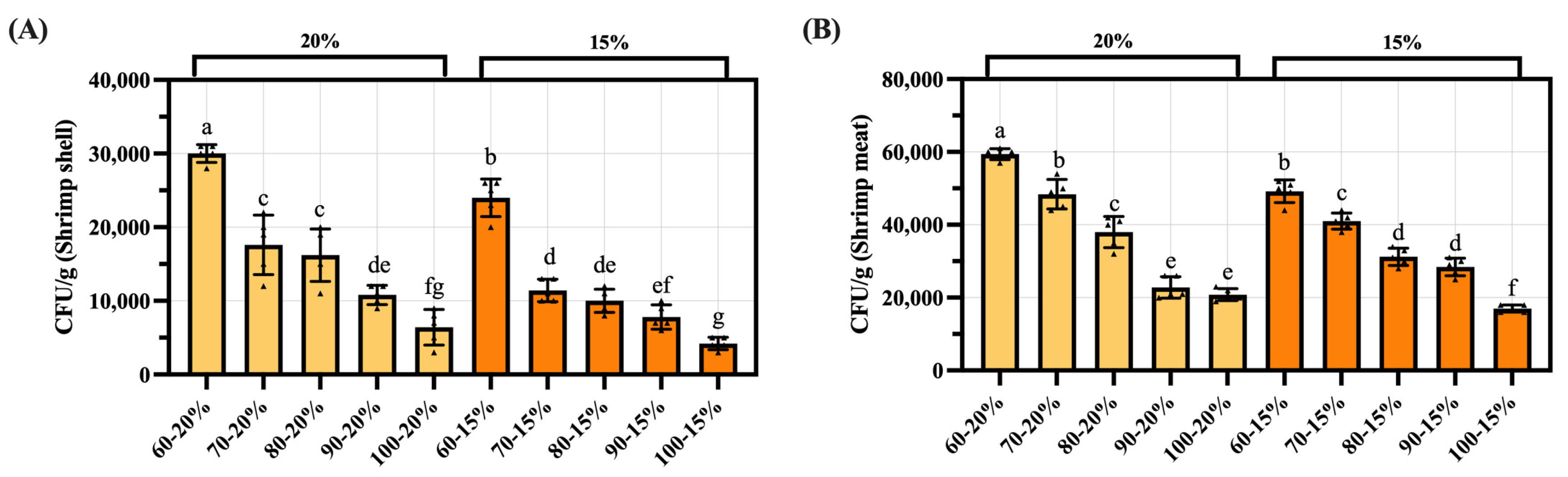
3.9. Analysis of Microbiological Indicator Test Results for Dried Shrimp Shell and Meat
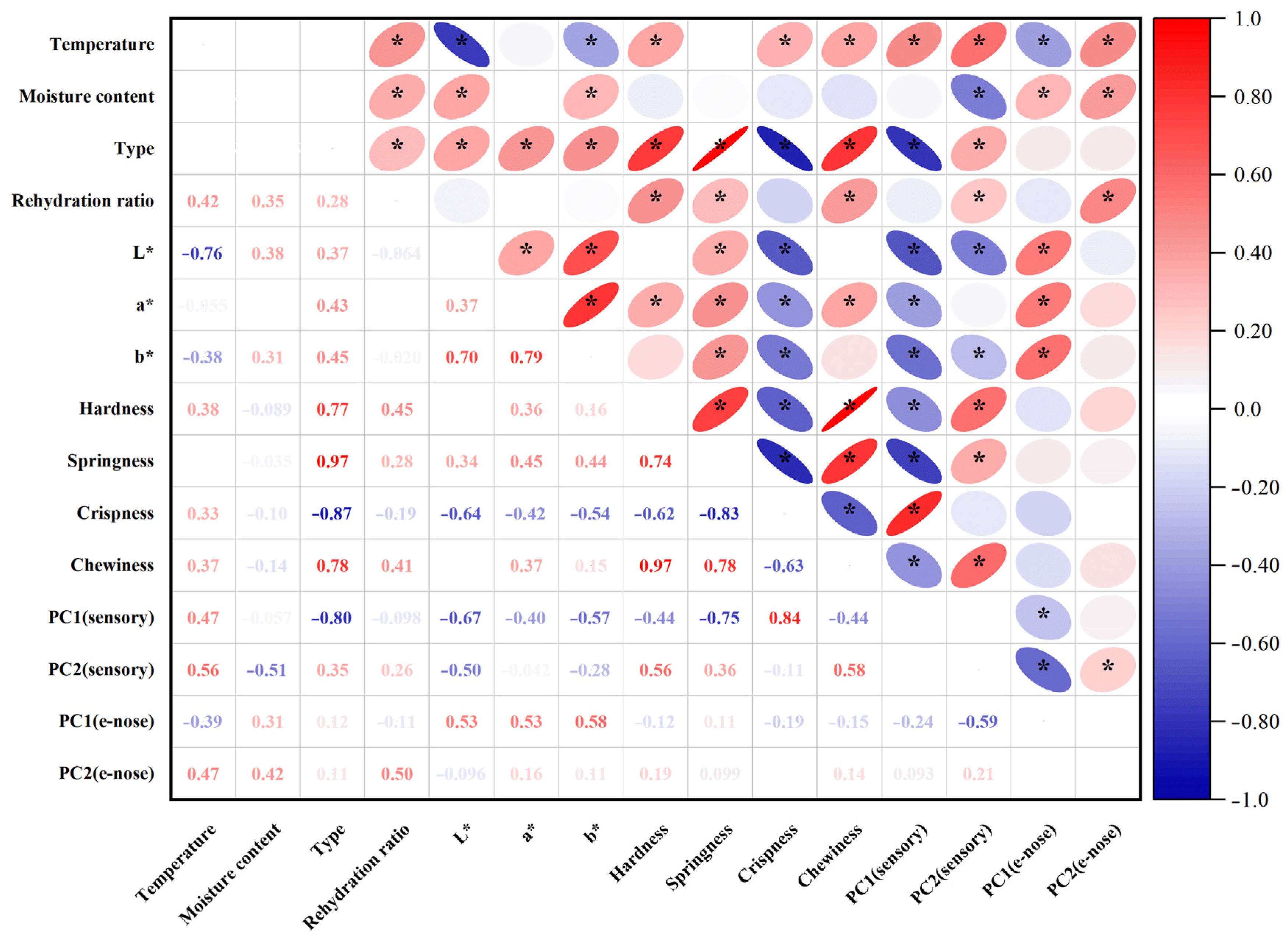
3.10. Correlation Analysis of Indices Between Dried Shrimp Shell and Meat
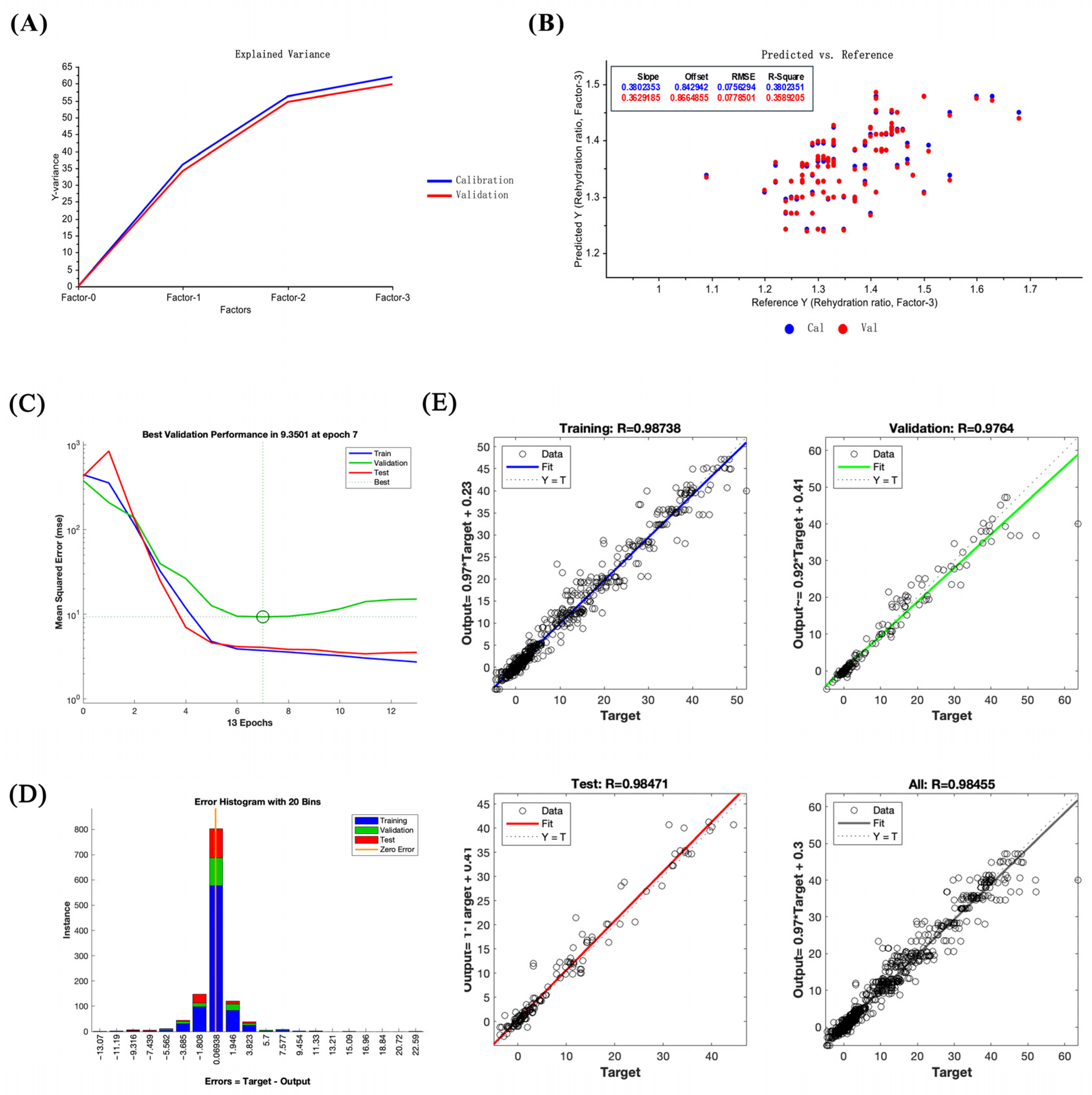
3.11. Comparison of Performance Indicators Between Least Squares and LM-ANN Models in Drying Process Prediction
3.12. Application of the LM-ANN Model in Predicting Quality Characteristics of Dried Shrimp
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ye, Y.; Zhu, B.; Zhang, J.; Yang, Y.; Tian, J.; Xu, W.; Du, X.; Huang, Y.; Li, Y.; Zhao, Y. Comparison of growth performance and biochemical components between low-salinity-tolerant hybrid and normal variety of pacific white shrimp (Penaeus vannamei). Animals 2023, 13, 2837. [Google Scholar] [CrossRef]
- Wei, Q.; Sun, Q.; Dong, X.; Kong, B.; Ji, H.; Liu, S. Effect of static magnetic field-assisted freezing at different temperatures on muscle quality of pacific white shrimp (Litopenaeus vannamei). Food Chem. 2024, 438, 138041. [Google Scholar] [CrossRef]
- Jiao, L.; Dai, T.; Tao, X.; Lu, J.; Zhou, Q. Influence of light/dark cycles on body color, hepatopancreas metabolism, and intestinal microbiota homeostasis in Litopenaeus vannamei. Front. Mar. Sci. 2021, 8, 750384. [Google Scholar] [CrossRef]
- Monsalve, E.R.; Quiroga, E. Farmed shrimp aquaculture in coastal wetlands of latin America—A review of environmental issues. Mar. Pollut. Bull. 2022, 183, 113956. [Google Scholar] [CrossRef]
- Siripatrawan, U.; Makino, Y. Rapid and chemical-free technique based on hyperspectral imaging combined with artificial intelligence for monitoring quality and shelf life of dried shrimp. Food Res. Int. 2025, 213, 116422. [Google Scholar] [CrossRef]
- Li, D.; Zhou, D.; Yin, F.; Dong, X.; Xie, H.; Liu, Z.; Li, A.; Li, J.; Rakariyatham, K.; Shahidi, F. Impact of different drying processes on the lipid deterioration and color characteristics of. J. Sci. Food Agric. 2020, 100, 2544–2553. [Google Scholar] [CrossRef]
- Carmen, P.-C.; Marlon, A.-T.; Luz, M.-N.; María, T.-Q.; Ricardo, A.-P. Influence of pre-treatment and drying process of the shrimp (Litopenaeus vannamei) exoskeleton for balanced feed production. Heliyon 2023, 9, e16712. [Google Scholar] [CrossRef]
- Pradhan, S.K.; Nayak, P.K.; Armitage, D. A social-ecological systems perspective on dried fish value chains. Curr. Res. Environ. Sustain. 2022, 4, 100128. [Google Scholar] [CrossRef]
- Belton, B.; Johnson, D.S.; Thrift, E.; Olsen, J.; Hossain, M.A.R.; Thilsted, S.H. Dried fish at the intersection of food science, economy, and culture: A global survey. Fish Fish. 2022, 23, 941–962. [Google Scholar] [CrossRef]
- Akonor, P.T.; Ofori, H.; Dziedzoave, N.T.; Kortei, N.K. Drying characteristics and physical and nutritional properties of shrimp meat as affected by different traditional drying techniques. Int. J. Food Sci. 2016, 2016, 7879097. [Google Scholar] [CrossRef] [PubMed]
- Akintola, S.L. Effects of smoking and sun-drying on proximate, fatty and amino acids compositions of southern pink shrimp (Penaeus notialis). J. Food Sci. Technol. 2015, 52, 2646–2656. [Google Scholar] [CrossRef]
- Alfiya, P.V.; Rajesh, G.K.; Murali, S.; Aniesrani Delfiya, D.S.; Samuel, M.P.; Prince, M.V. Quality evaluation of solar and microwave dried shrimps—A comparative study on renewable and dielectric heating methods. Sol. Energy 2022, 246, 234–244. [Google Scholar] [CrossRef]
- Rakib, R.J.; Jolly, Y.N.; Enyoh, C.E.; Khandaker, M.U.; Hossain, M.B.; Akther, S.; Alsubaie, A.; Almalki, A.S.A.; Bradley, D.A. Levels and health risk assessment of heavy metals in dried fish consumed in Bangladesh. Sci. Rep. 2021, 11, 14642. [Google Scholar] [CrossRef]
- Zhu, Y.; Chen, X.; Pan, N.; Liu, S.; Su, Y.; Xiao, M.; Shi, W.; Liu, Z. The effects of five different drying methods on the quality of semi-dried Takifugu obscurus fillets. LWT 2022, 161, 113340. [Google Scholar] [CrossRef]
- Pongsetkul, J.; Benjakul, S.; Boonchuen, P. The use of hot-air oven as an alternative drying method for salted shrimp paste production: Drying profile, fermentation rate, quality, and acceptability. Dry. Technol. 2024, 42, 79–89. [Google Scholar] [CrossRef]
- Teleken, J.T.; Amorim, S.M.; Rodrigues, S.S.S.; de Souza, T.W.P.; Ferreira, J.P.; Carciofi, B.A.M. Heat and mass transfer in shrimp hot-air drying: Experimental evaluation and numerical simulation. Foods 2025, 14, 428. [Google Scholar] [CrossRef]
- Wu, W.; Li, H.; Chen, Y.; Luo, Y.; Zeng, J.; Huang, J.; Gao, T. Recent advances in drying processing technologies for aquatic products. Processes 2024, 12, 942. [Google Scholar] [CrossRef]
- Xu, W.; Zhang, F.; Wang, J.; Ma, Q.; Sun, J.; Tang, Y.; Wang, J.; Wang, W. Real-time monitoring of the quality changes in shrimp (Penaeus vannamei) with hyperspectral imaging technology during hot air drying. Foods 2022, 11, 3179. [Google Scholar] [CrossRef] [PubMed]
- Indriani, S.; Srisakultiew, N.; Benjakul, S.; Boonchuen, P.; Petsong, K.; Pongsetkul, J. The impact of hot-air oven drying combined with Bacillus subtilis Ksingle bondC3 inoculation on quality characteristics and microbial profiles of salted shrimp paste. Int. J. Food Microbiol. 2024, 425, 110867. [Google Scholar] [CrossRef] [PubMed]
- Zheng, W.; Yang, R.; Shui, S.; Zhan, F.; Wang, L.; Lu, R.; Benjakul, S.; Zhang, B. Effects of hot air drying on muscle quality, volatile flavor compounds, and lipid profiles in sword prawn (Parapenaeopsis hardwickii) based on physicochemical, GC-IMS, and UHPLC-MS/MS-based lipidomics analysis. Food Chem. X 2025, 27, 102473. [Google Scholar] [CrossRef]
- Pan, X.; Wang, J.; Xu, W.; Wang, J.; Sun, J.; Wang, W.; Tang, Y. Uncovering quality changes of shrimp (Penaeus vannamei) during solar drying and its relationship with protein-related properties. Food Chem. X 2024, 23, 101696. [Google Scholar] [CrossRef]
- Nanan, K.; Eiamsa-ard, S.; Chokphoemphun, S.; Kumar, M.; Pimsarn, M.; Chuwattanakul, V. Influence of bed height and drying temperature on shrimp drying characteristics using a fluidized-bed dryer. Case Stud. Therm. Eng. 2023, 48, 103144. [Google Scholar] [CrossRef]
- Gonçalves Neto, J.; Vidal Ozorio, L.; Campos de Abreu, T.C.; Ferreira dos Santos, B.; Pradelle, F. Modeling of biogas production from food, fruits and vegetables wastes using artificial neural network (ANN). Fuel 2021, 285, 119081. [Google Scholar] [CrossRef]
- Zou, W.; Pan, F.; Yi, J.; Peng, W.; Tian, W.; Zhou, L. Targeted prediction of sensory preference for fermented pomegranate juice based on machine learning. LWT 2024, 201, 116260. [Google Scholar] [CrossRef]
- Liu, Y.; Zhu, Y.; Yang, Y.; Hu, S.; Jiang, W. Quality improvement of shrimp (Litopenaeus vannamei) during refrigerated storage by application of maillard peptides/water-soluble chitosan coating. Food Sci. Nutr. 2022, 10, 2980–2988. [Google Scholar] [CrossRef]
- Xuan, X.-T.; Yu, N.; Ding, T.; Shang, H.-T.; Cui, Y.; Lin, X.-D.; Zhu, L. Radio frequency-assisted hot air drying of pacific white shrimp (Litopenaeus vannamei): Drying kinetics, product quality and composition evaluation. CyTA J. Food 2024, 22, 2303445. [Google Scholar] [CrossRef]
- Gong, C.; Zhang, H.; Yue, J.; Miao, Y.; Jiao, S. Investigation of hot air-assisted radio frequency heating as a simultaneous dry-blanching and pre-drying method for carrot cubes. Innov. Food Sci. Emerg. Technol. 2019, 56, 102181. [Google Scholar] [CrossRef]
- Xue, X.; Wang, D.; Li, M.; Li, Y.; Guo, Y.; Ren, X.; Li, C. Effect of high-pressure processing treatment on the physicochemical properties and volatile flavor of Mercenaria mercenaria meat. Molecules 2024, 29, 4466. [Google Scholar] [CrossRef] [PubMed]
- Goula, A.M.; Adamopoulos, K.G. Modeling the rehydration process of dried tomato. Dry. Technol. 2009, 27, 1078–1088. [Google Scholar] [CrossRef]
- Jiang, P.; Jin, W.; Liu, Y.; Sun, N.; Zhu, K.; Bao, Z.; Dong, X. Hot-air drying characteristics of sea cucumber (Apostichopus japonicus) and its rehydration properties. J. Food Qual. 2022, 2022, 5147373. [Google Scholar] [CrossRef]
- Zhang, D.; Ji, H.; Liu, S.; Gao, J. Similarity of aroma attributes in hot-air-dried shrimp (Penaeus vannamei) and its different parts using sensory analysis and GC–MS. Food Res. Int. 2020, 137, 109517. [Google Scholar] [CrossRef]
- Sun, W.; Ji, H.; Zhang, D.; Zhang, Z.; Liu, S.; Song, W. Evaluation of aroma characteristics of dried shrimp (Litopenaeus vannamei) prepared by five different procedures. Foods 2022, 11, 3532. [Google Scholar] [CrossRef]
- Niamnuy, C.; Devahastin, S.; Soponronnarit, S. Effects of process parameters on quality changes of shrimp during drying in a jet-spouted bed dryer. J. Food Sci. 2007, 72, E553–E563. [Google Scholar] [CrossRef] [PubMed]
- Al-Hilphy, A.R.; Al-Mtury, A.A.A.; Al-Iessa, S.A.; Gavahian, M.; Al-Shatty, S.M.; Jassim, M.A.; Mohusen, Z.A.A.; Khaneghah, A.M. A pilot-scale rotary infrared dryer of shrimp (Metapenaeus affinis): Mathematical modeling and effect on physicochemical attributes. J. Food Process. Eng. 2022, 45, e13945. [Google Scholar] [CrossRef]
- Niamnuy, C.; Kerdpiboon, S.; Devahastin, S. Artificial neural network modeling of physicochemical changes of shrimp during boiling. LWT Food Sci. Technol. 2012, 45, 110–116. [Google Scholar] [CrossRef]
- Verma, S.K.; Sasikala, R.; Kishore, P.; Mohan, C.O.; Ganesan, P.; Padmavathy, P.; Muralidharan, N.; Jaganath, B.; Benjakul, S. Effects of different microwave power on the drying kinetics and physicochemical quality of brown shrimp (Metapenaeus dobsoni). Sustain. Food Technol. 2024, 2, 141–151. [Google Scholar] [CrossRef]
- Zhang, K.; Li, N.; Wang, Z.; Feng, D.; Liu, X.; Zhou, D.; Li, D. Recent advances in the color of aquatic products: Evaluation methods, discoloration mechanism, and protection technologies. Food Chem. 2024, 434, 137495. [Google Scholar] [CrossRef]
- Azizpour, M.; Mohebbi, M.; Khodaparast, M.H.H. Effects of foam-mat drying temperature on physico-chemical and microstructural properties of shrimp powder. Innov. Food Sci. Emerg. Technol. 2016, 34, 122–126. [Google Scholar] [CrossRef]
- Niamnuy, C.; Devahastin, S.; Soponronnarit, S.; Vijaya Raghavan, G.S. Kinetics of astaxanthin degradation and color changes of dried shrimp during storage. J. Food Eng. 2008, 87, 591–600. [Google Scholar] [CrossRef]
- Liu, F.; Chang, W.; Chen, M.; Xu, F.; Ma, J.; Zhong, F. Tailoring physicochemical properties of chitosan films and their protective effects on meat by varying drying temperature. Carbohydr. Polym. 2019, 212, 150–159. [Google Scholar] [CrossRef]
- Xiong, M.; Zhou, H.; Yu, R.-X.; Chen, J.-Q.; Wang, S.; Xu, W.; Tian, Y.; Su, L.-Y. Effects of thermal processing methods on the edible quality, nutrition, and metabolites of shrimp of Metapenaeus ensis. Food Prod. Process. Nutr. 2025, 7, 29. [Google Scholar] [CrossRef]
- Chen, F.; Shen, L.; Shi, X.; Deng, Y.; Qiao, Y.; Wu, W.; Xiong, G.; Wang, L.; Li, X.; Ding, A.; et al. Characterization of flavor perception and characteristic aroma of traditional dry-cured fish by flavor omics combined with multivariate statistics. LWT 2022, 173, 114240. [Google Scholar] [CrossRef]
- Zheng, X.; Ji, H.; Liu, S.; Shi, W.; Lu, Y. Shrimp lipids improve flavor by regulating characteristic aroma compounds in hot air-dried shrimp. Food Chem. 2025, 465, 142065. [Google Scholar] [CrossRef]
- Wu, J.; Chen, R.; Li, X.; Fu, Z.; Xian, C.; Zhao, W.; Zhao, C.; Wu, X. Comprehensive identification of key compounds in different quality grades of soy sauce-aroma type baijiu by HS-SPME-GC-MS coupled with electronic nose. Front. Nutr. 2023, 10, 1132527. [Google Scholar] [CrossRef] [PubMed]
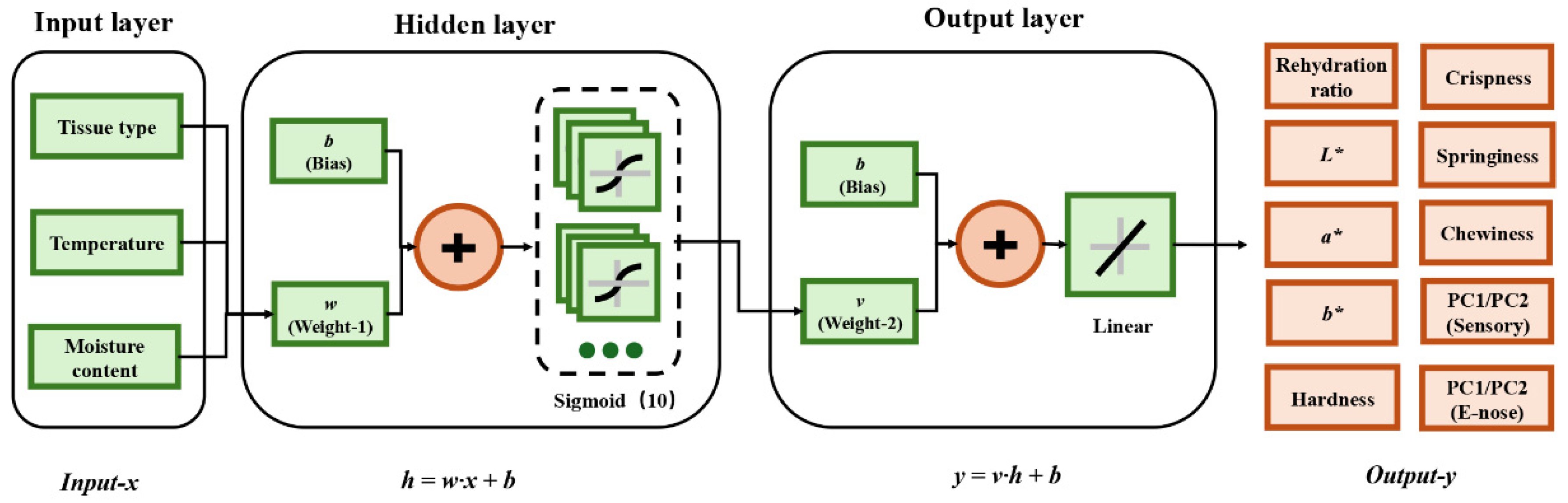

| Sample Type | Total Sample Size | Non-Destructive (Quantity) | Destructive (Quantity) |
|---|---|---|---|
| Shrimp shell | 25 | Visual inspection and color difference analysis (5) | Rehydration ratio, sensory evaluation, e-nose analysis, TPA (20, each 5) |
| Shrimp meat | 25 | Visual inspection and color difference analysis (5) | Rehydration ratio, sensory evaluation, e-nose analysis, TPA (20, each 5) |
| Parameter | Setting |
|---|---|
| Network type | Feed-forward backpropagation neural network |
| Neurons in hidden layers | 10 |
| Input variables | 3 |
| Output variables | 12 |
| Activation function (hidden layer) | Sigmoid (logsig) |
| Activation function (output layer) | Linear (purelin) |
| Epochs (maximum iterations) | 1000 |
| Training algorithm | Levenberg–Marquardt |
| Data division | 70% training, 15% validation, 15% testing |
| Random seed | 1 |
| Dataset Type | R2 | RMSE |
|---|---|---|
| Calibration | 0.38 | 0.08 |
| Validation | 0.32 | 0.08 |
| Dataset Type | R | R2 | RMSE |
|---|---|---|---|
| Training | 0.99 | 0.97 | 0.03 |
| Validation | 0.98 | 0.95 | 0.04 |
| Test | 0.98 | 0.96 | 0.04 |
| All | 0.98 | 0.97 | 0.04 |
| Input Parameters | Prediction Case A | Prediction Case B | Prediction Case C | Prediction Case D |
|---|---|---|---|---|
| Tissue type | 0 | 0 | 1 | 1 |
| Temperature (°C) | 75 | 87 | 64 | 93 |
| Moisture content (%) | 20 | 15 | 20 | 15 |
| Predictive indicators | Prediction Case A | Prediction Case B | Prediction Case C | Prediction Case D |
| Rehydration ratio | 1.34 | 1.26 | 1.38 | 1.34 |
| L* (lightness) | 37.44 | 28.71 | 42.91 | 31.04 |
| a* (redness) | 13.13 | 11.60 | 11.19 | 11.41 |
| b* (yellowness) | 15.57 | 12.36 | 18.32 | 11.35 |
| Hardness | 4.33 | 5.24 | 11.40 | 39.32 |
| Springiness | 0.33 | 0.48 | 0.63 | 0.79 |
| Crispness | 2.50 | 3.49 | 0.51 | 0.46 |
| Chewiness | 0.98 | 2.94 | 6.69 | 28.58 |
| PC1 (sensory) | 0.74 | 1.89 | −0.99 | −0.76 |
| PC2 (sensory) | −1.58 | 0.90 | −1.23 | 2.09 |
| PC1 (e-nose) | 2.39 | −2.34 | 0.86 | −4.08 |
| PC2 (e-nose) | 0.08 | −0.25 | −0.39 | −0.45 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, Z.; Zhang, Z.; Zheng, Z.; Hou, R.; Zhang, Y.; Zheng, B.; Sriboonvorakul, N.; Hu, J. Effects of Hot-Air Drying Conditions on Quality Attributes of Meat and Shell of Dried Shrimp. Foods 2025, 14, 4041. https://doi.org/10.3390/foods14234041
Lin Z, Zhang Z, Zheng Z, Hou R, Zhang Y, Zheng B, Sriboonvorakul N, Hu J. Effects of Hot-Air Drying Conditions on Quality Attributes of Meat and Shell of Dried Shrimp. Foods. 2025; 14(23):4041. https://doi.org/10.3390/foods14234041
Chicago/Turabian StyleLin, Zhongjing, Zhaorong Zhang, Zhipeng Zheng, Ruoting Hou, Yi Zhang, Baodong Zheng, Natthida Sriboonvorakul, and Jiamiao Hu. 2025. "Effects of Hot-Air Drying Conditions on Quality Attributes of Meat and Shell of Dried Shrimp" Foods 14, no. 23: 4041. https://doi.org/10.3390/foods14234041
APA StyleLin, Z., Zhang, Z., Zheng, Z., Hou, R., Zhang, Y., Zheng, B., Sriboonvorakul, N., & Hu, J. (2025). Effects of Hot-Air Drying Conditions on Quality Attributes of Meat and Shell of Dried Shrimp. Foods, 14(23), 4041. https://doi.org/10.3390/foods14234041









