Bioactive Compound Profiling of Agarophyte Seaweed (Gelidiella acerosa, Gracilaria arcuata, and Gracilaria verrucosa) Based on LC-HRMS Metabolomic and Molecular Networking Approach
Abstract
1. Introduction
2. Materials and Methods
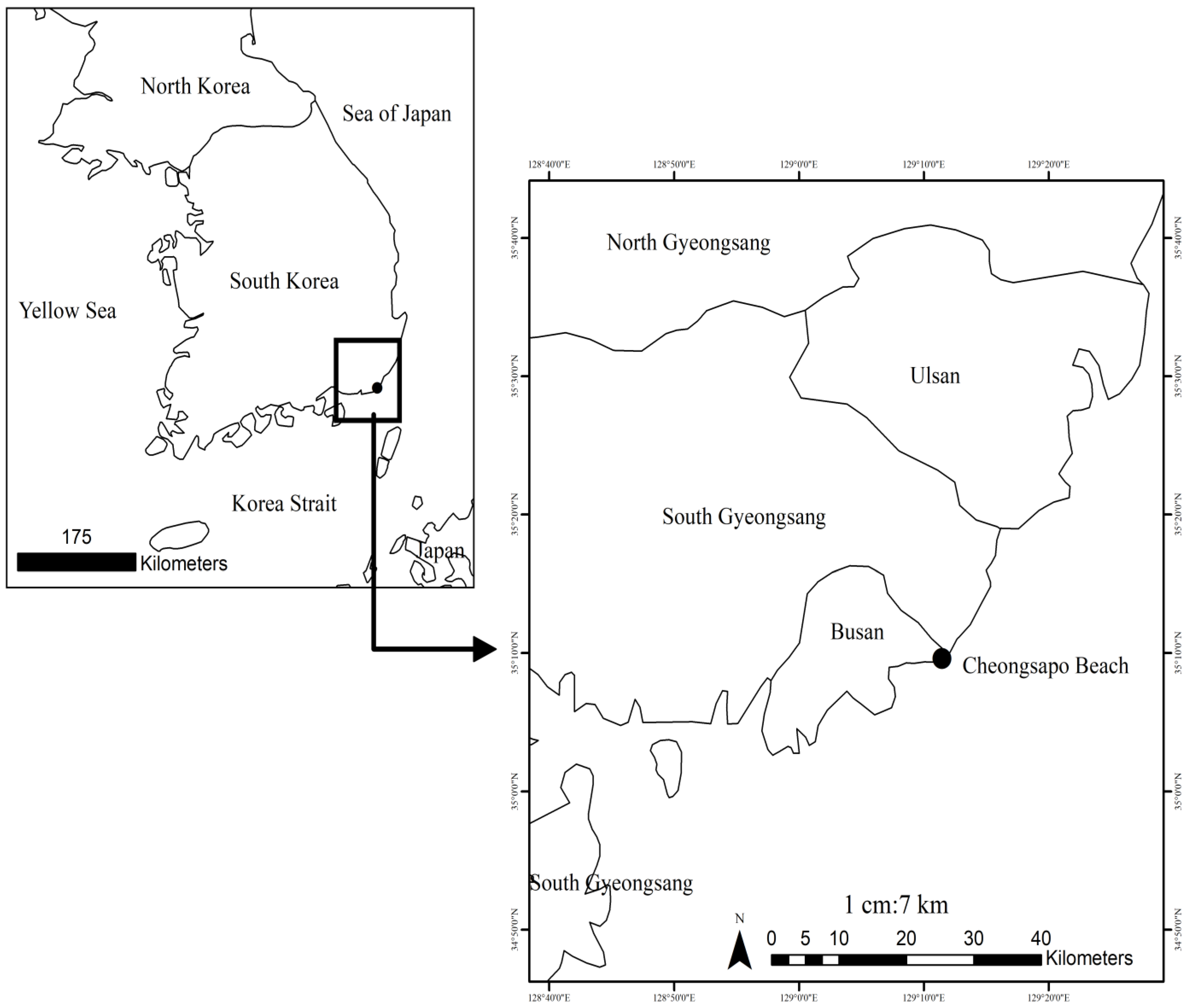
2.1. Sampling Site
2.2. Morphological and Molecular Identification
2.3. Pretreatment
2.4. Extraction of Bioactive Compounds
2.5. LC–HRMS Analysis
2.6. MS-Based Molecular Networking
2.7. Data Analysis
2.8. Feature Annotation
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- FAO. The State of World Fisheries and Aquaculture 2024. Blue Transformation in Action; Food and Agriculture Organization of the United Nations: Rome, Italy, 2024. [Google Scholar]
- Porse, H.; Rudolph, B. The Seaweed Hydrocolloid Industry: 2016 Updates, Requirements, and Outlook. J. Appl. Phycol. 2017, 29, 2187–2200. [Google Scholar] [CrossRef]
- Bixler, H.J.; Porse, H. A Decade of Change in the Seaweed Hydrocolloids Industry. J. Appl. Phycol. 2011, 23, 321–335. [Google Scholar] [CrossRef]
- McHugh, D.J. Worldwide Distribution of Commercial Resources of Seaweeds Including Gelidium. Hydrobiologia 1991, 221, 19–29. [Google Scholar] [CrossRef]
- Kumar, M.; Kuzhiumparambil, U.; Pernice, M.; Jiang, Z.; Ralph, P.J. Metabolomics: An Emerging Frontier of Systems Biology in Marine Macrophytes. Algal Res. 2016, 16, 76–92. [Google Scholar] [CrossRef]
- Gupta, V.; Thakur, R.S.; Baghel, R.S.; Reddy, C.R.K.; Jha, B. Seaweed Metabolomics: A New Facet of Functional Genomics; Elsevier: Amsterdam, The Netherlands, 2014; Volume 71, ISBN 9780124080621. [Google Scholar]
- Zwerger, M.J.; Hammerle, F.; Siewert, B.; Ganzera, M. Application of Feature-based Molecular Networking in the Field of Algal Research with Special Focus on Mycosporine-like Amino Acids. J. Apllied Phycol. 2023, 35, 1377–1392. [Google Scholar] [CrossRef]
- Davis, G.D.J.; Vasanthi, A.H.R. Seaweed Metabolite Database (SWMD): A Database of Natural Compounds from Marine Algae. Bioinformation 2011, 5, 361–364. [Google Scholar] [CrossRef] [PubMed]
- Meinita, M.D.N.; Harwanto, D.; Choi, J.S. Seaweed Exhibits Therapeutic Properties against Chronic Diseases: An Overview. Appl. Sci. 2022, 12, 2638. [Google Scholar] [CrossRef]
- Carriot, N.; Paix, B.; Greff, S.; Viguier, B.; Briand, J.F.; Culioli, G. Integration of LC/MS-Based Molecular Networking and Classical Phytochemical Approach Allows in-Depth Annotation of the Metabolome of Non-Model Organisms-The Case Study of the Brown Seaweed Taonia Atomaria. Talanta 2021, 225, 121925. [Google Scholar] [CrossRef] [PubMed]
- Chhetri, B.K.; Mojib, N.; Moore, S.G.; Delgadillo, D.A.; Burch, J.E.; Barrett, N.H.; Gaul, D.A.; Marquez, L.; Soapi, K.; Nelson, H.M.; et al. Cryptic Chemical Variation in a Marine Red Alga as Revealed by Nontargeted Metabolomics. ACS Omega 2023, 8, 13899–13910. [Google Scholar] [CrossRef]
- Tanna, B.; Mishra, A. Metabolomics of Seaweeds: Tools and Techniques; Elsevier Inc.: Amsterdam, The Netherlands, 2018; ISBN 9780128126899. [Google Scholar]
- Salunke, M.; Wakure, B.; Wakte, P. High-resolution liquid chromatography mass spectrometry (HR-LCMS) and 1H NMR analysis of methanol extracts from marine seaweed Gracilaria edulis. Nat. Prod. Res. 2024, 38, 1441–1444. [Google Scholar] [CrossRef]
- Salunke, M.A.; Wakure, B.S.; Wakte, P.S. High-resolution liquid chromatography and mass spectrometr(HR-LCMS) assisted phytochemical profiling and an assessment of anticancer activities of Gracilaria foliifera and Turbinaria conoides using in vitro and molecular docking analysis. J. Biomol. Struct. Dyn. 2023, 41, 6476–6491. [Google Scholar] [CrossRef]
- Gandhi, G.; Biswas, K.; Vaghela, P.; Nayak, J.; Nair, A.; Moradiya, K.; Gopalakrishnan, V.A.K.; Veeragurunathan, V.; Ghosh, A. In-depth metabolite characterization of seaweed-based plant biostimulants: Insights into bioactive components. Algal Res. 2024, 81, 103574. [Google Scholar] [CrossRef]
- Nisha, S.A.; Devi, K.P. Gelidiella acerosa protects against Aβ25–35 induced toxicity and memory impairment in Swiss albino mice: An in vivo report. Pharm. Biol. 2017, 55, 1423–1435. [Google Scholar] [CrossRef] [PubMed]
- Mahaboob Begum, S.F.M.; Chitra, K.; Joseph, B.; Sundararajan, R.; Hemalata, S. Gelidiella acerosa inhibits lung cancer proliferation. BMC Complement. Altern Med. 2018, 18, 104. [Google Scholar] [CrossRef]
- Meinita, M.D.N.; Harwanto, D.; Amron; Hannan, M.A.; Jeong, G.T.; Moon, I.S.; Choi, J.S. A Concise Review of the Potential Utilization Based on Bioactivity and Pharmacological Properties of the Genus Gelidium (Gelidiales, Rhodophyta). J. Appl. Phycol. 2023, 35, 1499–1523. [Google Scholar] [CrossRef]
- Meinita, M.D.N.; Akromah, N.; Andriyani, N.; Setijanto; Harwanto, D.; Liu, T. Molecular Identification of Gracilaria Species (Gracilariales, Rhodophyta) Obtained from the South Coast of Java Island, Indonesia. Biodiversitas 2021, 22, 3046–3056. [Google Scholar] [CrossRef]
- Riyanti; Marner, M.; Hartwig, C.; Patras, M.A.; Wodi, S.I.M.; Rieuwpassa, F.J.; Ijong, F.G.; Balansa, W.; Schäberle, T.F. Sustainable Low-Volume Analysis of Environmental Samples by Semi-Automated Prioritization of Extracts for Natural Product Research (SeaPEPR). Mar. Drugs 2020, 18, 649. [Google Scholar] [CrossRef]
- Vitale, G.A.; Sciarretta, M.; Cassiano, C.; Buonocore, C.; Festa, C.; Mazzella, V.; Pons, L.N.; D’auria, M.V.; de Pascale, D. Molecular Network and Culture Media Variation Reveal a Complex Metabolic Profile in Pantoea Cf. Eucrina D2 Associated with an Acidified Marine Sponge. Int. J. Mol. Sci. 2020, 21, 6307. [Google Scholar] [CrossRef]
- Hung, T.D.; Hye, J.L.; Eun, S.Y.; Shinde, P.B.; Yoon, M.L.; Hong, J.; Dong, K.K.; Jung, J.H. Anti-Inflammatory Constituents of the Red Alga Gracilaria Verrucosa and Their Synthetic Analogues. J. Nat. Prod. 2008, 71, 232–240. [Google Scholar] [CrossRef]
- Da Costa, E.; Melo, T.; Moreira, A.S.P.; Bernardo, C.; Helguero, L.; Ferreira, I.; Cruz, M.T.; Rego, A.M.; Domingues, P.; Calado, R.; et al. Valorization of Lipids from Gracilaria Sp. through Lipidomics and Decoding of Antiproliferative and Anti-Inflammatory Activity. Mar. Drugs 2017, 15, 62. [Google Scholar] [CrossRef]
- Devi, K.P.; Suganthy, N.; Kesika, P.; Pandian, S.K. Bioprotective Properties of Seaweeds: In Vitro Evaluation of Antioxidant Activity and Antimicrobial Activity against Food Borne Bacteria in Relation to Polyphenolic Content. BMC Complement. Altern. Med. 2008, 8, 38. [Google Scholar] [CrossRef]
- Islam, M.N.; Ishita, I.J.; Jin, S.E.; Choi, R.J.; Lee, C.M.; Kim, Y.S.; Jung, H.A.; Choi, J.S. Anti-Inflammatory Activity of Edible Brown Alga Saccharina Japonica and Its Constituents Pheophorbide a and Pheophytin a in LPS-Stimulated RAW 264.7 Macrophage Cells. Food Chem. Toxicol. 2013, 55, 541–548. [Google Scholar] [CrossRef]
- Rodríguez, M.; Marín, A.; Torres, M.; Béjar, V.; Campos, M.; Sampedro, I. Aphicidal Activity of Surfactants Produced by Bacillus Atrophaeus L193. Front. Microbiol. 2018, 9, 3114. [Google Scholar] [CrossRef] [PubMed]
- Khlupova, M.; Vasil’Eva, I.; Shumakovich, G.; Morozova, O.; Chertkov, V.; Shestakova, A.; Kisin, A.; Yaropolov, A. Laccase-Mediated Biotransformation of Dihydroquercetin (Taxifolin). J. Mol. Catal. B Enzym. 2016, 123, 62–66. [Google Scholar] [CrossRef]
- Zaynullin, R.A.; Kunakova, R.V.; Khusnutdinova, E.K.; Yalaev, B.I.; Segura-Ceniceros, E.P.; Chavez-Gonzalez, M.L.; Martínez-Hernández, J.L.; Gernet, M.V.; Batashov, E.S.; Ilyina, A. Dihydroquercetin: Known Antioxidant—New Inhibitor of Alpha-Amylase Activity. Med. Chem. Res. 2018, 27, 966–971. [Google Scholar] [CrossRef]
- Korzun, A.M.; Nurminskii, V.N.; Rozinov, S.V.; Stolbikov, A.S.; Slayaev, R.K. The Effect of Dihydroquercetin on Slow Vacuolar Channels. Dokl. Biochem. Biophys. 2006, 410, 297–299. [Google Scholar] [CrossRef]
- Vladimirov, Y.A.; Proskurnina, E.V.; Demin, E.M.; Matveeva, N.S.; Lubitskiy, O.B.; Novikov, A.A.; Izmailov, D.Y.; Osipov, A.N.; Tikhonov, V.P.; Kagan, V.E. Dihydroquercetin (Taxifolin) and Other Flavonoids as Inhibitors of Free Radical Formation at Key Stages of Apoptosis. Biochemistry 2009, 74, 301–307. [Google Scholar] [CrossRef]
- Chen, Y.; Deuster, P. Comparison of Quercetin and Dihydroquercetin: Antioxidant-Independent Actions on Erythrocyte and Platelet Membrane. Chem. Biol. Interact. 2009, 182, 7–12. [Google Scholar] [CrossRef]
- Valerio, F.; Di Biase, M.; Lattanzio, V.M.T.; Lavermicocca, P. Improvement of the Antifungal Activity of Lactic Acid Bacteria by Addition to the Growth Medium of Phenylpyruvic Acid, a Precursor of Phenyllactic Acid. Int. J. Food Microbiol. 2016, 222, 1–7. [Google Scholar] [CrossRef]
- Nylund, G.M.; Weinberger, F.; Rempt, M.; Pohnert, G. Metabolomic Assessment of Induced and Activated Chemical Defence in the Invasive Red Alga Gracilaria vermiculophylla. PLoS ONE 2011, 6, e29359. [Google Scholar] [CrossRef]
- Surget, G.; Le Lann, K.; Delebecq, G.; Kervarec, N.; Donval, A.; Poullaouec, M.; Bihannic, I.; Poupart, N. Seasonal Phenology and Metabolomics of the Introduced Red Macroalga Gracilaria vermiculophylla, Monitored in the Bay of Brest (France). J. Appl. Phycol. 2017, 29, 2651–2666. [Google Scholar] [CrossRef]
- Pillay, L.R.; Olasehinde, T.A.; Olofinsan, K.A.; Erukainure, O.L.; Islam, M.S.; Olaniran, A.O. Antidiabetic Potentials of Crude and Purified Sulphated Polysaccharides Isolated from Gracilaria gracilis, a Seaweed from South Africa. Heliyon 2024, 10, e35729. [Google Scholar] [CrossRef] [PubMed]
- Sakthivel, R.; Muniasamy, S.; Archunan, G.; Devi, K.P. Gracilaria edulis Exhibit Antiproliferative Activity Against Human Lung Adenocarcinoma Cell Line A549 Without Causing Adverse Toxic Effect In Vitro and In Vivo. Food Func. 2016, 7, 1155–1165. [Google Scholar] [CrossRef] [PubMed]
- Lopes, D.; Melo, T.; Rey, F.; Costa, E.; Moreira, A.S.P.; Abreu, M.H.; Domingues, P.; Lillebø, A.I.; Calado, R.; Rosário Domingues, M. Insights of Species-Specific Polar Lipidome Signatures of Seaweeds Fostering Their Valorization in the Blue Bioeconomy. Algal Res. 2021, 55, 102242. [Google Scholar] [CrossRef]
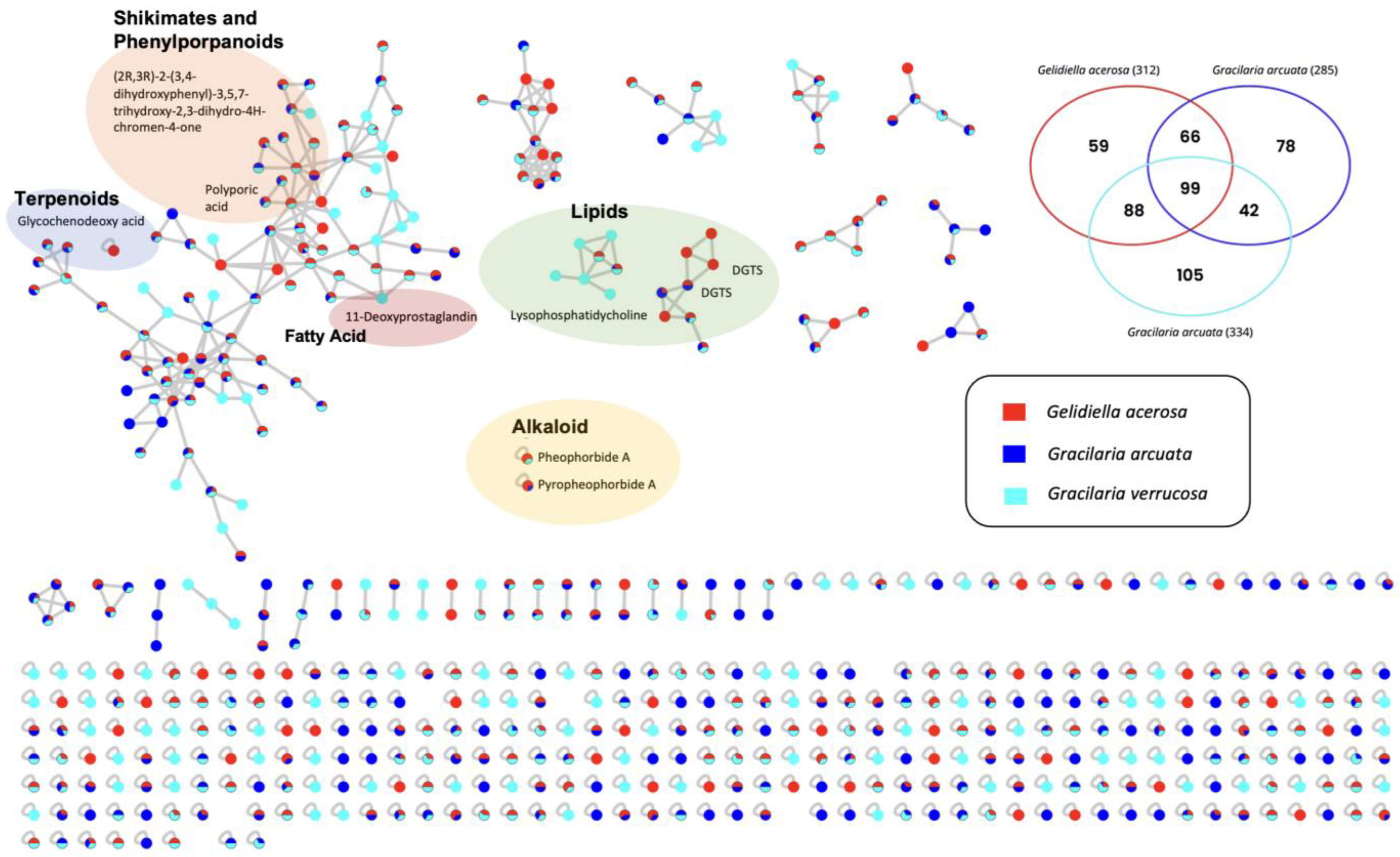
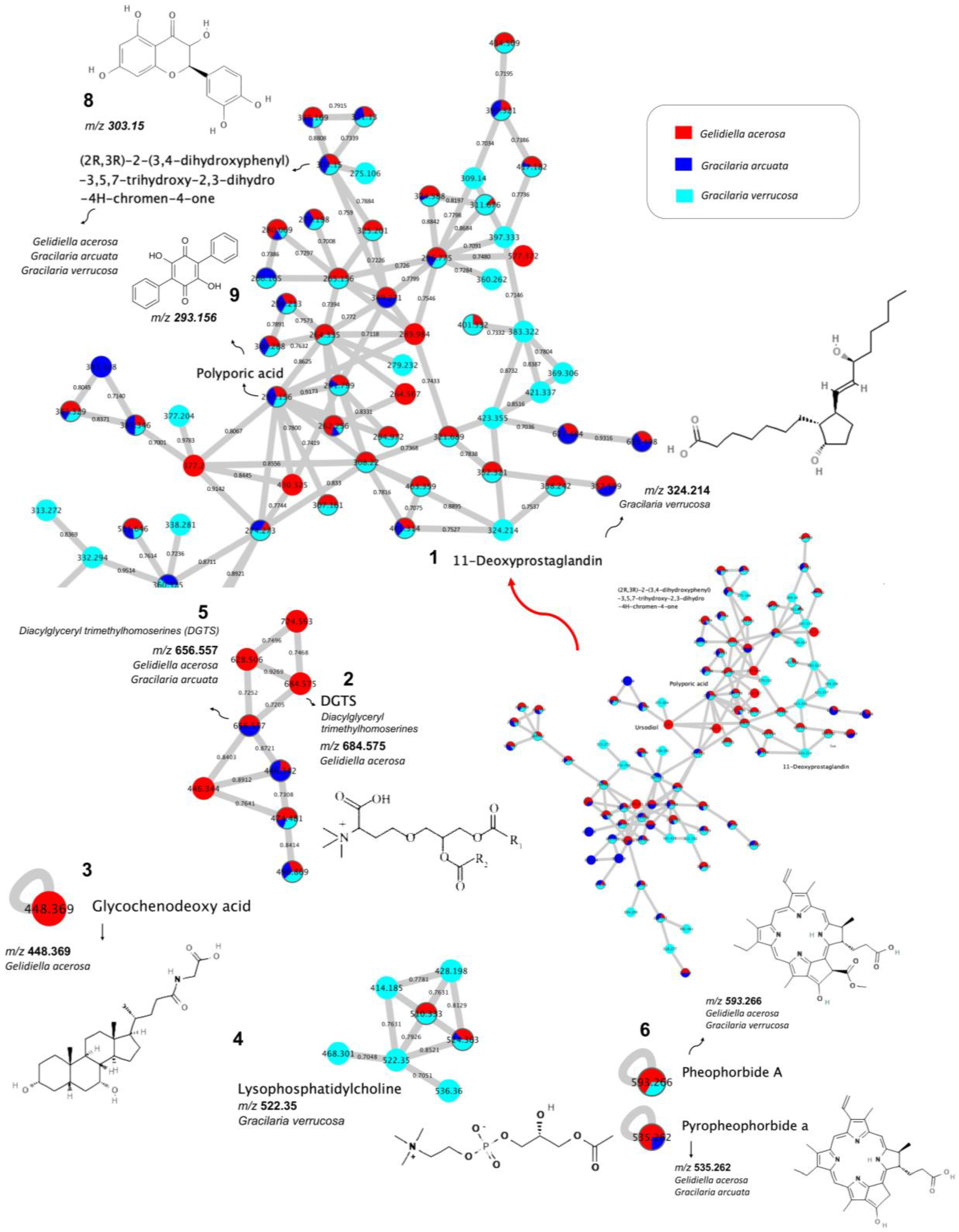
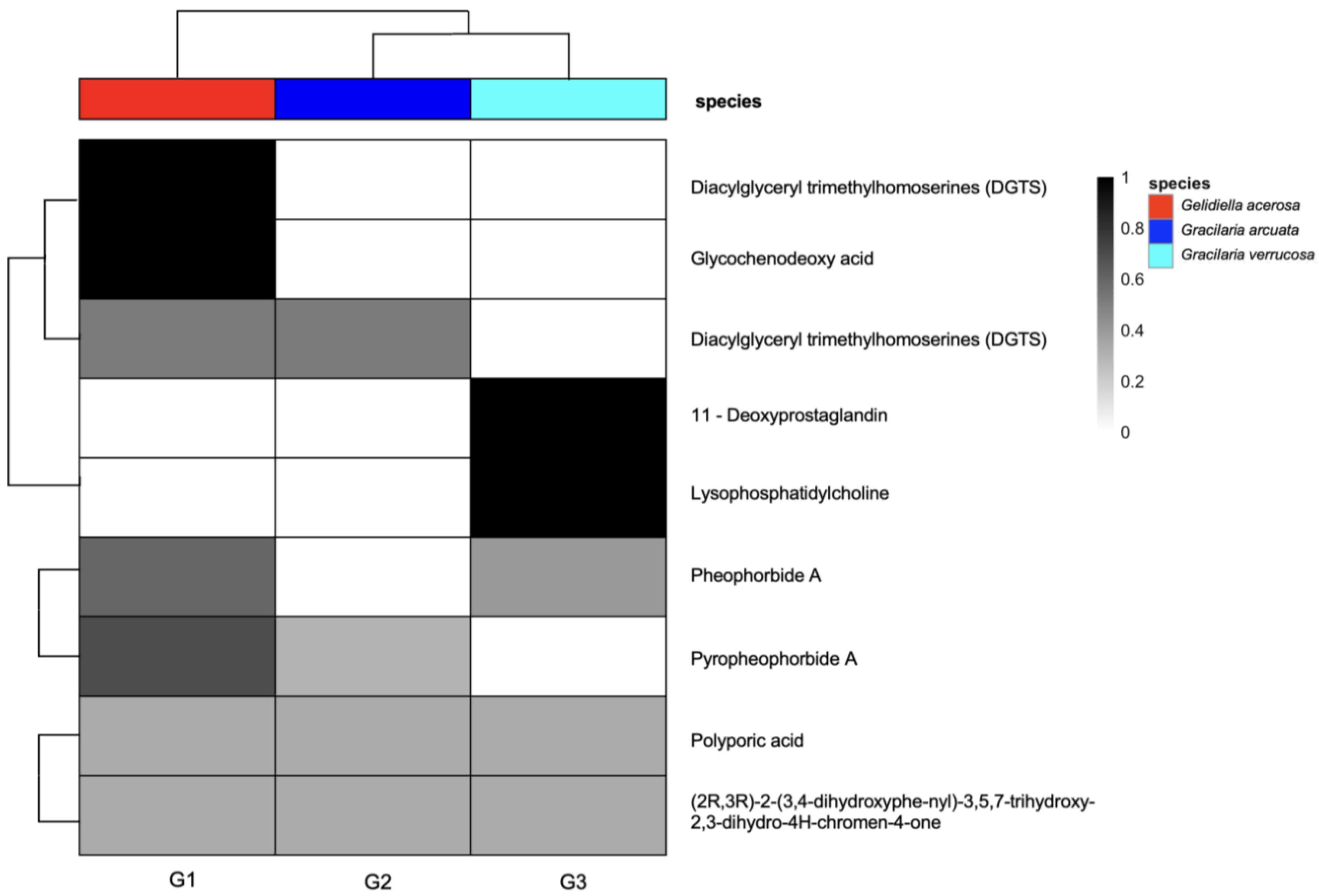
| Compound Code | Compound | Class | Parent Mass (m/z) | Adduct | Source |
|---|---|---|---|---|---|
| [1] | 11-Deoxyprostaglandin | Fatty Acids | 324.214 | M + H − H2O | Gracilaria verrucosa |
| [2] | Diacylglyceryl trimethylhomoserines (DGTSs) | Lipids | 684.575 | M + H | Gelidiella acerosa |
| [3] | Glycochenodeoxy acid | Terpenoids | 448.369 | M + H | Gelidiella acerosa |
| [4] | Lysophosphatidylcholine | Lipids | 522.350 | M + H | Gracilaria verrucosa |
| Compound Code | Compound | Class | Parent Mass (m/z) | Adduct | Source |
|---|---|---|---|---|---|
| [5] | Diacylglyceryl trimethylhomoserines (DGTS) | Lipids | 656.557 | M + H | Gelidiella acerosa Gracilaria arcuata |
| [6] | Pheophorbide A | Alkaloids | 593.266 | M + H | Gelidiella acerosa Gracilaria verrucosa |
| [7] | Pyropheophorbide A | Alkaloids | 535.262 | M + H | Gelidiella acerosa Gracilaria arcuata |
| Compound Code | Compound | Class | Parent Mass (m/z) | Adduct | Source |
|---|---|---|---|---|---|
| [8] | (2R,3R)-2-(3,4-dihydroxyphenyl)-3,5,7-trihydroxy-2,3-dihydro-4H-chromen-4-one | Shikimates and Phenylpropanoids | 303.15 | M + H | Gelidiella acerosa Gracilaria arcuata Gracilaria verrucosa |
| [9] | Polyporic acid | Alkaloids | 293.156 | M + H | Gelidiella acerosa Gracilaria arcuata Gracilaria verrucosa |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Meinita, M.D.N.; Riyanti; Sanjayasari, D.; Riviani; Harwanto, D.; Jiso, A.; Schäberle, T.F.; Mettal, U.; Moon, I.-S.; Choi, J.-S. Bioactive Compound Profiling of Agarophyte Seaweed (Gelidiella acerosa, Gracilaria arcuata, and Gracilaria verrucosa) Based on LC-HRMS Metabolomic and Molecular Networking Approach. Foods 2025, 14, 4042. https://doi.org/10.3390/foods14234042
Meinita MDN, Riyanti, Sanjayasari D, Riviani, Harwanto D, Jiso A, Schäberle TF, Mettal U, Moon I-S, Choi J-S. Bioactive Compound Profiling of Agarophyte Seaweed (Gelidiella acerosa, Gracilaria arcuata, and Gracilaria verrucosa) Based on LC-HRMS Metabolomic and Molecular Networking Approach. Foods. 2025; 14(23):4042. https://doi.org/10.3390/foods14234042
Chicago/Turabian StyleMeinita, Maria Dyah Nur, Riyanti, Dyahruri Sanjayasari, Riviani, Dicky Harwanto, Apisada Jiso, Till F. Schäberle, Ute Mettal, Il-Soo Moon, and Jae-Suk Choi. 2025. "Bioactive Compound Profiling of Agarophyte Seaweed (Gelidiella acerosa, Gracilaria arcuata, and Gracilaria verrucosa) Based on LC-HRMS Metabolomic and Molecular Networking Approach" Foods 14, no. 23: 4042. https://doi.org/10.3390/foods14234042
APA StyleMeinita, M. D. N., Riyanti, Sanjayasari, D., Riviani, Harwanto, D., Jiso, A., Schäberle, T. F., Mettal, U., Moon, I.-S., & Choi, J.-S. (2025). Bioactive Compound Profiling of Agarophyte Seaweed (Gelidiella acerosa, Gracilaria arcuata, and Gracilaria verrucosa) Based on LC-HRMS Metabolomic and Molecular Networking Approach. Foods, 14(23), 4042. https://doi.org/10.3390/foods14234042









