Development and Evaluation of a BCG/BCP-Based Cellulose Acetate Freshness Indicator for Beef Loin During Cold Storage
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
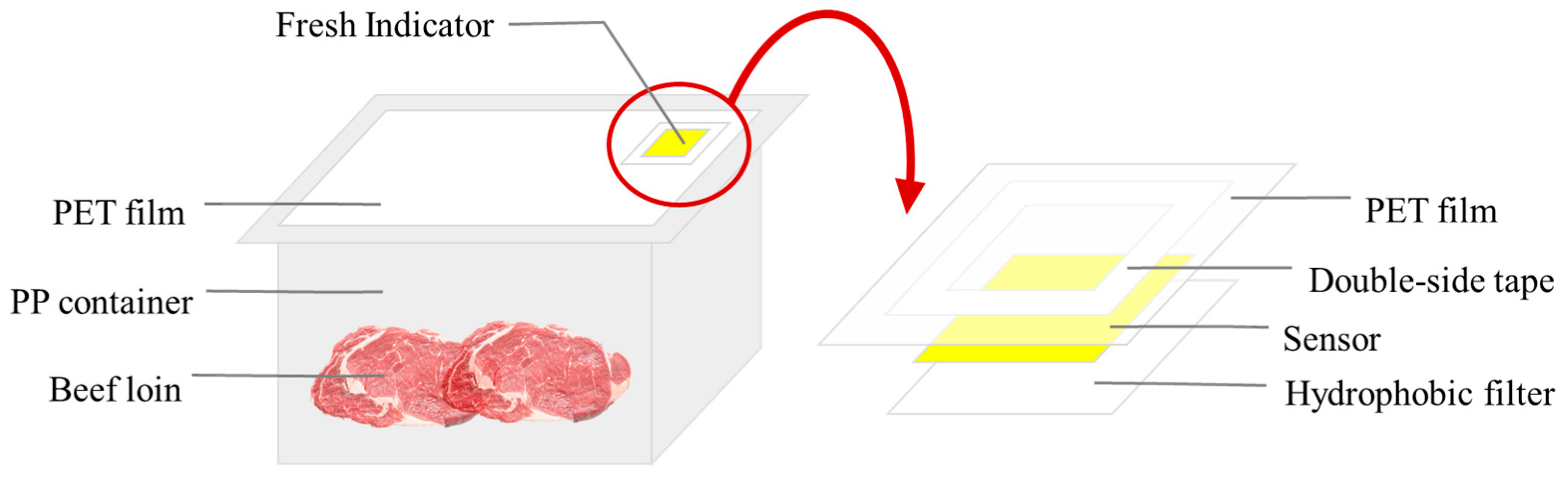
2.2. Fabrication of Freshness Indicator
2.3. Freshness Indicator Assessment
2.4. Colorimetric Analysis
2.5. Beef Quality Assessment
2.5.1. Microbial Analysis
2.5.2. TVB-N Measurement
2.5.3. pH Measurement
2.6. Headspace Analysis of Beef Packaging
2.7. Standardized Kinetic Framework for Cross-Indicator Comparison
2.8. Pearson’s Correlation Method
2.9. Statistical Analysis
3. Results and Discussion
3.1. Optimization and Colorimetric Response Under Simulated Volatile Amines
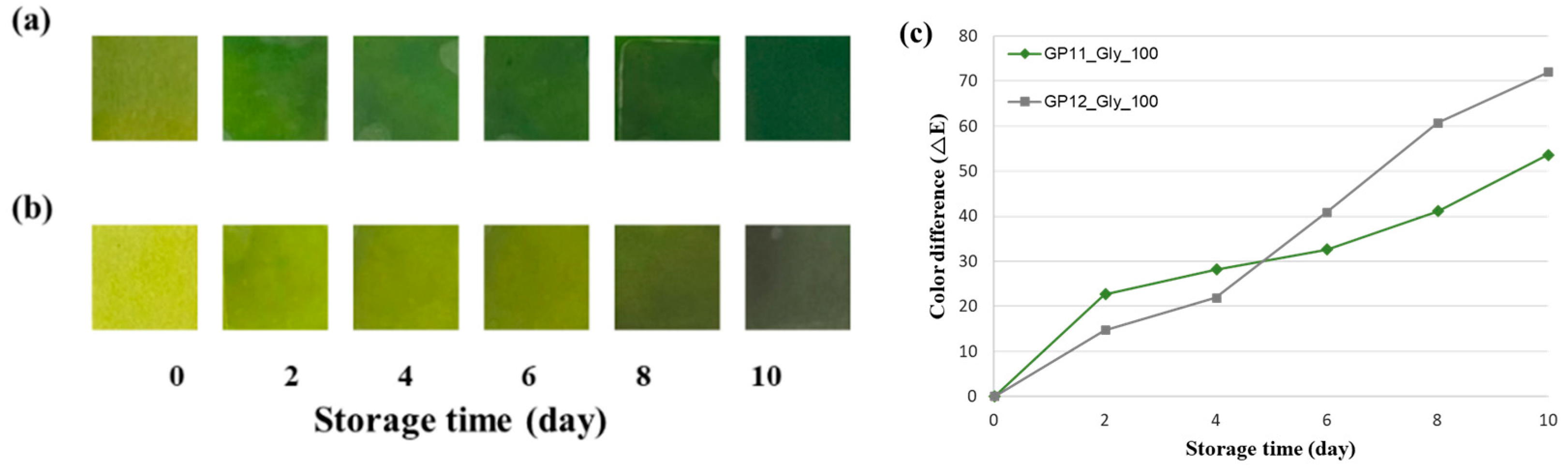
3.2. Application of Freshness Indicators to Beef Packaging During Storage
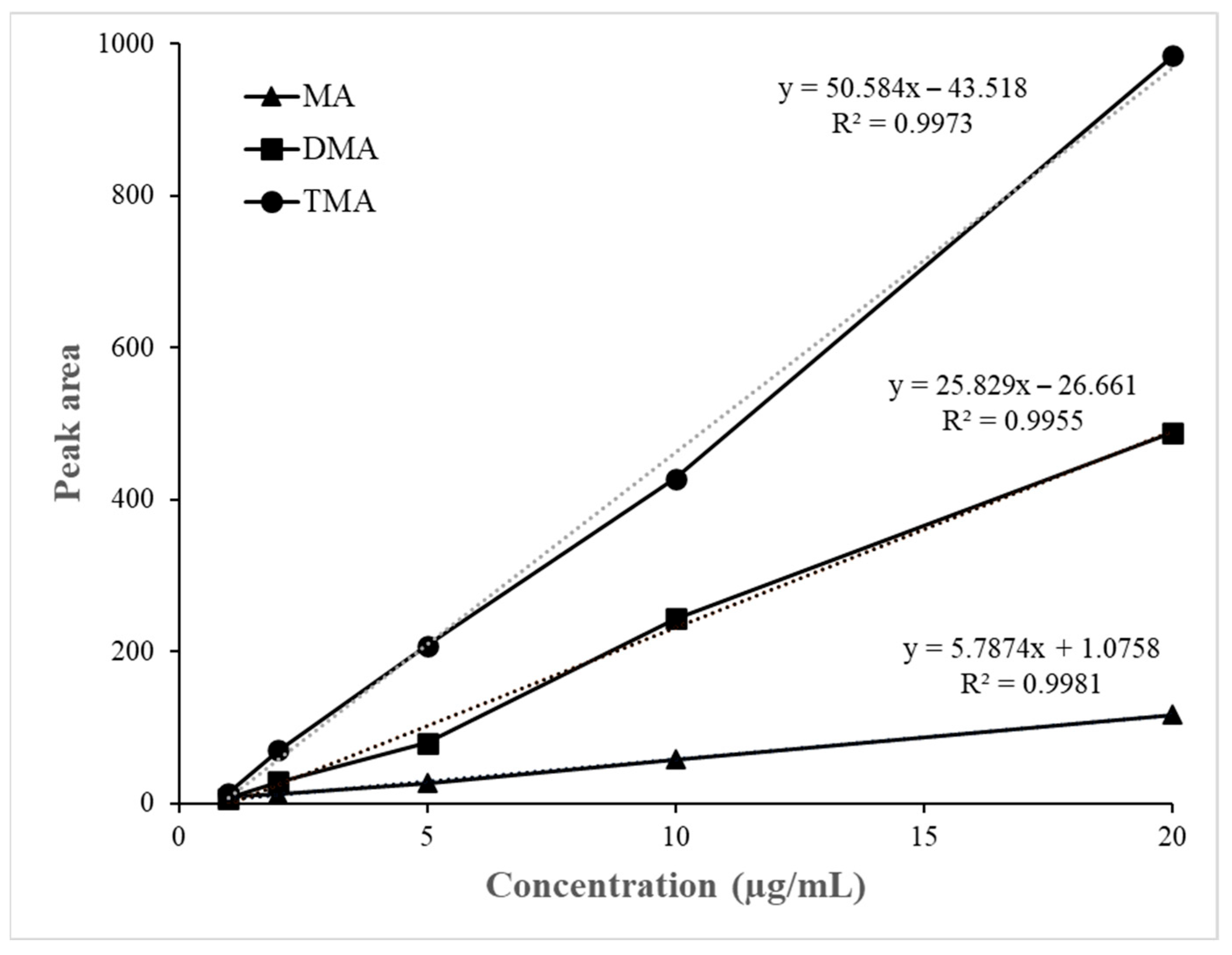
3.3. Headspace Volatile Amines Analysis
3.4. Pearson’s Correlation Between Label ΔE* and Headspace Amines
3.5. Linkage Between ΔE Thresholds and Spoilage Factors with Kinetics
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Robertson, G. Modified atmosphere packaging. In Food Packaging: Principles and Practice; CRC Press: Boca Raton, FL, USA, 2006; pp. 313–329. [Google Scholar]
- Kerry, J.; Butler, P. (Eds.) Smart Packaging Technologies for Fast Moving Consumer Goods; John Wiley & Sons: Chichester, UK, 2008. [Google Scholar]
- Biji, K.B.; Ravishankar, C.N.; Mohan, C.O.; Srinivasa Gopal, T.K. Smart packaging systems for food applications: A review. J. Food Sci. Technol. 2015, 52, 6125–6135. [Google Scholar] [CrossRef] [PubMed]
- Drago, E.; Campardelli, R.; Pettinato, M.; Perego, P. Innovations in smart packaging concepts for food: An extensive review. Foods 2020, 9, 1628. [Google Scholar] [CrossRef] [PubMed]
- Kuswandi, B.; Wicaksono, Y.; Jayus; Abdullah, A.; Heng, L.Y.; Ahmad, M. Real time on-package freshness indicator for guavas packaging. J. Food Meas. Charact. 2013, 7, 29–39. [Google Scholar] [CrossRef]
- Huang, J.; Liu, Q.; Xu, W.; Geng, L.; Wang, Q.; Lin, Y. Functional characteristics improvement by structural modification of hydroxypropyl methylcellulose modified polyvinyl alcohol films incorporating roselle anthocyanins for shrimp freshness monitoring. Int. J. Biol. Macromol. 2020, 162, 1250–1261. [Google Scholar] [CrossRef]
- Byrne, L.; Lau, K.T.; Diamond, D. Monitoring of headspace total volatile basic nitrogen from selected fish species using reflectance spectroscopic measurements of pH sensitive films. Analyst 2002, 127, 1338–1341. [Google Scholar] [CrossRef]
- Chen, H.; Zhang, M.; Bhandari, B.; Yang, C. Development of a novel colorimetric food package label for monitoring lean pork freshness. LWT 2019, 99, 43–49. [Google Scholar] [CrossRef]
- Gomes, V.; Teixeira, A.; Silva, A.M.S.; Freitas, V. Pyranoflavylium-cellulose acetate films and the glycerol effect towards the development of pH-freshness smart label for food packaging. Food Hydrocoll. 2022, 127, 107501. [Google Scholar] [CrossRef]
- Pham Le Khanh, H.; Nemes, D.; Rusznyák, Á.; Ujhelyi, Z.; Fehér, P.; Fenyvesi, F.; Bácskay, I. Comparative investigation of cellular effects of polyethylene glycol (PEG) derivatives. Polymers 2022, 14, 279. [Google Scholar] [CrossRef]
- Cheng, R.; Niu, B.; Fang, X.; Chen, H.; Chen, H.; Wu, W.; Gao, H. Preparation and characterization of water vapor-responsive methylcellulose–polyethylene glycol-400 composite membranes and an indication of freshness of shiitake mushrooms. Int. J. Biol. Macromol. 2024, 270, 132189. [Google Scholar] [CrossRef]
- Funnekotter, B.; Mancera, R.L.; Bunn, E. A simple but effective combination of pH indicators for plant tissue culture. Plants 2023, 12, 740. [Google Scholar] [CrossRef]
- Kuswandi, B.; Nurfawaidi, A. On-package dual sensors label based on pH indicators for real-time monitoring of beef freshness. Food Control 2017, 82, 91–100. [Google Scholar] [CrossRef]
- Erna, K.H.; Rovina, K.; Mantihal, S. Current detection techniques for monitoring the freshness of meat-based products: A review. J. Packag. Technol. Res. 2021, 5, 127–141. [Google Scholar] [CrossRef]
- Hopkins, D.; Holman, B.; Giteru, S. Total volatile basic nitrogen in meat products: Occurrence, method of determination and use as a freshness indicator. Foods 2020, 9, 1062. [Google Scholar]
- Aparicio-Ruiz, R.; García-González, D.L.; Morales, M.T.; Lobo-Prieto, A.; Romero, I. Comparison of two analytical methods validated for the determination of volatile compounds in virgin olive oil: GC-FID vs. GC-MS. Talanta 2018, 187, 133–141. [Google Scholar] [CrossRef]
- Kim, M.S.; Chen, Y.R.; Mehl, P.M. NIR spectroscopic sensing for point-of-need freshness assessment of meat, fish, vegetables and fruits. In Sensing for Agriculture and Food Quality and Safety IX; SPIE: Baltimore, MD, USA, 2017; Volume 102, pp. 1–10. [Google Scholar]
- Kataoka, H.; Lord, H.L.; Pawliszyn, J. Applications of solid-phase microextraction in food analysis. J. Chromatogr. A 2000, 880, 35–62. [Google Scholar] [CrossRef]
- Lyte, J.M.; Dawson, P.L.; Mitchell, G.E.; Woodward, C.L.; Diehl, K.C. Volatile compound characterization of modified atmosphere packaged ground beef held under temperature abuse. Food Control 2016, 59, 1–6. [Google Scholar] [CrossRef]
- Ioannidis, A.-G.; Walgraeve, C.; Vanderroost, M.; Van Langenhove, H.; Devlieghere, F.; De Meulenaer, B. Non-Destructive Measurement of Volatile Organic Compounds in Modified Atmosphere Packaged Poultry Using SPME-SIFT-MS in Tandem with Headspace TD-GC-MS. Food Anal. Methods 2017, 11, 848–861. [Google Scholar] [CrossRef]
- Lee, E.J.; Shin, H.S. Development of a freshness indicator for monitoring the quality of beef during storage. Food Sci. Biotechnol. 2019, 28, 1899–1906. [Google Scholar] [CrossRef] [PubMed]
- Dirpan, A.; Hidayat, S.H. Quality and shelf-life evaluation of fresh beef stored in smart packaging. Foods 2023, 12, 396. [Google Scholar] [CrossRef]
- Luo, X.; Lim, L.T. An inkjet-printed sulfonephthalein dye indicator array for volatile amine detection. J. Food Sci. 2020, 85, 442–454. [Google Scholar] [CrossRef]
- Ziyaina, M.; Rasco, B.; Coffey, T.; Ünlü, G.; Sablani, S.S. Colorimetric detection of volatile organic compounds for shelf-life monitoring of milk. Food Control 2019, 100, 220–226. [Google Scholar] [CrossRef]
- Mazunin, S.A.; Chechulin, V.L. Applied aspects of use of amines for the production of inorganic salts in systems with salting-out. Russ. J. Appl. Chem. 2010, 83, 1690–1697. [Google Scholar] [CrossRef]
- Lista, A.G.; Arce, L.; Ríos, A.; Valcárcel, M. Analysis of solid samples by capillary electrophoresis using a gas extraction sampling device in a flow system. Anal. Chim. Acta 2001, 438, 315–322. [Google Scholar] [CrossRef]
- Yin, C.; Wang, S.; Zhang, Y.; Chen, Z.; Lin, Z.; Fu, P.; Yao, L. Correlation between the pore resistance and water flux of the cellulose acetate membrane. Environ. Sci. Water Res. Technol. 2017, 3, 1037–1041. [Google Scholar] [CrossRef]
- Lindsey, D.T.; Wee, A.G. Perceptibility and acceptability of CIELAB color differences in computer-simulated teeth. J. Dent. 2007, 35, 593–599. [Google Scholar] [CrossRef]
- Paravina, R.D.; Ghinea, R.; Herrera, L.J.; Bona, A.D.; Igiel, C.; Linninger, M.; Sakai, M.; Takahashi, H.; Tashkandi, E.; del Mar Perez, M. Color difference thresholds in dentistry. J. Esthet. Restor. Dent. 2015, 27 (Suppl. 1), S1–S9. [Google Scholar] [CrossRef]
- Heo, W.; Kim, S.; Park, J. A review on gas indicators and sensors for smart food packaging. Foods 2024, 13, 3047. [Google Scholar] [CrossRef]
- Kuswandi, B.; Oktaviana, S.; Abdullah, A. Colorimetric paper-based dual indicator label for real-time monitoring of fish freshness. Food Technol. Biotechnol. 2022, 60, 497–508. [Google Scholar] [CrossRef]
- Eslami, Z.; Elkoun, S.; Robert, M.; Adjallé, K. A review of the effect of plasticizers on the physical and thermal properties of alginate-based films. Molecules 2023, 28, 6637. [Google Scholar] [CrossRef]
- Pires, A.F.; Díaz, O.; Cobos, A.; Pereira, C.D. A review of recent developments in edible films and coatings—Focus on whey-based materials. Foods 2024, 13, 2638. [Google Scholar] [CrossRef]
- Wang, G.; He, H.; Xu, J.; Wang, X.; Zhang, T.; Huang, S.; Li, H.; Zhao, P.; Liu, X. Preparation of fish freshness colorimetric indicator label based on the dye of BTB grafted on MOF carrier. Sens. Actuators B Chem. 2022, 354, 131230. [Google Scholar] [CrossRef]
- Merck/Sigma-Aldrich. Bromocresol Green Indicator—Transition Range pH 3.8–5.4. MilliporeSigma, n.d. Available online: https://www.sigmaaldrich.com/KR/ko/substance/bromocresolgreen6980176608 (accessed on 23 September 2025).
- Shokrollahi, A.; Firoozbakht, F. Determination of the acidity constants of neutral red and bromocresol green by solution scanometric method and comparison with spectrophotometric results. Beni-Suef Univ. J. Basic Appl. Sci. 2016, 5, 13–20. [Google Scholar] [CrossRef]
- ACS Reagent Chemicals. Bromocresol Purple—Visual Transition Interval pH 5.2–6.8; ACS Publications: Washington, DC, USA, 2017. [Google Scholar]
- PubChem. Bromocresol Purple—Indicator pH Range 5.2–6.8. PubChem, n.d. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/8273 (accessed on 23 September 2025).
- Yim, D.G.; Jo, C.; Kim, J.H. Microbial changes under packaging conditions during transport and comparison between sampling methods of boxed beef. J. Anim. Sci. Technol. 2019, 61, 47. [Google Scholar] [CrossRef] [PubMed]
- Holman, B.W.B.; Coombs, C.E.O.; Kerr, M.J.; Pearce, K.L.; Hopkins, D.L. The association between TVB-N concentration and other biomarkers of quality and spoilage for vacuum packaged beef. Meat Sci. 2021, 179, 108551. [Google Scholar] [CrossRef]
- Bekhit, A.E.D.A.; Giteru, S.G.; Holman, B.W.; Hopkins, D.L. Total volatile basic nitrogen and trimethylamine in muscle foods: Potential formation pathways and effects on human health. Compr. Rev. Food Sci. Food Saf. 2021, 20, 3620–3666. [Google Scholar] [CrossRef]
- Lee, G.Y.; Kwon, S.M.; Park, S.J. Development of a freshness indicator for assessing the quality of packaged pork products during refrigerated storage. Foods 2024, 13, 2097. [Google Scholar] [CrossRef]
- Australian Meat Processor Corporation. Total Volatile Basic Nitrogen in Meat Products (Final Report); Australian Meat Processor Corporation: Sydney, Australia, 2020; Available online: https://ampc.com.au/ (accessed on 8 August 2025).
- Pan, Y.; Xue, X.; Wang, Y.; Wang, J.; Teng, W.; Cao, J.; Zhang, Y. Effects of different preservation techniques on microbial and physicochemical quality characteristics of sauced beef under chilled storage. Foods 2025, 14, 1175. [Google Scholar] [CrossRef]
- Zhai, X.; Xue, Y.; Sun, Y.; Katona, J. Colorimetric food freshness indicators for intelligent packaging: Progress, shortcomings, and promising solutions. Foods 2025, 14, 2813. [Google Scholar] [CrossRef]
- Hwang, S.H.; Lee, J.; Nam, T.G.; Koo, M.; Cho, Y.S. Changes in physicochemical properties and bacterial communities in aged Korean native cattle beef during cold storage. Food Sci. Nutr. 2022, 10, 2590–2600. [Google Scholar] [CrossRef]
- Smith, C.L.; Geornaras, I.; Nair, M.N. Impact of spoilage bacterial populations on discoloration of beef steaks. Meat Muscle Biol. 2024, 8, 17796. [Google Scholar]
- Sallam, K.I.; Samejima, K. Microbiological and chemical quality of ground beef treated with sodium lactate and sodium chloride during refrigerated storage. LWT–Food Sci. Technol. 2004, 37, 865–871. [Google Scholar] [CrossRef]
- Ismail, A.; Park, S.; Kim, H.-J.; Jo, C. Evaluation of biomarkers that influence the freshness of beef during storage using VIS/NIR hyperspectral imaging. LWT 2025, 216, 117302. [Google Scholar] [CrossRef]
- Conte-Junior, C.A.; Monteiro, M.L.G.; Patrícia, R.; Mársico, E.T.; Lopes, M.M.; Alvares, T.S.; Mano, S.B. The effect of different packaging systems on the shelf life of beef: A review. Foods 2020, 9, 495. [Google Scholar] [CrossRef] [PubMed]
- Evans, M.; Gollasch, M.; Micha, R.; Wanner, C. The dietary source of trimethylamine-N-oxide and clinical outcomes: A review. Nutrients 2023, 15, 4068. [Google Scholar]
- Wang, Z.; Tang, W.H.W.; O’Connell, T.; Garcia, E.; Jeyarajah, E.J.; Li, X.S.; Jia, X.; Weeks, T.L.; Hazen, S.L. Circulating trimethylamine N-oxide levels following fish or seafood versus meat ingestion: A systematic review and meta-analysis. Adv. Nutr. 2022, 13, 1430–1446. [Google Scholar]
- Nguyen, T.T.T.; Huy, B.T.; Lee, Y.I. Disposable colorimetric paper-based probe for the detection of amine-containing gases in aquatic sediments. ACS Omega 2019, 4, 12665–12670. [Google Scholar] [CrossRef]
- Engel, L.; Benito-Altamirano, I.; Tarantik, K.R.; Pannek, C.; Dold, M.; Prades, J.D.; Wöllenstein, J. Printed sensor labels for colorimetric detection of ammonia, formaldehyde and hydrogen sulfide from the ambient air. Sens. Actuators B Chem. 2021, 330, 129281. [Google Scholar] [CrossRef]
- Zhang, Z.; Tang, H.; Cai, K.; Liang, R.; Tong, L.; Ou, C. A novel indicator based on polyacrylamide hydrogel and bromocresol green for food freshness monitoring. Foods 2023, 12, 3964. [Google Scholar] [CrossRef]
- Lin, Y.; Ma, J.; Cheng, J.-H.; Sun, D.-W. Visible detection of chilled beef freshness using a paper-based colourimetric sensor array combining with deep learning algorithms. Food Chem. 2024, 441, 138344. [Google Scholar] [CrossRef]
- Bhadury, D.; Rahman, S.; Karim, M.; Goon, S. Application of on-pack pH indicators to monitor freshness of raw beef during refrigerated storage. Food Qual. Saf. 2024, 8, fyae021. [Google Scholar] [CrossRef]
- Kaewprachu, P.; Jaisan, C.; Rawdkuen, S.; Osako, K. Colorimetric indicator films based on carboxymethyl cellulose and anthocyanins as a visual indicator for shrimp freshness tracking. Heliyon 2024, 10, e31527. [Google Scholar] [CrossRef] [PubMed]
- Qin, W.; Parzuchowski, P.; Zhang, W.; Meyerhoff, M.E. Optical sensor for amine vapors based on dimer–monomer equilibrium of indium(III) octaethylporphyrin in a polymeric film. Anal. Chem. 2003, 75, 332–340. [Google Scholar] [CrossRef] [PubMed]
- Evans, M.; Gollasch, M.; Micha, R.; Wanner, C. The dietary source of trimethylamine-N-oxide (TMAO) and clinical outcomes: An unexpected liaison. Clin. Kidney J. 2023, 16, 1804–1815. [Google Scholar] [CrossRef] [PubMed]
- Stavropoulou, E.; Bezirtzoglou, E. Predictive modeling of microbial behavior in food. Foods 2019, 8, 654. [Google Scholar] [CrossRef]
- Garre, A.; Koomen, J.; den Besten, H.M.; Zwietering, M.H. Modeling population growth in R with the biogrowth package. J. Stat. Softw. 2023, 107, 1–51. [Google Scholar] [CrossRef]
- Kim, S.J.; Kim, H.J.; Yun, Y.C.; Song, S.M.; Choi, J.H. Effects of aging methods and periods on quality traits and microbial characteristics of beef. Food Sci. Anim. Resour. 2022, 42, 953–967. [Google Scholar] [CrossRef]
- Savini, F.; Indio, V.; Giacometti, F.; Mekkonnen, Y.T.; De Cesare, A.; Prandini, L.; Marrone, R.; Seguino, A.; Di Paolo, M.; Vuoso, V.; et al. Microbiological safety dry-aged meat: A critical review of data gaps and research needs to define process hygiene and safety criteria. Ital. J. Food Saf. 2024, 13, 12438. [Google Scholar] [CrossRef]
- Luo, X.; Zaitoon, A.; Lim, L.T. A review on colorimetric indicators for monitoring product freshness in intelligent food packaging: Indicator dyes, preparation methods, and applications. Compr. Rev. Food Sci. Food Saf. 2022, 21, 2489–2519. [Google Scholar] [CrossRef]
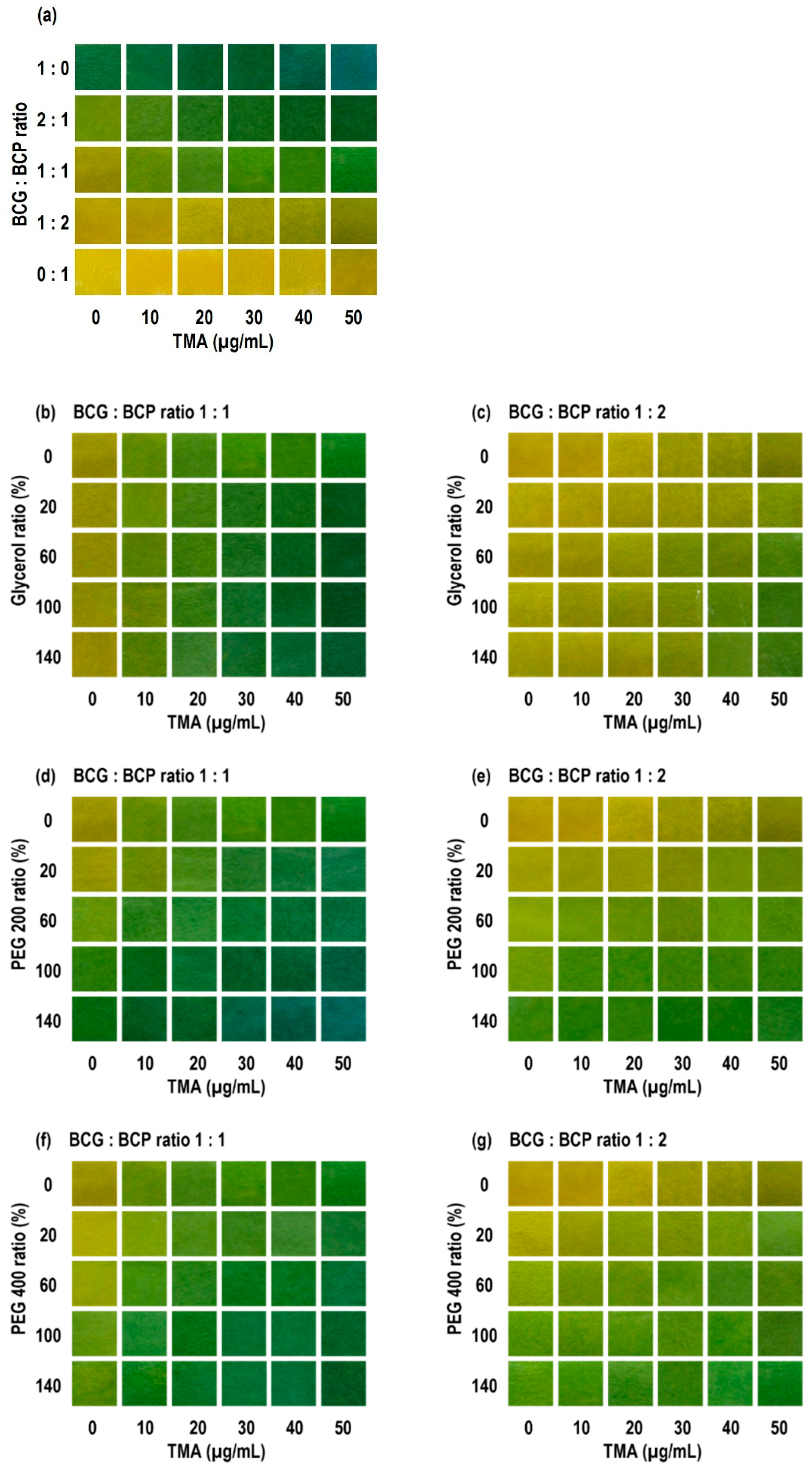
| Code | Matrix (Polymer/Substrate/Lamination) | BCG:BCP Ratio | Plasticizer Type | Initial Color at 0 µg/mL TMA | Plasticizer Conc. (% of CA) |
|---|---|---|---|---|---|
| G20-1 | CA-coated filter paper PTFE-D support PET lamination | 1:1 | Glycerol | Yellow (acidic form) at 0 µg/mL TMA | 20 |
| G60-1 | 60 | ||||
| G100-1 | 100 | ||||
| G140-1 | 140 | ||||
| 200P20-1 | PEG200 | Green (basic form) at 0 µg/mL TMA | 20 | ||
| 200P60-1 | 60 | ||||
| 200P100-1 | 100 | ||||
| 200P140-1 | 140 | ||||
| 400P20-1 | PEG400 | 20 | |||
| 400P60-1 | 60 | ||||
| 400P100-1 | 100 | ||||
| 400P140-1 | 140 | ||||
| G20-2 | 1:2 | Glycerol | Yellow (acidic form) at 0 µg/mL TMA | 20 | |
| G60-2 | 60 | ||||
| G100-2 | 100 | ||||
| G140-2 | 140 | ||||
| 200P20-2 | PEG200 | Green (basic form) at 0 µg/mL TMA | 20 | ||
| 200P60-2 | 60 | ||||
| 200P100-2 | 100 | ||||
| 200P140-2 | 140 | ||||
| 400P20-2 | PEG400 | 20 | |||
| 400P60-2 | 60 | ||||
| 400P100-2 | 100 | ||||
| 400P140-2 | 140 |
| Freshness Indicator | TMA Concentration (µg/mL) | |||||
|---|---|---|---|---|---|---|
| 0 µg/mL | 10 µg/mL | 20 µg/mL | 30 µg/mL | 40 µg/mL | 50 µg/mL | |
| ΔE | ||||||
| G20-1 | 0 | 13.87 ± 0.16 | 21.63 ± 0.09 | 21.63 ± 0.27 | 32.36 ± 0.18 | 34.53 ± 0.65 |
| G60-1 | 0 | 15.89 ± 1.49 | 21.43 ± 0.63 | 34.61 ± 0.94 | 39.61 ± 0.74 | 39.61 ± 0.34 |
| G100-1 | 0 | 12.17 ± 0.51 | 25.31 ± 0.05 | 35.00 ± 0.63 | 39.58 ± 1.21 | 47.80 ± 0.18 |
| G140-1 | 0 | 15.99 ± 1.77 | 27.96 ± 1.20 | 36.56 ± 0.65 | 41.38 ± 0.73 | 45.96 ± 2.23 |
| 200P20-1 | 0 | 13.97 ± 1.19 | 26.60 ± 1.17 | 38.15 ± 0.80 | 42.80 ± 0.89 | 45.70 ± 2.48 |
| 200P60-1 | 0 | 17.73 ± 0.28 | 22.39 ± 0.29 | 30.18 ± 0.07 | 30.18 ± 0.21 | 39.58 ± 0.04 |
| 200P100-1 | 0 | 16.79 ± 0.66 | 18.96 ± 0.54 | 25.53 ± 0.57 | 29.00 ± 0.73 | 35.21 ± 0.36 |
| 200P140-1 | 0 | 9.84 ± 0.18 | 9.33 ± 0.41 | 23.29 ± 0.20 | 26.48 ± 0.25 | 40.97 ± 0.48 |
| 400P20-1 | 0 | 14.11 ± 0.74 | 26.91 ± 0.46 | 33.01 ± 0.63 | 35.72 ± 0.79 | 39.60 ± 1.26 |
| 400P60-1 | 0 | 19.28 ± 0.35 | 27.60 ± 0.46 | 35.79 ± 0.44 | 36.79 ± 0.12 | 44.18 ± 1.43 |
| 400P100-1 | 0 | 17.69 ± 1.00 | 21.07 ± 1.62 | 28.95 ± 0.20 | 31.55 ± 0.48 | 35.77 ± 0.14 |
| 400P140-1 | 0 | 15.74 ± 0.78 | 26.64 ± 0.20 | 29.39 ± 0.29 | 32.36 ± 0.51 | 38.12 ± 0.44 |
| G20-2 | 0 | 1.40 ± 0.79 | 5.24 ± 0.44 | 9.46 ± 0.90 | 11.18 ± 0.66 | 18.86 ± 0.21 |
| G60-2 | 0 | 1.66 ± 0.37 | 8.81 ± 0.63 | 16.68 ± 1.23 | 20.48 ± 0.76 | 26.19 ± 0.73 |
| G100-2 | 0 | 7.68 ± 0.37 | 16.45 ± 0.48 | 18.57 ± 2.44 | 27.27 ± 0.28 | 33.03 ± 0.86 |
| G140-2 | 0 | 5.34 ± 1.75 | 10.42 ± 0.28 | 16.34 ± 0.04 | 16.34 ± 0.58 | 31.30 ± 0.61 |
| 200P20-2 | 0 | 5.23 ± 0.22 | 5.27 ± 1.59 | 8.92 ± 0.99 | 14.03 ± 0.86 | 18.88 ± 0.86 |
| 200P60-2 | 0 | 9.50 ± 0.09 | 13.90 ± 0.57 | 14.96 ± 0.29 | 13.98 ± 0.41 | 19.82 ± 0.75 |
| 200P100-2 | 0 | 14.92 ± 0.32 | 16.05 ± 0.72 | 16.05 ± 0.27 | 18.93 ± 1.32 | 18.43 ± 0.18 |
| 200P140-2 | 0 | 3.15 ± 0.46 | 2.92 ± 0.30 | 8.67 ± 0.51 | 10.19 ± 0.44 | 10.19 ± 1.61 |
| 400P20-2 | 0 | 11.12 ± 0.04 | 15.76 ± 0.45 | 19.23 ± 0.33 | 21.55 ± 0.30 | 31.30 ± 0.23 |
| 400P60-2 | 0 | 11.81 ± 0.24 | 14.27 ± 0.18 | 19.06 ± 1.01 | 24.15 ± 0.01 | 26.62 ± 0.05 |
| 400P100-2 | 0 | 4.39 ± 0.62 | 2.98 ± 0.05 | 5.84 ± 0.47 | 8.37 ± 2.38 | 17.02 ± 1.07 |
| 400P140-2 | 0 | 4.31 ± 0.27 | 10.64 ± 0.45 | 12.15 ± 0.21 | 14.15 ± 1.47 | 20.74 ± 0.50 |
| Parameter | Storage Time (Day) | |||||
|---|---|---|---|---|---|---|
| 0 Day | 2 Days | 4 Days | 6 Days | 8 Days | 10 Days | |
| TBC (log CFU/g) | 3 | 4.51 | 5.92 | 6.92 | 8.36 | 8.64 |
| TVB-N (mg/100 g) | 7.00 ± 0.00 | 10.20 ± 0.80 | 14.00 ± 0.00 | 14.40 ± 0.80 | 18.20 ± 1.40 | 23.80 ± 1.40 |
| pH | 5.72 ± 0.02 | 5.86 ± 0.01 | 5.92 ± 0.01 | 5.96 ± 0.01 | 6.05 ± 0.17 | 6.24 ± 0.08 |
| Parameter | Storage Time (Day) | |||||
|---|---|---|---|---|---|---|
| 0 Day | 2 Days | 4 Days | 6 Days | 8 Days | 10 Days | |
| MA (µg/kg) | - | n.d. * | n.d. | n.d. | 3.19 ± 0.21 | 6.96 ± 0.34 |
| CV (%) | - | - | - | - | 6.6 | 4.9 |
| DMA (µg/kg) | - | 2.28 ± 0.12 | 2.35 ± 0.18 | 2.83 ± 0.15 | 4.87 ± 0.27 | 7.37 ± 0.32 |
| CV (%) | 5.3 | 7.7 | 5.3 | 5.5 | 4.3 | |
| TMA (µg/kg) | - | n.d. | n.d. | n.d. | 2.03 ± 0.14 | 3.29 ± 0.22 |
| CV (%) | - | - | - | - | 6.9 | 6.7 |
| Freshness Indicator | DMA | Total Volatile Amines |
|---|---|---|
| GP11_Gly_100 | 0.956 * | 0.945 * |
| p = 0.003 | p = 0.004 | |
| GP12_Gly_100 | 0.959 * | 0.936 * |
| p = 0.002 | p = 0.006 |
| Analyte | k (Normalized) | t0 (Day) | R2 (Norm) | RMSE (Norm) |
|---|---|---|---|---|
| ΔE (G100-1) | 0.405 | 4.111 | 0.892 | 0.101 |
| ΔE (G100-2) | 0.583 | 5.211 | 0.981 | 0.049 |
| TBC (log CFU/g) | 0.601 | 4.065 | 0.98 | 0.051 |
| TVB-N (mg/100 g) | 0.447 | 5.726 | 0.927 | 0.087 |
| DMA (μg/kg) | 1.381 | 7.913 | 0.993 | 0.032 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lim, K.-J.; Kim, J.-S.; Heo, Y.-J.; Shin, H.-S. Development and Evaluation of a BCG/BCP-Based Cellulose Acetate Freshness Indicator for Beef Loin During Cold Storage. Foods 2025, 14, 4017. https://doi.org/10.3390/foods14234017
Lim K-J, Kim J-S, Heo Y-J, Shin H-S. Development and Evaluation of a BCG/BCP-Based Cellulose Acetate Freshness Indicator for Beef Loin During Cold Storage. Foods. 2025; 14(23):4017. https://doi.org/10.3390/foods14234017
Chicago/Turabian StyleLim, Kyung-Jik, Jun-Seo Kim, Yu-Jin Heo, and Han-Seung Shin. 2025. "Development and Evaluation of a BCG/BCP-Based Cellulose Acetate Freshness Indicator for Beef Loin During Cold Storage" Foods 14, no. 23: 4017. https://doi.org/10.3390/foods14234017
APA StyleLim, K.-J., Kim, J.-S., Heo, Y.-J., & Shin, H.-S. (2025). Development and Evaluation of a BCG/BCP-Based Cellulose Acetate Freshness Indicator for Beef Loin During Cold Storage. Foods, 14(23), 4017. https://doi.org/10.3390/foods14234017







