Sequential Inoculation with Selected Indigenous Yeasts Enhances the Aroma Profiles and Typicity of White Wines from Yantai, China
Abstract
1. Introduction
2. Materials and Methods
2.1. Grape Processing and Fermentation Protocol
2.2. Determination of Oenological Parameters
2.3. Analysis of Phenolic Compounds
2.4. Volatile Compound Analysis via HS–SPME–GC–MS
2.5. Sensory Evaluation
2.6. Statistical Analysis and Data Processing
3. Results and Discussion
3.1. Impact of Yeast Strains on the Fermentation Dynamics of Must
3.2. Physicochemical Parameters of the Wines
3.3. Phenolic Content of White Wines
3.4. Volatile Compounds of White Wines
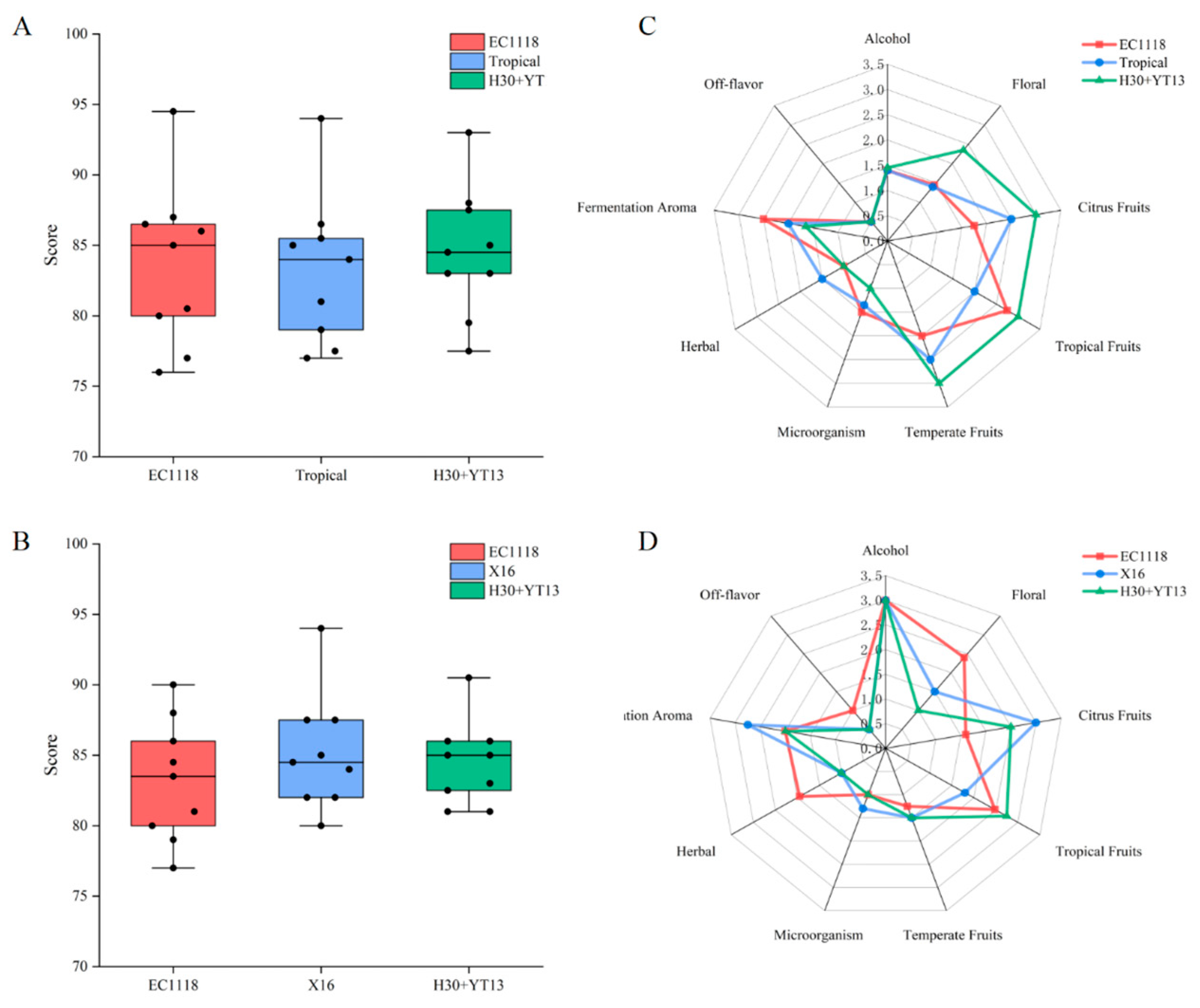
3.5. Sensory Evaluation
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gambetta, J.M.; Cozzolino, D.; Bastian, S.E.P.; Jeffery, D.W. Exploring the Effects of Geographical Origin on the Chemical Composition and Quality Grading of Vitis vinifera L. Cv. Chardonnay Grapes. Molecules 2017, 22, 218. [Google Scholar] [CrossRef]
- Wang, L.; Yin, H.; Shao, X.; Zhang, Z.; Zhong, X.; Wei, R.; Ding, Y.; Wang, H.; Li, H. Improving the Aging Aroma Profiles of Italian Riesling and Petit Verdot Wines: Impact of Spontaneous and Inoculated Fermentation Processes. Food Chem. X 2023, 20, 100978. [Google Scholar] [CrossRef]
- Ting, J.H.; Surratt, A.A.; Moccio, L.E.; Sandbrook, A.M.; Chang, E.A.; Cladis, D.P. Ripening Kinetics and Grape Chemistry of Virginia Petit Manseng. Beverages 2025, 11, 108. [Google Scholar] [CrossRef]
- Silva, V.; Brito, I.; Alexandre, A. The Vineyard Microbiome: How Climate and the Main Edaphic Factors Shape Microbial Communities. Microorganisms 2025, 13, 1092. [Google Scholar] [CrossRef]
- Li, R.; Yang, D.; Li, Z.; Tang, X.; Zhong, K.; Ding, Y.; Han, X.; Guan, X.; Sun, Y. The Effect of Stem Contact Fermentation on the Quality of Cabernet Sauvignon and Merlot Wines from Yantai, China. Food Biosci. 2025, 64, 105872. [Google Scholar] [CrossRef]
- Noguer-Juncà, E.; Crespi-Vallbona, M. Tourism and Coexistence between Branded and Natural Wines: The Case of Calonge i Sant Antoni. J. Rural Stud. 2025, 119, 103726. [Google Scholar] [CrossRef]
- Liu, S.; Lou, Y.; Li, Y.; Zhao, Y.; Laaksonen, O.; Li, P.; Zhang, J.; Battino, M.; Yang, B.; Gu, Q. Aroma Characteristics of Volatile Compounds Brought by Variations in Microbes in Winemaking. Food Chem. 2023, 420, 136075. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Zhu, X.-L.; Ullah, N.; Tao, Y.-S. Aroma Glycosides in Grapes and Wine. J. Food Sci. 2017, 82, 248–259. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Zhang, R.; Sirisena, S.; Gan, R.; Fang, Z. Beta-Glucosidase Activity of Wine Yeasts and Its Impacts on Wine Volatiles and Phenolics: A Mini-Review. Food Microbiol. 2021, 100, 103859. [Google Scholar] [CrossRef]
- Han, X.; Qing, X.; Yang, S.; Li, R.; Zhan, J.; You, Y.; Huang, W. Study on the Diversity of Non-Saccharomyces Yeasts in Chinese Wine Regions and Their Potential in Improving Wine Aroma by β-Glucosidase Activity Analyses. Food Chem. 2021, 360, 129886. [Google Scholar] [CrossRef]
- de Ovalle, S.; Brena, B.; González-Pombo, P. Influence of Beta Glucosidases from Native Yeast on the Aroma of Muscat and Tannat Wines. Food Chem. 2021, 346, 128899. [Google Scholar] [CrossRef]
- Villena, M.A.; Iranzo, J.F.Ú.; Pérez, A.I.B. β-Glucosidase Activity in Wine Yeasts: Application in Enology. Enzym. Microb. Technol. 2007, 40, 420–425. [Google Scholar] [CrossRef]
- Huang, R.; Zhang, F.; Yan, X.; Qin, Y.; Jiang, J.; Liu, Y.; Song, Y. Characterization of the β-Glucosidase Activity in Indigenous Yeast Isolated from Wine Regions in China. J. Food Sci. 2021, 86, 2327–2345. [Google Scholar] [CrossRef]
- Souid, I.; Hassene, Z.; Palomo, E.S.; Pérez-Coello, M.S.; Ghorbel, A. Varietal Aroma Compounds of Vitis vinifera Cv. Khamri Grown in Tunisia. J. Food Qual. 2007, 30, 718–730. [Google Scholar] [CrossRef]
- Rodríguez-Bencomo, J.J.; Cabrera-Valido, H.M.; Pérez-Trujillo, J.P.; Cacho, J. Bound Aroma Compounds of Gual and Listán Blanco Grape Varieties and Their Influence in the Elaborated Wines. Food Chem. 2011, 127, 1153–1162. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, M.; Li, W.; Wang, X.; Kong, W.; Huang, W.; Zhan, J.; Xia, G.; You, Y. Indigenous Yeast Can Increase the Phenolic Acid and Volatile Ester Compounds in Petit Manseng Wine. Front. Nutr. 2022, 9, 1031594. [Google Scholar] [CrossRef]
- Parker, M.; Capone, D.L.; Francis, I.L.; Herderich, M.J. Aroma Precursors in Grapes and Wine: Flavor Release during Wine Production and Consumption. J. Agric. Food Chem. 2018, 66, 2281–2286. [Google Scholar] [CrossRef]
- Niedźwiedź, I.; Płotka-Wasylka, J.; Kapusta, I.; Simeonov, V.; Stój, A.; Waśko, A.; Pawłat, J.; Polak-Berecka, M. The Impact of Cold Plasma on the Phenolic Composition and Biogenic Amine Content of Red Wine. Food Chem. 2022, 381, 132257. [Google Scholar] [CrossRef] [PubMed]
- Bai, X.; Chen, X.; Li, X.; Tan, F.; Sam, F.E.; Tao, Y. Wine Polyphenol Oxidation Mechanism and the Effects on Wine Quality: A Review. Compr. Rev. Food Sci. Food Saf. 2024, 23, e70035. [Google Scholar] [CrossRef]
- Paup, V.D.; Barton, T.L.; Edwards, C.G.; Lange, I.; Lange, B.M.; Lee, J.; Ross, C.F. Improving the Chemical and Sensory Characteristics of Red and White Wines with Pectinase-Producing Non-Saccharomyces Yeasts. J. Food Sci. 2022, 87, 5402–5417. [Google Scholar] [CrossRef] [PubMed]
- Rogerson, F.; Symington, C. A Method for the Estimation of Alcohol in Fortified Wines Using Hydrometer Baume and Refractometer Brix. Am. J. Enol. Vitic. 2006, 57, 486–490. [Google Scholar] [CrossRef]
- Han, X.; Qin, Q.; Li, C.; Zhao, X.; Song, F.; An, M.; Chen, Y.; Wang, X.; Huang, W.; Zhan, J.; et al. Application of Non-Saccharomyces Yeasts with High β-Glucosidase Activity to Enhance Terpene-Related Floral Flavor in Craft Beer. Food Chem. 2023, 404, 134726. [Google Scholar] [CrossRef]
- Garcia, L.; Poulain, B.; Douliez, A.; Naud, E.; Valls-Fonayet, J.; Nioi, C. White Wine Lees as a Source of Antioxidants: Insights into Their Chemical Profile. Food Chem. 2025, 494, 146118. [Google Scholar] [CrossRef]
- dos Santos Lima, M.; da Silva Monteiro, L.I.; de Brito Araújo Carvalho, A.J.; Bastos, D.C.; Pimentel, T.C.; Magnani, M. A Robust Method for Quantifying 42 Phenolic Compounds by RP-HPLC/DAD: Columns Performance and Characterization of Brazilian Citrus Peels. Food Chem. 2024, 460, 140807. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Wang, Z.; Li, Y.; Liu, Q.; Yuan, C. Survey of the Phenolic Content and Antioxidant Properties of Wines from Five Regions of China According to Variety and Vintage. LWT 2022, 169, 114004. [Google Scholar] [CrossRef]
- Bai, W.; Sun, S.; Zhao, W.; Qian, M.; Liu, X.; Chen, W. Determination of Ethyl Carbamate (EC) by GC-MS and Characterization of Aroma Compounds by HS-SPME-GC-MS During Wine Frying Status in Hakka Yellow Rice Wine. Food Anal. Methods 2017, 10, 2068–2077. [Google Scholar] [CrossRef]
- Han, B.; Tang, Y.; Xie, Y.; Liu, H.; Zhou, H.; Zheng, X.; Zhan, J.; Huang, W.; You, Y. Use of Starmerella bacillaris and Hanseniaspora uvarum Sequential Fermentation with Saccharomyces cerevisiae to Reduce Putrescine and Cadaverine and Improve Aroma Profiles of Wines. Food Res. Int. 2025, 210, 116409. [Google Scholar] [CrossRef]
- Maicas, S. The Role of Yeasts in Fermentation Processes. Microorganisms 2020, 8, 1142. [Google Scholar] [CrossRef]
- Naghshbandi, M.P.; Tabatabaei, M.; Aghbashlo, M.; Gupta, V.K.; Sulaiman, A.; Karimi, K.; Moghimi, H.; Maleki, M. Progress toward Improving Ethanol Production through Decreased Glycerol Generation in Saccharomyces cerevisiae by Metabolic and Genetic Engineering Approaches. Renew. Sustain. Energy Rev. 2019, 115, 109353. [Google Scholar] [CrossRef]
- Laguna, L.; Bartolomé, B.; Moreno-Arribas, M.V. Mouthfeel Perception of Wine: Oral Physiology, Components and Instrumental Characterization. Trends Food Sci. Technol. 2017, 59, 49–59. [Google Scholar] [CrossRef]
- Robles, A.; Fabjanowicz, M.; Chmiel, T.; Płotka-Wasylka, J. Determination and Identification of Organic Acids in Wine Samples. Problems and Challenges. TrAC Trends Anal. Chem. 2019, 120, 115630. [Google Scholar] [CrossRef]
- Yang, W.; Lv, Z.; Liu, H.; Zhang, Q.; Qiao, C.; Nawaz, M.; Jiao, Z.; Liu, J. Effect of Organic Acid Addition Before Fermentation on the Physicochemical and Sensory Properties of Cherry Wine. Foods 2024, 13, 3902. [Google Scholar] [CrossRef]
- Zeng, Q.; Ha, S.; Chen, M.; Zhang, C.; Yang, H. Common Organic Acids in Fruit Wine and the Deacidification Strategies. Syst. Microbiol. Biomanuf. 2025, 5, 489–499. [Google Scholar] [CrossRef]
- Hornedo-Ortega, R.; González-Centeno, M.R.; Chira, K.; Jourdes, M.; Teissedre, P.-L.; Hornedo-Ortega, R.; González-Centeno, M.R.; Chira, K.; Jourdes, M.; Teissedre, P.-L. Phenolic Compounds of Grapes and Wines: Key Compounds and Implications in Sensory Perception. In Chemistry and Biochemistry of Winemaking, Wine Stabilization and Aging; IntechOpen: London, UK, 2020; ISBN 978-1-83962-576-3. [Google Scholar]
- Garrido, J.; Borges, F. Wine and Grape Polyphenols—A Chemical Perspective. Food Res. Int. 2013, 54, 1844–1858. [Google Scholar] [CrossRef]
- Enogieru, A.B.; Haylett, W.; Hiss, D.C.; Bardien, S.; Ekpo, O.E. Rutin as a Potent Antioxidant: Implications for Neurodegenerative Disorders. Oxid Med. Cell Longev. 2018, 2018, 6241017. [Google Scholar] [CrossRef]
- Bai, S.; Tao, X.; Hu, J.; Chen, H.; Wu, J.; Zhang, F.; Cai, J.; Wu, G.; Meng, J. Flavonoids Profile and Antioxidant Capacity of Four Wine Grape Cultivars and Their Wines Grown in the Turpan Basin of China, the Hottest Wine Region in the World. Food Chem. X 2025, 26, 102301. [Google Scholar] [CrossRef]
- Ambigaipalan, P.; Oh, W.Y.; Shahidi, F. Epigallocatechin (EGC) Esters as Potential Sources of Antioxidants. Food Chem. 2020, 309, 125609. [Google Scholar] [CrossRef] [PubMed]
- McGinty, D.; Letizia, C.S.; Api, A.M. Fragrance Material Review on Cyclopentadecanone. Food Chem. Toxicol. 2011, 49, S142–S148. [Google Scholar] [CrossRef] [PubMed]
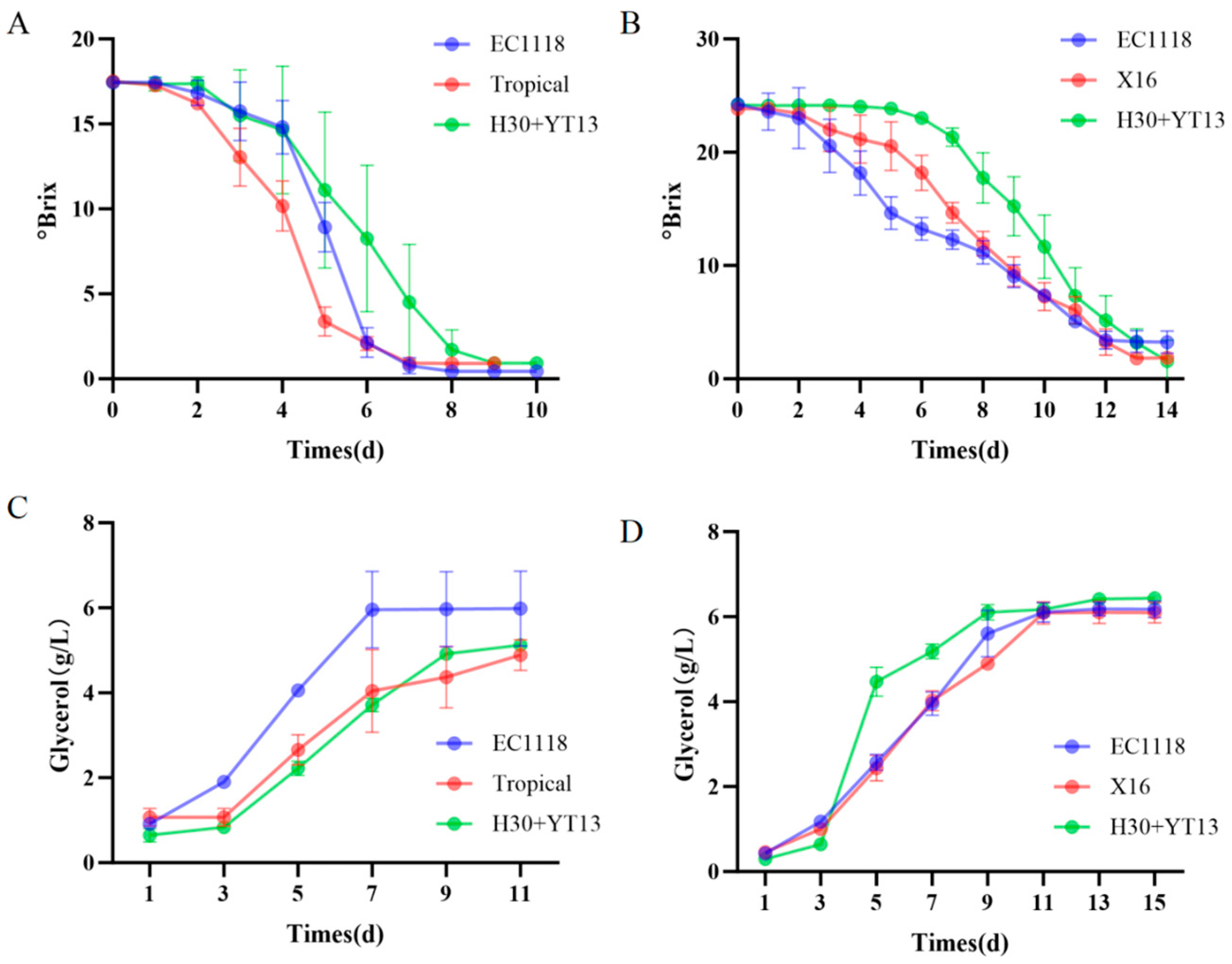
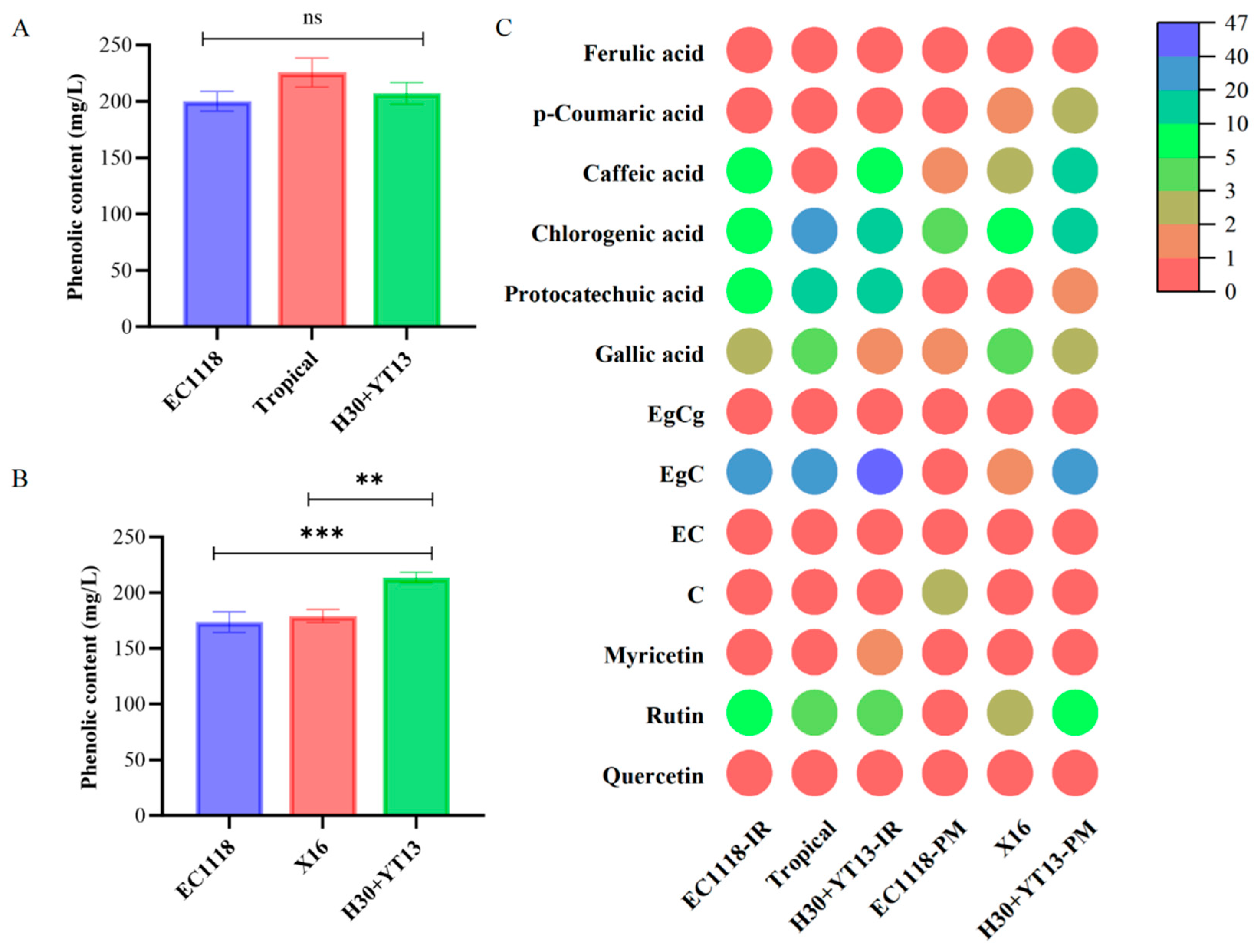
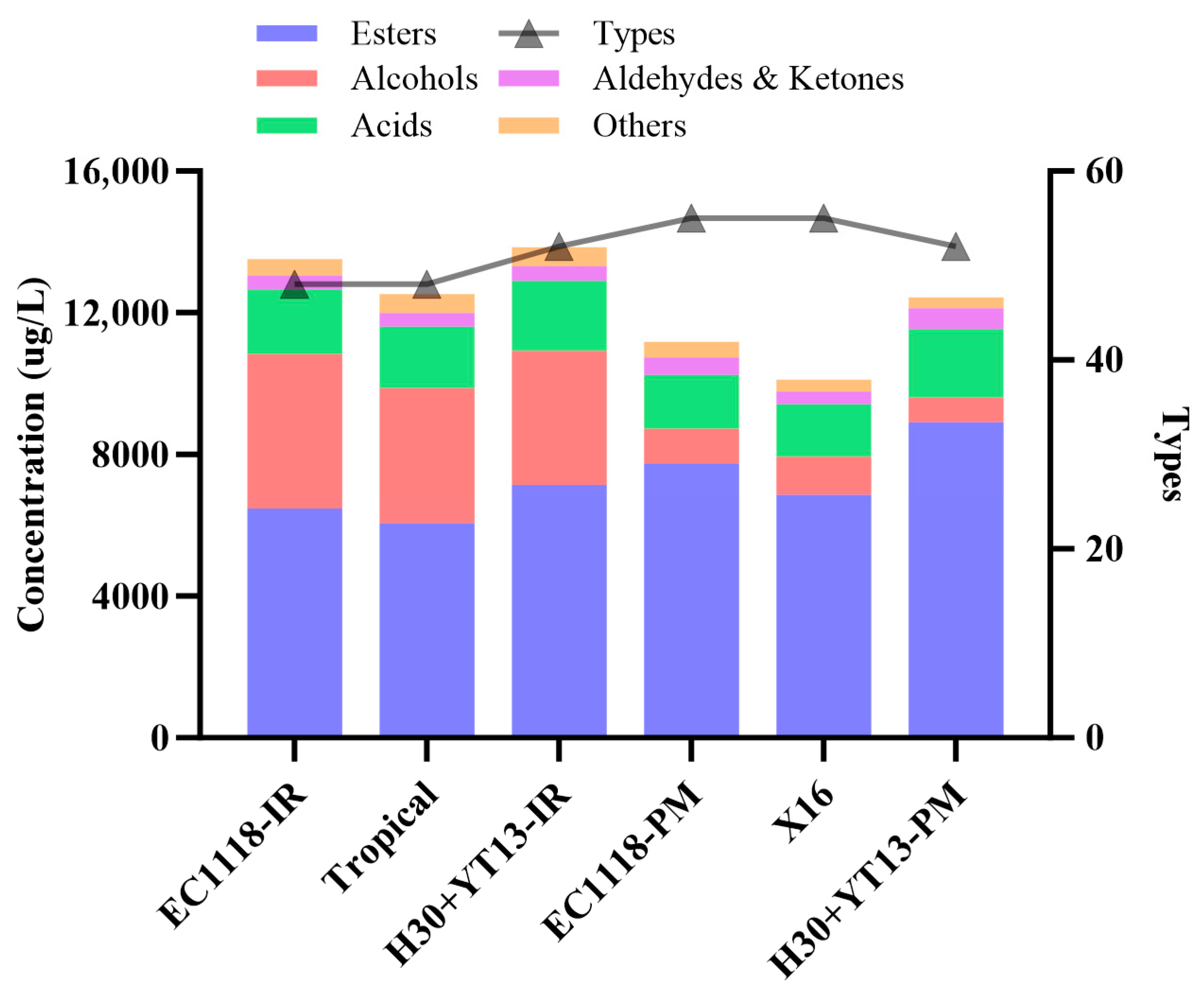
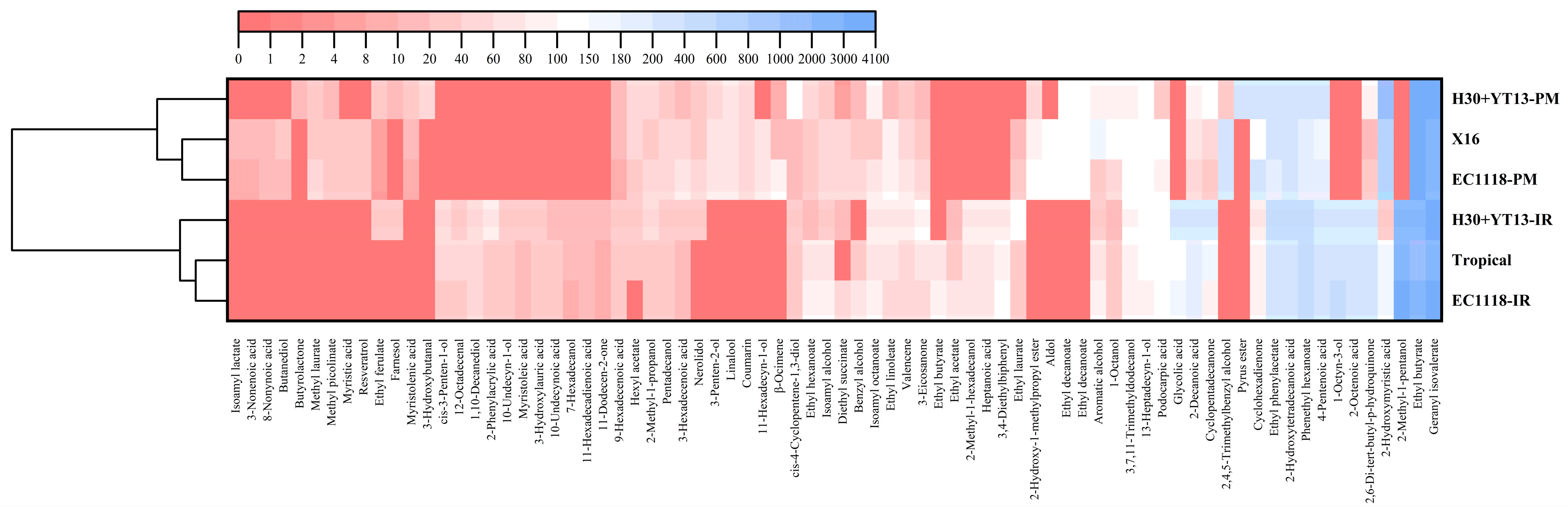
| Grape Variety | Fermentation Groups |
|---|---|
| Italian Riesling | EC1118 |
| Tropical | |
| H30 + YT13 | |
| Petit Manseng | EC1118 |
| X16 | |
| H30 + YT13 |
| Grape Variety | Yeast | Basic Physicochemical Parameters | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Residual Sugar (g/L) | Alcohol Content% (vol) | pH Value | Volatile Acids (g/L) | Malic Acid (g/L) | Succinic Acid (g/L) | Tartaric Acid (g/L) | Citric Acid (g/L) | Lactic Acid (g/L) | ||
| Italian Riesling | EC1118 | 3.96 ± 0.11 a | 9.90 ± 0.10 a | 2.96 ± 0.02 a | 0.48 ± 0.25 a | 2.07 ± 0.05 a | 1.12 ± 0.43 b | 13.79 ± 1.53 a | 0.86 ± 0.17 a | 0.77 ± 0.04 a |
| Tropical | 3.76 ± 0.20 a | 9.93 ± 0.05 a | 2.86 ± 0.10 a | 0.21 ± 0.10 a | 1.86 ± 0.15 a | 1.99 ± 0.10 a | 12.74 ± 2.80 a | 0.90 ± 0.20 a | 0.95 ± 0.06 a | |
| H30 + YT13 | 3.23 ± 0.25 a | 9.80 ± 0.43 a | 2.94 ± 0.05 a | 0.23 ± 0.00 a | 2.23 ± 0.47 a | 1.48 ± 0.13 ab | 11.40 ± 1.98 a | 0.70 ± 0.01 a | 0.71 ± 0.11 a | |
| Petit Manseng | EC1118 | 3.90 ± 1.31 a | 13.37 ± 0.40 a | 2.89 ± 0.11 a | 0.93 ± 0.06 a | 3.80 ± 0.17 a | 0.69 ± 0.15 b | 19.40 ± 1.72 a | 0.62 ± 0.24 a | 0.30 ± 0.05 b |
| X16 | 3.43 ± 1.38 a | 13.10 ± 0.45 a | 2.88 ± 0.10 a | 0.96 ± 0.17 a | 3.33 ± 0.20 ab | 4.55 ± 2.17 a | 16.01 ± 1.17 b | 0.35 ± 0.01 a | 0.60 ± 0.18 ab | |
| H30 + YT13 | 3.13 ± 1.54 a | 12.57 ± 1.72 a | 2.85 ± 0.03 a | 0.84 ± 0.08 a | 3.00 ± 0.26 b | 0.67 ± 0.06 b | 15.67 ± 0.51 b | 0.34 ± 0.05 a | 0.83 ± 0.11 a | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhai, Z.; Zhang, P.; Huang, W.; Zhan, J.; Xia, G.; Kong, W.; You, Y. Sequential Inoculation with Selected Indigenous Yeasts Enhances the Aroma Profiles and Typicity of White Wines from Yantai, China. Foods 2025, 14, 4015. https://doi.org/10.3390/foods14234015
Zhai Z, Zhang P, Huang W, Zhan J, Xia G, Kong W, You Y. Sequential Inoculation with Selected Indigenous Yeasts Enhances the Aroma Profiles and Typicity of White Wines from Yantai, China. Foods. 2025; 14(23):4015. https://doi.org/10.3390/foods14234015
Chicago/Turabian StyleZhai, Zihao, Piaoran Zhang, Weidong Huang, Jicheng Zhan, Guangli Xia, Weifu Kong, and Yilin You. 2025. "Sequential Inoculation with Selected Indigenous Yeasts Enhances the Aroma Profiles and Typicity of White Wines from Yantai, China" Foods 14, no. 23: 4015. https://doi.org/10.3390/foods14234015
APA StyleZhai, Z., Zhang, P., Huang, W., Zhan, J., Xia, G., Kong, W., & You, Y. (2025). Sequential Inoculation with Selected Indigenous Yeasts Enhances the Aroma Profiles and Typicity of White Wines from Yantai, China. Foods, 14(23), 4015. https://doi.org/10.3390/foods14234015








