Toward Functional Oil Blends: Physicochemical and Nutritional Evaluation of Rapeseed–Hazelnut Oil Mixtures
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Determination of Fatty Acid Composition by Gas Chromatography
2.2.2. Health-Related Lipid Indices
2.2.3. Distribution of Fatty Acids in Triacylglycerols Using Enzymatic Hydrolysis
2.2.4. Determination of Acid and Peroxide Values by Potentiometric Titration Method
2.2.5. Determination of Oxidative Stability Using Pressure Differential Scanning Calorimetry
2.3. Statistical Analysis
3. Results and Discussion
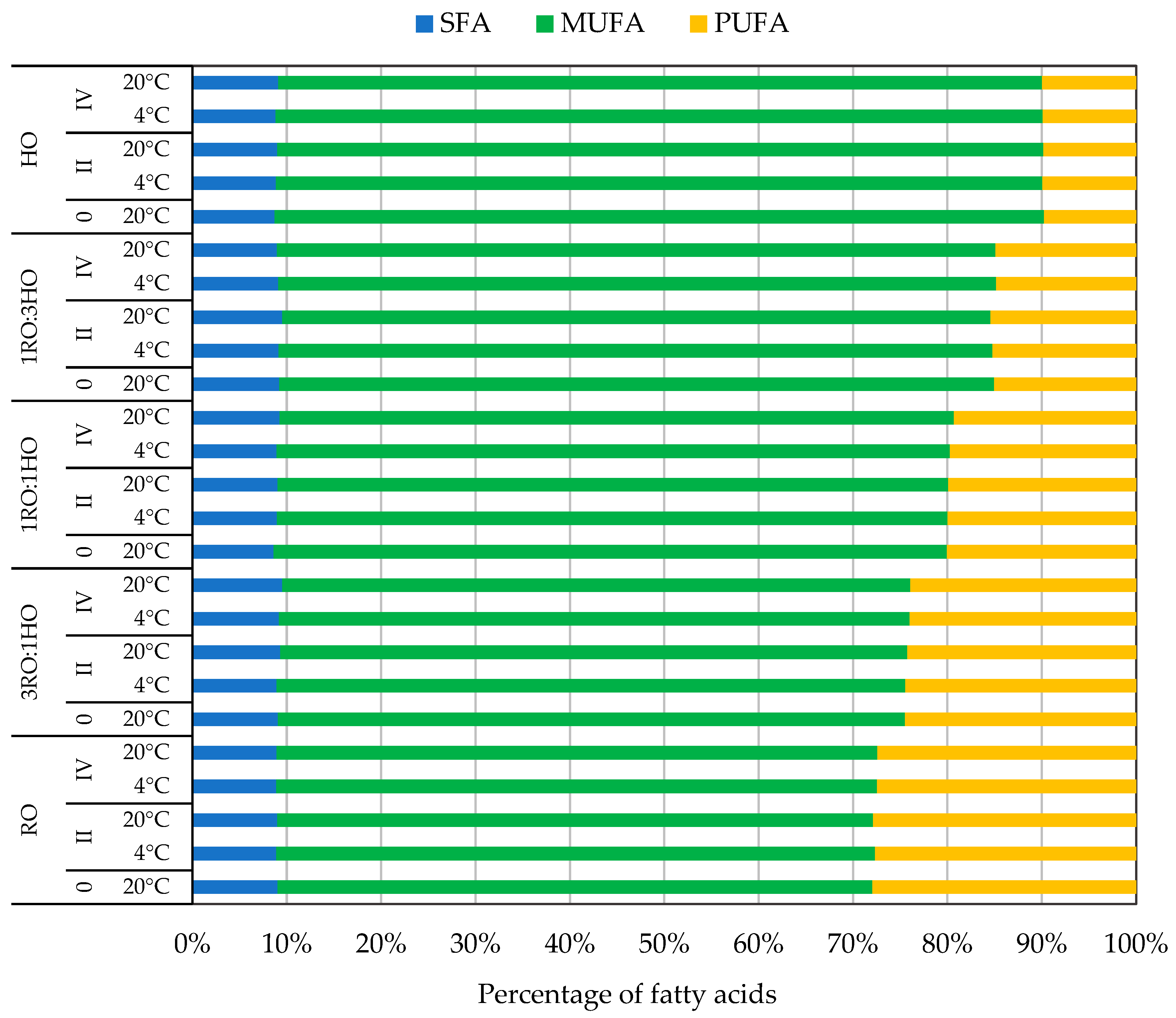
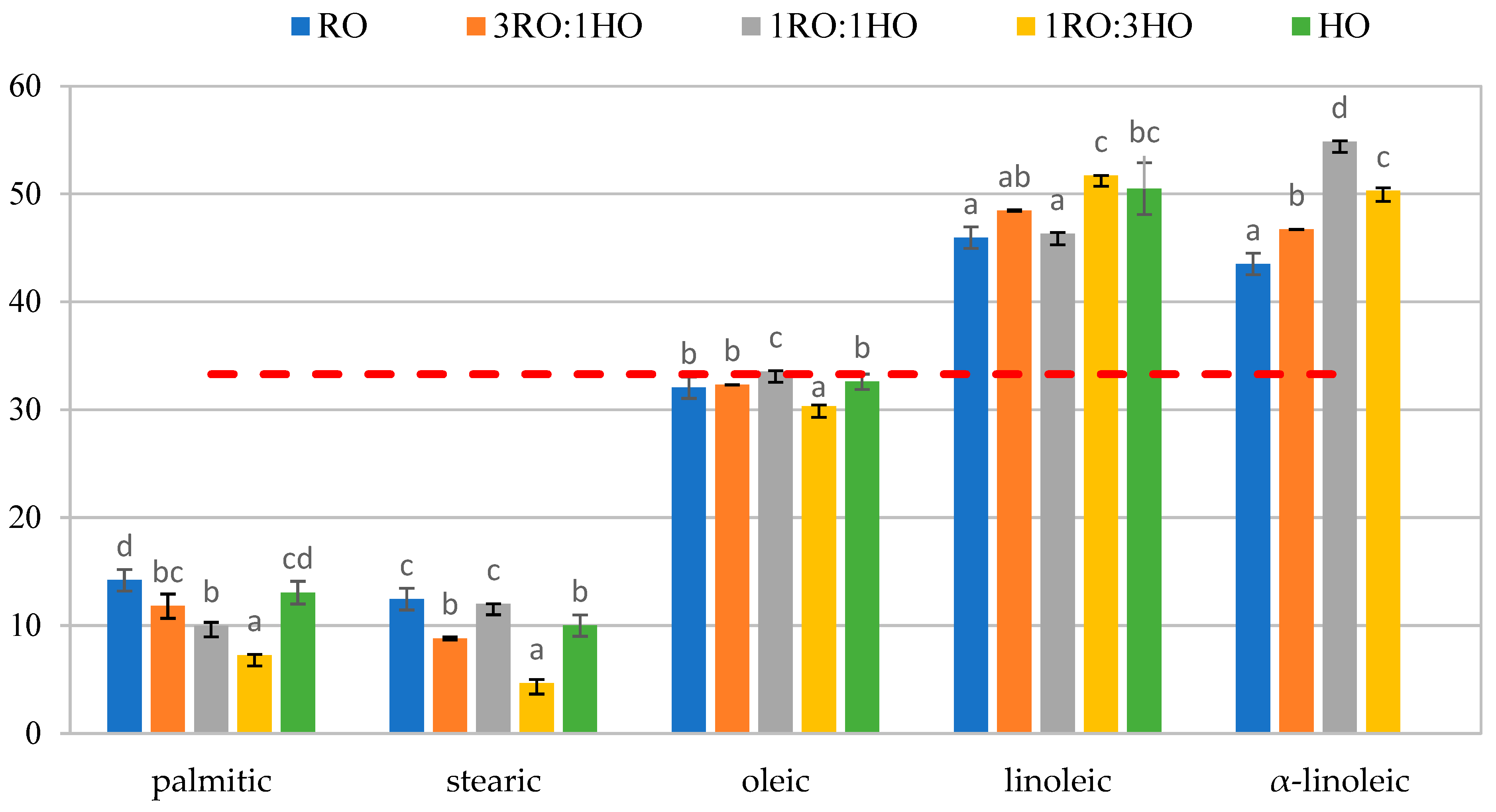
3.1. Fatty Acid Profile
3.2. Fatty Acid Distribution
3.3. Evaluation of Oil Quality
3.3.1. The Acid and Peroxide Values
3.3.2. Evaluation of Oxidative Stability
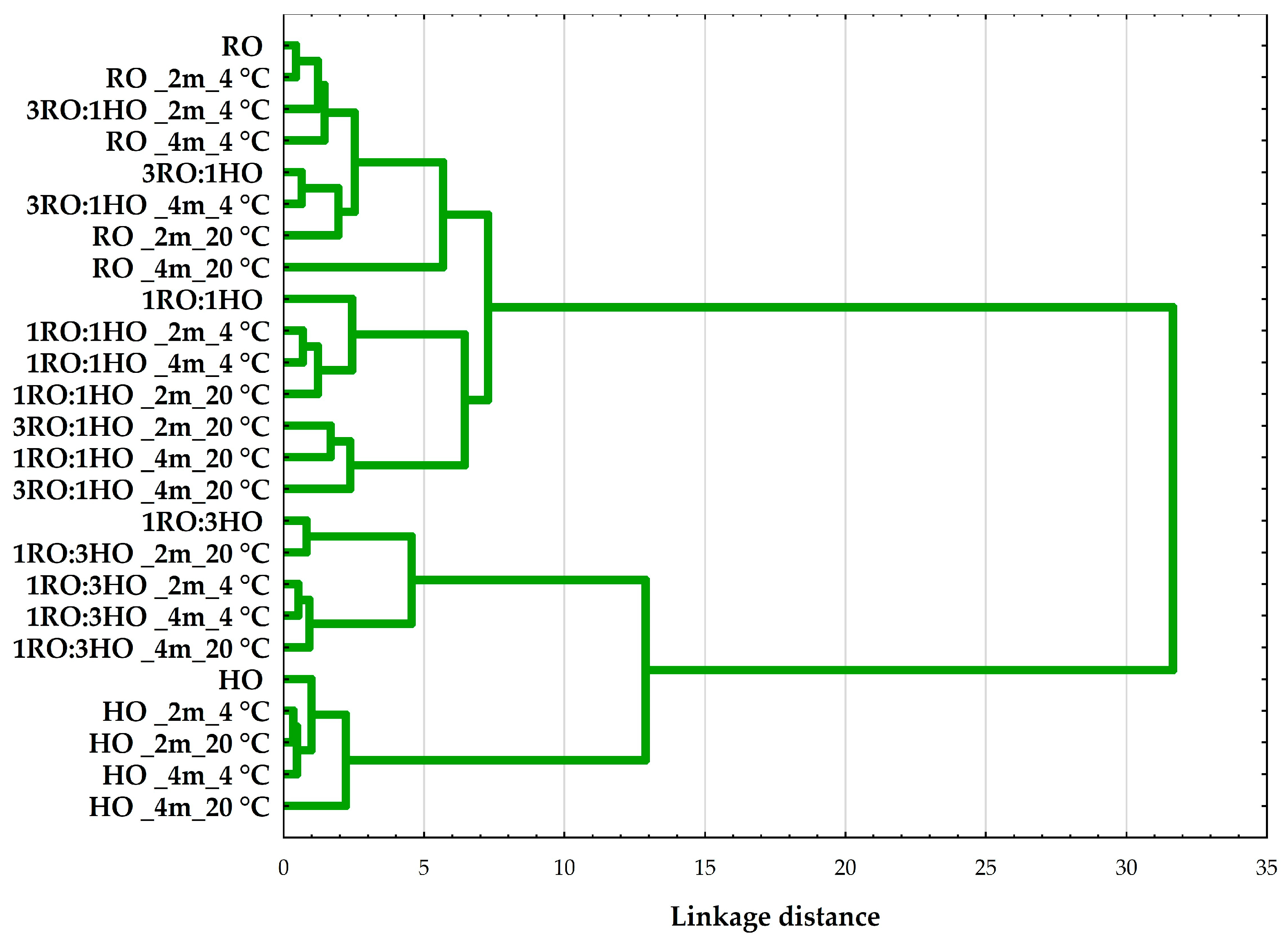
3.3.3. Multivariate Analysis of Quality Parameters in Rapeseed–Hazelnut Oil Blends
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Xiao, Z.; Pan, Y.; Wang, C.; Li, X.; Lu, Y.; Tian, Z.; Kuang, L.; Wang, X.; Dun, X.; Wang, H. Multi-functional Development and Utilization of Rapeseed: Comprehensive Analysis of the Nutritional Value of Rapeseed Sprouts. Foods 2022, 11, 778. [Google Scholar] [CrossRef]
- Marcinkowski, D.; Bochniak, M.; Wereńska, M.; Czwartkowski, K. The Influence of Storage Conditions of Cold-Pressed Rapeseed Oil on Its Quality Parameters. Appl. Sci. 2023, 13, 11746. [Google Scholar] [CrossRef]
- Lewinska, A.; Zebrowski, J.; Duda, M.; Gorka, A.; Wnuk, M. Fatty Acid Profile and Biological Activities of Linseed and Rapeseed Oils. Molecules 2015, 20, 22872–22880. [Google Scholar] [CrossRef] [PubMed]
- Siger, A.; Józefiak, M.; Górnaś, P. Cold-pressed and Hot-pressed Rapeseed Oil: The Effects of Roasting and Seed Moisture on the Antioxidant Activity, Canolol, and Tocopherol Level. Acta Sci. Pol. Technol. Aliment. 2017, 16, 69–81. [Google Scholar] [CrossRef] [PubMed]
- Kasprzak, M.; Rudzińska, M.; Przybylski, R.; Kmiecik, D.; Siger, A.; Olejnik, A. The Degradation of Bioactive Compounds and Formation of Their Oxidation Derivatives in Refined Rapeseed Oil during Heating in Model System. LWT 2020, 123, 109078. [Google Scholar] [CrossRef]
- Gracka, A.; Raczyk, M.; Hradecký, J.; Hajslova, J.; Jeziorski, S.; Karlovits, G.; Michalak, B.; Bąkowska, N.; Jeleń, H. Volatile Compounds and other Indicators of Quality for Cold-pressed Rapeseed Oils Obtained from Peeled, Whole, Flaked and Roasted Seeds. Eur. J. Lipid Sci. Technol. 2017, 119, 1600328. [Google Scholar] [CrossRef]
- Tang, D.; Dong, Y.; Ren, H.; Li, L.; He, C. A Review of Phytochemistry, Metabolite Changes, and Medicinal Uses of the Common Food Mung Bean and its Sprouts (Vigna radiata). Chem. Cent. J. 2014, 8, 4. [Google Scholar] [CrossRef]
- Şahin, S.; Topçu, C.Ü. Evaluation of Oxidation Stability of Organic and Conventional Hazelnut Oils. J. Agric. Sci. 2025, 31, 126–136. [Google Scholar] [CrossRef]
- Topkafa, M.; Ayyildiz, H.F.; Kara, H. Hazelnut (Corylus avellana) Oil. In Fruit Oils: Chemistry and Functionality; Ramadan, M.F., Ed.; Springer International Publishing: Cham, Switzerland, 2019; pp. 223–241. [Google Scholar] [CrossRef]
- Sun, J.; Feng, X.; Lyu, C.; Zhou, S.; Liu, Z. Effects of Different Processing Methods on the Lipid Composition of Hazelnut Oil: A Lipidomics Analysis. Food Sci. Hum. Wellness 2022, 11, 427–435. [Google Scholar] [CrossRef]
- Karaosmanoğlu, H. Lipid Characteristics, Bioactive Properties, and Mineral Content in Hazelnut Grown under Different Cultivation Systems. Food Process. Preserv. 2022, 46, e16717. [Google Scholar] [CrossRef]
- Matthäus, B.; Özcan, M.M. The Comparison of Properties of the Oil and Kernels of Various Hazelnuts from Germany and Turkey. Eur. J. Lipid Sci. Technol. 2012, 114, 801–806. [Google Scholar] [CrossRef]
- Kishimoto, N.; Takano, N. Enhanced Moisture Loss and Oil Absorption of Deep-Fried Food by Blending Extra Virgin Olive Oil in Rapeseed Oil. Food Sci. Technol. Res. 2021, 27, 63–68. [Google Scholar] [CrossRef]
- Mukhametov, A.; Dautkanova, D.; Kazhymurat, A.; Yerbulekova, M.; Aitkhozhayeva, G. The Effects of Heat Treatment on the Oxidation Resistance and Fatty Acid Composition of the Vegetable Oil Blend. J. Oleo Sci. 2023, 72, 597–604. [Google Scholar] [CrossRef] [PubMed]
- Akpinar, M.; Bauer Estrada, K.; Tekin, A.; Quintanilla-Carvajal, M.X.; Gumus-Bonacina, C.E. Oxidative Stability of High Oleic Palm and Hazelnut Skin Oil Blends. J. Am. Oil Chem. Soc. 2022, 99, 1127–1135. [Google Scholar] [CrossRef]
- Ghosh, M.; Upadhyay, R.; Mahato, D.K.; Mishra, H.N. Thermal and Oxidative Stability Assessment of Synergistic Blends of Sunflower and Sesame Oils Tailored for Nutritionally Stable Composition of Omega Fatty Acids. J. Therm. Anal. Calorim. 2019, 135, 2389–2398. [Google Scholar] [CrossRef]
- Mazaheri, Y.; Torbati, M.; Azadmard-Damirchi, S.; Savage, G.P. Oil Extraction from Blends of Sunflower and Black Cumin Seeds by Cold Press and Evaluation of Its Physicochemical Properties. J. Food Process. Preserv. 2019, 43, e14154. [Google Scholar] [CrossRef]
- Siddeeg, A.; Xia, W. Oxidative Stability, Chemical Composition and Organoleptic Properties of Seinat (Cucumis melo var. tibish) Seed Oil Blends with Peanut Oil from China. J. Food Sci. Technol. 2015, 52, 8172–8179. [Google Scholar] [CrossRef]
- Zielinska, M.; Rutkowska, J.; Antoniewska, A. Lipid Oxidation Products—Nutritional and Health Consequences. Probl. Hig. Epidemiol. 2017, 98, 203–211. [Google Scholar]
- Hashempour-Baltork, F.; Torbati, M.; Azadmard-Damirchi, S.; Savage, G.P. Vegetable Oil Blending: A Review of Physicochemical, Nutritional and Health Effects. Trends Food Sci. Technol. 2016, 57, 52–58. [Google Scholar] [CrossRef]
- PN-EN ISO 5509:2001; Vegetable and Animal Oils and Fats. Preparation of Fatty Acid Methyl Esters. Polish Committee for Standardization: Warsaw, Poland, 2001.
- Ulbricht, T.L.V.; Southgate, D.A.T. Coronary Heart Disease: Seven Dietary Factors. Lancet 1991, 338, 985–992. [Google Scholar] [CrossRef]
- Santos-Silva, J.; Bessa, R.J.B.; Santos-Silva, F. Effect of Genotype, Feeding System and Slaughter Weight on the Quality of Light Lambs: II. Fatty Acid Composition of Meat. Livest. Prod. Sci. 2022, 77, 187–194. [Google Scholar] [CrossRef]
- Bandara, R.R.; Louis-Gavet, C.; Bryś, J.; Mańko-Jurkowska, D.; Górska, A.; Brzezińska, R.; Siol, M.; Makouie, S.; Palani, B.K.; Obranović, M.; et al. Enzymatic Interesterification of Coconut and Hemp Oil Mixtures to Obtain Modified Structured Lipids. Foods 2024, 13, 2722. [Google Scholar] [CrossRef] [PubMed]
- AOCS Official Method Te 1a-64. Acid value. In Official Methods and Recommended Practices of the AOCS; AOCS Official: Champaign, IL, USA, 2009. [Google Scholar]
- AOCS Official Method Cd 8b-90. Peroxide Value Acetic Acid-Isooctane Method Official Methods and Recommended Practices of the AOCS; AOCS Official: Champaign, IL, USA, 2009. [Google Scholar]
- Kostik, V.; Memeti, S.; Bauer, B. Fatty Acid Composition of Edible Oils and Fats. J. Hyg. Eng. Des. 2013, 4, 112–116. [Google Scholar]
- Dijkstra, A.J. Controlling Physical and Chemical Properties of Fat Blends through Their Triglyceride Compositions. In Trans Fatty Acids; Dijkstra, A.J., Hamilton, R.J., Hamm, W., Eds.; CRC Press: New York, NY, USA, 2008; pp. 132–146. [Google Scholar] [CrossRef]
- Zambiazi, R.C.; Przybylski, R.; Zambiazi, M.W.; Mendonça, C.B. Fatty Acid Composition of Vegetable Oils and Fats. B. Ceppa Curitiba 2007, 25, 111–120. [Google Scholar]
- Benitez-Sánchez, P.L.; León-Camacho, M.; Aparicio, R. A Comprehensive Study of Hazelnut Oil Composition with Comparisons to other Vegetable Oils, Particularly Olive Oil. Eur. Food Res. Technol. 2003, 218, 13–19. [Google Scholar] [CrossRef]
- Khalili Tilami, S.; Kouřimská, L. Assessment of the Nutritional Quality of Plant Lipids Using Atherogenicity and Thrombogenicity Indices. Nutrients 2022, 14, 3795. [Google Scholar] [CrossRef]
- Alam, A.M.M.N.; Hwang, Y.-H.; Samad, A.; Joo, S.-T. Fabrication of Gelatin-based Hybrid Films using Solvent-casting and Electrospinning to Enhance the Quality Characteristics and Shelf Life of Meat Analog. Food Biosci. 2025, 72, 107415. [Google Scholar] [CrossRef]
- Frančáková, H.; Ivanišová, E.; Dráb, Š.; Krajčovič, T.; Tokár, M.; Mareček, J.; Musilová, J. Composition of Fatty Acids in Selected Vegetable Oils. Potravinarstvo 2015, 9, 538–542. [Google Scholar] [CrossRef]
- FAO/WHO. Fats and Fatty Acids in Human Nutrition: Report of an Expert Consultation; FAO Food and Nutrition Paper, No. 91; Food and Agriculture Organization of the United Nations: Rome, Italy, 2010; Available online: https://openknowledge.fao.org/server/api/core/bitstreams/2cf62dbc-07b1-4f80-af83-43089b3c55fc/content (accessed on 17 November 2025).
- Bishehkolaei, M.; Pathak, Y. Influence of Omega n-6/n-3 Ratio on Cardiovascular Disease and Nutritional Interventions. Hum. Nutr. Metab. 2024, 37, 200275. [Google Scholar] [CrossRef]
- Alfieri, A.; Imperlini, E.; Nigro, E.; Vitucci, D.; Orrù, S.; Daniele, A.; Buono, P.; Mancini, A. Effects of Plant Oil Interesterified Triacylglycerols on Lipemia and Human Health. Int. J. Mol. Sci. 2017, 19, 104. [Google Scholar] [CrossRef]
- Berry, S.E.E. Triacylglycerol Structure and Interesterification of Palmitic and Stearic Acid-Rich Fats: An Overview and Implications for Cardiovascular Disease. Nutr. Res. Rev. 2009, 22, 3–17. [Google Scholar] [CrossRef] [PubMed]
- Ciemniewska-Żytkiewicz, H.; Pasini, F.; Verardo, V.; Bryś, J.; Koczoń, P.; Caboni, M.F. Changes of the Lipid Fraction During Fruit Development in Hazelnuts (Corylus avellana L.) Grown in Poland. Eur. J. Lipid Sci. Technol. 2015, 117, 710–717. [Google Scholar] [CrossRef]
- Schmidt, Š.; Vajdák, M.; Sekretár, S.; Koman, V. Effect of Some Refining Steps on Rapeseed Oil Triacylglycerol Structures. Czech J. Food Sci. 2004, 22, S346–S348. [Google Scholar] [CrossRef]
- Gharby, S.; Asbbane, A.; Ahmed, M.N.; Gagour, J.; Hallouch, O.; Oubannin, S.; Bijla, L.; Goh, K.W.; Bouyahya, A.; Ibourki, M. Vegetable Oil Oxidation: Mechanisms, Impacts on Quality, and Approaches to Enhance Shelf Life. Food Chem. X 2025, 28, 102541. [Google Scholar] [CrossRef]
- Choe, E.; Min, D.B. Mechanisms and Factors for Edible Oil Oxidation. Compr. Rev. Food Sci. Food Saf. 2006, 5, 169–186. [Google Scholar] [CrossRef]
- Lo Turco, V.; Litrenta, F.; Nava, V.; Albergamo, A.; Rando, R.; Bartolomeo, G.; Potortì, A.G.; Di Bella, G. Effect of Filtration Process on Oxidative Stability and Minor Compounds of the Cold-Pressed Hempseed Oil during Storage. Antioxidants 2023, 12, 1231. [Google Scholar] [CrossRef]
- Kozłowska, M.; Mańko-Jurkowska, D.; Zieniuk, B.; Rudzińska, M. Influence of Extraction Techniques on Almond Oil Quality: A Comparative Study of Solvent-Extracted and Commercial Products. Molecules 2025, 30, 3519. [Google Scholar] [CrossRef]
- Codex-ALINORM 09/32/17; Codex Alimentarius 2009. Codex Standard for Named Vegetable Oils. Codex Alimentarius Commission: Rome, Italy, 2009.
- Bielecka, M.; Ziajka, J.; Staniewski, B.; Nowak, H. Oxidative Stability and Health-Related Indices of Anhydrous Milk Fat and Vegetable Oil Blends. Int. Dairy J. 2023, 137, 105529. [Google Scholar] [CrossRef]
- Ottaway, J.M.; Chance Carter, J.; Adams, K.L.; Camancho, J.; Lavine, B.K.; Booksh, K.S. Comparison of Spectroscopic Techniques for Determining the Peroxide Value of 19 Classes of Naturally Aged, Plant-Based Edible Oils. Appl. Spectrosc. 2021, 75, 781–794. [Google Scholar] [CrossRef]
- Ahmed, M.; Pickova, J.; Ahmad, T.; Liaquat, M.; Farid, A.; Jahangir, M. Oxidation of Lipids in Foods. Sarhad J. Agric. 2016, 32, 230–238. [Google Scholar] [CrossRef]
- Szydłowska-Czerniak, A.; Tułodziecka, A.; Momot, M.; Stawicka, B. Physicochemical, Antioxidative, and Sensory Properties of Refined Rapeseed Oils. J. Am. Oil Chem. Soc. 2019, 96, 405–419. [Google Scholar] [CrossRef]
- Zheng, C.; Yang, M.; Zhou, Q.; Liu, C.S.; Huang, F.H. Changes in the Content of Canolol and Total Phenolics, Oxidative Stability of Rapeseed Oil during Accelerated Storage. Eur. J. Lipid Sci. Technol. 2014, 116, 1675–1684. [Google Scholar] [CrossRef]
- Król, K.; Gantner, M.; Piotrowska, A. The Quality Characteristic and Fatty Acid Profile of Cold-Pressed Hazelnut Oils during Nine Months of Storage. Agronomy 2021, 11, 2045. [Google Scholar] [CrossRef]
- Konuskan, D.B.; Arslan, M.; Oksuz, A. Physicochemical Properties of Cold Pressed Sunflower, Peanut, Rapeseed, Mustard and Olive Oils Grown in the Eastern Mediterranean Region. Saudi J. Biol. Sci. 2019, 26, 340–344. [Google Scholar] [CrossRef]
- Ciemniewska-Żytkiewicz, H.; Bryś, J.; Sujka, K.; Koczoń, P. Assessment of the Hazelnuts Roasting Process by Pressure Differential Scanning Calorimetry and MID-FT-IR Spectroscopy. Food Anal. Methods 2015, 8, 2465–2473. [Google Scholar] [CrossRef]
- Ayyildiz, H.F.; Topkafa, M.; Kara, H.; Sherazi, S.T.H. Evaluation of Fatty Acid Composition, Tocols Profile, and Oxidative Stability of Some Fully Refined Edible Oils. Int. J. Food Prop. 2015, 18, 2064–2076. [Google Scholar] [CrossRef]
- Symoniuk, E.; Ratusz, K.; Krygier, K. Comparison of the Oxidative Stability of Cold-Pressed Rapeseed Oil using Pressure Differential Scanning Calorimetry and Rancimat Methods. Eur. J. Lipid Sci. Technol. 2017, 119, 1600182. [Google Scholar] [CrossRef]
- Siol, M.; Witkowska, B.; Mańko-Jurkowska, D.; Makouie, S.; Bryś, J. Comprehensive Evaluation of the Nutritional Quality of Stored Watermelon Seed Oils. Appl. Sci. 2025, 15, 830. [Google Scholar] [CrossRef]
- Ciemniewska-Żytkiewicz, H.; Ratusz, K.; Bryś, J.; Reder, M.; Koczon, P. Determination of the Oxidative Stability of Hazelnut Oils by PDSC and Rancimat Methods. J. Therm. Anal. Calorim. 2014, 118, 875–881. [Google Scholar] [CrossRef]
- Symoniuk, E.; Ratusz, K.; Krygier, K. Evaluation of the Oxidative Stability of Cold-Pressed Rapeseed Oil by Rancimat and Pressure Differential Scanning Calorimetry Measurements. Eur. J. Lipid Sci. Technol. 2019, 121, 1800017. [Google Scholar] [CrossRef]
- Siol, M.; Chołuj, N.; Mańko-Jurkowska, D.; Bryś, J. Assessment of the Stability and Nutritional Quality of Hemp Oil and Pumpkin Seed Oil Blends. Foods 2024, 13, 3813. [Google Scholar] [CrossRef]
- Talbot, G. 16-The Stability and Shelf Life of Fats and Oils. In Woodhead Publishing Series in Food Science, Technology and Nutrition, The Stability and Shelf Life of Food, 2nd ed.; Subramaniam, P., Ed.; Woodhead Publishing: Cambridge, UK, 2016; pp. 461–503. [Google Scholar] [CrossRef]
- Symoniuk, E.; Hryńko, M.; Kalisz, M.; Kruszewski, B.; Szymańska, I. Natural Antioxidant Enrichment of Linseed Oil: Ultrasound-Assisted Maceration with Mullein Flowers (Verbascum thapsus L.). Food Biophys. 2025, 20, 125. [Google Scholar] [CrossRef]
- Ahmad, I.; Ullah, J.; Khan, R.; Ahmad, W. Antioxidant Performance of Bio-Oils in Oxidative Stability of Base Lubricating Oil Determined through TGA and PDSC Techniques. Thermochim. Acta 2022, 713, 179241. [Google Scholar] [CrossRef]
- Grajzer, M.; Szmalcel, K.; Kuźmiński, Ł.; Witkowski, M.; Kulma, A.; Prescha, A. Characteristics and Antioxidant Potential of Cold-Pressed Oils—Possible Strategies to Improve Oil Stability. Foods 2020, 9, 1630. [Google Scholar] [CrossRef] [PubMed]
- Grosshagauer, S.; Steinschaden, R.; Pignitter, M. Strategies to Increase the Oxidative Stability of Cold Pressed Oils. LWT 2019, 106, 72–77. [Google Scholar] [CrossRef]
- Szydłowska-Czerniak, A.; Łaszewska, A. Effect of Refining Process on Antioxidant Capacity, Total Phenolics and Prooxidants Contents in Rapeseed Oils. LWT 2015, 64, 853–859. [Google Scholar] [CrossRef]
- Chew, S.C. Cold-pressed Rapeseed (Brassica napus) Oil: Chemistry and Functionality. Food Res. Int. 2020, 131, 108997. [Google Scholar] [CrossRef]
- Celenk, V.U.; Argon, Z.U.; Gumus, Z.P. Chapter 20—Cold Pressed Hazelnut (Corylus avellana) Oil. In Cold Pressed Oils; Ramadan, M.F., Ed.; Academic Press: Cambridge, MA, USA, 2020; pp. 241–254. [Google Scholar] [CrossRef]
- Olszowy-Tomczyk, M. Synergistic, Antagonistic and Additive Antioxidant Effects in the Binary Mixtures. Phytochem. Rev. 2020, 19, 63–103. [Google Scholar] [CrossRef]
- Ghirardello, D.; Contessa, C.; Valentini, N.; Zeppa, G.; Rolle, L.; Gerbi, V.; Botta, R. Effect of Storage Conditions on Chemical and Physical Characteristics of Hazelnut (Corylus avellana L.). Postharvest Biol. Technol. 2013, 81, 37–43. [Google Scholar] [CrossRef]
- Suri, K.; Singh, B.; Kaur, A.; Singh, N. Impact of Roasting and Extraction Methods on Chemical Properties, Oxidative Stability and Maillard Reaction Products of Peanut Oils. J. Food Sci. Technol. 2019, 56, 2436–2445. [Google Scholar] [CrossRef]
- Wroniak, M.; Raczyk, M.; Kruszewski, B.; Symoniuk, E.; Dach, D. Effect of Deep Frying of Potatoes and Tofu on Thermo-Oxidative Changes of Cold Pressed Rapeseed Oil, Cold Pressed High Oleic Rapeseed Oil and Palm Olein. Antioxidants 2021, 10, 1637. [Google Scholar] [CrossRef]
- Altaee, R.T.; Aldabbagh, M.G.; Rashan, A.I. Detection of Fatty Acids and Some Secondary Metabolites in Macadamia and Hazelnut Fruits and their Shells, with Studying the Physiochemical Properties of its Extracted Oil: A Comparative Study. Acad. Open 2024, 9, 10–21070. [Google Scholar] [CrossRef]
- Chen, X.; Sun, S. Color Reversion of Refined Vegetable Oils: A Review. Molecules 2023, 28, 5177. [Google Scholar] [CrossRef]

| Sample | Storage (Month) | Temperature (°C) | n-6/n-3 | AI | TI | h/H |
|---|---|---|---|---|---|---|
| RO | 0 | - | 2.09 | 0.06 | 0.12 | 16.45 |
| 2 | 4 | 2.06 | 0.06 | 0.12 | 16.74 | |
| 20 | 2.07 | 0.06 | 0.12 | 16.09 | ||
| 4 | 4 | 2.04 | 0.06 | 0.12 | 17.38 | |
| 20 | 2.04 | 0.06 | 0.12 | 16.90 | ||
| 3RO:1HO | 0 | - | 2.23 | 0.06 | 0.13 | 16.30 |
| 2 | 4 | 2.22 | 0.06 | 0.12 | 16.82 | |
| 20 | 2.26 | 0.07 | 0.13 | 15.22 | ||
| 4 | 4 | 2.28 | 0.06 | 0.13 | 15.99 | |
| 20 | 2.34 | 0.07 | 0.14 | 14.88 | ||
| 1RO:1HO | 0 | - | 2.71 | 0.06 | 0.13 | 17.27 |
| 2 | 4 | 2.77 | 0.06 | 0.14 | 16.06 | |
| 20 | 2.76 | 0.07 | 0.14 | 15.58 | ||
| 4 | 4 | 2.76 | 0.06 | 0.14 | 16.47 | |
| 20 | 2.83 | 0.07 | 0.15 | 14.77 | ||
| 1RO:3HO | 0 | - | 4.51 | 0.07 | 0.17 | 14.39 |
| 2 | 4 | 4.36 | 0.07 | 0.17 | 15.16 | |
| 20 | 4.27 | 0.07 | 0.17 | 13.97 | ||
| 4 | 4 | 4.39 | 0.07 | 0.17 | 15.42 | |
| 20 | 4.32 | 0.06 | 0.16 | 15.77 | ||
| HO | 0 | - | 56.41 | 0.06 | 0.18 | 15.91 |
| 2 | 4 | 61.06 | 0.07 | 0.19 | 15.51 | |
| 20 | 60.44 | 0.07 | 0.19 | 15.44 | ||
| 4 | 4 | 65.00 | 0.07 | 0.19 | 15.62 | |
| 20 | 65.47 | 0.07 | 0.19 | 14.75 |
| Sample | Storage (Month) | Temperature (°C) | AV (mg KOH/g Oil) | PV (meq O2/kg Oil) |
|---|---|---|---|---|
| RO | 0 | - | 5.81 b ± 0.01 | 6.21 e ± 0.14 |
| 2 | 4 | 5.86 b ± 0.05 | 6.54 e ± 0.28 | |
| 20 | 5.99 ab ± 0.04 | 11.06 c ± 0.95 | ||
| 4 | 4 | 5.90 b ± 0.06 | 8.46 d ± 0.21 | |
| 20 | 6.21 a ± 0.27 | 23.72 a ± 0.22 | ||
| 3RO:1HO | 0 | - | 4.98 d ± 0.21 | 4.84 fg ± 0.05 |
| 2 | 4 | 5.09 cd ± 0.08 | 5.02 f ± 0.14 | |
| 20 | 5.16 cd ± 0.21 | 5.93 e ± 0.05 | ||
| 4 | 4 | 5.20 cd ± 0.03 | 6.31 e ± 0.62 | |
| 20 | 5.29 c ± 0.11 | 14.61 b ± 0.57 | ||
| 1RO:1HO | 0 | - | 4.00 f ± 0.01 | 3.85 h ± 0.14 |
| 2 | 4 | 4.23 ef ± 0.20 | 4.04 gh ± 0.16 | |
| 20 | 4.24 ef ± 0.07 | 4.71 fg ± 0.60 | ||
| 4 | 4 | 4.22 f ± 0.07 | 4.86 fg ± 0.06 | |
| 20 | 4.48 e ± 0.24 | 6.44 e ± 0.13 | ||
| 1RO:3HO | 0 | - | 3.17 g ± 0.01 | 2.54 kl ± 0.11 |
| 2 | 4 | 3.23 g ± 0.04 | 2.62 jkl ± 0.04 | |
| 20 | 3.25 g ± 0.04 | 3.56 hi ± 0.56 | ||
| 4 | 4 | 3.24 g ± 0.03 | 3.27 hijk ± 0.69 | |
| 20 | 3.30 g ± 0.06 | 3.71 hi ± 0.28 | ||
| HO | 0 | - | 1.98 i ± 0.01 | 2.28 l ± 0.58 |
| 2 | 4 | 1.98 i ± 0.01 | 2.34 l ± 0.08 | |
| 20 | 2.16 hi ± 0.01 | 3.45 hij ± 0.08 | ||
| 4 | 4 | 2.00 i ± 0.05 | 2.88 ijkl ± 0.16 | |
| 20 | 2.31 h ± 0.09 | 3.56 hi ± 0.21 |
| Sample | Storage (Month) | Temperature (°C) | τmax (min) |
|---|---|---|---|
| RO | 0 | - | 66.28 jk ± 2.64 |
| 2 | 4 | 62.61 kl ± 2.22 | |
| 20 | 51.58 n ± 1.32 | ||
| 4 | 4 | 60.41 lm ± 0.45 | |
| 20 | 35.99 p ± 0.01 | ||
| 3RO:1HO | 0 | - | 69.32 j ± 0.83 |
| 2 | 4 | 64.02 kl ± 0.23 | |
| 20 | 56.89 m ± 3.63 | ||
| 4 | 4 | 74.95 i ± 4.72 | |
| 20 | 45.48 o ± 2.67 | ||
| 1RO:1HO | 0 | - | 84.05 h ± 0.11 |
| 2 | 4 | 82.87 h ± 0.75 | |
| 20 | 81.55 h ± 1.73 | ||
| 4 | 4 | 89.54 g ± 0.18 | |
| 20 | 79.64 hi ± 1.13 | ||
| 1RO:3HO | 0 | - | 115.31 ef ± 1.10 |
| 2 | 4 | 118.16 de ± 0.91 | |
| 20 | 116.17 def ± 2.17 | ||
| 4 | 4 | 120.85 d ± 1.63 | |
| 20 | 111.86 f ± 2.35 | ||
| HO | 0 | - | 160.32 c ± 3.84 |
| 2 | 4 | 167.02 b ± 1.07 | |
| 20 | 164.68 bc ± 1.73 | ||
| 4 | 4 | 177.07 a ± 2.23 | |
| 20 | 174.99 a ± 3.44 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Siol, M.; Mańko-Jurkowska, D.; Stanaszek, I.; Zieniuk, B.; Bryś, A.; Bryś, J. Toward Functional Oil Blends: Physicochemical and Nutritional Evaluation of Rapeseed–Hazelnut Oil Mixtures. Foods 2025, 14, 4008. https://doi.org/10.3390/foods14234008
Siol M, Mańko-Jurkowska D, Stanaszek I, Zieniuk B, Bryś A, Bryś J. Toward Functional Oil Blends: Physicochemical and Nutritional Evaluation of Rapeseed–Hazelnut Oil Mixtures. Foods. 2025; 14(23):4008. https://doi.org/10.3390/foods14234008
Chicago/Turabian StyleSiol, Marta, Diana Mańko-Jurkowska, Izabela Stanaszek, Bartłomiej Zieniuk, Andrzej Bryś, and Joanna Bryś. 2025. "Toward Functional Oil Blends: Physicochemical and Nutritional Evaluation of Rapeseed–Hazelnut Oil Mixtures" Foods 14, no. 23: 4008. https://doi.org/10.3390/foods14234008
APA StyleSiol, M., Mańko-Jurkowska, D., Stanaszek, I., Zieniuk, B., Bryś, A., & Bryś, J. (2025). Toward Functional Oil Blends: Physicochemical and Nutritional Evaluation of Rapeseed–Hazelnut Oil Mixtures. Foods, 14(23), 4008. https://doi.org/10.3390/foods14234008









