Bioactive, Functional, and Technological Properties of Gluten-Free Pasta Enriched with Mango (Mangifera indica L.) Leaf Powder
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Preparation of Mango Leaf Powders and Flour Blends
2.2. Techno-Functional Characterization of Blends
2.3. Determination of Total Polyphenol Content and Antioxidant Capacity
2.4. Determination of Reducing Sugars
2.5. Rheological (Pasting) Properties of Selected Blends
2.6. Preparation of Gluten-Free Pasta
2.7. Evaluation of Pasta Quality
2.7.1. Color Analysis
2.7.2. Texture Analysis
2.7.3. Determination of Total Polyphenols and Antioxidant Activity in Pasta
2.8. Statistical Analysis
3. Results and Discussion
3.1. Absorptive Profile of Mango Leaves and Chestnut Flour Blends
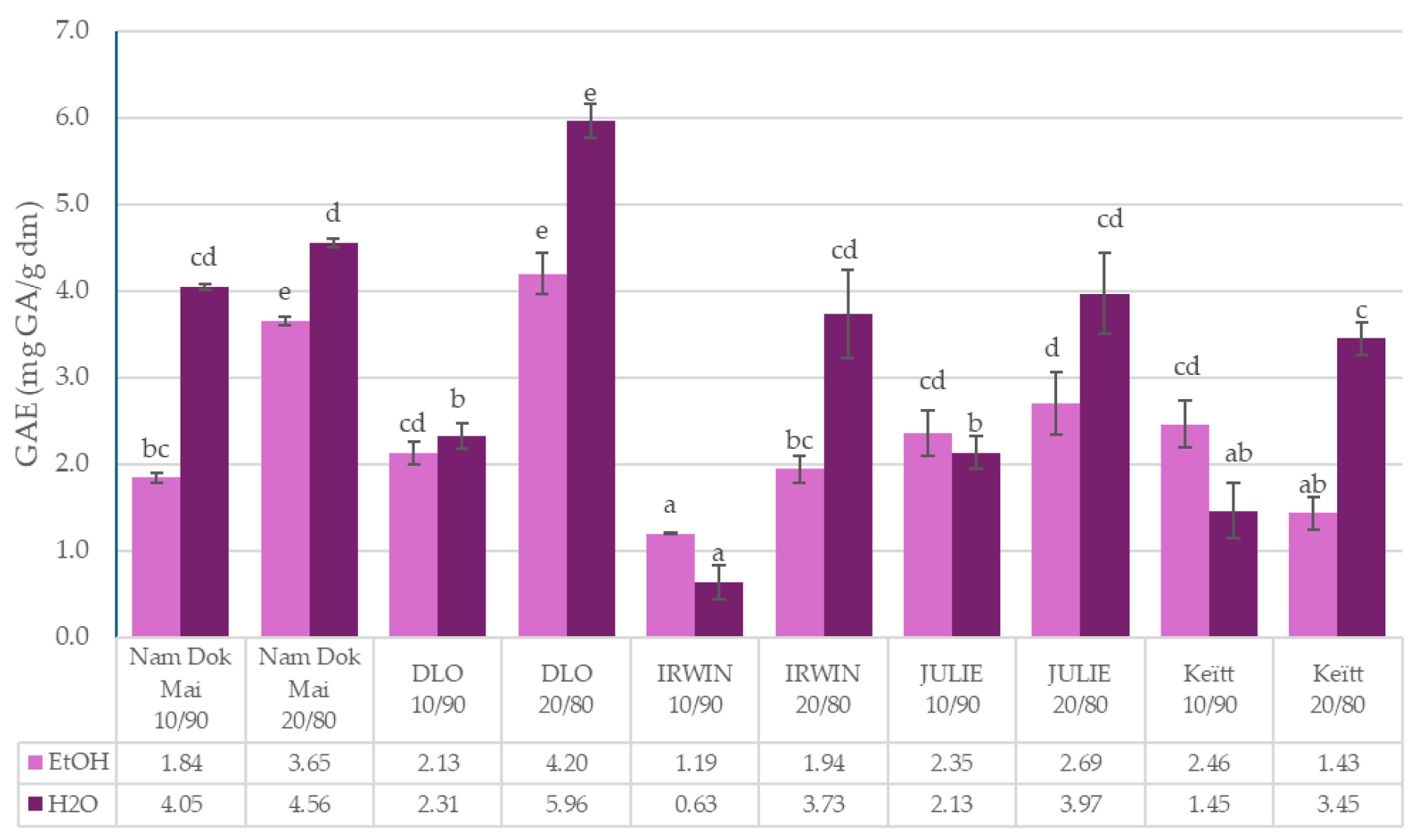
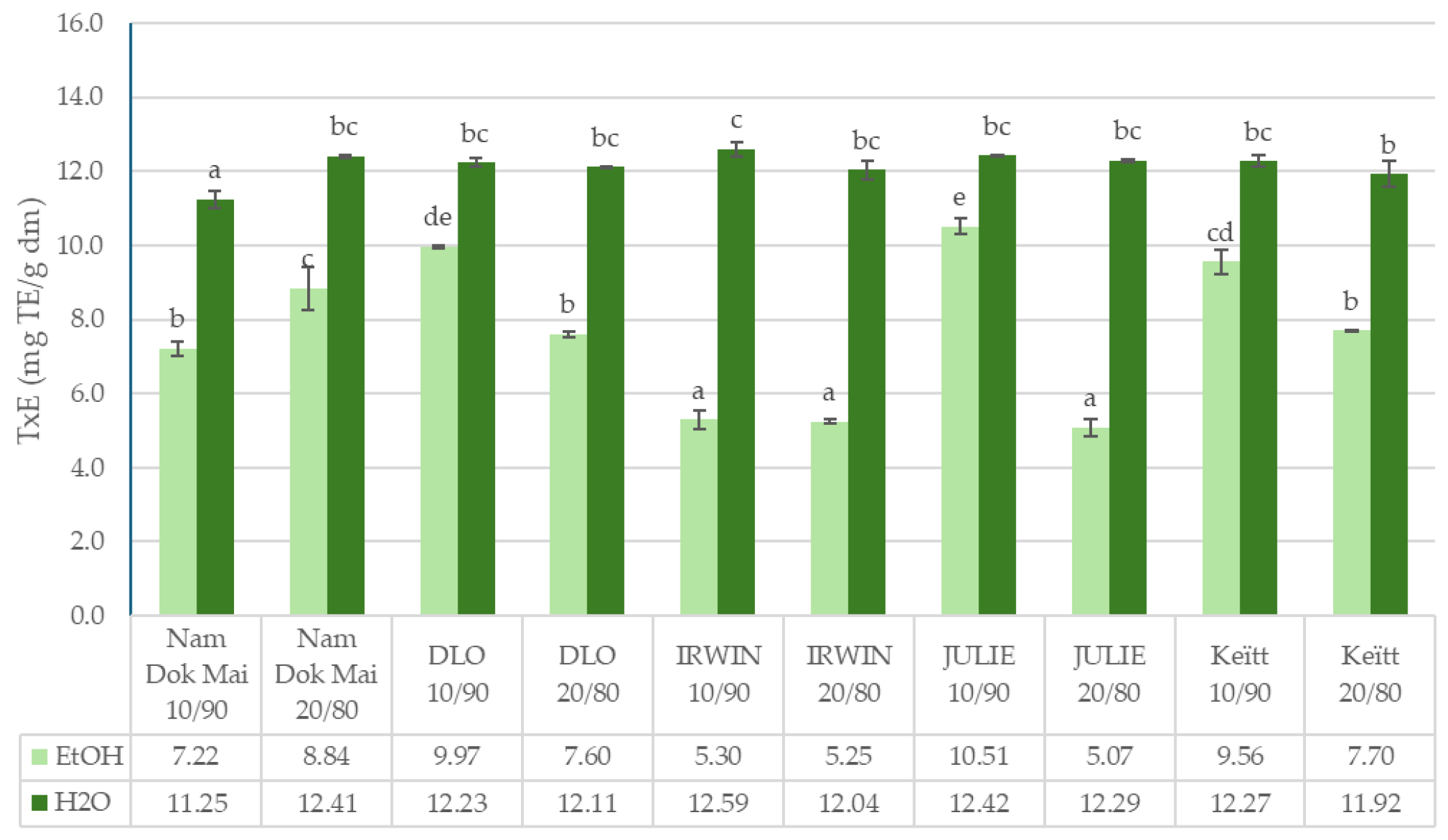
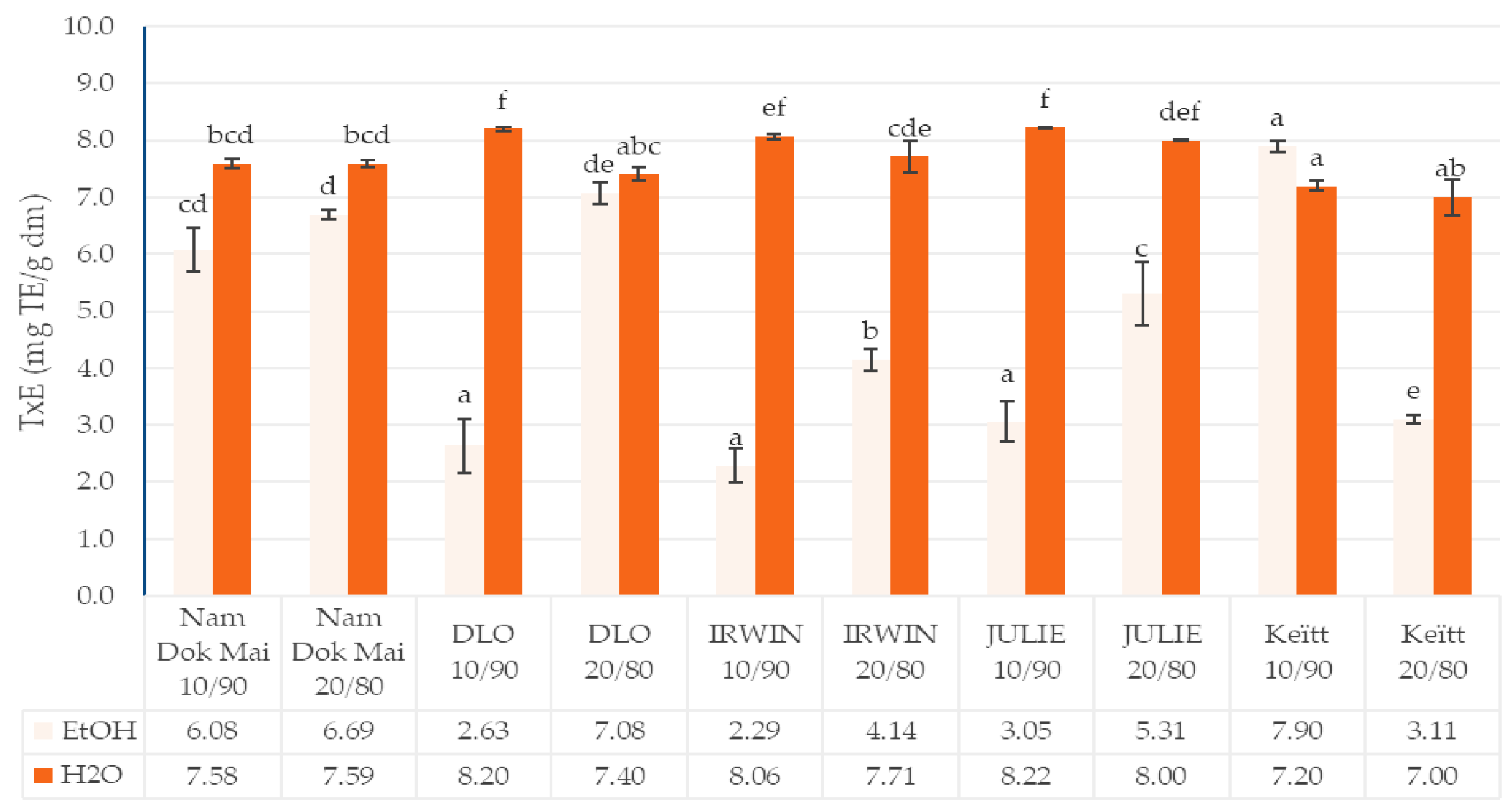
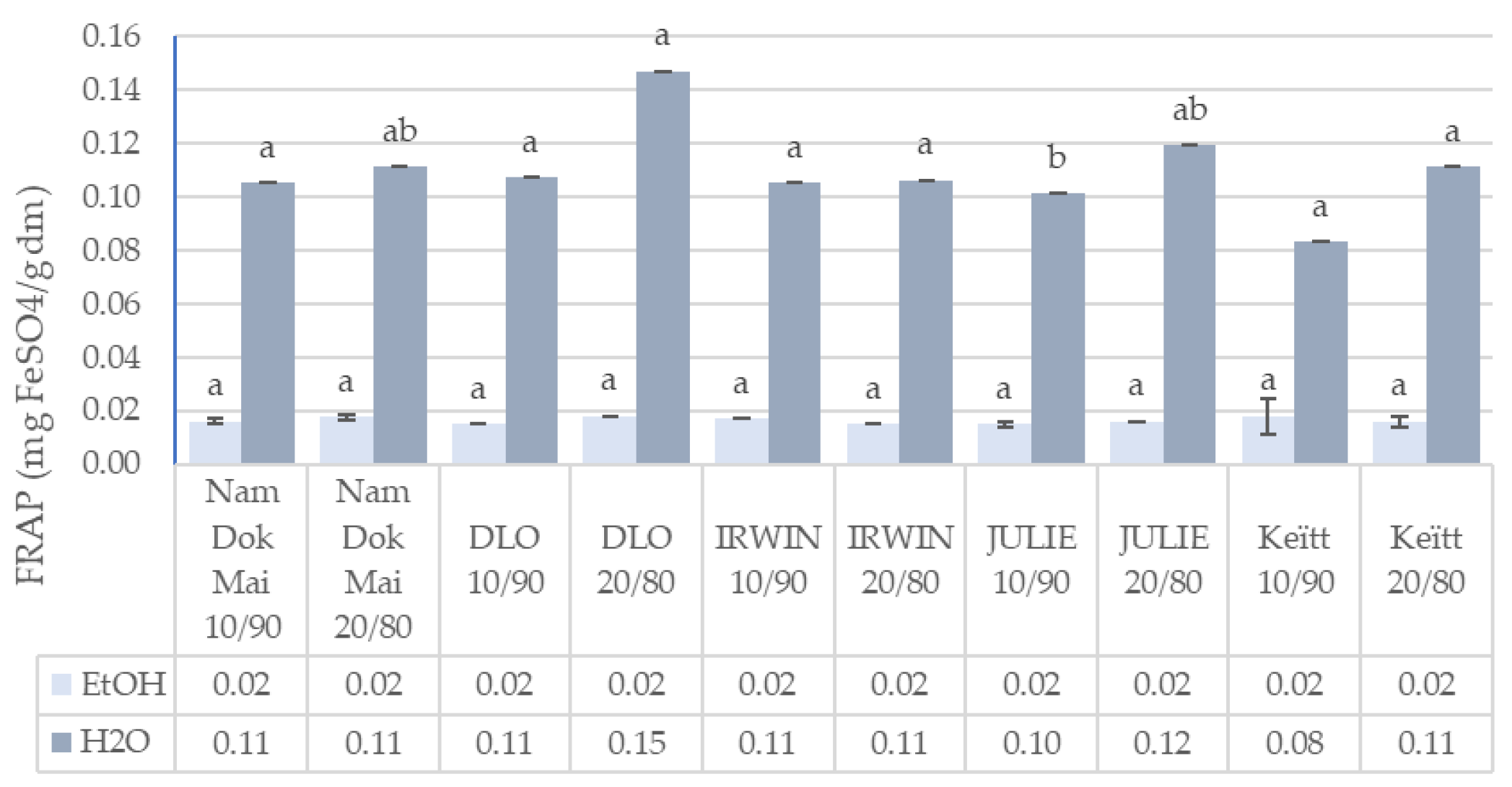
3.2. Total Polyphenols Content and Antioxidant Capacity of Mango Leaves and Chestnut Flour Blends
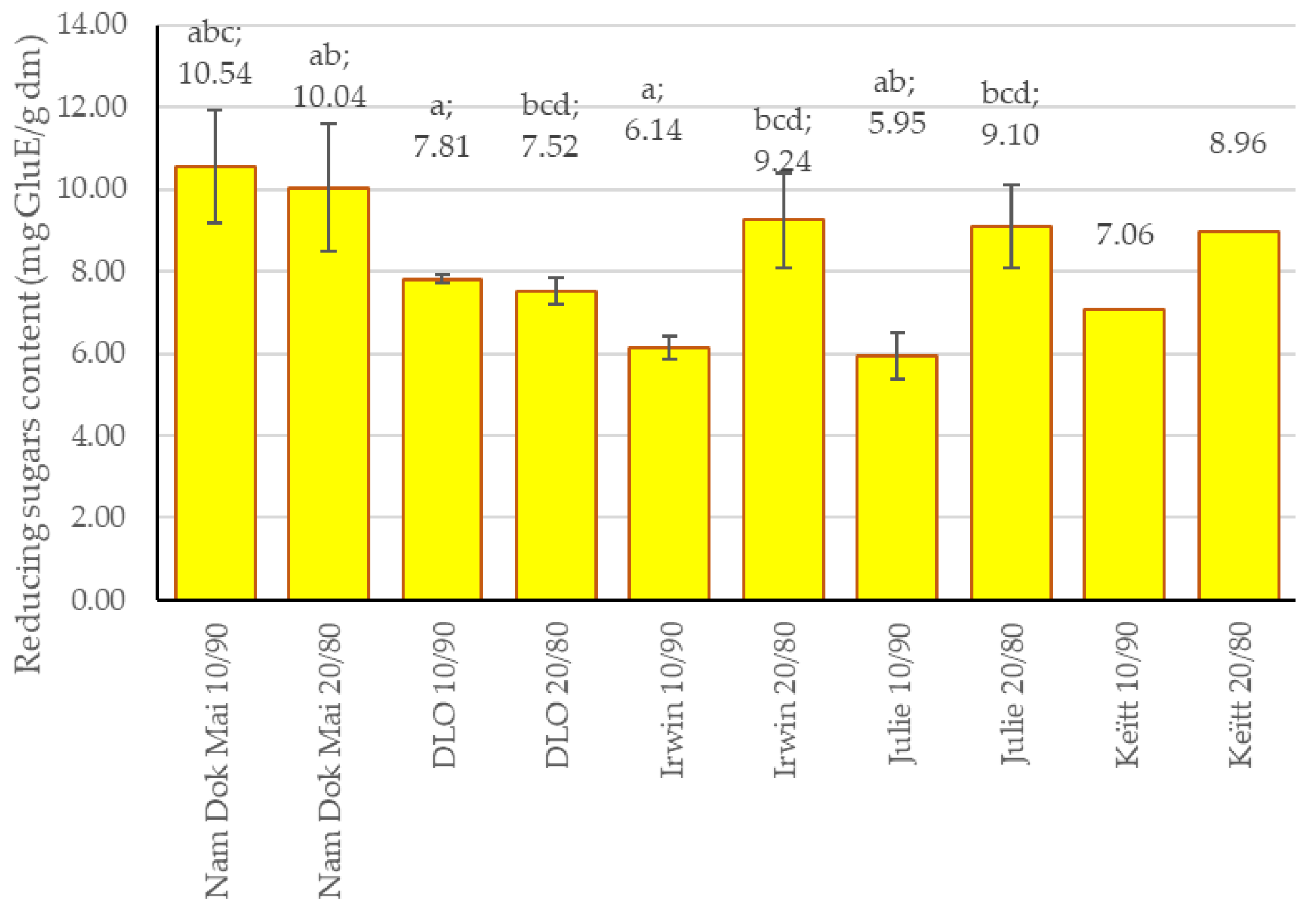
3.3. Reducing Sugar Content in Mango Leaves and Chestnut Flour Blends
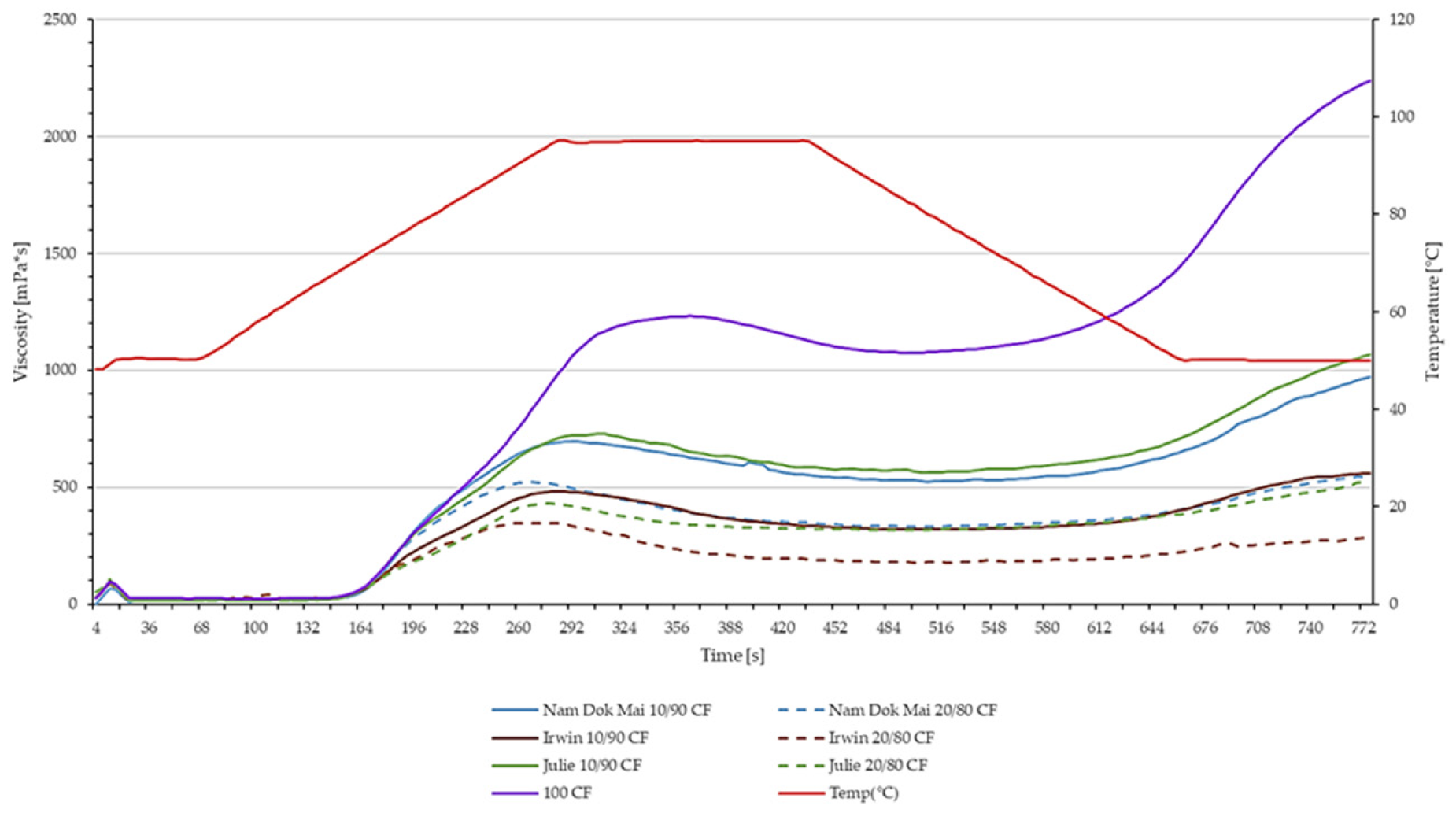
3.4. Viscometric Properties of Chosen Mango Leaves and Chestnut Flour Blends
3.5. Quality Features of Spaghetti Made from Chosen Mango Leaves Blends
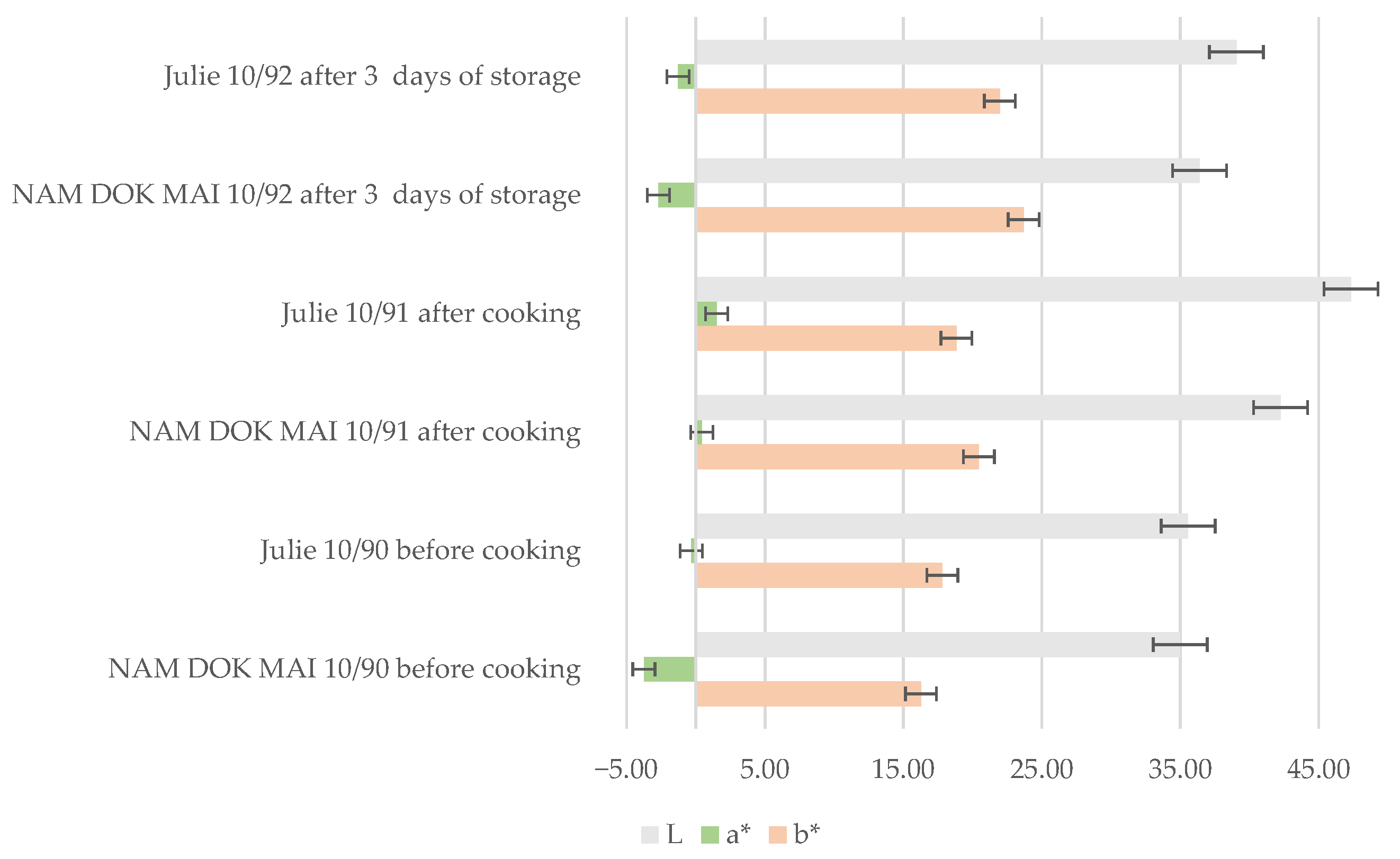
3.5.1. Color Changes in Spaghetti During Processing and Storage
3.5.2. Cutting Force of Spaghetti
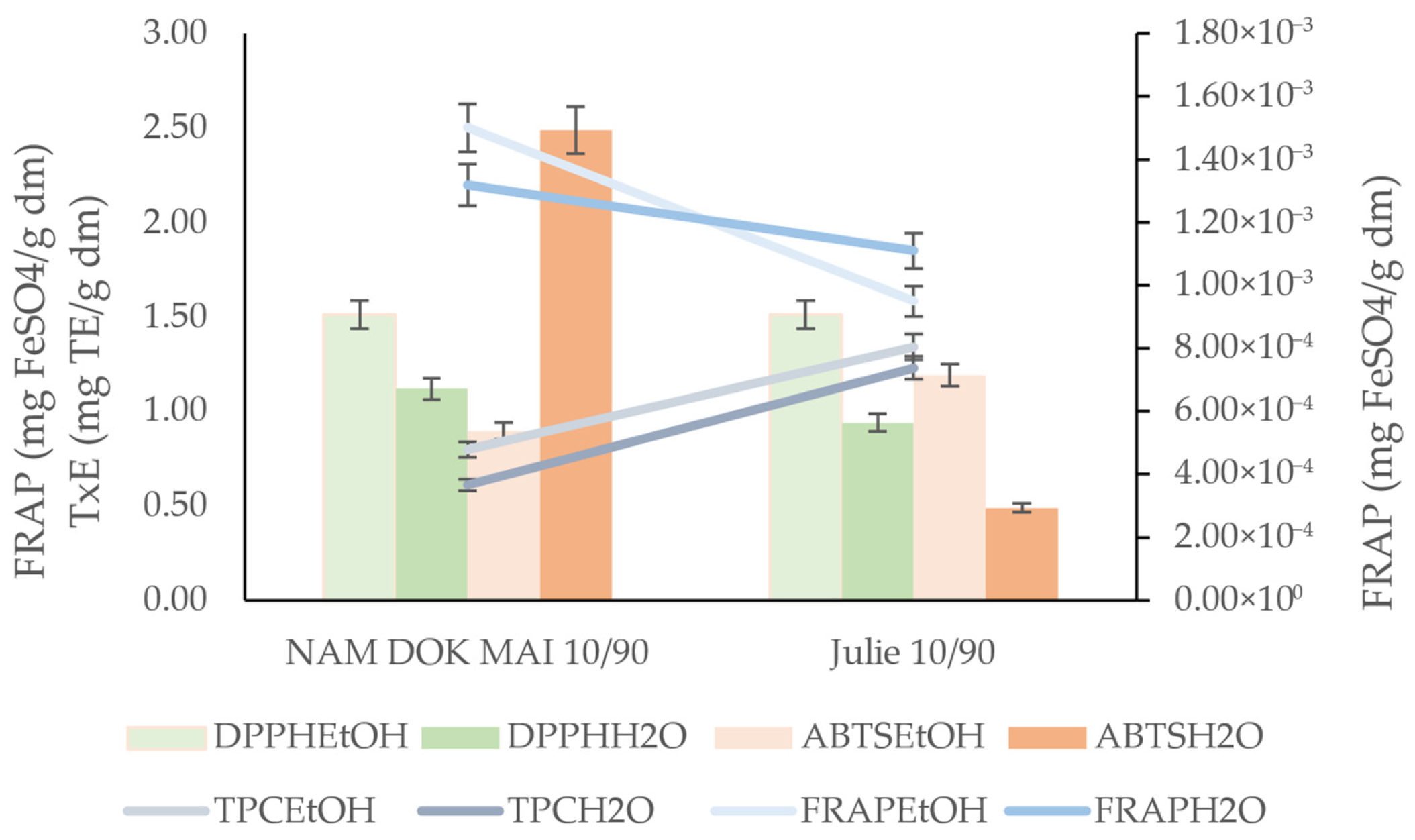
3.5.3. Antioxidant Activity of Spaghetti
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- FAO. The Future of Food and Agriculture—Alternative Pathways to 2050; FAO: Rome, Italy, 2018. [Google Scholar]
- El Khoury, D.; Balfour-Ducharme, S.; Joye, I.J. A Review on the Gluten-Free Diet: Technological and Nutritional Challenges. Nutrients 2018, 10, 1410. [Google Scholar] [CrossRef]
- Park, J.; Kim, H.-S. Rice-Based Gluten-Free Foods and Technologies: A Review. Foods 2023, 12, 4110. [Google Scholar] [CrossRef]
- Gobbetti, M.; Pontonio, E.; Filannino, P.; Rizzello, C.G.; De Angelis, M.; Di Cagno, R. How to Improve the Gluten-Free Diet: The State of the Art from a Food Science Perspective. Food Res. Int. 2018, 110, 22–32. [Google Scholar] [CrossRef] [PubMed]
- Padalino, L.; Conte, A.; Del Nobile, M.A. Overview on the General Approaches to Improve Gluten-Free Pasta and Bread. Foods 2016, 5, 87. [Google Scholar] [CrossRef]
- Mishra, R.; Garg, S.K.; Singh, M. Gluten-Free Pasta: A Comprehensive Review of Alternative Grains and Formulation Approaches. Biol. Forum Int. J. 2023, 15, 349–354, Available online: https://www.researchtrend.net/bfij/pdf/Gluten-Free-Pasta-A-Comprehensive-Review-of-Alternative-Grains-and-Formulation-Approaches-Roopal-Mishra-61.pdf. (accessed on 19 April 2025). [Google Scholar]
- Romano, A.; Ferranti, P.; Gallo, V.; Masi, P. New Ingredients and Alternatives to Durum Wheat Semolina for High-Quality Pasta. Curr. Opin. Food Sci. 2021, 41, 249–259. [Google Scholar] [CrossRef]
- Levent, H.; Koyuncu, M.; Bilgiçli, N.; Adıgüzel, E.; Dedeoğlu, M. Improvement of Chemical Properties of Noodle and Pasta Using Dephytinized Cereal Brans. LWT 2020, 128, 109470. [Google Scholar] [CrossRef]
- Tudorica, C.M.; Kuri, V.; Brennan, C.S. Nutritional and Physicochemical Characteristics of Dietary Fiber-Enriched Pasta. J. Agric. Food Chem. 2002, 50, 347–356. [Google Scholar] [CrossRef]
- Brennan, C.S.; Brennan, M.A.; Derbyshire, E.; Tiwari, B.K. Effects of Extrusion on the Polyphenols, Vitamins, and Antioxidant Activity of Foods. Trends Food Sci. Technol 2011, 22, 570–575. [Google Scholar] [CrossRef]
- Melini, V.; Melini, F.; Acquistucci, R. Phenolic Compounds and Bioaccessibility Thereof in Functional Pasta. Antioxidants 2020, 9, 343. [Google Scholar] [CrossRef] [PubMed]
- Chillo, S.; Laverse, J.; Falcone, P.M.; Protopapa, A.; Del Nobile, M.A. Influence of the addition of buckwheat flour and durum wheat bran on spaghetti quality. J. Cereal Sci. 2008, 47, 144–152. [Google Scholar] [CrossRef]
- Rosan, C.A.; Bei, M.F.; Teusdea, A.C.; Costea, T.O.; Cavalu, S.D.; Domocos, D.; Vicas, S.I. Pasta Fortified with Wild Garlic (Allium ursinum L.) as a Functional Food Rich in Phenolic Compounds. Pol. J. Food Nutr. Sci. 2025, 75, 193–207. [Google Scholar] [CrossRef]
- Kumar, M.; Saurabh, V.; Tomar, M.; Hasan, M.; Changan, S.; Sasi, M.; Maheshwari, C.; Prajapati, U.; Singh, S.; Prajapat, R.K.; et al. Mango (Mangifera indica L.) Leaves: Nutritional Composition, Phytochemical Profile, and Health-Promoting Bioactivities. Antioxidants 2021, 10, 299. [Google Scholar] [CrossRef]
- Mirza, B.; Croley, C.R.; Ahmad, M.; Pumarol, J.; Das, N.; Sethi, G.; Bishayee, A. Mango (Mangifera indica L.): A Magnificent plant with cancer preventive and anticancer therapeutic potential. Crit. Rev. Food Sci. Nutr. 2021, 61, 2125–2151. [Google Scholar] [CrossRef]
- Lawrence, G.; Marchaux, I.; Pejcz, E.; Wojciechowicz-Budzisz, A.; Olędzki, R.; Zając, A.; Paroń, O.; Aurore, G.; Harasym, J. Prescreening of Mango (Mangifera indica L.) Leaves as a Potential Functional Food Ingredient: Techno-Functional and Antioxidative Characteristics. Molecules 2025, 30, 3381. [Google Scholar] [CrossRef] [PubMed]
- Vingadassalon, A.; Pejcz, E.; Wojciechowicz-Budzisz, A.; Olędzki, R.; Groton, K.; Aurore, G.; Harasym, J. Terminalia catappa kernel flour characterization as a functional and bioactive ingredient for cookies formulation. Appl. Sci. 2024, 14, 11201. [Google Scholar] [CrossRef]
- Harasym, J.; Satta, E.; Kaim, U. Ultrasound treatment of buckwheat grains impacts important functional properties of resulting flour. Molecules 2020, 25, 3012. [Google Scholar] [CrossRef] [PubMed]
- Olędzki, R.; Lutosławski, K.; Nowicka, P.; Wojdyło, A.; Harasym, J. Non-Commercial Grapevines Hybrids Fruits as a Novel Food of High Antioxidant Activity. Foods 2022, 11, 2216. [Google Scholar] [CrossRef]
- Lamuela-Raventós, R.M. Folin—Ciocalteu method for the measurement of total phenolic content and antioxidant capacity. In Measurement of Antioxidant Activity & Capacity; John Wiley and Sons: Hoboken, NJ, USA, 2018; pp. 107–115. [Google Scholar] [CrossRef]
- Sridhar, K.; Charles, A.L. In Vitro Antioxidant Activity of Kyoho Grape Extracts in DPPH [Rad] and ABTS [Rad] Assays: Estimation Methods for EC 50 Using Advanced Statistical Programs. Food Chem. 2019, 275, 41–49. [Google Scholar] [CrossRef]
- Cano, A.; Maestre, A.B.; Hernández-Ruiz, J.; Arnao, M.B. ABTS/TAC Methodology: Main Milestones and Recent Applications. Processes 2023, 11, 185. [Google Scholar] [CrossRef]
- Fernandes, R.P.P.; Trindade, M.A.; Tonin, F.G.; Lima, C.G.; Pugine, S.M.P.; Munekata, P.E.S.; Lorenzo, J.M.; de Melo, M.P. Evaluation of antioxidant capacity of 13 plant extracts by three different methods: Cluster analyses applied for selection of the natural extracts with higher antioxidant capacity to replace synthetic antioxidant in lamb burgers. J. Food Sci. Technol. 2016, 53, 451–460. [Google Scholar] [CrossRef]
- Hu, R.; Lin, L.; Liu, T.; Ouyang, P.; He, B.; Liu, S. Reducing Sugar Content in Hemicellulose Hydrolysate by DNS Method: A Revisit. J. Biobased Mater. Bioenergy 2008, 2, 156–161. [Google Scholar] [CrossRef]
- Masiala, A.; Vingadassalon, A.; Lemoyne, S.; Pejcz, E.; Wojciechowicz-Budzisz, A.; Olędzki, R.; Aurore, G.; Harasym, J. Sustainable valorization of breadfruit (Artocarpus altilis leaves) as a pasta ingredient. Sustainability 2024, 16, 11030. [Google Scholar] [CrossRef]
- Kaur, J.; Kaushik, D.; Kumar, M.; Babagil, G.E.; Amarowicz, R.; Proestos, C.; Oz, E.; Oz, F.; Esatbeyoglu, T.; Bordiga, M. Comprehensive Analysis and Characterization of Mangifera indica L. Leaf Powder. Food Sci Nutr. 2025, 13, e70083. [Google Scholar] [CrossRef]
- Bhardwaj, K.; Najda, A.; Sharma, R.; Nurzyńska-Wierdak, R.; Dhanjal, D.S.; Sharma, R.; Manickam, S.; Kabra, A.; Kuča, K.; Bhardwaj, P. Fruit and Vegetable Peel-Enriched Functional Foods: Potential Avenues and Health Perspectives. Evid Based Complement Altern. Med. 2022, 2022, 8543881. [Google Scholar] [CrossRef]
- Vingadassalon, A.; Pejcz, E.; Vinceslas, L.; Wojciechowicz-Budzisz, A.; Olędzki, R.; Zając, A.; Aurore, G.; Harasym, J. Impact of Coconut Copra Byproducts Incorporation on Granola Quality Characteristics. Appl. Sci. 2025, 15, 2108. [Google Scholar] [CrossRef]
- Ola, E.; Hogan, V.J.; Seo, H.-S. Descriptive Sensory Analysis of Gluten-Containing and Gluten-Free Chocolate Chip Cookies Available in the Marketplace. Foods 2025, 14, 2233. [Google Scholar] [CrossRef]
- Kakar, A.; Miano, T.F.; Soomro, A.H.; Yar, A.; Memon, S.A.; Khan, B.; Miano, F. Oil and water absorption capacity of wheat, rice and gram flour powders. Int. J. Ecosyst. Ecol. Sci. (IJEES) 2022, 12, 585–594. [Google Scholar] [CrossRef]
- Oluwole, S.O.; Farhan, M.S.; Nur Fathin, S.D.; Fatmawati, A. Advancing gluten-free noodles development through sustainable and nutritional interventions. Appl. Food Res. 2025, 5, 101209. [Google Scholar] [CrossRef]
- Güven, Ö.; Şensoy, İ. Effect of fibers on starch structural changes during hydrothermal treatment: Multiscale analyses, and evaluation of dilution effects on starch digestibility. J Sci Food Agric. 2024, 104, 5724–5734. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Zhao, S.; Jia, C.; Xu, Y.; Zhang, B.; Niu, M. Textural Properties of Chinese Water Chestnut (Eleocharis dulcis) during Steam Heating Treatment. Foods 2022, 11, 1175. [Google Scholar] [CrossRef]
- Sabouni, R.; Himed, L.; Akachat, B.; Wójtowicz, A.; Kasprzak-Drozd, K.; Namoune, H.; Merniz, S.; D’Elia, M.; Rastrelli, L.; Oniszczuk, A. Physiochemical Characterization and Antioxidant Potential of Sorghum and Cork Oak as Valuable Additives to Traditional Trida Pasta. Foods 2025, 14, 2832. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Huang, S.; Chao, C.; Yu, J.; Copeland, L.; Wang, S. Changes of starch during thermal processing of foods: Current status and future directions. Trends Food Sci. Technol. 2022, 119, 320–337. [Google Scholar] [CrossRef]
- Prommajak, T.; Kim, S.M.; Pan, C.-H.; Kim, S.M.; Surawang, S.; Rattanapanone, N. Identification of Antioxidants in Young Mango Leaves by LC-ABTS and LC-MS. Chiang Mai Univ. J. Nat. Sci. 2014, 13, 317–328. [Google Scholar] [CrossRef]
- Muruganandan, S.; Srinivasan, K.; Gupta, S.; Gupta, P.K.; Lal, J. Effect of mangiferin on hyperglycemia and athero-genicity in streptozotocin diabetic rats. J. Ethnopharmacol. 2005, 97, 497–501. [Google Scholar] [CrossRef]
- Al-Madhagi, H. From Nature to Nanotechnology: The Bioactivities of Mangiferin Explored. Nanotechnol. Sci. Appl. 2025, 18, 277–294. [Google Scholar] [CrossRef] [PubMed]
- Abdullahi, D.M.; Kirawa, T.; Tame, V.T.; Jakusko, B.B. Physicochemical Properties of the Keith and Julie Varieties of Mango (Mangifera Indica L) in Yola, Nigeria. Res. J. Agric. 2024, 12, 45–50. [Google Scholar]
- Alo’s, E.; Rey, F.; Gil, J.V.; Rodrigo, M.J.; Zacarias, L. Ascorbic Acid Content and Transcriptional Profiling of Genes Involved in Its Metabolism during Development of Petals, Leaves, and Fruits of Orange (Citrus sinensis cv. Valencia Late). Plants 2021, 10, 2590. [Google Scholar]
- Nowak, M.; Tryniszewski, W.; Sarniak, A.; Wlodarczyk, A.; Nowak, P.J.; Nowak, D. Concentration Dependence of Anti- and Pro-Oxidant Activity of Polyphenols as Evaluated with a Light-Emitting Fe2+-Egta-H2O2 System. Molecules 2022, 27, 3453. [Google Scholar] [CrossRef]
- Minniti, G.; Laurindo, L.F.; Machado, N.M.; Duarte, L.G.; Guiguer, E.L.; Araujo, A.C.; Dias, J.A.; Lamas, C.B.; Nunes, Y.C.; Bechara, M.D.; et al. Mangifera indica L., By-Products, and Mangiferin on Car-dio-Metabolic and Other Health Conditions: A Systematic Review. Life 2023, 13, 2270. [Google Scholar] [CrossRef]
- Robards, K.; Prenzler, P.D.; Tucker, G.; Swatsitang, P.; Glover, W. Phenolic Compounds and Their Role in Oxidative Processes in Fruits. Food Chem. 1999, 66, 401–436. [Google Scholar] [CrossRef]
- Tomec-zko, P.M.; Królicka, A. Verification of the Conditions for Determination of Antioxidant Activity by ABTS and DPPH Assays—A Practical Approach. Molecules 2022, 27, 50. [Google Scholar]
- Gershenzon, J.; McConkey, M.E.; Croteau, R.B. Regulation of Monoterpene Accumulation in Leaves of Peppermint. Plant Physiol 2000, 122, 205–214. [Google Scholar] [CrossRef] [PubMed]
- da Cruz Francisco, J.; Sivik, B. Solubility of Three Monoterpenes, Their Mixtures and Eucalyptus Leaf Oils in Dense Carbon Dioxide. J. Supercrit. Fluids 2002, 23, 11–19. [Google Scholar] [CrossRef]
- Yamada, K.; Kanai, M.; Osakabe, Y.; Ohiraki, H.; Shinozaki, K.; Yamaguchi-Shinozaki, K. Monosaccharide Absorp-tion Activity of Arabidopsis Roots Depends on Expression Profiles of Transporter Genes under High Salinity Condi-tions. J. Biol. Chem. 2011, 286, 43577–43586. [Google Scholar] [CrossRef] [PubMed]
- Williams, L.E.; Lemoine, R.; Sauer, N. Sugar Transporters in Higher Plants—A Diversity of Roles and Complex Reg-ulation. Trends Plant Sci. 2000, 5, 283–290. [Google Scholar] [CrossRef] [PubMed]
- Sami, F.; Yusuf, M.; Faizan, M.; Faraz, A.; Hayat, S. Role of Sugars under Abiotic Stress. Plant Physiol. Biochem. 2016, 109, 54–61. [Google Scholar] [CrossRef]
- Kuhn, D.N.; Bally, I.S.E.; Dillon, N.L.; Innes, D.; Groh, A.M.; Rahaman, J.; Ophir, R.; Cohen, Y.; Sherman, A. Ge-netic Map of Mango: A Tool for Mango Breeding. Front. Plant Sci. 2017, 8, 577. [Google Scholar] [CrossRef]
- Santamaria, M.; Garzon, R.; Skendi, A.; Papageorgiou, M.; Rosell, C.M. Examining the role of polyphenols in the in vitro starch hydrolysis of gluten free enriched flatbreads. Food Chem. 2025, 486, 144456. [Google Scholar] [CrossRef]
- Delgado-Ospina, J.; Lucas-González, R.; Viuda-Martos, M.; Fernández-López, J.; Pérez-Álvarez, J.Á.; Martuscelli, M.; Chaves-López, C. Bioactive compounds and techno-functional properties of high-fiber co-products of the cacao agro-industrial chain. Heliyon. 2021, 7, e06799. [Google Scholar] [CrossRef]
- Karim, A.; Raji, Z.; Habibi, Y.; Khalloufi, S. A review on the hydration properties of dietary fibers derived from food waste and their interactions with other ingredients: Opportunities and challenges for their application in the food industry. Crit. Rev. Food Sci. Nutr. 2023, 64, 11722–11756. [Google Scholar] [CrossRef]
- Paternina-Contreras, A.L.; Andrade-Pizarro, R.D.; Figueroa-Flórez, J.A. Physical Modification of Starch in Plant-Based Flours: Structural, Physicochemical, and Pasting Property Changes and Potential Applications in Baked and Extruded Products. Compr. Rev. Food Sci. Food Saf. 2025, 24, e70184. [Google Scholar] [CrossRef]
- Wu, Y.; Liu, Y.; Jia, Y.; Zhang, H.; Ren, F. Formation and Application of Starch–Polyphenol Complexes: Influencing Factors and Rapid Screening Based on Chemometrics. Foods 2024, 13, 1557. [Google Scholar] [CrossRef]
- Ribeiro, A.R.; Botelho, G.; Gaspar, A.; Costa, R. Storage Stability of Durum Wheat Pasta Enriched with Seaweeds Flours. Foods 2021, 10, 2450. [Google Scholar] [CrossRef] [PubMed]
- Cavazza, A.; Corradini, C.; Rinaldi, M.; Salvadeo, P.; Borromei, C.; Massini, R. Evaluation of Pasta Thermal Treatment By Determination of Carbohydrates, Furosine, and Color Indices. Food Bioprocess Technol 2013, 6, 2721–2731. [Google Scholar] [CrossRef]
- Iacobellis, I.; Lisi, A.; Vacca, M.; Apa, C.A.; Celano, G.; Mancini, L.; Minervini, F.; Calasso, M.; De Angelis, M. Nutritional, Biochemical, and Functional Properties of Spinach Leaf-Enriched Dough: A Healthier Alternative to Conventional Pasta. Foods 2024, 13, 3608. [Google Scholar] [CrossRef]
- Stuknytė, M.; Cattaneo, S.; Pagani, M.A.; Marti, A.; Micard, V.; Hogenboom, J.; De Noni, I. Spaghetti from Durum Wheat: Effect of Drying Conditions on Heat Damage, Ultrastructure and In Vitro Digestibility. Food Chem. 2014, 149, 40–46. [Google Scholar] [CrossRef] [PubMed]
- Lisiecka, K.; Wójtowicz, A.; Dziki, D.; Gawlik-Dziki, U. The influence of Cistus incanus L. leaves on wheat pasta quality. J. Food Sci. Technol. 2019, 56, 4311–4322. [Google Scholar] [CrossRef] [PubMed]
- Cao, H.; Saroglu, O.; Karadag, A.; Diaconeasa, Z.; Zoccatelli, G.; Conte-Junior, C.A.; Gonzalez-Aguilar, G.A.; Ou, J.; Bai, W.; Zamarioli, C.M.; et al. Available Technologies on Improving the Stability of Polyphenols in Food Processing. Food Front. 2021, 2, 109–139. [Google Scholar] [CrossRef]
- Turturică, M.; Oancea, A.M.; Râpeanu, G.; Bahrim, G. Anthocyanins: Naturally Occurring Fruit Pigments with Functional Properties. Ann. Univ. Dunarea Jos Galati. Fascicle VI-Food Technol. 2015, 39, 9–24. [Google Scholar]
- Martins, C.C.; Rodrigues, R.C.; Mercali, G.D.; Rodrigues, E. New Insights into Non-Extractable Phenolic Com-pounds Analysis. Food Res. Int. 2022, 157, 111487. [Google Scholar] [CrossRef] [PubMed]
- Tilley, A.; McHenry, M.P.; McHenry, J.A.; Solah, V.; Bayliss, K. Enzymatic Browning: The Role of Substrates in Polyphenol Oxidase Mediated Browning. Curr. Res. Food Sci. 2023, 7, 100623. [Google Scholar] [CrossRef] [PubMed]

| Sample | WHC | WAC | OAC | HLI | WAI | WSI | SP |
|---|---|---|---|---|---|---|---|
| (g H2O/g dm) | (g H2O/g dm) | (g Oil/g dm) | (g H2O/g dm) | (g H2O/g dm) | (g/g dm) | (g H2O/g dm) | |
| Nam Dok Mai 10/90 | 3.67 ± 0.02 f | 2.25 ± 0.09 bcd | 1.91 ± 0.02 a | 1.18 ± 0.06 bcd | 5.92 ± 0.11 cd | 18.34 ± 0.39 a | 6.48 ± 0.12 ab |
| Nam Dok Mai 20/80 | 3.55 ± 0.02 def | 2.42 ± 0.06 ef | 1.96 ± 0.06 ab | 1.23 ± 0.04 cd | 6.06 ± 0.03 cd | 07.55 ± 1.44 bc | 6.53 ± 0.23 ab |
| DLO 10/90 | 3.20 ± 0.07 a | 2.31 ± 0.08 de | 1.81 ± 0.15 a | 1.28 ± 0.06 d | 5.69 ± 0.07 c | 18.78 ± 1.76 bc | 6.05 ± 0.37 ab |
| DLO 20/80 | 3.43 ± 0.09 cd | 2.44 ± 0.08 f | 1.93 ± 0.18 ab | 1.28 ± 0.06 d | 5.62 ± 0.07 c | 15.12 ± 1.47 b | 5.96 ± 0.36 ab |
| IRWIN 10/90 | 3.47 ± 0.17 cd | 2.13 ± 0.01 ab | 1.89 ± 0.13 a | 1.13 ± 0.08 abc | 6.68 ± 0.25 e | 16.34 ± 0.43 b | 6.85 ± 0.36 b |
| IRWIN 20/80 | 3.29 ± 0.09 ab | 2.26 ± 0.08 cd | 1.88 ± 0.06 a | 1.20 ± 0.03 cd | 5.72 ± 0.17 c | 08.44 ± 0.94 a | 6.50 ± 0.52 ab |
| JULIE 10/90 | 3.62 ± 0.02 ef | 2.16 ± 0.05 abc | 2.14 ± 0.22 b | 1.02 ± 0.13 a | 6.35 ± 0.23 de | 24.78 ± 3.60 d | 6.84 ± 0.31 b |
| JULIE 20/80 | 3.43 ± 0.06 cd | 2.28 ± 0.07 cd | 1.81 ± 0.09 a | 1.26 ± 0.03 d | 4.48 ± 0.76 a | 17.16 ± 0.78 b | 5.49 ± 1.69 a |
| Keitt 10/90 | 3.40 ± 0.04 bc | 2.05 ± 0.01 a | 1.90 ± 0.06 a | 1.08 ± 0.04 ab | 5.11 ± 0.14 b | 21.95 ± 4.99 cd | 5.94 ± 0.69 ab |
| Keitt 20/80 | 3.53 ± 0.03 cde | 2.25 ± 0.10 bcd | 1.93 ± 0.07 a | 1.17 ± 0.05 bcd | 4.95 ± 0.19 ab | 15.62 ± 2.25 b | 5.45 ± 0.63 a |
| variety | *** | *** | ns | ** | *** | *** | ns |
| share | ns | *** | ns | ** | *** | *** | ns |
| varietyxshare | *** | ns | * | * | *** | ns | ns |
| Sample | Peak Viscosity (mPa·s) | Trough Viscosity (mPa·s) | Breakdown (mPa·s) | Final Viscosity (mPa·s) | Setback (mPa·s) | Pasting Time (s) | Pasting Temperature (°C) |
|---|---|---|---|---|---|---|---|
| CF | 1233.5 ± 55.5 e | 1075.5 ± 46.5 | 158.0 ± 9.0 | 2237.0 ± 63.0 | 1161.5 ± 16.5 | 6.00 ± 0.13 | 71.88 ± 0.03 |
| Nam Dok Mai 10/90 | 698.0 ± 6.0 d | 524.5 ± 7.5 | 173.5 ± 1.5 | 970.0 ± 21.0 | 445.5 ± 13.5 | 4.87 ± 0.06 | 72.10 ± 0.25 |
| Nam Dok Mai 20/80 | 521.5 ± 18.5 c | 332.0 ± 6.0 | 189.5 ± 12.5 | 546.5 ± 5.5 | 214.5 ± 0.5 | 4.46 ± 0.06 | 71.72 ± 0.02 |
| Irwin 10/90 | 481.5 ± 0.5 c | 318.5 ± 2.5 | 163.0 ± 2.0 | 559.0 ± 8.0 | 240.5 ± 5.5 | 4.70 ± 0.03 | 72.70 ± 0.00 |
| Irwin 20/80 | 350.0 ± 1.0 a | 176.5 ± 2.5 | 173.5 ± 3.5 | 277.0 ± 5.0 | 100.5 ± 7.5 | 4.50 ± 0.10 | 65.97 ± 7.57 |
| Julie 10/90 | 732.5 ± 2.5 d | 564.0 ± 1.0 | 168.5 ± 3.5 | 1066.0 ± 29.0 | 502.0 ± 28.0 | 5.04 ± 0.17 | 71.82 ± 0.02 |
| Julie 20/80 | 431.0 ± 27.0 b | 316.0 ± 20.0 | 115.0 ± 7.0 | 525.5 ± 51.5 | 209.5 ± 31.5 | 4.60 ± 0.07 | 73.08 ± 0.38 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lawrence, G.; Pejcz, E.; Marchaux, I.; Wojciechowicz-Budzisz, A.; Olędzki, R.; Aurore, G.; Harasym, J. Bioactive, Functional, and Technological Properties of Gluten-Free Pasta Enriched with Mango (Mangifera indica L.) Leaf Powder. Foods 2025, 14, 4006. https://doi.org/10.3390/foods14234006
Lawrence G, Pejcz E, Marchaux I, Wojciechowicz-Budzisz A, Olędzki R, Aurore G, Harasym J. Bioactive, Functional, and Technological Properties of Gluten-Free Pasta Enriched with Mango (Mangifera indica L.) Leaf Powder. Foods. 2025; 14(23):4006. https://doi.org/10.3390/foods14234006
Chicago/Turabian StyleLawrence, Génica, Ewa Pejcz, Ingrid Marchaux, Agata Wojciechowicz-Budzisz, Remigiusz Olędzki, Guylène Aurore, and Joanna Harasym. 2025. "Bioactive, Functional, and Technological Properties of Gluten-Free Pasta Enriched with Mango (Mangifera indica L.) Leaf Powder" Foods 14, no. 23: 4006. https://doi.org/10.3390/foods14234006
APA StyleLawrence, G., Pejcz, E., Marchaux, I., Wojciechowicz-Budzisz, A., Olędzki, R., Aurore, G., & Harasym, J. (2025). Bioactive, Functional, and Technological Properties of Gluten-Free Pasta Enriched with Mango (Mangifera indica L.) Leaf Powder. Foods, 14(23), 4006. https://doi.org/10.3390/foods14234006









