Incorporation of Edible Plant Extracts as Natural Food Preservatives: Green Extraction Methods, Antibacterial Mechanisms and Applications
Abstract
1. Introduction
2. Green Extraction Methods for Bioactive-Rich Plant Extracts
2.1. Green Solvents
2.2. Ultrasound-Assisted Extraction
2.3. Microwave-Assisted Extraction
2.4. Enzyme-Assisted Extraction
2.5. Supercritical Fluid Extraction
2.6. High Hydrostatic Pressure
2.7. Polarity and Extraction Compatibility
2.8. Comparative Evaluation of Green Extraction Technologies
3. Antibacterial Assays
3.1. Agar Diffusion Assays
3.2. Broth Dilution Assays
3.3. Time-Kill Kinetics
3.4. Thin-Layer Chromatography Bioautography
3.5. Antibiofilm Assay
3.6. Flow Cytometry Assay
3.7. Plate Count Methods
3.8. Microbiome Profiling
4. Antibacterial Activity of Plant Extracts Against Foodborne Pathogen Microorganisms
5. Antibacterial Mechanisms of Plant Extracts
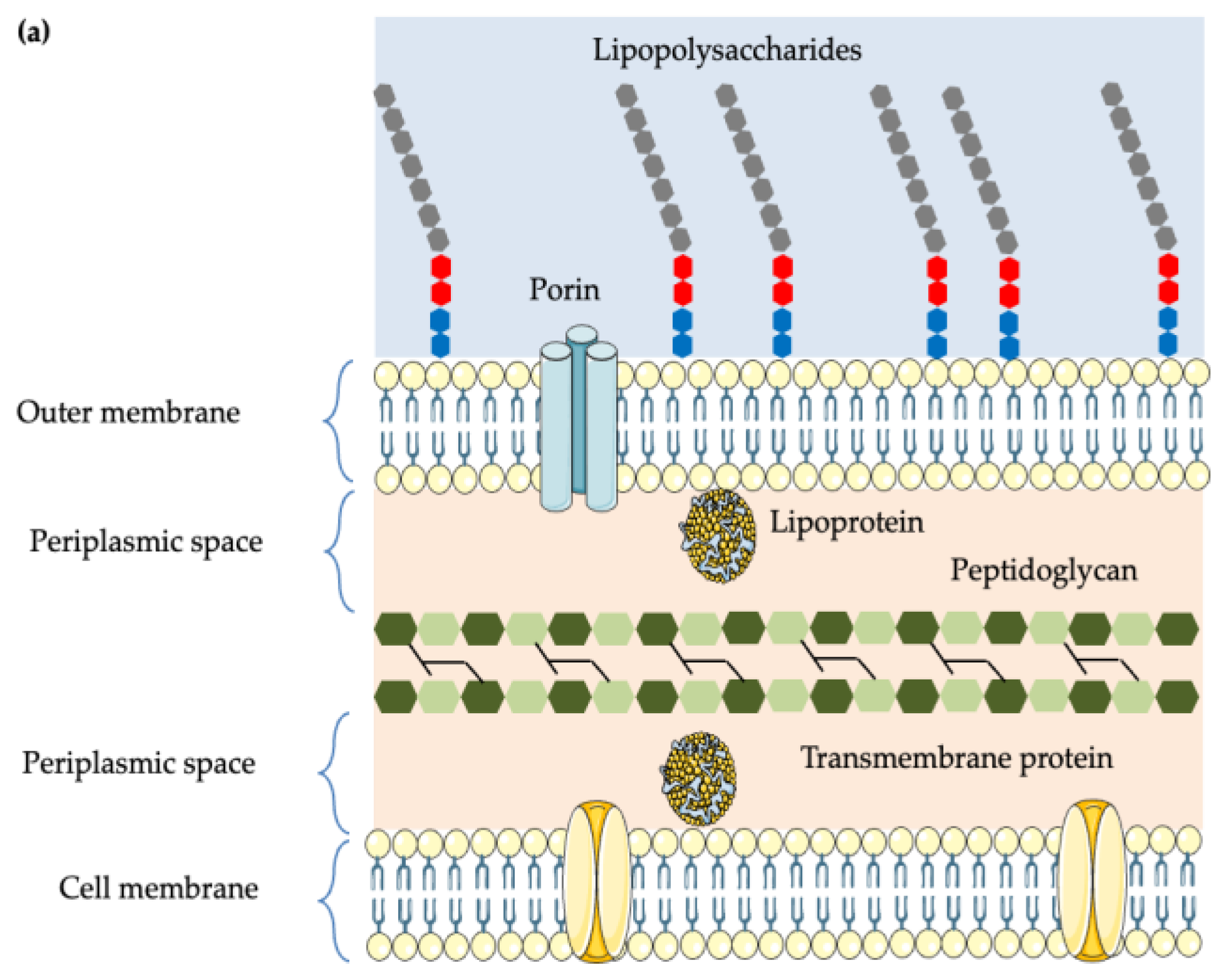
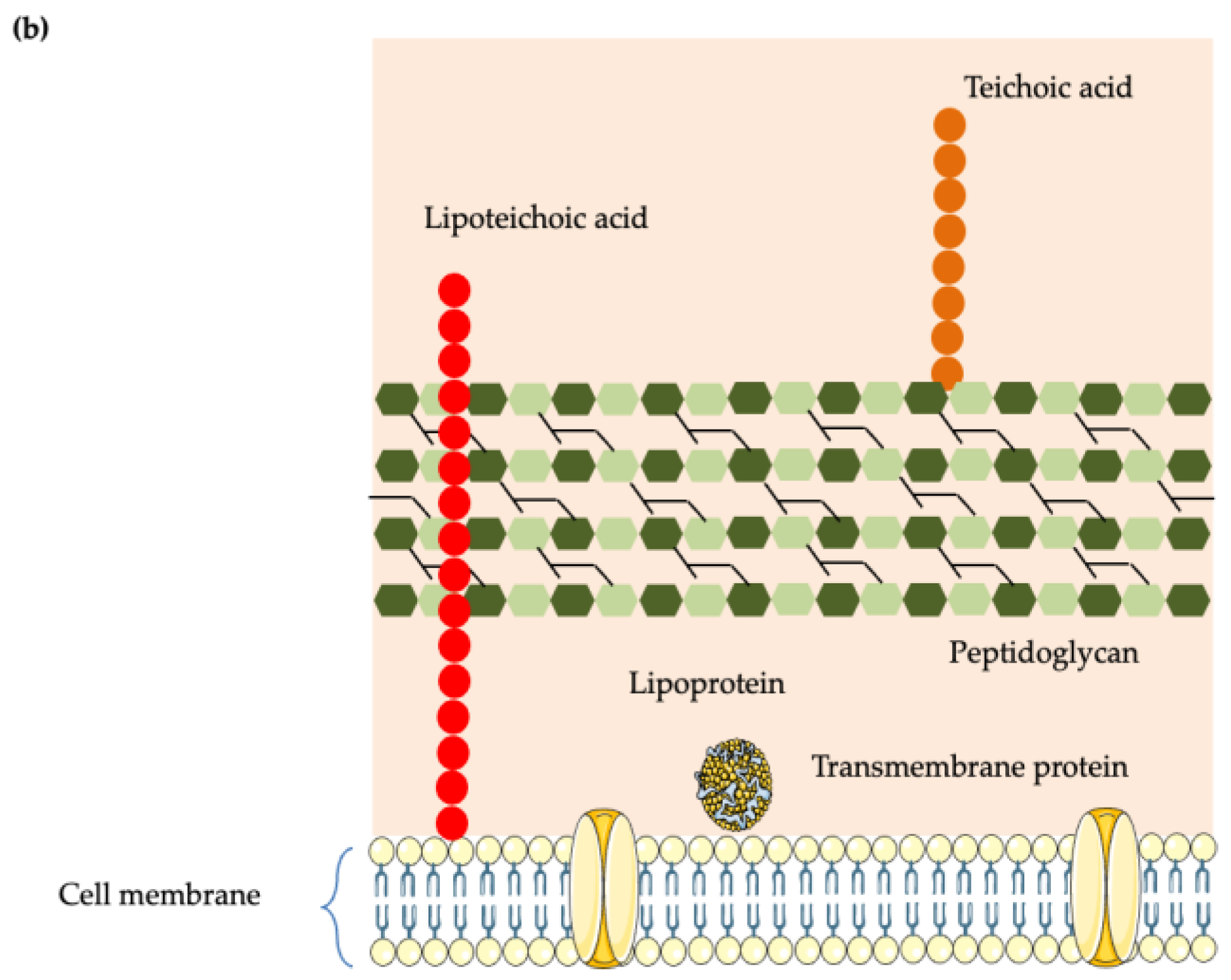
5.1. Cell Wall Structure of Gram-Positive and Gram-Negative Bacteria
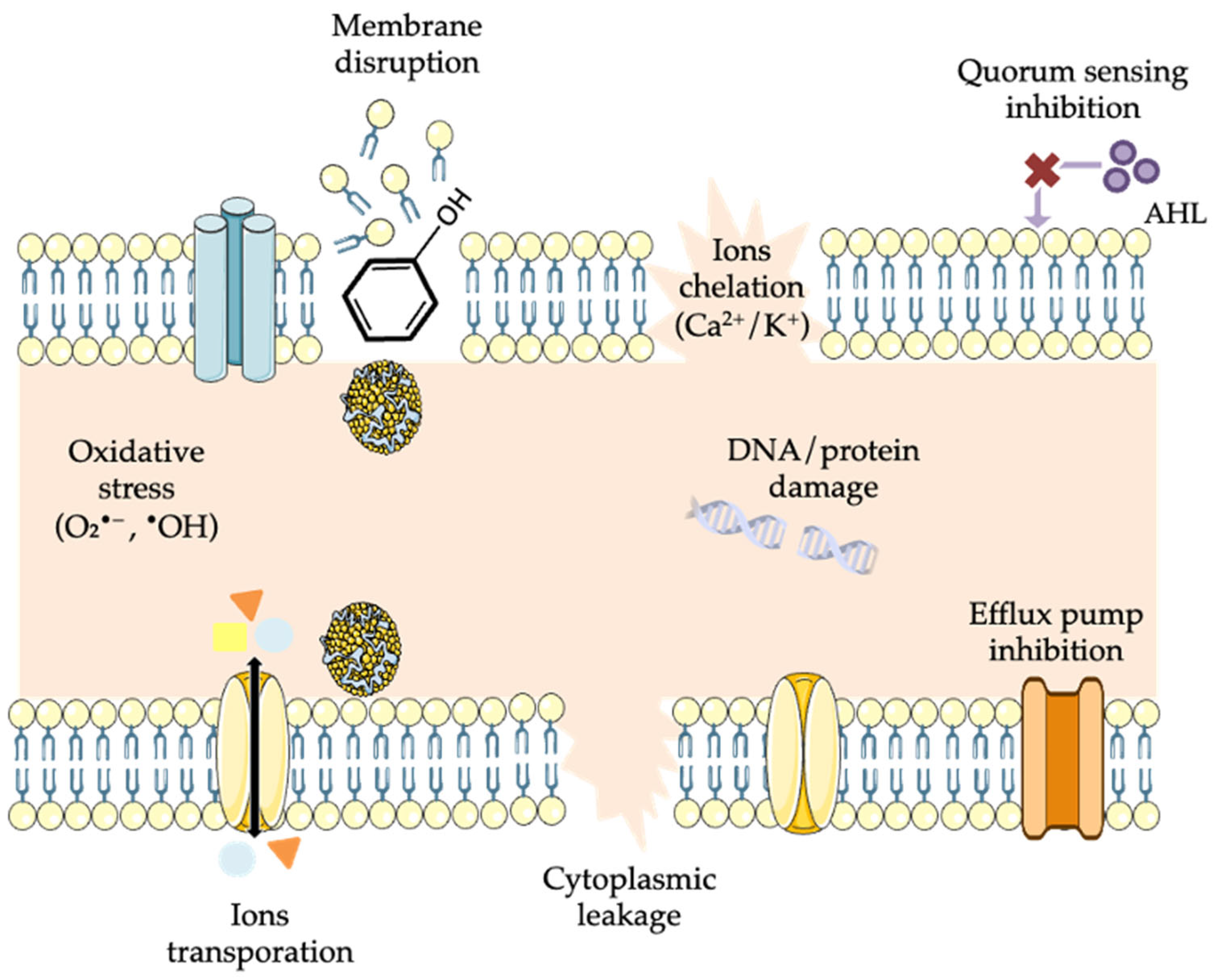
5.2. Disruption of the Cell Membrane and Cell Wall Integrity
5.3. Interaction with Enzymes, Microbial Proteins, and Nucleic Acids
5.4. Induction of Oxidative Stress
5.5. Inhibition of Quorum Sensing and Biofilm Formation
5.6. Efflux Pump and β-Lactamase Inhibition
6. Plant Extracts in Food Matrices as Preservatives
| Plant (Part) | Food Matrix | Application (Including Extraction Method) | Target Microorganisms | Main Result | Ref. |
|---|---|---|---|---|---|
| Eugenia uniflora L. (Pitanga) (Leaves) | Pork Burgers | Hydroethanolic (40:60 H2O/EtOH) UAE/Stirring (80 °C). Mixed with minced meat (250–1000 mg/kg) | TVC, LAB, Pseudomonas spp. | Significantly ↓ microbial counts, mainly at the end of 18 d shelf-life | [146] |
| Cymbopogon citratus (Lemongrass) (Leaves) | Cooked and Shredded Chicken Breast | Hydro-ethanolic (95% EtOH) extract. Added to meat (1% v/w) | TCC, Staphylococci, Salmonella sp. | Staph, Salmonella, and Coliforms Not Detected at 45 °C during 60 d storage | [162] |
| Rosmarinus officinalis L. (Rosemary) (Aerial parts) | Beef meat | Nano-encapsulated (Soybean Protein Isolate/Basil Gum). Immersion (60 min). 1600 ppm | TVC | 1600 ppm extract maintained TVC < 7 logCFU g−1 until d21 | [87] |
| Olea europaea (Olive)/Urtica dioica (Stinging Nettle)/Camellia sinensis (Green Tea) (Leaves) | Frankfurter type sausage | EtOH (95%) extraction. Incorporated at 500 ppm before cooking/stuffing | TVC, TCC, yeasts/molds | TVC reduced (Stinging Nettle extracts best). Coliforms Not Detected | [143] |
| Citrus reticulata/Citrus sinensis/Citrus bigarradia/Citrus macrocarpa (Citrus) (Peel) | Beef tenderloin | Hydrodistilled extracts (100 °C, 6 h). Boiling in 50 g/L of the corresponding Citrus peel extract. | TBC, TAC | Significant ↓ microbial counts especially at d8. Citrus reticulata performed best | [145] |
| Castanea sativa (Chestnut) (Nut)/Vitis vinifera (Grape) (Seeds) | Italian Cinta Senese dry-fermented sausages | CHE and GSE mixed with tocopherol/hydroxytyrosol, replacing sodium nitrate in sausage | TVC, Prokaryotic communities (Illumina MiSeq) | Spoilage Photobacterium genus >30x lower. CHE/GSE. Extracts did not alter the prokaryotic community | [163] |
| Citrus sinesis L. (Orange)/Rosmarinus officinalis L. (Rosemary)/Malpighia emarginata (Acerola) | Spanish Chorizo (Fermented) | Combined with natural nitrate sources/spices. Mixed with meat paste | TVC, TCC, Clostridium perfringens | No growth of C. perfringens. Citric extracts showed the lowest viable growth | [144] |
| Pistacia vera (Pistachio) (Hull) | Fermented beef sausage | Water extract (1:15, 8 h stirring). Added to meat dough (500, 750, 1000 ppm) | TVC, LAB, staphylococci, yeasts & molds | The highest dose (1000 ppm) showed the lowest TVC (d28) | [164] |
| Coriandrum sativum L. (Coriander) (Seed) | Poultry meatballs | Commercial extract. Added to minced meat (200 ppm and 500 ppm) | TAM | 500 ppm inhibited aerobic growth after d6. 200 ppm had no influence | [165] |
| Prunus cerasus (Cherry) (Leaves)/Ribes nigrum (Blackcurrant) (Leaves) | Pork meat sausages | Water extracts. Added to meat (0.5–1.0 g/100 g) before stuffing | TMC, PTC, LAB, Brochothrix, Pseudomonas, Enterobacteriaceae | Mesophiles, psychrotrophs, LAB, Brochothrix ↓ after 14 d | [166] |
| Castanea sativa (Chestnut) (Leaves, Bur, Hull) | Beef patties | Leaf: Acidified water. (25 °C, 90 mins) Bur and Hull: Water in pressurized reactor (220 °C–Bur/130 °C–Hull), non-ionic polymer resins, 96%EtOH (35 °C) | TVC, Psychotropic bacteria, LAB, Pseudomonas spp. | Leaf extract showed the lowest CFU for TVC, Psych., Pseudomonas. Bur extract showed higher CFU than the control | [167] |
| Salicornia perennans (Glassworts) (Leaves) | Beef patties | UAE EtOH (70%) extraction. Mixed with minced meat. | TAM, TPC, yeasts & molds | 1.0–1.5% extract significantly ↓ all microbial counts for 15 d | [147] |
| Punica granatum L. (Pomegranate) (Peel)/Cynara cardunculus L. (Artichoke) (Leaves) | Sardine Fillets | Water extract (95 °C). Marinated in 5% solution (72 h) with 4% Acetic Acid/10% NaCl | TVC, LAB, TCC, S. aureus | LAB growth inhibited post-marination; Significant ↓ TVC/Coliforms after d30 (Pomegranate best) | [161] |
| Cuminum Cyminum L. (Cumin) (Seeds)/Mentha Longifolia L. (Wild mint) (Leaves) | Rainbow Trout fillets | EtOH extracts. Dipped in 3.0% and 6.0% (w/v) aqueous solution | TVC, PTC, E. coli, S. Aureus, L. monocytogenes | Mint showed lower TVC/PTC than Cumin. All treated samples < limit until d18 | [168] |
| Solanum lycopersicum (Tomato) (Plant) | Sierra fillets | EtOH/Acetic acid (95:5 v/v) extract. Dipped in 0.3% TPE or TPE-C (Chitosan coating) | TAM | TPE/TPE-C delayed bacterial growth for 15 d | [169] |
| Gracilaria sp. (Red Algae) (Plant) | Pangas Fillets | EtOH (99%) extraction. Dipped in 3 concs (2% best) for 10 min | APC, Pychrophillic bacteria, Enterobacteriacease, Staphylococcus | 2% GE allowed 6 more days of storage | [170] |
| Allium ascalonicum L. (Shallot) (Fruit)/Trachyspermum ammi (Ajwain) (Seed) | Rainbow trout (semi-fried) | EtOH (85%) extraction. Mixed into edible coating (1.5%/3% v/v). Semi-fried | TVC, PTC, total aerobic count, Pseudomonas spp. | 3% Ajwain extract extended storage up to 9 more days. Ajwain consistently lower counts than Shallot | [171] |
| Satureja hortensis (Summer savory) (Leaves and stems) | Spangled Emperor fillets | EtOH (80%) extract combined with CMC coating. Immersion (10 min) | TVC, PTC | CMC + 1% SHE and CMC + 1.5% SHE extended storage life by 3 days | [152] |
| Chenopodium quinoa (Quinoa) (Grain) | Atlantic Chub Mackerel | EtOH (80%v/v) extract. Used as ice flakes for chill storage | Lipolytic bacteria | Quinoa extract inhibited the growth of lipolytic bacteria proportional to concentration | [149] |
| Punica granatum (Pomegranate) (Peel) | Nile tilapia fillets | EtOH (70%) extract. Added to Chitosan coating (1% PPE). Immersion (1 min) | TVC, Psychrotrophs, Yeasts/Molds, Coliforms, E. coli, Salmonella spp. | Complete Inhibition of most groups (30 d). TVC; ↓ 73.2%; Psychrotrophs ↓53.9%. | [172] |
| Mentha arvensis (Mint) (Leaves)/Citrus aurantium (Citrus) (Peel) | Indian mackerel | EtOH (60%) extraction. Dipped in 0.5% (Mint) or 1% (Citrus) solution (30 min) | APC | Mint extract extended acceptable limit by 5 days (to d16) | [173] |
| Stevia rebaudiana Bertoni (stevia) (Leaves) | Catla fillets | EtOH (80%v/v) extract. Dipped to form edible coating (2% best) | APC, PBC, LAB, Enterobacteriaceae, Staphyloccocus | 2% Stevia leaf extract extended shelf life for 8 more days | [174] |
| Allium paradoxum (Few-flowered leek) (Leaves)/Eryngium caucasicum (Leaves) | Silver carp fillets | EtOH (80%) extraction. Dipped in 2% and 5% concs (30 min) | TVC, PTC | Extended storage life by 3 to 9 days. A. paradoxum (4%) showed lowest values | [175] |
| Foeniculum vulgare (Fennel) (Plant) | Silver carp fillets | EtOH extract. Used alone and liposome-encapsulated. Dipped for 15 min | TVC, TPC | Encapsulated extracts presented the best results by day 15 | [148] |
| Morinda citrifolia (Noni) (Leaves) | Striped Catfish slices | EtOH (70%v/v) extract. Used as-is and dechlorophyllized. Mixed with fish slices | PBC, TVC | Extracts doubled storage period. DE consistently lower counts | [153] |
| Punica granatum (Pomegranate) (Fruit)/Rosmarinus officinalis L. (Rosemary) (De-oiled Leaf)/Olea europaea (Olive) (Leaf/Fruit) | Fish patties | Hydroethanolic extracts mixed into fish paste | TVC, TCC, E. Coli, S. Aureus, L. monocytogenes | HYT-F/HYT-L (Olive) had lowest TVC (d11). Pomegranate/Rosemary diterpene extracts (NOS/NOVS/RA) had lowest TCC | [176] |
| Posidonia Oceanica (Leaves) | Peach slices (Fresh-cut) | 50% EtOH extraction. Dipped in 2% w/v solution (3 min) | TAC, Pseudomonas, Yeasts & molds, Enterobacteriaceae | Significant ↓ in yeasts/molds (3 d), Pseudomonas, and TAC. No change in Enterobacteriaceae | [158] |
| Vitis vinifera (Grape) (Seeds) | Snakehead fillets | 60% EtOH UAE. Immersed in GSE solution (0.52 mg GAE/mL) for 20 min | TVC, Microbiota (Illumina MiSeq) | Slower TVC growth (extended shelf-life by 4 days). Inhibited Aeromonas growth | [154] |
| Porphyra yezoensis (Red Algae, Nori) (Plant) | Pacific white shrimp | Polyphenolic: EtOH (70%) & UAE. Polysaccharide: Water & UAE. Dipping (5 g/L extract for 60 min) | TVC | Extract mixture slowed TVC increase, reaching limit 4 days after control sample | [156] |
| Punica granatum (Pomegranate) (Peels) | Wounded “Satsuma” Mandarins | PPE (60% EtOH w/citric acid). Dipped for 1 min (Curative/Preventative) | Penicillium italicum, Penicillium digitatum (Molds) | Increased conc. showed an 80–90% ↓ infection rate and reduced lesion diameter | [157] |
| Arbutus unedo L. (Strawberry tree) (Leaf) | Quark cheese | Ultrasonic Assisted (UAE) and Dynamic Maceration (DM) extracts. Mixed into cheese (0.1 g/100 g) | TAM, Enterobacteriaceae, Molds, Yeasts, LAB, S. aureus | DM extracts performed better than UAE/Sorbate, significantly ↓ Molds, Yeast, TAM | [159] |
| Rhus coriaria (Sumac) (Fruits)/Tamarindus indica (Tamarind) (Pods)/Rosmarinus officinalis L. (Rosemary) (Aerial Parts)/Hibiscus sabdariffa (Roselle) (Red calyces)/Citrus limon (Lemon) (Fruits) | Raw Cow milk | EtOH (80%) extraction. Added at 3000 ppm to milk | TVC, TCC | Sumac most effective in ↓ total bacteria. Coliforms Not Detected in any treated sample | [160] |
7. Applications in Industry & Challenges
8. Future Perspectives
- •
- Optimizing green extraction methods to improve yield, selectivity, scalability, and cost efficiency.
- •
- Standardizing analytical and extraction protocols to ensure consistent identification, quantification, and comparison of active compounds across studies and species.
- •
- Investigating synergistic and antagonistic interactions within whole extracts and assessing their antibacterial and antioxidant performance in real food matrices.
- •
- Conducting application-focused studies including sensory evaluation and shelf-life testing to support commercial feasibility.
- •
- Developing multifunctional or synergistic extract combinations to enhance antimicrobial spectra and reduce required dosages.
- •
- Advancing industrial upscaling strategies, including solvent recovery, process reproducibility, and energy-efficient operation.
- •
- Establishing regulatory frameworks and clear labeling guidelines specific to green-extracted plant preservatives.
- •
- Improving consumer acceptance and communication, emphasizing natural, sustainable, and clean-label benefits.
9. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AMR | Antimicrobial Resistance |
| UAE | Ultrasound-Assisted Extraction |
| MAE | Microwave-Assisted Extraction |
| EAE | Enzyme-Assisted Extraction |
| NADES | Natural Deep Eutectic Solvents-Assisted Extraction |
| ILs | Ionic Liquids |
| DES | Deep Eutectic Solvents |
| HBA | Hydrogen Bond Acceptor |
| HBD | Hydrogen Bond Donor |
| SFE | Supercritical Fluid Extraction |
| HHP | High Hydrostatic Pressure |
| MIC | Minimum Inhibitory Concentration |
| MBC | Minimum Bactericidal Concentration |
| CFUs | Colony-Forming Units |
| TLC | Thin-Layer Chromatography |
| HPLC | High-Performance Liquid Chromatography |
| OPLC | Over-Pressured-Layer Chromatography |
| CV | Crystal Violet |
| cFDA | Carboxyfluorescein Diacetate |
| STC | Standard Plate Count |
| TVC | Total Viable Count |
| ACC | Aerobic Colony Count |
| AMC | Aerobic Mesophilic Count |
| CFU·g−1 or CFU·mL−1 | Colony-Forming Units per gram or milliliter |
| FDA | Food and Drug Administration |
| NGS | Next-Generation Sequencing |
| PCR | Polymerase Chain Reaction |
| EGCG | Epigallocatechin Gallate |
| DHFR | Dihydrofolate Reductase |
| THF | Tetrahydrofolate |
| ROS | Reactive Oxygen Species |
| QS | Quorum Sensing |
| ESM | Extracellular Signaling Molecules |
| AIs | Auto-Inducers |
| EPS | Extracellular Polymeric Substance |
| Quorum Quenching | |
| AHL | Acyl-homoserine lactones |
| ICMSF | International Commission on Microbiological Specifications for Foods |
| List of microorganisms and their abbreviations | |
| A. baumannii | Acinetobacter baumannii |
| B. cereus | Bacillus cereus |
| B. pumilus | Bacillus pumilus |
| B. subtilis | Bacillus subtilis |
| C. albicans | Candida albicans |
| E. asburiae | Enterobacter asburiae |
| E. coli | Escherichia coli |
| E. faecalis | Enterococcus faecalis |
| K. pneumoniae | Klebsiella pneumoniae |
| L. innocua | Listeria innocua |
| L. monocytogenes | Listeria monocytogenes |
| M. luteus | Micrococcus luteus |
| M.R.S. aureus | Methicillin-Resistant Staphylococcus aureus (MRSA) |
| P. aeruginosa | Pseudomonas aeruginosa |
| P. mirabilis | Proteus mirabilis |
| S. aureus | Staphylococcus aureus |
| S. enterica | Salmonella enterica |
| S. enterica sv. Typhimurium | Salmonella enterica serovar Typhimurium |
| S. Enteritidis | Salmonella Enteritidis |
| S. Typhimurium | Salmonella Typhimurium (synonym for S. enterica sv. Typhimurium) |
| V. parahaemolyticus | Vibrio parahaemolyticus |
| Y. enterocolitica | Yersinia enterocolitica |
References
- Murray, C.J.; Ikuta, K.S.; Sharara, F.; Swetschinski, L.; Robles Aguilar, G.; Gray, A.; Han, C.; Bisignano, C.; Rao, P.; Wool, E.; et al. Global Burden of Bacterial Antimicrobial Resistance in 2019: A Systematic Analysis. Lancet 2022, 399, 629–655. [Google Scholar] [CrossRef]
- Drug-Resistant Infections: A Threat to Our Economic Future. Available online: https://www.worldbank.org/en/topic/health/publication/drug-resistant-infections-a-threat-to-our-economic-future (accessed on 5 October 2025).
- Chauhan, K.; Rao, A. Clean-Label Alternatives for Food Preservation: An Emerging Trend. Heliyon 2024, 10, e35815. [Google Scholar] [CrossRef]
- Pinto, L.; Tapia-Rodríguez, M.R.; Baruzzi, F.; Ayala-Zavala, J.F. Plant Antimicrobials for Food Quality and Safety: Recent Views and Future Challenges. Foods 2023, 12, 2315. [Google Scholar] [CrossRef]
- Matei, A.T.; Visan, A.I. Mechanism, Efficacy, and Safety of Natural Antibiotics. Antibiotics 2025, 14, 981. [Google Scholar] [CrossRef]
- Duda-Madej, A.; Viscardi, S.; Niezgódka, P.; Szewczyk, W.; Wińska, K. The Impact of Plant-Derived Polyphenols on Combating Efflux-Mediated Antibiotic Resistance. Int. J. Mol. Sci. 2025, 26, 4030. [Google Scholar] [CrossRef] [PubMed]
- De Rossi, L.; Rocchetti, G.; Lucini, L.; Rebecchi, A. Antimicrobial Potential of Polyphenols: Mechanisms of Action and Microbial Responses—A Narrative Review. Antioxidants 2025, 14, 200. [Google Scholar] [CrossRef]
- Wang, L.; Li, T.; Wu, C.; Fan, G.; Zhou, D.; Li, X. Unlocking the Potential of Plant Polyphenols: Advances in Extraction, Antibacterial Mechanisms, and Future Applications. Food Sci. Biotechnol. 2025, 34, 1235–1259. [Google Scholar] [CrossRef] [PubMed]
- Panja, P. Green Extraction Methods of Food Polyphenols from Vegetable Materials. Curr. Opin. Food Sci. 2018, 23, 173–182. [Google Scholar] [CrossRef]
- Kohnen-Johannsen, K.L.; Kayser, O. Tropane Alkaloids: Chemistry, Pharmacology, Biosynthesis and Production. Molecules 2019, 24, 796. [Google Scholar] [CrossRef]
- Bolt, H.M.; Hengstler, J.G. Ricin: An Ancient Toxicant, but Still an Evergreen. Arch. Toxicol. 2023, 97, 909–911. [Google Scholar] [CrossRef]
- Nabi, M.H.B.; Ahmed, M.M.; Mia, M.S.; Islam, S.; Zzaman, W. Essential Oils: Advances in Extraction Techniques, Chemical Composition, Bioactivities, and Emerging Applications. Food Chem. Adv. 2025, 8, 101048. [Google Scholar] [CrossRef]
- Soulaimani, B. Comprehensive Review of the Combined Antimicrobial Activity of Essential Oil Mixtures and Synergism with Conventional Antimicrobials. Nat. Prod. Commun. 2025, 20, 1934578X251328241. [Google Scholar] [CrossRef]
- da Silva, B.D.; Bernardes, P.C.; Pinheiro, P.F.; Fantuzzi, E.; Roberto, C.D. Chemical Composition, Extraction Sources and Action Mechanisms of Essential Oils: Natural Preservative and Limitations of Use in Meat Products. Meat Sci. 2021, 176, 108463. [Google Scholar] [CrossRef] [PubMed]
- Wińska, K.; Mączka, W.; Łyczko, J.; Grabarczyk, M.; Czubaszek, A.; Szumny, A. Essential Oils as Antimicrobial Agents—Myth or Real Alternative? Molecules 2019, 24, 2130. [Google Scholar] [CrossRef]
- Twaij, B.M.; Hasan, M.N. Bioactive Secondary Metabolites from Plant Sources: Types, Synthesis, and Their Therapeutic Uses. Int. J. Plant Biol. 2022, 13, 4–14. [Google Scholar] [CrossRef]
- Reshi, Z.A.; Ahmad, W.; Lukatkin, A.S.; Javed, S.B. From Nature to Lab: A Review of Secondary Metabolite Biosynthetic Pathways, Environmental Influences, and In Vitro Approaches. Metabolites 2023, 13, 895. [Google Scholar] [CrossRef]
- Edo, G.I.; Nwachukwu, S.C.; Ali, A.B.M.; Yousif, E.; Jikah, A.N.; Zainulabdeen, K.; Ekokotu, H.A.; Isoje, E.F.; Igbuku, U.A.; Opiti, R.A.; et al. A Review on the Composition, Extraction and Applications of Phenolic Compounds. Ecol. Front. 2025, 45, 7–23. [Google Scholar] [CrossRef]
- Mark, R.; Lyu, X.; Lee, J.J.L.; Parra-Saldívar, R.; Chen, W.N. Sustainable Production of Natural Phenolics for Functional Food Applications. J. Funct. Foods 2019, 57, 233–254. [Google Scholar] [CrossRef]
- Albuquerque, B.R.; Heleno, S.A.; Oliveira, M.B.P.P.; Barros, L.; Ferreira, I.C.F.R. Phenolic Compounds: Current Industrial Applications, Limitations and Future Challenges. Food Funct. 2021, 12, 14–29. [Google Scholar] [CrossRef]
- Das, A.K.; Islam, M.N.; Faruk, M.O.; Ashaduzzaman, M.; Dungani, R. Review on Tannins: Extraction Processes, Applications and Possibilities. S. Afr. J. Bot. 2020, 135, 58–70. [Google Scholar] [CrossRef]
- Masyita, A.; Mustika Sari, R.; Dwi Astuti, A.; Yasir, B.; Rahma Rumata, N.; Emran, T.B.; Nainu, F.; Simal-Gandara, J. Terpenes and Terpenoids as Main Bioactive Compounds of Essential Oils, Their Roles in Human Health and Potential Application as Natural Food Preservatives. Food Chem. X 2022, 13, 100217. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.; Wang, Y.; Tian, W.; Cui, X.; Tu, P.; Li, J.; Shi, S.; Liu, X. Biosynthesis Investigations of Terpenoid, Alkaloid, and Flavonoid Antimicrobial Agents Derived from Medicinal Plants. Antibiotics 2022, 11, 1380. [Google Scholar] [CrossRef] [PubMed]
- Bilal, M.; Iqbal, H.M.N. Biologically Active Macromolecules: Extraction Strategies, Therapeutic Potential and Biomedical Perspective. Int. J. Biol. Macromol. 2020, 151, 1–18. [Google Scholar] [CrossRef]
- da Silva, R.F.; Carneiro, C.N.; Cheila, C.B.; Gomez, F.J.V.; Espino, M.; Boiteux, J.; de los Á. Fernández, M.; Silva, M.F.; de S. Dias, F. Sustainable Extraction Bioactive Compounds Procedures in Medicinal Plants Based on the Principles of Green Analytical Chemistry: A Review. Microchem. J. 2022, 175, 107184. [Google Scholar] [CrossRef]
- Bucar, F.; Wube, A.; Schmid, M. Natural Product Isolation-How to Get from Biological Material to Pure Compounds. Nat. Prod. Rep. 2013, 30, 525–545. [Google Scholar] [CrossRef] [PubMed]
- Alara, O.R.; Abdurahman, N.H.; Ukaegbu, C.I. Extraction of Phenolic Compounds: A Review. Curr. Res. Food. Sci. 2021, 4, 200–214. [Google Scholar] [CrossRef]
- Das, S.; Nadar, S.S.; Rathod, V.K. Integrated Strategies for Enzyme Assisted Extraction of Bioactive Molecules: A Review. Int. J. Biol. Macromol. 2021, 191, 899–917. [Google Scholar] [CrossRef] [PubMed]
- Mungwari, C.P.; King’ondu, C.K.; Sigauke, P.; Obadele, B.A. Conventional and Modern Techniques for Bioactive Compounds Recovery from Plants: Review. Sci. Afr. 2025, 27, e02509. [Google Scholar] [CrossRef]
- Welton, T. Solvents and Sustainable Chemistry. Proc. R. Soc. A Math. Phys. Eng. Sci. 2015, 471, 502. [Google Scholar] [CrossRef]
- Gomez, F.J.V.; Espino, M.; Fernández, M.A.; Silva, M.F. A Greener Approach to Prepare Natural Deep Eutectic Solvents. ChemistrySelect 2018, 3, 6122–6125. [Google Scholar] [CrossRef]
- Werner, S.; Haumann, M.; Wasserscheid, P. Ionic Liquids in Chemical Engineering. Annu. Rev. Chem. Biomol. Eng. 2010, 1, 203–230. [Google Scholar] [CrossRef] [PubMed]
- Abbott, A.P.; Boothby, D.; Capper, G.; Davies, D.L.; Rasheed, R.K. Deep Eutectic Solvents Formed between Choline Chloride and Carboxylic Acids: Versatile Alternatives to Ionic Liquids. J. Am. Chem. Soc. 2004, 126, 9142–9147. [Google Scholar] [CrossRef]
- Ruesgas-Ramón, M.; Figueroa-Espinoza, M.C.; Durand, E. Application of Deep Eutectic Solvents (DES) for Phenolic Compounds Extraction: Overview, Challenges, and Opportunities. J. Agric. Food. Chem. 2017, 65, 3591–3601. [Google Scholar] [CrossRef] [PubMed]
- Płotka-Wasylka, J.; de la Guardia, M.; Andruch, V.; Vilková, M. Deep Eutectic Solvents vs Ionic Liquids: Similarities and Differences. Microchem. J. 2020, 159, 105539. [Google Scholar] [CrossRef]
- Choi, Y.H.; van Spronsen, J.; Dai, Y.; Verberne, M.; Hollmann, F.; Arends, I.W.C.E.; Witkamp, G.J.; Verpoorte, R. Are Natural Deep Eutectic Solvents the Missing Link in Understanding Cellular Metabolism and Physiology? Plant Physiol. 2011, 156, 1701–1705. [Google Scholar] [CrossRef]
- Radošević, K.; Čanak, I.; Panić, M.; Markov, K.; Bubalo, M.C.; Frece, J.; Srček, V.G.; Redovniković, I.R. Antimicrobial, Cytotoxic and Antioxidative Evaluation of Natural Deep Eutectic Solvents. Environ. Sci. Pollut. Res. 2018, 25, 14188–14196. [Google Scholar] [CrossRef]
- Emami, S.; Shayanfar, A. Deep Eutectic Solvents for Pharmaceutical Formulation and Drug Delivery Applications. Pharm. Dev. Technol. 2020, 25, 779–796. [Google Scholar] [CrossRef]
- Liu, Y.; Friesen, J.B.; McAlpine, J.B.; Lankin, D.C.; Chen, S.N.; Pauli, G.F. Natural Deep Eutectic Solvents: Properties, Applications, and Perspectives. J. Nat. Prod. 2018, 81, 679–690. [Google Scholar] [CrossRef]
- Shen, L.; Pang, S.; Zhong, M.; Sun, Y.; Qayum, A.; Liu, Y.; Rashid, A.; Xu, B.; Liang, Q.; Ma, H.; et al. A Comprehensive Review of Ultrasonic Assisted Extraction (UAE) for Bioactive Components: Principles, Advantages, Equipment, and Combined Technologies. Ultrason. Sonochem. 2023, 101, 106646. [Google Scholar] [CrossRef]
- Kumar, K.; Srivastav, S.; Sharanagat, V.S. Ultrasound Assisted Extraction (UAE) of Bioactive Compounds from Fruit and Vegetable Processing by-Products: A Review. Ultrason. Sonochem. 2021, 70, 105325. [Google Scholar] [CrossRef] [PubMed]
- Mehta, N.; S, J.; Kumar, P.; Verma, A.K.; Umaraw, P.; Khatkar, S.K.; Khatkar, A.B.; Pathak, D.; Kaka, U.; Sazili, A.Q. Ultrasound-Assisted Extraction and the Encapsulation of Bioactive Components for Food Applications. Foods 2022, 11, 2973. [Google Scholar] [CrossRef]
- Yusoff, I.M.; Mat Taher, Z.; Rahmat, Z.; Chua, L.S. A Review of Ultrasound-Assisted Extraction for Plant Bioactive Compounds: Phenolics, Flavonoids, Thymols, Saponins and Proteins. Food Res. Int. 2022, 157, 111268. [Google Scholar] [CrossRef]
- Lopez-Avila, V.; Luque de Castro, M.D. Microwave-Assisted Extraction. Ref. Modul. Chem. Mol. Sci. Chem. Eng. 2014, 1389–1398. [Google Scholar] [CrossRef]
- Sadeghi, A.; Hakimzadeh, V.; Karimifar, B. Microwave Assisted Extraction of Bioactive Compounds from Food: A Review. Int. J. Food Sci. Nutr. Eng 2017, 7, 19–27. [Google Scholar] [CrossRef]
- Veggi, P.C.; Martinez, J.; Meireles, M.A.A. Fundamentals of microwave extraction. In Microwave-Assisted Extraction for Bioactive Compounds: Theory and Practice; Chemat, F., Cravotto, G., Eds.; Springer: Boston, MA, USA, 2012; pp. 15–52. [Google Scholar]
- Destandau, E.; Michel, T.; Elfakir, C. Natural product extraction. In Natural Product Extraction: Principles and Applications; Rostagno, M.A., Prado, J.M., Eds.; The Royal Society of Chemistry: London, UK, 2013; pp. 113–156. [Google Scholar]
- Vovk, H.; Karnpakdee, K.; Ludwig, R.; Nosenko, T. Enzymatic Pretreatment of Plant Cells for Oil Extraction. Food Technol. Biotechnol. 2023, 61, 160–178. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Le Bourvellec, C.; Renard, C.M.G.C. Interactions between Cell Wall Polysaccharides and Polyphenols: Effect of Molecular Internal Structure. Compr. Rev. Food Sci. Food Saf. 2020, 19, 3574–3617. [Google Scholar] [CrossRef]
- Iacono, R.; Slavov, G.T.; Davey, C.L.; Clifton-Brown, J.; Allison, G.; Bosch, M. Variability of Cell Wall Recalcitrance and Composition in Genotypes of Miscanthus from Different Genetic Groups and Geographical Origin. Front. Plant. Sci. 2023, 14, 1155188. [Google Scholar] [CrossRef]
- Puri, M.; Sharma, D.; Barrow, C.J. Enzyme-Assisted Extraction of Bioactives from Plants. Trends Biotechnol. 2012, 30, 37–44. [Google Scholar] [CrossRef]
- Uwineza, P.A.; Waśkiewicz, A. Recent Advances in Supercritical Fluid Extraction of Natural Bioactive Compounds from Natural Plant Materials. Molecules 2020, 25, 3847. [Google Scholar] [CrossRef]
- Wrona, O.; Rafińska, K.; Możeński, C.; Buszewski, B. Supercritical Fluid Extraction of Bioactive Compounds from Plant Materials. J. AOAC Int. 2017, 100, 1624–1635. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.W.; Hsu, C.P.; Yang, B.B.; Wang, C.Y. Advances in the Extraction of Natural Ingredients by High Pressure Extraction Technology. Trends Food Sci. Technol. 2013, 33, 54–62. [Google Scholar] [CrossRef]
- Grassino, A.N.; Pedisić, S.; Dragović-Uzelac, V.; Karlović, S.; Ježek, D.; Bosiljkov, T. Insight into High-Hydrostatic Pressure Extraction of Polyphenols from Tomato Peel Waste. Plant Foods Hum. Nutr. 2020, 75, 427–433. [Google Scholar] [CrossRef]
- Khan, S.A.; Aslam, R.; Makroo, H.A. High Pressure Extraction and Its Application in the Extraction of Bio-Active Compounds: A Review. J. Food Process. Eng. 2019, 42, e12896. [Google Scholar] [CrossRef]
- Galanakis, C.M.; Goulas, V.; Tsakona, S.; Manganaris, G.A.; Gekas, V. A Knowledge Base for the Recovery of Natural Phenols with Different Solvents. Int. J. Food Prop. 2013, 16, 382–396. [Google Scholar] [CrossRef]
- Jiang, Z.; Kempinski, C.; Chappell, J. Extraction and Analysis of Terpenes/Terpenoids. Curr. Protoc. Plant Biol. 2016, 1, 345–358. [Google Scholar] [CrossRef]
- Zimmermann, J.; Chiaberge, S.; Iversen, S.B.; Raffelt, K.; Dahmen, N. Sequential Extraction and Characterization of Nitrogen Compounds after Hydrothermal Liquefaction of Sewage Sludge. Energy Fuels 2022, 36, 14292–14303. [Google Scholar] [CrossRef] [PubMed]
- Oro, C.E.D.; Wancura, J.H.C.; Santos, M.S.N.d.; Venquiaruto, L.D.; Dallago, R.M.; Tres, M.V. High-Pressure Extraction Techniques for Efficient Recovery of Flavonoids and Coumarins from Flower Seeds. Processes 2025, 13, 300. [Google Scholar] [CrossRef]
- Carpentieri, S.; Soltanipour, F.; Ferrari, G.; Pataro, G.; Donsì, F. Emerging Green Techniques for the Extraction of Antioxidants from Agri-Food By-Products as Promising Ingredients for the Food Industry. Antioxidants 2021, 10, 1417. [Google Scholar] [CrossRef]
- Alvarez-Yanamango, E.; Obregon, D.; Ibañez, A. A Comprehensive Review of the Development of Green Extraction Methods and Encapsulation of Theobromine from Cocoa Bean Shells for Nutraceutical Applications. Food Eng. Rev. 2025, 1–22. [Google Scholar] [CrossRef]
- Chemat, F.; Vian, M.A.; Cravotto, G. Green Extraction of Natural Products: Concept and Principles. Int. J. Mol. Sci. 2012, 13, 8615–8627. [Google Scholar] [CrossRef]
- Gonelimali, F.D.; Lin, J.; Miao, W.; Xuan, J.; Charles, F.; Chen, M.; Hatab, S.R. Antimicrobial Properties and Mechanism of Action of Some Plant Extracts against Food Pathogens and Spoilage Microorganisms. Front. Microbiol. 2018, 9, 1639. [Google Scholar] [CrossRef]
- Hossain, T.J. Methods for Screening and Evaluation of Antimicrobial Activity: A Review of Protocols, Advantages, and Limitations. Eur. J. Microbiol. Immunol. 2024, 14, 97–115. [Google Scholar] [CrossRef]
- Balouiri, M.; Sadiki, M.; Ibnsouda, S.K. Methods for in Vitro Evaluating Antimicrobial Activity: A Review. J. Pharm. Anal. 2016, 6, 71–79. [Google Scholar] [CrossRef]
- Bubonja-Šonje, M.; Knezević, S.; Abram, M. Challenges to Antimicrobial Susceptibility Testing of Plant-Derived Polyphenolic Compounds. Arh. Hig. Rada. Toksikol. 2020, 71, 300–311. [Google Scholar] [CrossRef]
- Elshikh, M.; Ahmed, S.; Funston, S.; Dunlop, P.; McGaw, M.; Marchant, R.; Banat, I.M. Resazurin-Based 96-Well Plate Microdilution Method for the Determination of Minimum Inhibitory Concentration of Biosurfactants. Biotechnol. Lett. 2016, 38, 1015–1019. [Google Scholar] [CrossRef]
- Wiegand, I.; Hilpert, K.; Hancock, R.E.W. Agar and Broth Dilution Methods to Determine the Minimal Inhibitory Concentration (MIC) of Antimicrobial Substances. Nat. Protoc. 2008, 3, 163–175. [Google Scholar] [CrossRef] [PubMed]
- Barnes, V.L.; Heithoff, D.M.; Mahan, S.P.; House, J.K.; Mahan, M.J. Antimicrobial Susceptibility Testing to Evaluate Minimum Inhibitory Concentration Values of Clinically Relevant Antibiotics. STAR Protoc. 2023, 4, 102512. [Google Scholar] [CrossRef]
- Hulankova, R. Methods for Determination of Antimicrobial Activity of Essential Oils In Vitro—A Review. Plants 2024, 13, 2784. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Pastor, R.; Carrera-Pacheco, S.E.; Zúñiga-Miranda, J.; Rodríguez-Pólit, C.; Mayorga-Ramos, A.; Guamán, L.P.; Barba-Ostria, C. Current Landscape of Methods to Evaluate Antimicrobial Activity of Natural Extracts. Molecules 2023, 28, 1068. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Zhang, Y.; Wang, R.; Wang, Z.; Yang, B.; Kuang, H. An Evolving Technology That Integrates Classical Methods with Continuous Technological Developments: Thin-Layer Chromatography Bioautography. Molecules 2021, 26, 4647. [Google Scholar] [CrossRef]
- O’Toole, G.A. Microtiter Dish Biofilm Formation Assay. J. Vis. Exp. 2010, 47, 2437. [Google Scholar] [CrossRef]
- Diouchi, J.; Marinković, J.; Nemoda, M.; El Rhaffari, L.; Toure, B.; Ghoul, S. In Vitro Methods for Assessing the Antibacterial and Antibiofilm Properties of Essential Oils as Potential Root Canal Irrigants—A Simplified Description of the Technical Steps. Methods Protoc. 2024, 7, 50. [Google Scholar] [CrossRef]
- Qian, W.; Wang, W.; Zhang, J.; Wang, T.; Liu, M.; Yang, M.; Sun, Z.; Li, X.; Li, Y. Antimicrobial and Antibiofilm Activities of Ursolic Acid against Carbapenem-Resistant Klebsiella Pneumoniae. J. Antibiot. 2020, 73, 382–391. [Google Scholar] [CrossRef] [PubMed]
- Ton, T.P.; Bright, R.; Truong, V.K.; Vasilev, K. Fluorescent Antibiotics: Bridging Diagnostic and Therapy in the Fight against Bacterial Infections. Small Sci. 2025, 5, 2500138. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Wang, Y.; Zhou, J.; Wang, J.; Zhang, M.; Chen, H. Structural Characterization, Cytotoxicity, and the Antifungal Mechanism of a Novel Peptide Extracted from Garlic (Allium sativa L.). Molecules 2023, 28, 3098. [Google Scholar] [CrossRef]
- Nuding, S.; Fellermann, K.; Wehkamp, J.; Mueller, H.A.G.; Stange, E.F. A Flow Cytometric Assay to Monitor Antimicrobial Activity of Defensins and Cationic Tissue Extracts. J. Microbiol. Methods 2006, 65, 335–345. [Google Scholar] [CrossRef] [PubMed]
- Diez-Gonzalez, F. Total Viable Counts: Specific Techniques. Encycl. Food Microbiol. Second. Ed. 2014, 630–635. [Google Scholar] [CrossRef]
- Ratajczak, K.; Piotrowska-Cyplik, A.; Cyplik, P. Analysis of the Effect of Various Potential Antimicrobial Agents on the Quality of the Unpasteurized Carrot Juice. Molecules 2023, 28, 6297. [Google Scholar] [CrossRef]
- U.S. Food and Drug Administration. Guidelines for the Validation of Microbiological Methods for the FDA Foods Program, 3rd ed.; FDA: Silver Spring, MD, USA, 2019. Available online: https://www.fda.gov/media/83812/download (accessed on 15 September 2025).
- Health Protection Agency. Guidelines for assessing the microbiological safety of ready-to-eat foods placed on the market. Hyg. Indic. Org. 2009, 3, 12. [Google Scholar]
- Ge, B.; McDonald, R.C.; Yang, Q.; Domesle, K.J.; Sarria, S.; Li, X.; Hsu, C.H.; Jarvis, K.G.; Tadesse, D.A. Exploring Animal Food Microbiomes and Resistomes via 16S RRNA Gene Amplicon Sequencing and Shotgun Metagenomics. Appl. Environ. Microbiol. 2025, 91, e02230-24. [Google Scholar] [CrossRef]
- Tian, Y.; Puganen, A.; Alakomi, H.L.; Uusitupa, A.; Saarela, M.; Yang, B. Antioxidative and Antibacterial Activities of Aqueous Ethanol Extracts of Berries, Leaves, and Branches of Berry Plants. Food Res. Int. 2018, 106, 291–303. [Google Scholar] [CrossRef]
- Criste, A.; Urcan, A.C.; Bunea, A.; Furtuna, F.R.P.; Olah, N.K.; Madden, R.H.; Corcionivoschi, N. Phytochemical Composition and Biological Activity of Berries and Leaves from Four Romanian Sea Buckthorn (Hippophae rhamnoides L.) Varieties. Molecules 2020, 25, 1170. [Google Scholar] [CrossRef]
- Rashidaie Abandansarie, S.S.; Ariaii, P.; Charmchian Langerodi, M. Effects of Encapsulated Rosemary Extract on Oxidative and Microbiological Stability of Beef Meat during Refrigerated Storage. Food Sci. Nutr. 2019, 7, 3969–3978. [Google Scholar] [CrossRef] [PubMed]
- Urbanavičiūte, I.; Liaudanskas, M.; Bobinas, Č.; Šarkinas, A.; Rezgiene, A.; Viskelis, P. Japanese Quince (Chaenomeles japonica) as a Potential Source of Phenols: Optimization of the Extraction Parameters and Assessment of Antiradical and Antimicrobial Activities. Foods 2020, 9, 132. [Google Scholar] [CrossRef] [PubMed]
- Oyedemi, S.O.; Oyedemi, B.O.; Prieto, J.M.; Coopoosamy, R.M.; Stapleton, P.; Gibbons, S. In Vitro Assessment of Antibiotic-Resistance Reversal of a Methanol Extract from Rosa canina L. S. Afr. J. Bot. 2016, 105, 337–342. [Google Scholar] [CrossRef]
- Peršurić, Ž.; Martinović, L.S.; Malenica, M.; Gobin, I.; Pedisić, S.; Dragović-Uzelac, V.; Pavelić, S.K. Assessment of the Biological Activity and Phenolic Composition of Ethanol Extracts of Pomegranate (Punica granatum L.) Peels. Molecules 2020, 25, 5916. [Google Scholar] [CrossRef]
- Grillo, G.; Capaldi, G.; Radošević, K.; Jakopović, Ž.; Markov, K.; Brncic, M.; Gallina, L.; Calcio Gaudino, E.; Cravotto, G. Unlocking the Bioactive Potential of Pomegranate Peels: A Green Extraction Approach. Antioxidants 2023, 12, 1796. [Google Scholar] [CrossRef]
- Abutayeh, R.F.; Ayyash, M.A.K.; Alwany, R.A.; Abuodeh, A.; Jaber, K.; Al-Najjar, M.A.A. Exploring the Antimicrobial Potential of Pomegranate Peel Extracts (PPEs): Extraction Techniques and Bacterial Susceptibility. PLoS ONE 2024, 19, e0315173. [Google Scholar] [CrossRef]
- Singh, T.; Shafi, Z.; Singh, R.; Bisht, B.; Yadav, K.K.; Algethami, J.S.; Albakri, G.S.; Alreshidi, M.A. Microwave-Assisted Extraction of Phytochemicals from Piper betle L.: Optimization, Characterization, and Bioactivity Evaluation. Food Chem. X 2025, 29, 102672. [Google Scholar] [CrossRef] [PubMed]
- Bodea, I.M.; Cătunescu, G.M.; David, A.P.; Vidican, R.; Pop, C.R.; Stănilă, A.; Rotar, A.M.; Palop Gómez, A. Optimization of Microwave Assisted Extraction of Bioactive Compounds in Oregano and Lovage Ethanolic Extracts. LWT 2024, 212, 116973. [Google Scholar] [CrossRef]
- Ürgeová, E.; Uváčková, Ľ.; Vaneková, M.; Maliar, T. Antibacterial Potential of Microwave-Assisted Extraction Prepared Hydrolates from Different Salvia Species. Plants 2023, 12, 1325. [Google Scholar] [CrossRef]
- Sánchez-Gutiérrez, M.; Bascón-Villegas, I.; Rodríguez, A.; Pérez-Rodríguez, F.; Fernández-Prior, Á.; Rosal, A.; Carrasco, E. Article Valorisation of Olea Europaea l. Olive Leaves through the Evaluation of Their Extracts: Antioxidant and Antimicrobial Activity. Foods 2021, 10, 966. [Google Scholar] [CrossRef]
- Januskevice, V.; Gomes, A.M.; Sousa, S.; Barbosa, J.C.; Vedor, R.; Martusevice, P.; Liaudanskas, M.; Zvikas, V.; Viskelis, P.; Cesoniene, L.; et al. Phytochemical and Functional Diversity of Enzyme-Assisted Extracts from Hippophae Rhamnoides L., Aralia Cordata Thunb, and Cannabis Sativa L. Antioxidants 2024, 13, 950. [Google Scholar] [CrossRef]
- Lemoni, Z.; Kalantzi, S.; Lymperopoulou, T.; Tzani, A.; Stavropoulos, G.; Detsi, A.; Mamma, D. Kinetic Modeling and Biological Activities of Rosa canina L. Pseudo-Fruit Extracts Obtained via Enzyme-Assisted Extraction. Antioxidants 2025, 14, 588. [Google Scholar] [CrossRef]
- Lemoni, Z.; Kalantzi, S.; Lymperopoulou, T.; Tzani, A.; Stavropoulos, G.; Detsi, A.; Mamma, D. Optimization of Bioactive Compounds Extraction from Rosa canina L. Pseudofruit through the Action of Two Hydrolytic Enzyme Preparations. J. Chem. Technol. Biotechnol. 2025. [Google Scholar] [CrossRef]
- Alexandre, E.M.C.; Silva, S.; Santos, S.A.O.; Silvestre, A.J.D.; Duarte, M.F.; Saraiva, J.A.; Pintado, M. Antimicrobial Activity of Pomegranate Peel Extracts Performed by High Pressure and Enzymatic Assisted Extraction. Food Res. Int. 2019, 2019. 115, 167–176. [Google Scholar] [CrossRef] [PubMed]
- Žitek, T.; Borjan, D.; Golle, A.; Knez, Ž.; Knez, M. Optimization of Extraction of Phenolic Compounds with Antimicrobial Properties from Origanum Vulgare. Processes 2021, 9, 1032. [Google Scholar] [CrossRef]
- Jurić, T.; Mićić, N.; Potkonjak, A.; Milanov, D.; Dodić, J.; Trivunović, Z.; Popović, B.M. The Evaluation of Phenolic Content, in Vitro Antioxidant and Antibacterial Activity of Mentha Piperita Extracts Obtained by Natural Deep Eutectic Solvents. Food Chem. 2021, 362, 130226. [Google Scholar] [CrossRef] [PubMed]
- Memdueva, N.; Tzanova, M.; Yaneva, Z.; Rusenova, N.; Grozeva, N.; Dinev, T. Natural Deep Eutectic Solvent-Based Extraction of Malva Sylvestris L.: Phytochemical Content, Antioxidant and Antimicrobial Potential. Separations 2025, 12, 187. [Google Scholar] [CrossRef]
- Silhavy, T.J.; Kahne, D.; Walker, S. The bacterial cell envelope. Cold Spring Harb. Perspect. Biol. 2010, 2, a000414. [Google Scholar] [CrossRef]
- Breijyeh, Z.; Jubeh, B.; Karaman, R. Resistance of Gram-Negative Bacteria to Current Antibacterial Agents and Approaches to Resolve It. Molecules 2020, 25, 1340. [Google Scholar] [CrossRef] [PubMed]
- Uddin, T.M.; Chakraborty, A.J.; Khusro, A.; Zidan, B.R.M.; Mitra, S.; Emran, T.B.; Dhama, K.; Ripon, M.K.H.; Gajdács, M.; Sahibzada, M.U.K.; et al. Antibiotic Resistance in Microbes: History, Mechanisms, Therapeutic Strategies and Future Prospects. J. Infect. Public Health 2021, 14, 1750–1766. [Google Scholar] [CrossRef] [PubMed]
- Rajput, P.; Nahar, K.S.; Rahman, K.M. Evaluation of Antibiotic Resistance Mechanisms in Gram-Positive Bacteria. Antibiotics 2024, 13, 1197. [Google Scholar] [CrossRef] [PubMed]
- Sweet, R.; Kroon, P.A.; Webber, M.A. Activity of Antibacterial Phytochemicals and Their Potential Use as Natural Food Preservatives. Crit. Rev. Food Sci. Nutr. 2024, 64, 2076–2087. [Google Scholar] [CrossRef]
- Lobiuc, A.; Pavăl, N.E.; Mangalagiu, I.I.; Gheorghiță, R.; Teliban, G.C.; Amăriucăi-Mantu, D.; Stoleru, V. Future Antimicrobials: Natural and Functionalized Phenolics. Molecules 2023, 28, 1114. [Google Scholar] [CrossRef]
- Chen, X.; Lan, W.; Xie, J. Natural Phenolic Compounds: Antimicrobial Properties, Antimicrobial Mechanisms, and Potential Utilization in the Preservation of Aquatic Products. Food Chem. 2024, 440, 138198. [Google Scholar] [CrossRef]
- Mickymaray, S. Efficacy and Mechanism of Traditional Medicinal Plants and Bioactive Compounds against Clinically Important Pathogens. Antibiotics 2019, 8, 257. [Google Scholar] [CrossRef]
- Gradišar, H.; Pristovšek, P.; Plaper, A.; Jerala, R. Green Tea Catechins Inhibit Bacterial DNA Gyrase by Interaction with Its ATP Binding Site. J. Med. Chem. 2007, 50, 264–271. [Google Scholar] [CrossRef]
- Khan, T.; Sankhe, K.; Suvarna, V.; Sherje, A.; Patel, K.; Dravyakar, B. DNA Gyrase Inhibitors: Progress and Synthesis of Potent Compounds as Antibacterial Agents. Biomed. Pharmacother. 2018, 103, 923–938. [Google Scholar] [CrossRef]
- Sánchez-del-Campo, L.; Sáez-Ayala, M.; Chazarra, S.; Cabezas-Herrera, J.; Rodríguez-López, J.N. Binding of Natural and Synthetic Polyphenols to Human Dihydrofolate Reductase. Int. J. Mol. Sci. 2009, 10, 5398–5410. [Google Scholar] [CrossRef]
- Navarro-Perán, E.; Cabezas-Herrera, J.; García-Cánovas, F.; Durrant, M.C.; Thorneley, R.N.F.; Neptuno Rodríguez-López, J. The Antifolate Activity of Tea Catechins. Cancer Res. 2005, 65, 2059–2064. [Google Scholar] [CrossRef]
- Aslan, E.; Adem, S. Investigation of the Effects of Some Drugs and Phenolic Compounds on Human Dihydrofolate Reductase Activity. J. Biochem. Mol. Toxicol. 2015, 29, 135–139. [Google Scholar] [CrossRef]
- Dembińska, K.; Shinde, A.H.; Pejchalová, M.; Richert, A.; Swiontek Brzezinska, M. The Application of Natural Phenolic Substances as Antimicrobial Agents in Agriculture and Food Industry. Foods 2025, 14, 1893. [Google Scholar] [CrossRef]
- Alfei, S.; Schito, G.C.; Schito, A.M.; Zuccari, G. Reactive Oxygen Species (ROS)-Mediated Antibacterial Oxidative Therapies: Available Methods to Generate ROS and a Novel Option Proposal. Int. J. Mol. Sci. 2024, 25, 7182. [Google Scholar] [CrossRef]
- Mourenza, Á.; Gil, J.A.; Mateos, L.M.; Letek, M. Oxidative Stress-Generating Antimicrobials, a Novel Strategy to Overcome Antibacterial Resistance. Antioxidants 2020, 9, 361. [Google Scholar] [CrossRef]
- Block, E. The Organosulfur Chemistry of the Genus Allium—Implications for the Organic Chemistry of Sulfur. Angew. Chem. Int. Ed. Engl. 1992, 31, 1135–1178. [Google Scholar] [CrossRef]
- Olivito, F.; Amodio, N.; Di Gioia, M.L.; Nardi, M.; Oliverio, M.; Juli, G.; Tassone, P.; Procopio, A. Synthesis and Preliminary Evaluation of the Anti-Cancer Activity on A549 Lung Cancer Cells of a Series of Unsaturated Disulfides. MedChemComm 2019, 10, 116–119. [Google Scholar] [CrossRef] [PubMed]
- Bastaki, S.M.A.; Ojha, S.; Kalasz, H.; Adeghate, E. Chemical constituents and medicinal properties of Allium species. Mol. Cell. Biochem. 2021, 476, 4301–4321. [Google Scholar] [CrossRef] [PubMed]
- Sathiya Deepika, M.; Thangam, R.; Sakthidhasan, P.; Arun, S.; Sivasubramanian, S.; Thirumurugan, R. Combined Effect of a Natural Flavonoid Rutin from Citrus Sinensis and Conventional Antibiotic Gentamicin on Pseudomonas Aeruginosa Biofilm Formation. Food Control 2018, 90, 282–294. [Google Scholar] [CrossRef]
- Jomova, K.; Alomar, S.Y.; Valko, R.; Liska, J.; Nepovimova, E.; Kuca, K.; Valko, M. Flavonoids and Their Role in Oxidative Stress, Inflammation, and Human Diseases. Chem. Biol. Interact. 2025, 413, 111489. [Google Scholar] [CrossRef]
- Ecevit, K.; Barros, A.A.; Silva, J.M.; Reis, R.L. Preventing Microbial Infections with Natural Phenolic Compounds. Fut. Pharmacol. 2022, 2, 460–498. [Google Scholar] [CrossRef]
- Hetta, H.F.; Ramadan, Y.N.; Rashed, Z.I.; Alharbi, A.A.; Alsharef, S.; Alkindy, T.T.; Alkhamali, A.; Albalawi, A.S.; Battah, B.; Donadu, M.G. Quorum Sensing Inhibitors: An Alternative Strategy to Win the Battle against Multidrug-Resistant (MDR) Bacteria. Molecules 2024, 29, 3466. [Google Scholar] [CrossRef]
- Alum, E.U.; Gulumbe, B.H.; Izah, S.C.; Uti, D.E.; Aja, P.M.; Igwenyi, I.O.; Offor, C.E. Natural Product-Based Inhibitors of Quorum Sensing: A Novel Approach to Combat Antibiotic Resistance. Biochem. Biophys. Rep. 2025, 43, 102111. [Google Scholar] [CrossRef]
- Fydrych, D.; Jeziurska, J.; Wełna, J.; Kwiecińska-Piróg, J. Potential Use of Selected Natural Compounds with Anti-Biofilm Activity. Int. J. Mol. Sci. 2025, 26, 607. [Google Scholar] [CrossRef]
- Chadha, J.; Harjai, K.; Chhibber, S. Repurposing Phytochemicals as Anti-Virulent Agents to Attenuate Quorum Sensing-Regulated Virulence Factors and Biofilm Formation in Pseudomonas Aeruginosa. Microb. Biotechnol. 2022, 15, 1695–1718. [Google Scholar] [CrossRef]
- Patel, K.; Panchal, R.; Sakariya, B.; Gevariya, M.; Raiyani, R.; Soni, R.; Goswami, D. Combatting Antibiotic Resistance by Exploring the Promise of Quorum Quenching in Targeting Bacterial Virulence. Microbe 2025, 6, 100224. [Google Scholar] [CrossRef]
- Yan, Y.; Li, X.; Zhang, C.; Lv, L.; Gao, B.; Li, M. Research Progress on Antibacterial Activities and Mechanisms of Natural Alkaloids: A Review. Antibiotics 2021, 10, 318. [Google Scholar] [CrossRef] [PubMed]
- Nouh, H.S.; El-Zawawy, N.A.; Halawa, M.; Shalamesh, E.M.; Ali, S.S.; Korbecka-Glinka, G.; Shala, A.Y.; El-Sapagh, S. Endophytic Penicillium Oxalicum AUMC 14898 from Opuntia Ficus-Indica: A Novel Source of Tannic Acid Inhibiting Virulence and Quorum Sensing of Extensively Drug-Resistant Pseudomonas Aeruginosa. Int. J. Mol. Sci. 2024, 25, 11115. [Google Scholar] [CrossRef] [PubMed]
- Donadio, G.; Mensitieri, F.; Santoro, V.; Parisi, V.; Bellone, M.L.; De Tommasi, N.; Izzo, V.; Piaz, F.D. Interactions with Microbial Proteins Driving the Antibacterial Activity of Flavonoids. Pharmaceutics 2021, 13, 660. [Google Scholar] [CrossRef] [PubMed]
- Mulat, M.; Banicod, R.J.S.; Tabassum, N.; Javaid, A.; Karthikeyan, A.; Jeong, G.-J.; Kim, Y.-M.; Jung, W.-K.; Khan, F. Multiple Strategies for the Application of Medicinal Plant-Derived Bioactive Compounds in Controlling Microbial Biofilm and Virulence Properties. Antibiotics 2025, 14, 555. [Google Scholar] [CrossRef]
- Selvaraj, A.; Valliammai, A.; Sivasankar, C.; Suba, M.; Sakthivel, G.; Pandian, S.K. Antibiofilm and Antivirulence Efficacy of Myrtenol Enhances the Antibiotic Susceptibility of Acinetobacter Baumannii. Sci. Rep. 2020, 10, 21975. [Google Scholar] [CrossRef] [PubMed]
- Burt, S.A.; Ojo-Fakunle, V.T.A.; Woertman, J.; Veldhuizen, E.J.A. The Natural Antimicrobial Carvacrol Inhibits Quorum Sensing in Chromobacterium violaceum and Reduces Bacterial Biofilm Formation at Sub-Lethal Concentrations. PLoS ONE 2014, 9, e93414. [Google Scholar] [CrossRef]
- Braga, P.C.; Culici, M.; Alfieri, M.; Dal Sasso, M. Thymol Inhibits Candida Albicans Biofilm Formation and Mature Biofilm. Int. J. Antimicrob. Agents 2008, 31, 472–477. [Google Scholar] [CrossRef]
- Zai, M.J.; Cock, I.E.; Cheesman, M.J. Plant Metabolites as Potential Agents That Potentiate or Block Resistance Mechanisms Involving β-Lactamases and Efflux Pumps. Int. J. Mol. Sci. 2025, 26, 5550. [Google Scholar] [CrossRef]
- Davidova, S.; Galabov, A.S.; Satchanska, G. Antibacterial, Antifungal, Antiviral Activity, and Mechanisms of Action of Plant Polyphenols. Microorganisms 2024, 12, 2502. [Google Scholar] [CrossRef] [PubMed]
- Tooke, C.L.; Hinchliffe, P.; Bragginton, E.C.; Colenso, C.K.; Hirvonen, V.H.A.; Takebayashi, Y.; Spencer, J. β-Lactamases and β-Lactamase Inhibitors in the 21st Century. J. Mol. Biol. 2019, 431, 3472–3500. [Google Scholar] [CrossRef]
- Shah, M.A.; Mir, S.A. Plant Extracts as Food Preservatives. In Plant Extracts: Applications in the Food Industry; Elsevier: Amsterdam, The Netherlands, 2022; pp. 127–141. [Google Scholar] [CrossRef]
- Tran, V.T.; Nguyen, T.B.; Nguyen, H.C.; Do, N.H.N.; Le, P.K. Recent Applications of Natural Bioactive Compounds from Piper Betle (L.) Leaves in Food Preservation. Food Control 2023, 154, 110026. [Google Scholar] [CrossRef]
- Alirezalu, K.; Hesari, J.; Eskandari, M.H.; Valizadeh, H.; Sirousazar, M. Effect of Green Tea, Stinging Nettle and Olive Leaves Extracts on the Quality and Shelf Life Stability of Frankfurter Type Sausage. J. Food Process. Preserv. 2017, 41, e13100. [Google Scholar] [CrossRef]
- Martínez, L.; Castillo, J.; Ros, G.; Nieto, G. Antioxidant and Antimicrobial Activity of Rosemary, Pomegranate and Olive Extracts in Fish Patties. Antioxidants 2019, 8, 86. [Google Scholar] [CrossRef]
- Liu, C.; Xu, S.; Liu, X.; Wang, W.; Liao, W.; Yang, X.; He, Q. Preservation of Beef with Limonene-Rich Citrus Peel Extracts: Antioxidant, Antimicrobial and Textural Benefits. Foods 2025, 14, 3506. [Google Scholar] [CrossRef] [PubMed]
- Lorenzo, J.M.; Vargas, F.C.; Strozzi, I.; Pateiro, M.; Furtado, M.M.; Sant’Ana, A.S.; Rocchetti, G.; Barba, F.J.; Dominguez, R.; Lucini, L.; et al. Influence of Pitanga Leaf Extracts on Lipid and Protein Oxidation of Pork Burger during Shelf-Life. Food Res. Int. 2018, 114, 47–54. [Google Scholar] [CrossRef]
- Tokysheva, G.; Konysbayeva, D.; Myrzabayeva, M.; Ospankulova, G.; Dairova, K.; Makangali, K. Effects of Salicornia Extract on the Quality, Shelf-Life, and Functional Properties of Beef Patties During Refrigerated Storage. Appl. Sci. 2025, 15, 11751. [Google Scholar] [CrossRef]
- Mazandrani, H.A.; Javadian, S.; Bahram, S. The Effect of Encapsulated Fennel Extracts on the Quality of Silver Carp Fillets during Refrigerated Storage. Food Sci. Nutr. 2016, 4, 298–304. [Google Scholar] [CrossRef]
- Miranda, J.M.; Carrera, M.; Pastén, A.; Vega-Gálvez, A.; Barros-Velázquez, J.; Aubourg, S.P. The Impact of Quinoa (Chenopodium quinoa Willd.) Ethanolic Extracts in the Icing Medium on Quality Loss of Atlantic Chub Mackerel (Scomber colias) Under Chilling Storage. Eur. J. Lipid Sci. Technol. 2018, 120, ejlt.201800280. [Google Scholar] [CrossRef]
- Duan, J.; Cherian, G.; Zhao, Y. Quality Enhancement in Fresh and Frozen Lingcod (Ophiodon elongates) Fillets by Employment of Fish Oil Incorporated Chitosan Coatings. Food Chem. 2010, 119, 524–532. [Google Scholar] [CrossRef]
- International Commission on Microbiological Specifications for Foods. Sampling for Microbiological Analysis: Principles and Specific Applications; University of Toronto Press: Toronto, Japan, 1974; Volume 2. [Google Scholar]
- Baghlani, N.; Hosseini, S.M.; Jafarpour, S.A.; Mousavi, S.M.; Khodanazary, A. Effect of Carboxymethyl Cellulose Edible Coating Enriched with Summer Savory Extract on Quality Parameters of Spangled Emperor (Lethrinus nebulosus) Fillets During Refrigerated Storage. J. Package. Technol. Res. 2019, 3, 149–160. [Google Scholar] [CrossRef]
- Olatunde, O.O.; Benjakul, S.; Huda, N.; Zhang, B.; Deng, S. Ethanolic Noni (Morinda citrifolia L.) Leaf Extract Dechlorophyllised Using Sedimentation Process: Antioxidant, Antibacterial Properties and Efficacy in Extending the Shelf-Life of Striped Catfish Slices. Int. J. Food Sci. Technol. 2021, 56, 2804–2819. [Google Scholar] [CrossRef]
- Li, Y.; Zhuang, S.; Liu, Y.; Zhang, L.; Liu, X.; Cheng, H.; Liu, J.; Shu, R.; Luo, Y. Effect of Grape Seed Extract on Quality and Microbiota Community of Container-Cultured Snakehead (Channa argus) Fillets during Chilled Storage. Food Microbiol. 2020, 91, 103492. [Google Scholar] [CrossRef]
- Shan, B.; Cai, Y.; Brooks, J.D.; Corke, H. Antibacterial and Antioxidant Effects of Five Spice and Herb Extracts as Natural Preservatives of Raw Pork. J. Sci. Food Agric. 2009, 89, 1879–1885. [Google Scholar] [CrossRef]
- Li, Y.; Yang, Z.; Li, J. Shelf-life Extension of Pacific White Shrimp Using Algae Extracts during Refrigerated Storage. J. Sci. Food Agric. 2017, 97, 291–298. [Google Scholar] [CrossRef]
- Givi, F.; Gholami, M.; Massah, A. Application of Pomegranate Peel Extract and Essential Oil as a Safe Botanical Preservative for the Control of Postharvest Decay Caused by Penicillium Italicum and Penicillium Digitatum on “Satsuma” Mandarin. J. Food Saf. 2019, 39, e12639. [Google Scholar] [CrossRef]
- Piva, G.; Fracassetti, D.; Tirelli, A.; Mascheroni, E.; Musatti, A.; Inglese, P.; Piergiovanni, L.; Rollini, M. Evaluation of the Antioxidant/Antimicrobial Performance of Posidonia Oceanica in Comparison with Three Commercial Natural Extracts and as a Treatment on Fresh-Cut Peaches (Prunus persica Batsch). Postharvest. Biol. Technol. 2017, 124, 54–61. [Google Scholar] [CrossRef]
- Derbassi, N.; Pedrosa, M.C.; Heleno, S.; Fernandes, F.; Dias, M.I.; Calhelha, R.C.; Rodrigues, P.; Carocho, M.; Ferreira, I.C.F.R.; Barros, L. Arbutus Unedo Leaf Extracts as Potential Dairy Preservatives: Case Study on Quark Cheese. Food Funct. 2022, 13, 5442–5454. [Google Scholar] [CrossRef] [PubMed]
- Atwaa, E.S.H.; Shahein, M.R.; Radwan, H.A.; Mohammed, N.S.; Aloraini, M.A.; Albezrah, N.K.A.; Alharbi, M.A.; Sayed, H.H.; Daoud, M.A.; Elmahallawy, E.K. Antimicrobial Activity of Some Plant Extracts and Their Applications in Homemade Tomato Paste and Pasteurized Cow Milk as Natural Preservatives. Fermentation 2022, 8, 428. [Google Scholar] [CrossRef]
- Essid, I.; Tajine, S.; Gharbi, S.; Bellagha, S. Use of Pomegranate Peel and Artichoke Leaf Extracts to Improve the Quality of Marinated Sardine (Sardinella aurita) Fillets. J. Food Sci. Technol. 2020, 57, 713–722. [Google Scholar] [CrossRef]
- Kieling, D.D.; Delarco, M.F.; Prudencio, S.H. Lemongrass Extract as a Natural Preservative of Cooked and Shredded Chicken Breast during Storage. J. Culin. Sci. Technol. 2021, 19, 55–66. [Google Scholar] [CrossRef]
- Pini, F.; Aquilani, C.; Giovannetti, L.; Viti, C.; Pugliese, C. Characterization of the Microbial Community Composition in Italian Cinta Senese Sausages Dry-Fermented with Natural Extracts as Alternatives to Sodium Nitrite. Food Microbiol. 2020, 89, 103417. [Google Scholar] [CrossRef]
- Lashgari, S.S.; Noorolahi, Z.; Sahari, M.A.; Ahmadi Gavlighi, H. Improvement of Oxidative Stability and Textural Properties of Fermented Sausage via Addition of Pistachio Hull Extract. Food Sci. Nutr. 2020, 8, 2920–2928. [Google Scholar] [CrossRef]
- Gantner, M.; Guzek, D.; Najda, A.; Brodowska, M.; Górska-Horczyczak, E.; Wojtasik-Kalinowska, I.; Godziszewska, J. Oxidative and Microbial Stability of Poultry Meatballs Added with Coriander Extracts and Packed in Cold Modified Atmosphere. Int. J. Food Prop. 2017, 20, 2527–2537. [Google Scholar] [CrossRef]
- Nowak, A.; Czyzowska, A.; Efenberger, M.; Krala, L. Polyphenolic Extracts of Cherry (Prunus cerasus L.) and Blackcurrant (Ribes nigrum L.) Leaves as Natural Preservatives in Meat Products. Food Microbiol. 2016, 59, 142–149. [Google Scholar] [CrossRef]
- Zamuz, S.; López-Pedrouso, M.; Barba, F.J.; Lorenzo, J.M.; Domínguez, H.; Franco, D. Application of Hull, Bur and Leaf Chestnut Extracts on the Shelf-Life of Beef Patties Stored under MAP: Evaluation of Their Impact on Physicochemical Properties, Lipid Oxidation, Antioxidant, and Antimicrobial Potential. Food Res. Int. 2018, 112, 263–273. [Google Scholar] [CrossRef]
- Raeisi, S.; Quek, S.Y.; Ojagh, S.M.; Alishahi, A.R. Effects of cumin (Cuminum cyminum L.) seed and wild mint (Mentha longifolia L.) leaf extracts on the shelf life and quality of rainbow trout (Oncorhynchus mykiss) fillets stored at 4C ± 1. J. Food Saf. 2016, 36, 271–281. [Google Scholar] [CrossRef]
- Ramírez-Guerra, H.E.; Castillo-Yañez, F.J.; Montaño-Cota, E.A.; Ruíz-Cruz, S.; Márquez-Ríos, E.; Canizales-Rodríguez, D.F.; Torres-Arreola, W.; Montoya-Camacho, N.; Ocaño-Higuera, V.M. Protective Effect of an Edible Tomato Plant Extract/Chitosan Coating on the Quality and Shelf Life of Sierra Fish Fillets. J. Chem. 2018, 2018, 2436045. [Google Scholar] [CrossRef]
- Dulal, M.A.; Islam, R.; Ahmed, S.; Yuan, C.; Shah, A.K.M.A. Preservative Effects of Gracilaria Sp. Extract in Maintaining the Quality and Shelf Life of Refrigerated Pangas (Pangasius hypophthalmus) Fillets. Food Sci. Nutr. 2025, 13, e70683. [Google Scholar] [CrossRef]
- Raeisi, S.; Sharifi-Rad, M.; Quek, S.Y.; Shabanpour, B.; Sharifi-Rad, J. Antimicrobial Effects of Shallot (Allium Ascalonicum L.) Fruit and Ajwain (Trachyspermum Ammi (L.) Sprague) Seed Extracts in Semi-Fried Coated Rainbow Trout (Oncorhynchus mykiss) Fillets for Shelf-Life Extension. LWT 2016, 65, 112–121. [Google Scholar] [CrossRef]
- Alsaggaf, M.S.; Moussa, S.H.; Tayel, A.A. Application of Fungal Chitosan Incorporated with Pomegranate Peel Extract as Edible Coating for Microbiological, Chemical and Sensorial Quality Enhancement of Nile Tilapia Fillets. Int. J. Biol. Macromol. 2017, 99, 499–505. [Google Scholar] [CrossRef]
- Viji, P.; Binsi, P.K.; Visnuvinayagam, S.; Bindu, J.; Ravishankar, C.N.; Srinivasa Gopal, T.K. Efficacy of Mint (Mentha arvensis) Leaf and Citrus (Citrus aurantium) Peel Extracts as Natural Preservatives for Shelf Life Extension of Chill Stored Indian Mackerel. J. Food Sci. Technol. 2015, 52, 6278–6289. [Google Scholar] [CrossRef] [PubMed]
- Kundu, A.; Chakma, A.; Dulal, M.A.; Rasul, M.G.; Mondal, M.N.; Shah, A.K.M.A. Effects of Stevia (Stevia rebaudiana Bertoni) Leaf Extracts on the Quality and Shelf Life of Refrigerated Catla (Gibelion catla) Fillets. J. Agric. Food Res. 2024, 15, 101058. [Google Scholar] [CrossRef]
- Raeisi, S.; Ojagh, S.M.; Sharifi-Rad, M.; Sharifi-Rad, J.; Quek, S.Y. Evaluation of Allium paradoxum (M.B.) G. Don. and Eryngium Caucasicum Trauve. Extracts on the Shelf--life and Quality of Silver Carp (Hypophthalmichthys molitrix) Fillets during Refrigerated Storage. J. Food Saf. 2017, 37, e12321. [Google Scholar] [CrossRef]
- Martínez, L.; Bastida, P.; Castillo, J.; Ros, G.; Nieto, G. Green Alternatives to Synthetic Antioxidants, Antimicrobials, Nitrates, and Nitrites in Clean Label Spanish Chorizo. Antioxidants 2019, 8, 184. [Google Scholar] [CrossRef]
- Herbalox® Natural Rosemary Extract for Extended Shelf Life|Kalsec. Available online: https://www.kalsec.com/natural-food-protection/antioxidants/herbalox-rosemary-extract (accessed on 8 October 2025).
- StabilEnhance OSR 4 Rosemary (BA201187)-Givaudan-Naturex. Available online: https://www.knowde.com/stores/givaudan-naturex/products/stabilenhance-osr-4-rosemary-ba201187 (accessed on 8 October 2025).
- Rosemary Extract Based Natural Antioxidants to Extend Shelf Life: Oxikan-Mane Kancor Ingredients Ltd. Available online: https://manekancor.com/natural-antioxidants/rosemary-extracts-oxikan/ (accessed on 8 October 2025).
- FORTIUMTM R Rosemary-Based Antioxidant for Food Shelf Life Extension. Available online: https://www.kemin.com/na/en-us/markets/food/products/fortium-r (accessed on 8 October 2025).
- NaturFORTTM|Rosemary and Green Tea Blend|Kemin Europe. Available online: https://www.kemin.com/eu/en/markets/food/products/naturfort (accessed on 8 October 2025).
- OLESSENCETM|Discover Nature’s Flavour and Freshness|Kemin EMENA. Available online: https://www.kemin.com/eu/en/markets/food/products/olessence (accessed on 8 October 2025).
- REGULATION (EC) No 1333/2008 of the European Parliament and of the Council of 16 December 2008 on Food Additives (Text with EEA Relevance). Available online: https://eur-lex.europa.eu/eli/reg/2008/1333/oj/eng (accessed on 20 September 2025).
- Food Ingredient & Packaging Inventories|FDA. Available online: https://www.fda.gov/food/food-ingredients-packaging/food-ingredient-packaging-inventories (accessed on 11 November 2025).
- Younes, M.; Aggett, P.; Aguilar, F.; Crebelli, R.; Dusemund, B.; Filipič, M.; Frutos, M.J.; Galtier, P.; Gundert-Remy, U.; Kuhnle, G.G.; et al. Guidance on Safety Evaluation of Sources of Nutrients and Bioavailability of Nutrient from the Sources (Revision 1)1. EFSA J. 2021, 19, e06552. [Google Scholar] [CrossRef] [PubMed]
- Campolina, G.A.; Cardoso, M.D.G.; Caetano, A.R.S.; Nelson, D.L.; Ramos, E.M. Essential Oil and Plant Extracts as Preservatives and Natural Antioxidants Applied to Meat and Meat Products: A Review. Food Technol. Biotechnol. 2023, 61, 212–225. [Google Scholar] [CrossRef] [PubMed]
| Method | Extraction Efficiency | Yield | Solvent Use | Energy Consumption | Key Limitations | Industrial Scalability | Future Directions | Regulatory Acceptance |
|---|---|---|---|---|---|---|---|---|
| NADES | polar (phenolics)/some terpenoids | very high | very low (no toxic solvents) | low | high viscosity; solvent recovery; regulatory gaps | emerging (strong potential but not yet widely adopted) | developing scalable and cost-effective recovery and purification methods; toxicological studies | emerging/Low |
| UAE | polar (phenolics)/ non-polar (terpenes) | high | low–moderate (aqueous/ ethanolic) | low | localized heat spots; compounds degradation | high (economical & scalable) | real-time monitoring for efficiency and energy use optimization; focus on hybrid systems | high widely accepted established safety guidelines for ultrasound exposure |
| MAE | polar (phenolics) | very high | low (polar solvents needed) | moderate | expensive equipment; limited microwave penetration depth in large volumes | moderate (scaling requires specialized reactors) | optimization of continuous-flow reactors with lower frequencies | moderate accepted for specific applications |
| EAE | bound phenolics | high | low (water-based) | low | enzyme cost; variability; potential enzyme deactivation | moderate (cost-limited) | creation of enzymes with enhanced stability and activity for specific industrial conditions | Very high well-established and accepted technology in food processing and pharmaceuticals |
| SFE (CO2) | non-polar (terpenes/ terpenoids) | very high | very low | moderate–high (pressurized CO2) | high capital cost; trained operators | moderate–high(used in food & pharma) | exploring SFE as a hybrid method for targeted fractionation of extracts | very high well-established, non-toxic, widely used in food and pharmaceutical industries |
| HHP | polar (phenolics) | high | moderate (aqueous/ ethanolic) | low-moderate | batch process; high capital cost | commercially viable and highly scalable | development of continuous systems to improve throughput | high well-regulated for safety and efficacy in food industry; growing acceptance in pharmaceutical industry |
| Plant Official Name, Common Name, Plant Part | Extraction Method & Conditions SLR (g/mL); T (min) | Antibacterial Assay | Microorganism | Antibacterial Activity | Ref. | |
|---|---|---|---|---|---|---|
| Inhibition Zone (mm) | MIC | |||||
| Hibiscus sabdariffa, Roselle | UAE EtOH:Water (90:10 v/v) 53 kHz, 1:18, 30 min | Agar well diffusion | E. coli B. cereus | 21.1 22.2 | - | [64] |
| Rosmarinus officinalis, Rosemary | E. coli B. cereus | 17.4 16.7 | ||||
| Syzygium aromaticum, Clove | E. coli B. cereus | 21.1 19.8 | ||||
| Thymus vulgaris, Thyme | E. coli B. cereus | 15.9 17.3 | ||||
| Vaccinium vitis-idaea, Lingonberry, leaves | UAE EtOH:Water:Acetic acid (70:30:1 v/v/v), 1:10, 30 min | Broth microdilution | B. cereus S. enterica sv. Typhimurium | - | 100 71 | [85] |
| Ribes rubrum var. alba, White currant, leaves | B. cereus S. enterica sv. Typhimurium | 90 78 | ||||
| Crataegus spp., Hawthorn, leaves | B. cereus S. enterica sv. Typhimurium | 100 86 | ||||
| Hippophae rhamnoides, Sea buckthorn, leaves | B. cereus S. enterica sv. Typhimurium | 100 100 | ||||
| Amelanchier alnifolia, Saskatoon, leaves | B. cereus S. enterica sv. Typhimurium | 890 | ||||
| Rubus idaeus, Raspberry leaves | B. cereus S. enterica sv. Typhimurium | 96 81 3 | ||||
| Hippophae rhamnoides, sea buckthorn, leaves | UAE EtOH:Water (50:50 v/v) 40 kHz, 1:5 w/v, 1 h | Broth microdilution | S. aureus B. cereus P. aeruginosa | - | 6.20 12.5 6.20 1 | [86] |
| Rosmarinus officinalis L., Rosemary leaves | UAE EtOH:Water (50:50 v/v) 20 kHz, 1:10, 20 min | Broth microdilution | S. aureus P. aeruginosa E. coli | - | 0.140 0.360 0.340 1 | [87] |
| Chaenomeles japonica (Thunb.) Lindl. ex Spach, Japanese quince, fruits | UAE EtOH:Water (50:50 v/v) 480 W, 1:20, 20 min | B. subtilis E. faecalis S. aureus E. coli | 21.7 30.7 18.7 19.6 | - | [88] | |
| Rosa canina L., Dog rose, pseudofruit | UAE Methanol, 1:6, 45 min | Broth microdilution | P. aeruginosa E. coli | - | 0.256 >0.512 1 | [89] |
| Punica granatum L., Pomegranate, Peels | UAE EtOH:Water (30:70 v/v) with 1% formic acid, 1:10, 30 min | Broth microdilution | A. baumannii S. aureus P. aeruginosa E. coli | - | 3.2 0.8 6.4 12.8 1 | [90] |
| Punica granatum L., Pomegranate, Peels | MAE 1500 W, 1:30, 10 min | Agar disk diffusion | P. aeruginosa E. coli S. aureus | 15 19 22 | - | [91] |
| Punica granatum L., Pomegranate, Peels | MAE 900 W, 1:8, 8 min | Broth microdilution | S. aureus E. coli P. aeruginosa Proteus mirabilis | - | 12.5 25 25 50 2 | [92] |
| Piper betle L., Betel, leaves | MAE 239.6 W, 1:22, 1.6 min | Agar well diffusion | B. pumilus B. cereus K. pneumoniae E. coli | 3.2 2.6 2.5 2.8 | - | [93] |
| Levisticum officinale, Lovage, leaves | MAE 53% EtOH, 800 W | Broth microdilution | S. aureus E. coli | - | 13.5 13.5 1 | [94] |
| Origanum vulgare, Oregano, leaves | MAE 49% EtOH, 160 W | 3.06 13.5 1 | ||||
| Salvia officinalis, Sage leaves | MAE 800 W, 8 min | Broth microdilution | E. coli E. asburiae M. luteus B. subtilis | - | 27.50 56.65 5.69 18.43 4 | [95] |
| Olea europaea L., Olive, leaves | MAE Water, 800 W, 1:8, 10 min | Broth microdilution | S. aureus S. enterica sv. Typhimurium E. coli L. monocytogenes | - | 2.5 40 40 30 1 | [96] |
| Hippophae rhamnoides, Sea buckthorn, leaves | EAE Viscozyme L and Cellulase 1% (v/w), 1:20 (w/v), 3.15 h | Agar well diffusion | S. aureus | 4.17 | - | [97] |
| Rosa canina L., Dog rose, pseudofruit | EAE Phosphate buffer pH 5.5, Cellic Ctec3 1% v/v, 1:16.67, 360 min | Broth microdilution | E. coli | - | 80 3 | [98] |
| Rosa canina L., Dog rose, pseudofruit | EAE Phosphate buffer pH 5.5, Pectinex Ultra color 0.59% v/v, Viscoferm 0.51%, 1:16.67, 96 min | Broth microdilution | E. coli | - | 55 3 | [99] |
| Punica granatum L., Pomegranate, Peels | HHP Water, 600 MPa, 1:62.5, 15 min | Agar well diffusion | S. aureus B. cereus P. aeruginosa E. coli | 20 18 29 10 | 7.82 15.63 62.5 62.5 1 | [100] |
| Origanum vulgare, Oregano | SFE CO2 (purity 2.5%), 25 MPa | Broth microdilution | S. aureus E. coli C. albicans | - | 0.147 0.728 0.311 1 | [101] |
| Mentha piperita, Peppermint, leaves | NADES Choline chloride: citric acid (1:1) + 30% water, 75:1, 30 min | Broth microdilution | P. aeruginosa S. aureus E. coli S. enterica sv. Typhimurium | - | 0.39 0.39 0.78 0.78 3 | [102] |
| Malva sylvestris L., mallow, flower | NADES Choline chloride: citric acid (1:1) + 30% water, 1:13.3, 60 min | Agar well diffusion | S. aureus E. coli P. aeruginosa B. cereus | 33.3 30.0 32.0 31.3 | - | [103] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lemoni, Z.; Evangeliou, K.; Lymperopoulou, T.; Mamma, D. Incorporation of Edible Plant Extracts as Natural Food Preservatives: Green Extraction Methods, Antibacterial Mechanisms and Applications. Foods 2025, 14, 4000. https://doi.org/10.3390/foods14234000
Lemoni Z, Evangeliou K, Lymperopoulou T, Mamma D. Incorporation of Edible Plant Extracts as Natural Food Preservatives: Green Extraction Methods, Antibacterial Mechanisms and Applications. Foods. 2025; 14(23):4000. https://doi.org/10.3390/foods14234000
Chicago/Turabian StyleLemoni, Zafeiria, Konstantinos Evangeliou, Theopisti Lymperopoulou, and Diomi Mamma. 2025. "Incorporation of Edible Plant Extracts as Natural Food Preservatives: Green Extraction Methods, Antibacterial Mechanisms and Applications" Foods 14, no. 23: 4000. https://doi.org/10.3390/foods14234000
APA StyleLemoni, Z., Evangeliou, K., Lymperopoulou, T., & Mamma, D. (2025). Incorporation of Edible Plant Extracts as Natural Food Preservatives: Green Extraction Methods, Antibacterial Mechanisms and Applications. Foods, 14(23), 4000. https://doi.org/10.3390/foods14234000








