Polyphenols Bioactive Metabolites, and Their Anti-Biofilm and Neuroprotective Potential
Abstract
1. Introduction
2. Polyphenols: Chemical Classification and Dietary Sources
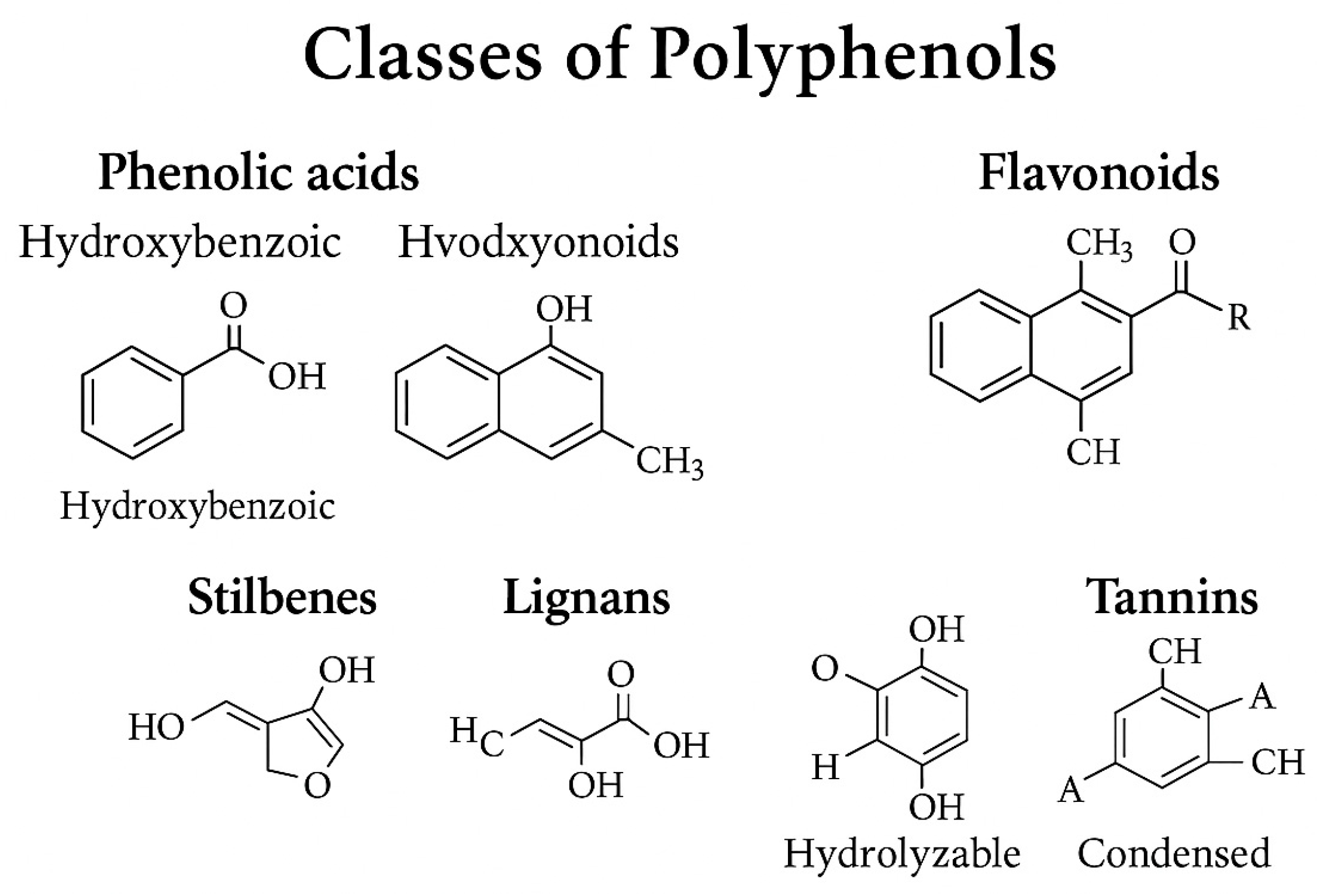
2.1. Chemical Classification of Polyphenols
2.1.1. Phenolic Acids
2.1.2. Flavonoids
2.1.3. Stilbenes
2.1.4. Lignans
2.1.5. Tannins
2.2. Factors Affecting Polyphenol Content
2.3. Polyphenol Structural Diversity and Biological Relevance
2.4. Microbial Transformation of Polyphenols
2.5. Biofunctional Properties of LMWPMs
- −
- Quorum Sensing Inhibition and Anti-Biofilm Activity
- −
- Neuroactive Potential and Blood–Brain Barrier Interaction
- −
- Enhancement of Gut Barrier Function
- −
- Epigenetic Modulation and Gene Regulation
2.6. Relationship Between Dietary Polyphenols and the Gut Microbiota
2.7. Polyphenol Bioavailability and Microbiota-Driven Biotransformation
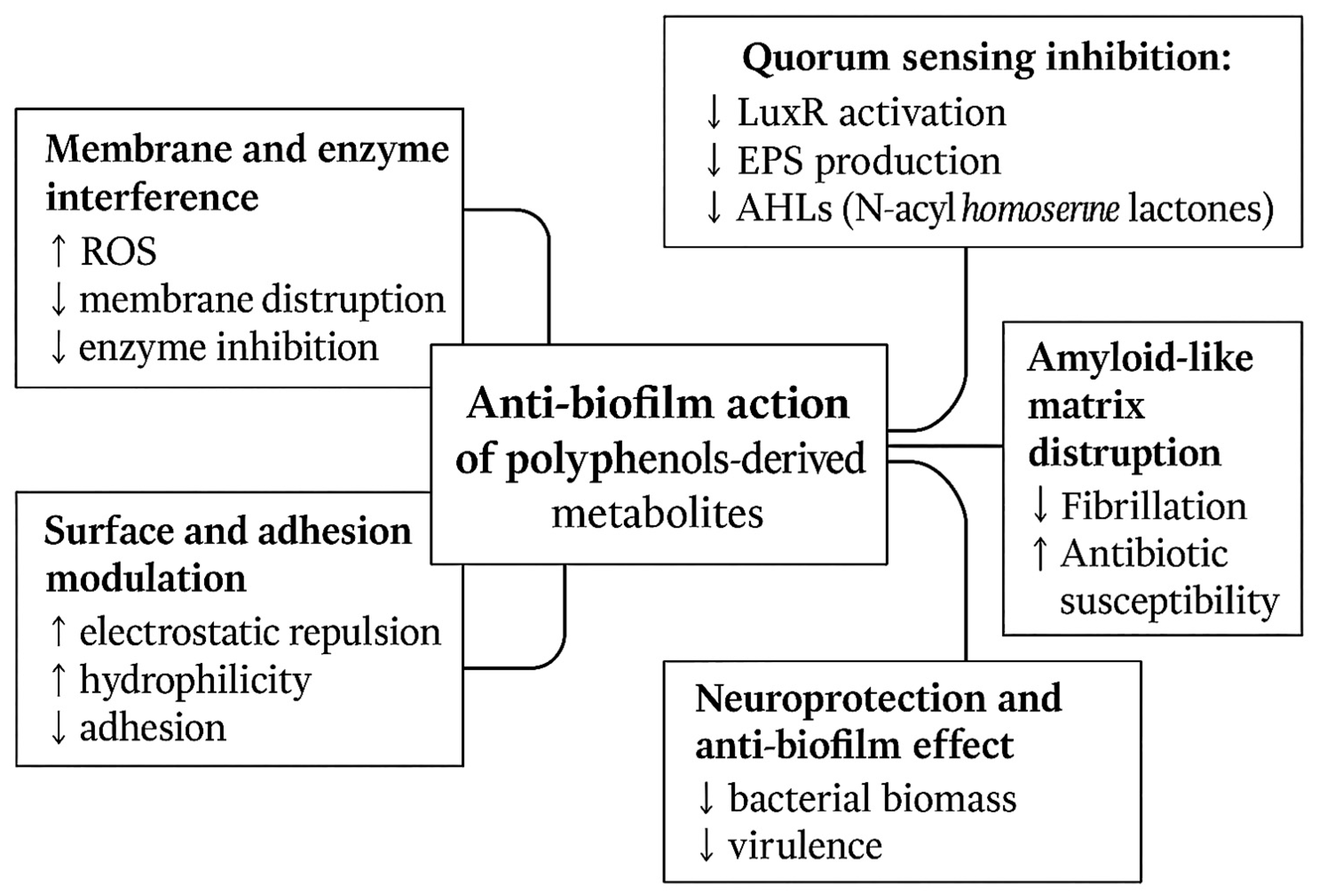
3. Anti-Biofilm Activity of Polyphenol-Derived Metabolites
3.1. Antimicrobial Resistance and Foodborne Pathogens
3.2. Pathogenic Biofilms
3.3. Mechanisms of Action of Polyphenol-Derived Metabolites on Foodborne Biofilms
3.4. Quorum Sensing Modulation
3.5. Polyphenol-Derived Metabolites Most Effectively Target Which Food-Borne Pathogens?
3.6. Anti-Biofilm Activity of Polyphenol-Derived Microbial Metabolites
3.7. Synergy with Antimicrobials
4. Neuroprotective Properties of Polyphenol-Derived Metabolites and the Gut–Brain Axis
4.1. The Gut–Brain Axis and Foodborne Pathogens
4.2. Potential Mechanism of Pathogen-Associated Neurotoxicity
4.3. Foodborne Pathogens and Their Neurological Impact
4.4. Neurotoxic Effects of Foodborne Toxins
4.5. Foodborne Pathogens and BBB Disruption
4.6. Role of Polyphenols and Their Metabolites in Central Nervous System Protection
4.7. Core Neuroprotective Mechanisms
4.8. Alzheimer’s Disease (AD)
4.9. Parkinson’s Disease (PD)
4.10. Multiple Sclerosis (MS)
4.11. Huntington’s Disease (HD)
4.12. Vascular Cognitive Impairment and Mixed Dementias
4.13. Anxiety and Mood Regulation
4.14. Key Mechanisms of Neuroprotection by Polyphenol-Derived Metabolites
- −
- Mitochondrial Biogenesis and Autophagy Activation. Mitochondrial impairment and defective autophagy render neurons vulnerable, leading to energy failure and the formation of dysfunctional organelles. Polyphenol metabolites modulate mitochondrial quality control. Urolithin A activates PINK1/Parkin mitophagy to remove damaged mitochondria and stimulates PGC-1α-driven biogenesis, boosting ATP and reducing ROS. Increased LC3-II, BNIP3, and TFEB indicate greater lysosomal–autophagic flux. These effects prevent neuronal energy failure, key in early neurodegeneration [95,246].
- −
- Inhibition of Amyloid and Protein Aggregation. Protein misfolding and aggregation are pivotal in the progression of Alzheimer’s disease (AD) and Parkinson’s disease (PD). Accumulation of amyloid-β (Aβ) fibrils or α-synuclein aggregates drives synaptic dysfunction and neuronal loss. Urolithin A Inhibits Aβ1–42 fibrillogenesis, destabilizes preformed fibrils, and reduces their cytotoxicity in neuronal cultures, while attenuating oligomer-mediated synaptic impairment in hippocampal neurons [234,246]. Urolithin B and dihydroresveratrol inhibit α-synuclein aggregation by interfering with hydrophobic interactions within the NAC (non-amyloid component) domain, thereby preventing the formation of toxic oligomers and promoting the proteasomal degradation of misfolded proteins [247,248,249].
- −
- Modulation of neuroinflammation. Chronic neuroinflammation, driven by persistent activation of microglia and astrocytes, contributes to blood–brain barrier disruption and neuronal injury. Phenylacetic acid and phenylpropionic acid derivatives suppress IL-6, TNF-α, and IL-1β production in activated microglia, inhibit NF-κB translocation and MAPK phosphorylation, and promote a shift toward M2-like microglia expressing Arg1 and IL-10. This immunomodulatory effect reduces glial scarring, protects the BBB, and limits bystander neuronal damage [240,250].
- −
- Antioxidant and Redox-Modulating Effects. Oxidative stress is a shared mechanism of neuronal injury in AD, PD, and related disorders. Excessive ROS and RNS damage lipids, proteins, and DNA, disrupting synaptic function. 3,4-Dihydroxyphenylacetic acid (DOPAC) and protocatechuic acid activate the Nrf2/ARE pathway, thereby upregulating HO-1, SOD1, and catalase, which in turn increase intracellular GSH and reduce lipid hydroperoxide accumulation. These metabolites also stabilize the mitochondrial membrane potential (Δψm), lower mtROS production, and preserve synaptic plasticity and neurotransmitter balance [212,247,251,252].
- ✓
- Block amyloid and α-synuclein aggregation.
- ✓
- Restore mitochondrial homeostasis through biogenesis and mitophagy.
- ✓
- Reprogram neuroinflammation toward a protective phenotype.
- ✓
- Reinforce endogenous antioxidant and redox-balancing systems.
5. Critical Perspective and Limitations
6. Conclusions and Future Directions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Santhiravel, S.; Bekhit, A.E.-D.A.; Mendis, E.; Jacobs, J.L.; Dunshea, F.R.; Rajapakse, N.; Ponnampalam, E.N. The impact of plant phytochemicals on the gut microbiota of humans for a balanced life. Int. J. Mol. Sci. 2022, 23, 8124. [Google Scholar] [CrossRef]
- Scalbert, A.; Williamson, G. Dietary intake and bioavailability of polyphenols. J. Nutr. 2000, 130, 2073S–2085S. [Google Scholar] [CrossRef]
- Mosele, J.I.; Macià, A.; Motilva, M.-J. Metabolic and microbial modulation of the large intestine ecosystem by non-absorbed diet phenolic compounds: A review. Molecules 2015, 20, 17429–17468. [Google Scholar] [CrossRef] [PubMed]
- Cai, Y.; Xu, J.; Wang, B. Gut Microbiota-Targeted Polyphenol Interventions: A Novel Paradigm and Synergistic Strategies for Obesity Management. Phytother. Res. 2025, 39, 4913–4933. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Gomez, Á.; Cantone, M.; Garcia-Muñoz, A.M.; Victoria-Montesinos, D.; Lucas-Abellán, C.; Serrano-Martínez, A.; Muñoz-Morillas, A.M.; Morillas-Ruiz, J.M. Effect of Polyphenol-Rich Interventions on Gut Microbiota and Inflammatory or Oxidative Stress Markers in Adults Who Are Overweight or Obese: A Systematic Review and Meta-Analysis. Nutrients 2025, 17, 2468. [Google Scholar] [CrossRef]
- Davidova, S.; Galabov, A.S.; Satchanska, G. Antibacterial, antifungal, antiviral activity, and mechanisms of action of plant polyphenols. Microorganisms 2024, 12, 2502. [Google Scholar] [CrossRef] [PubMed]
- Jadaun, S.; Sharma, U.; Khapudang, R.; Siddiqui, S. Polyphenolic Compounds from Diet: Potential Role in Regulation of Gut Microbiota and Effects on Human Body. In Sustainable Food Systems (Volume I) SFS: Framework, Sustainable Diets, Traditional Food Culture & Food Production; Springer: Berlin/Heidelberg, Germany, 2024; pp. 275–296. [Google Scholar]
- Ávila-Gálvez, M.Á.; Garay-Mayol, B.; Marín, A.; Brito, M.A.; Giménez-Bastida, J.A.; Espín, J.C.; González-Sarrías, A. Metabolic Profiling of a Mediterranean-Inspired (Poly) phenol-Rich Mixture in the Brain: Perfusion Effect and In Vitro Blood– Brain Barrier Transport Validation. J. Agric. Food Chem. 2025, 73, 11056–11066. [Google Scholar] [CrossRef]
- Tavan, M.; Hanachi, P.; de la Luz Cádiz-Gurrea, M.; Segura Carretero, A.; Mirjalili, M.H. Natural phenolic compounds with neuroprotective effects. Neuroch. Res. 2024, 49, 306–326. [Google Scholar] [CrossRef]
- Li, W.; Chen, H.; Xu, B.; Wang, Y.; Zhang, C.; Cao, Y.; Xing, X. Research progress on classification, sources and functions of dietary polyphenols for prevention and treatment of chronic diseases. J. Fut. Foods 2023, 3, 289–305. [Google Scholar] [CrossRef]
- Shahidi, F.; Yeo, J. Bioactivities of phenolics by focusing on suppression of chronic diseases: A review. Int. J. Mol. Sci. 2018, 19, 1573. [Google Scholar] [CrossRef]
- Diwan, A.; Panche, A. Flavonoids: An overview. J. Nutr. Sci. 2016, 5, e47. [Google Scholar] [CrossRef]
- Baur, J.A.; Sinclair, D.A. Therapeutic potential of resveratrol: The in vivo evidence. Nat. Rev. Drug Discov. 2006, 5, 493–506. [Google Scholar] [CrossRef] [PubMed]
- Landete, J.M. Plant and mammalian lignans: A review of source, intake, metabolism, intestinal bacteria and health. Food Res. Int. 2012, 46, 410–424. [Google Scholar] [CrossRef]
- Quideau, S.; Deffieux, D.; Douat-Casassus, C.; Pouységu, L. Plant polyphenols: Chemical properties, biological activities, and synthesis. Angewandte Chemie Int. Edit. 2011, 50, 586–621. [Google Scholar] [CrossRef]
- Kiokias, S.; Oreopoulou, V. A review of the health protective effects of phenolic acids against a range of severe pathologic conditions (including coronavirus-based infections). Molecules 2021, 26, 5405. [Google Scholar] [CrossRef]
- Rathod, N.B.; Elabed, N.; Punia, S.; Ozogul, F.; Kim, S.-K.; Rocha, J.M. Recent developments in polyphenol applications on human health: A review with current knowledge. Plants 2023, 12, 1217. [Google Scholar] [CrossRef] [PubMed]
- Teka, T.; Zhang, L.; Ge, X.; Li, Y.; Han, L.; Yan, X. Stilbenes: Source plants, chemistry, biosynthesis, pharmacology, application and problems related to their clinical application-A comprehensive review. Phytochem. 2022, 197, 113128. [Google Scholar] [CrossRef]
- Rodríguez-García, C.; Sánchez-Quesada, C.; Toledo, E.; Delgado-Rodríguez, M.; Gaforio, J.J. Naturally lignan-rich foods: A dietary tool for health promotion? Molecules 2019, 24, 917. [Google Scholar] [CrossRef]
- Perez-Jimenez, J.; Neveu, V.; Vos, F.; Scalbert, A. Systematic analysis of the content of 502 polyphenols in 452 foods and beverages: An application of the phenol-explorer database. J. Agric. Food Chem. 2010, 58, 4959–4969. [Google Scholar] [CrossRef]
- Manach, C.; Scalbert, A.; Morand, C.; Rémésy, C.; Jiménez, L. Polyphenols: Food sources and bioavailability. Am. J. Clin. Nutr. 2004, 79, 727–747. [Google Scholar] [CrossRef]
- Pinasseau, L.; Vallverdú-Queralt, A.; Verbaere, A.; Roques, M.; Meudec, E.; Le Cunff, L.; Péros, J.-P.; Ageorges, A.; Sommerer, N.; Boulet, J.-C. Cultivar diversity of grape skin polyphenol composition and changes in response to drought investigated by LC-MS based metabolomics. Front. Plant Sci. 2017, 8, 1826. [Google Scholar] [CrossRef]
- Vermerris, W.; Nicholson, R. Phenolic Compound Biochemistry; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2007. [Google Scholar]
- Muñoz-Ulecia, E.; Bernués, A.; Ondé, D.; Ramanzin, M.; Soliño, M.; Sturaro, E.; Martín-Collado, D. People’ s attitudes towards the agrifood system influence the value of ecosystem services of mountain agroecosystems. Plos ONE 2022, 17, e0267799. [Google Scholar] [CrossRef] [PubMed]
- D’ Archivio, M.; Filesi, C.; Di Benedetto, R.; Gargiulo, R.; Giovannini, C.; Masella, R. Polyphenols, dietary sources and bioavailability. Annali-Istituto Superiore di Sanità 2007, 43, 348. [Google Scholar]
- Fabbrini, M.; D’Amico, F.; Barone, M.; Conti, G.; Mengoli, M.; Brigidi, P.; Turroni, S. Polyphenol and tannin nutraceuticals and their metabolites: How the human gut microbiota influences their properties. Biomolecules 2022, 12, 875. [Google Scholar] [CrossRef]
- Prakash, V.; Bose, C.; Sunilkumar, D.; Cherian, R.M.; Thomas, S.S.; Nair, B.G. Resveratrol as a promising nutraceutical: Implications in gut microbiota modulation, inflammatory disorders, and colorectal cancer. Int. J. Mol. Sci. 2024, 25, 3370. [Google Scholar] [CrossRef]
- Akinwumi, B.C.; Bordun, K.-A.M.; Anderson, H.D. Biological activities of stilbenoids. Int. J. Mol. Sci. 2018, 19, 792. [Google Scholar] [CrossRef] [PubMed]
- Kaşıkcı, M.B.; Bağdatlıoğlu, N. Bioavailability of quercetin. Curr. Res. Nutr. Food Sci. 2016, 4, 146–151. [Google Scholar] [CrossRef]
- Baldi, S.; Tristán Asensi, M.; Pallecchi, M.; Sofi, F.; Bartolucci, G.; Amedei, A. Interplay between lignans and gut microbiota: Nutritional, functional and methodological aspects. Molecules 2023, 28, 343. [Google Scholar] [CrossRef]
- Smeriglio, A.; Barreca, D.; Bellocco, E.; Trombetta, D. Proanthocyanidins and hydrolysable tannins: Occurrence, dietary intake and pharmacological effects. Brit. J. Pharmacol. 2017, 174, 1244–1262. [Google Scholar] [CrossRef]
- Kawabata, K.; Yoshioka, Y.; Terao, J. Role of intestinal microbiota in the bioavailability and physiological functions of dietary polyphenols. Molecules 2019, 24, 370. [Google Scholar] [CrossRef]
- Mena, P.; Bresciani, L.; Brindani, N.; Ludwig, I.A.; Pereira-Caro, G.; Angelino, D.; Llorach, R.; Calani, L.; Brighenti, F.; Clifford, M.N. Phenyl-γ-valerolactones and phenylvaleric acids, the main colonic metabolites of flavan-3-ols: Synthesis, analysis, bioavailability, and bioactivity. Nat. Prod. Rep. 2019, 36, 714–752. [Google Scholar] [CrossRef]
- Rodríguez-Daza, M.C.; Pulido-Mateos, E.C.; Lupien-Meilleur, J.; Guyonnet, D.; Desjardins, Y.; Roy, D. Polyphenol-mediated gut microbiota modulation: Toward prebiotics and further. Front. Nutr. 2021, 8, 689456. [Google Scholar] [CrossRef]
- Caballero Fernández, V.; Estévez García, M.; Tomás Barberán, F.A.; Morcuende Sánchez, D.; Martín Tornero, I.; Delgado Perón, J. Biodegradation of Punicalagin into ellagic acid by selected probiotic bacteria: A study of the underlying mechanisms by MS-Based proteomics. J. Agric. Food Chem. 2022, 70, 16273–16285. [Google Scholar] [CrossRef]
- Hassan, M.H.U.; Shahbaz, M.; Momal, U.; Naeem, H.; Imran, M.; Abdelgawad, M.A.; Ghoneim, M.M.; Mostafa, E.M.; El-Ghorab, A.H.; Alsagaby, S.A. Exploring Punicalagin Potential Against Cancers: A Comprehensive Review. Food Sci. Nutr. 2025, 13, e70072. [Google Scholar] [CrossRef] [PubMed]
- García-Villalba, R.; Beltrán, D.; Frutos, M.D.; Selma, M.V.; Espín, J.C.; Tomás-Barberán, F.A. Metabolism of different dietary phenolic compounds by the urolithin-producing human-gut bacteria Gordonibacter urolithinfaciens and Ellagibacter isourolithinifaciens. Food Funct. 2020, 11, 7012–7022. [Google Scholar] [CrossRef] [PubMed]
- da, C.; Pinaffi-Langley, A.C.; Tarantini, S.; Hord, N.G.; Yabluchanskiy, A. Polyphenol-derived microbiota metabolites and cardiovascular health: A concise review of human studies. Antioxidants 2024, 13, 1552. [Google Scholar]
- La Rosa, G.; Lonardo, M.S.; Cacciapuoti, N.; Muscariello, E.; Guida, B.; Faraonio, R.; Santillo, M.; Damiano, S. Dietary polyphenols, microbiome, and multiple sclerosis: From molecular anti-inflammatory and neuroprotective mechanisms to clinical evidence. Int. J. Mol. Sci. 2023, 24, 7247. [Google Scholar] [CrossRef]
- Tremaroli, V.; Bäckhed, F. Functional interactions between the gut microbiota and host metabolism. Nature 2012, 489, 242–249. [Google Scholar] [CrossRef]
- Moco, S.; Martin, F.-P.J.; Rezzi, S. Metabolomics view on gut microbiome modulation by polyphenol-rich foods. J. Proteome Res. 2012, 11, 4781–4790. [Google Scholar] [CrossRef] [PubMed]
- Favari, C.; de Alvarenga, J.F.R.; Sánchez-Martínez, L.; Tosi, N.; Mignogna, C.; Cremonini, E.; Manach, C.; Bresciani, L.; Del Rio, D.; Mena, P. Factors driving the inter-individual variability in the metabolism and bioavailability of (poly) phenolic metabolites: A systematic review of human studies. Redox Biol. 2024, 71, 103095. [Google Scholar] [CrossRef]
- Dong, X.; Bae, M.; Le, C.; Aguilar Ramos, M.A.; Balskus, E.P. Enantiocomplementary Gut Bacterial Enzymes Metabolize Dietary Polyphenols. J. Am. Chem. Soc. 2025, 147, 7231–7244. [Google Scholar] [CrossRef] [PubMed]
- Stevens, J.F.; Maier, C.S. The chemistry of gut microbial metabolism of polyphenols. Phytochem. Rev. 2016, 15, 425–444. [Google Scholar] [CrossRef]
- Duda-Chodak, A.; Tarko, T.; Satora, P.; Sroka, P. Interaction of dietary compounds, especially polyphenols, with the intestinal microbiota: A review. Eur. J. Nutr. 2015, 54, 325–341. [Google Scholar] [CrossRef]
- Tomás-Barberán, F.A.; Selma, M.V.; Espin, J.C. Interactions of gut microbiota with dietary polyphenols and consequences to human health. Curr. Opin. Clin. Nutr. Metab. Care 2016, 19, 471–476. [Google Scholar] [CrossRef]
- Kim, Y.J.; Jung, J.; Kim, D.H. Gut microbiota-mediated biotransformation of polyphenols: Recent advances and future perspectives. Food Res. Int. 2023, 167, 112675. [Google Scholar]
- Selma, M.V.; Espin, J.C.; Tomas-Barberan, F.A. Interaction between phenolics and gut microbiota: Role in human health. J. Agric. Food Chem. 2009, 57, 6485–6501. [Google Scholar] [CrossRef] [PubMed]
- Mena, P.; de Llano, D.G.; Brindani, N.; Esteban-Fernández, A.; Curti, C.; Moreno-Arribas, M.V.; Del Rio, D.; Bartolomé, B. 5-(3′, 4′-Dihydroxyphenyl)-γ-valerolactone and its sulphate conjugates, representative circulating metabolites of flavan-3-ols, exhibit anti-adhesive activity against uropathogenic Escherichia coli in bladder epithelial cells. J. Funct. Foods 2017, 29, 275–280. [Google Scholar] [CrossRef]
- Espin, J.C.; Larrosa, M.; García-Conesa, M.T.; Tomás-Barberán, F. Biological significance of urolithins, the gut microbial ellagic acid-derived metabolites: The evidence so far. Evidence-Based Compl. Altern. Med. 2013, 2013, 270418. [Google Scholar] [CrossRef]
- Espin, J.C.; Gonzalez-Sarrias, A.; Tomas-Barberan, F.A. The gut microbiota: A key factor in the therapeutic effects of (poly) phenols. Biochem. Pharmacol. 2017, 139, 82–93. [Google Scholar] [CrossRef]
- Cardona, F.; Andrés-Lacueva, C.; Tulipani, S.; Tinahones, F.J.; Queipo-Ortuño, M.I. Benefits of polyphenols on gut microbiota and implications in human health. J. Nutr. Biochem. 2013, 24, 1415–1422. [Google Scholar] [CrossRef]
- Aura, A.-M. Microbial metabolism of dietary phenolic compounds in the colon. Phytochem. Rev. 2008, 7, 407–429. [Google Scholar] [CrossRef]
- Bode, L.M.; Bunzel, D.; Huch, M.; Cho, G.-S.; Ruhland, D.; Bunzel, M.; Bub, A.; Franz, C.M.; Kulling, S.E. In vivo and in vitro metabolism of trans-resveratrol by human gut microbiota. Am. J. Clin. Nutr. 2013, 97, 295–309. [Google Scholar] [CrossRef] [PubMed]
- Clavel, T.; Henderson, G.; Alpert, C.A.; Philippe, C.; Rigottier-Gois, L.; Doré, J.; Blaut, M. Intestinal bacterial communities that produce active estrogen-like compounds enterodiol and enterolactone in humans. Appl. Environ. Microbiol 2005, 71, 6077–6085. [Google Scholar] [CrossRef]
- Landete, J. Ellagitannins, ellagic acid and their derived metabolites: A review about source, metabolism, functions and health. Food Res. Int. 2011, 44, 1150–1160. [Google Scholar] [CrossRef]
- Mingshu, L.; Kai, Y.; Qiang, H.; Dongying, J. Biodegradation of gallotannins and ellagitannins. J. Basic Microbiol. 2006, 46, 68–84. [Google Scholar] [CrossRef]
- Piwowarski, J.P.; Stanisławska, I.; Granica, S.; Stefańska, J.; Kiss, A.K. Phase II conjugates of urolithins isolated from human urine and potential role of β -glucuronidases in their disposition. Drug Met. Disp. 2017, 45, 657–665. [Google Scholar] [CrossRef]
- Feng, W.; Liu, J.; Cheng, H.; Zhang, D.; Tan, Y.; Peng, C. Dietary compounds in modulation of gut microbiota-derived metabolites. Front. Nutr. 2022, 9, 939571. [Google Scholar] [CrossRef]
- Speckmann, B.; Ehring, E.; Hu, J.; Rodriguez Mateos, A. Exploring substrate–microbe interactions: A metabiotic approach toward developing targeted synbiotic compositions. Gut Microbes 2024, 16, 2305716. [Google Scholar] [CrossRef] [PubMed]
- Martinelli, F.; Thiele, I. Microbial metabolism marvels: A comprehensive review of microbial drug transformation capabilities. Gut Microbes 2024, 16, 2387400. [Google Scholar] [CrossRef]
- Selma, M.V.; Beltrán, D.; García-Villalba, R.; Espín, J.C.; Tomás-Barberán, F.A. Description of urolithin production capacity from ellagic acid of two human intestinal Gordonibacter species. Food Funct. 2014, 5, 1779–1784. [Google Scholar] [CrossRef] [PubMed]
- Beltrán, D.; Romo-Vaquero, M.; Espín, J.C.; Tomás-Barberán, F.A.; Selma, M.V. Ellagibacter isourolithinifaciens gen. nov., sp. nov., a new member of the family Eggerthellaceae, isolated from human gut. Int. J. Syst. Evol. Microbiol. 2018, 68, 1707–1712. [Google Scholar]
- Kipshidze, M. Age-Related Changes in Proportions of Urolithins A, B, and 0. Junior Res. 2023, 1, 17–29. [Google Scholar] [CrossRef]
- Monagas, M.; Urpi-Sarda, M.; Sánchez-Patán, F.; Llorach, R.; Garrido, I.; Gómez-Cordovés, C.; Andres-Lacueva, C.; Bartolomé, B. Insights into the metabolism and microbial biotransformation of dietary flavan-3 -ols and the bioactivity of their metabolites. Food Funct. 2010, 1, 233–253. [Google Scholar] [CrossRef] [PubMed]
- Chourasia, R.; Phukon, C.L.; Abedin, M.M.; Sahoo, D.; Rai, A.K. Microbial Transformation during Gut Fermentation. In Bioactive Compounds in Fermented Foods; CRC Press: Boca Raton, FL, USA, 2021; pp. 365–402. [Google Scholar]
- Wang, M.; Li, J.; Hu, T.; Zhao, H. Metabolic fate of tea polyphenols and their crosstalk with gut microbiota. Food Sci. Hum. Wellness 2022, 11, 455–466. [Google Scholar] [CrossRef]
- Hidalgo, M.; Oruna-Concha, M.J.; Kolida, S.; Walton, G.E.; Kallithraka, S.; Spencer, J.P.; de Pascual-Teresa, S. Metabolism of anthocyanins by human gut microflora and their influence on gut bacterial growth. J. Agric. Food Chem. 2012, 60, 3882–3890. [Google Scholar] [CrossRef]
- Gade, A.; Kumar, M.S. Gut microbial metabolites of dietary polyphenols and their potential role in human health and diseases. J. Physiol. Biochem. 2023, 79, 695–718. [Google Scholar] [CrossRef]
- Sallam, I.E.; Abdelwareth, A.; Attia, H.; Aziz, R.K.; Homsi, M.N.; von Bergen, M.; Farag, M.A. Effect of gut microbiota biotransformation on dietary tannins and human health implications. Microorganisms 2021, 9, 965. [Google Scholar] [CrossRef]
- Le Sayec, M.; Xu, Y.; Laiola, M.; Gallego, F.A.; Katsikioti, D.; Durbidge, C.; Kivisild, U.; Armes, S.; Lecomte, M.; Fança-Berthon, P. The effects of Aronia berry (poly) phenol supplementation on arterial function and the gut microbiome in middle aged men and women: Results from a randomized controlled trial. Clin. Nutr. 2022, 41, 2549–2561. [Google Scholar] [CrossRef]
- Loo, Y.T.; Howell, K.; Chan, M.; Zhang, P.; Ng, K. Modulation of the human gut microbiota by phenolics and phenolic fiber-rich foods. Compr. Rev. Food Sci. Food Safety 2020, 19, 1268–1298. [Google Scholar] [CrossRef] [PubMed]
- Istas, G.; Wood, E.; Le Sayec, M.; Rawlings, C.; Yoon, J.; Dandavate, V.; Cera, D.; Rampelli, S.; Costabile, A.; Fromentin, E. Effects of aronia berry (poly) phenols on vascular function and gut microbiota: A double-blind randomized controlled trial in adult men. Am. J. Clin. Nutr. 2019, 110, 316–329. [Google Scholar] [CrossRef]
- Selma, M.V.; Tomás-Barberán, F.A.; Romo-Vaquero, M.; Cortés-Martín, A.; Espín, J.C. Understanding polyphenols’ health effects through the gut microbiota. In Dietary Polyphenols: Their Metabolism and Health Effects; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2020; pp. 497–531. [Google Scholar]
- Ortiz, C.; Manta, B. Advances in equol production: Sustainable strategies for unlocking soy isoflavone benefits. Results Chem. 2024, 7, 101288. [Google Scholar] [CrossRef]
- Kim, M.; Kim, S.-I.; Han, J.; Wang, X.-L.; Song, D.-G.; Kim, S.-U. Stereospecific biotransformation of dihydrodaidzein into (3 S)-equol by the human intestinal bacterium Eggerthella strain Julong 732. Appl. Environ. Microbiol. 2009, 75, 3062–3068. [Google Scholar] [CrossRef] [PubMed]
- Landete, J.M.; Arqués, J.; Medina, M.; Gaya, P.; De Las Rivas, B.; Muñoz, R. Bioactivation of phytoestrogens: Intestinal bacteria and health. Crit. Rev. Food Sci. Nutr. 2016, 56, 1826–1843. [Google Scholar] [CrossRef]
- Clavel, T.; Borrmann, D.; Braune, A.; Doré, J.; Blaut, M. Occurrence and activity of human intestinal bacteria involved in the conversion of dietary lignans. Anaerobe 2006, 12, 140–147. [Google Scholar] [CrossRef]
- Li, Y.; Wang, F.; Li, J.; Ivey, K.L.; Wilkinson, J.E.; Wang, D.D.; Li, R.; Liu, G.; Eliassen, H.A.; Chan, A.T. Dietary lignans, plasma enterolactone levels, and metabolic risk in men: Exploring the role of the gut microbiome. BMC Microbiol. 2022, 22, 82. [Google Scholar] [CrossRef] [PubMed]
- Peirotén, Á.; Gaya, P.; Álvarez, I.; Bravo, D.; Landete, J.M. Influence of different lignan compounds on enterolignan production by Bifidobacterium and Lactobacillus strains. Int. J. Food Microbiol. 2019, 289, 17–23. [Google Scholar] [CrossRef]
- Cueva, C.; Moreno-Arribas, M.V.; Martín-Álvarez, P.J.; Bills, G.; Vicente, M.F.; Basilio, A.; Rivas, C.L.; Requena, T.; Rodríguez, J.M.; Bartolomé, B. Antimicrobial activity of phenolic acids against commensal, probiotic and pathogenic bacteria. Res. Microbiol. 2010, 161, 372–382. [Google Scholar] [CrossRef]
- Kiriyama, Y.; Tokumaru, H.; Sadamoto, H.; Kobayashi, S.; Nochi, H. Effects of phenolic acids produced from food-derived flavonoids and amino acids by the gut microbiota on health and disease. Molecules 2024, 29, 5102. [Google Scholar] [CrossRef]
- Kan, J.; Wu, F.; Wang, F.; Zheng, J.; Cheng, J.; Li, Y.; Yang, Y.; Du, J. Phytonutrients: Sources, bioavailability, interaction with gut microbiota, and their impacts on human health. Front. Nutr. 2022, 9, 960309. [Google Scholar] [CrossRef]
- Moens, E.; Bolca, S.; Van de Wiele, T.; Van Landschoot, A.; Goeman, J.L.; Possemiers, S.; Verstraete, W. Exploration of isoxanthohumol bioconversion from spent hops into 8 -prenylnaringenin using resting cells of Eubacterium limosum. AMB Express 2020, 10, 79. [Google Scholar] [CrossRef] [PubMed]
- Possemiers, S.; Rabot, S.; Espín, J.C.; Bruneau, A.; Philippe, C.; González-Sarrías, A.; Heyerick, A.; Tomás-Barberán, F.A.; De Keukeleire, D.; Verstraete, W. Eubacterium limosum activates isoxanthohumol from hops (Humulus lupulus L.) into the potent phytoestrogen 8-prenylnaringenin in vitro and in rat intestine. J. Nutr. 2008, 138, 1310–1316. [Google Scholar] [CrossRef]
- Kwon, C.; Ediriweera, M.K.; Kim Cho, S. Interplay between phytochemicals and the colonic microbiota. Nutrients 2023, 15, 1989. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.C.; Jenner, A.M.; Low, C.S.; Lee, Y.K. Effect of tea phenolics and their aromatic fecal bacterial metabolites on intestinal microbiota. Res. Microbiol. 2006, 157, 876–884. [Google Scholar] [CrossRef]
- Wang, X.; Qi, Y.; Zheng, H. Dietary polyphenol, gut microbiota, and health benefits. Antioxidants 2022, 11, 1212. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Q.; Wang, Z.; Wang, X.; Yan, X.; Guo, Q.; Yue, Y.; Yue, T.; Yuan, Y. The bioaccessibility, bioavailability, bioactivity, and prebiotic effects of phenolic compounds from raw and solid-fermented mulberry leaves during in vitro digestion and colonic fermentation. Food Res. Int. 2023, 165, 112493. [Google Scholar] [CrossRef]
- Makarewicz, M.; Drozdz, I.; Tarko, T.; Duda-Chodak, A. The interactions between polyphenols and microorganisms, especially gut microbiota. Antioxidants 2021, 10, 188. [Google Scholar] [CrossRef]
- Dokic, J.; Dinic, M.; Sokovic Bajic, S.; Bisenic, A.; Mitrovic, H.; Jakovljevic, S.; Radojevic, D.; Brdaric, E.; Lukic, J.; Zivkovic, M. High-throughput workflow for cultivation and characterization of gut microbiota strains with anti-inflammatory properties and metabolite signature associated with gut-brain communication. Sci. Rep. 2025, 15, 8741. [Google Scholar] [CrossRef]
- Hu, J.; Chen, J.; Xu, X.; Hou, Q.; Ren, J.; Yan, X. Gut microbiota-derived 3 -phenylpropionic acid promotes intestinal epithelial barrier function via AhR signaling. Microbiome 2023, 11, 102. [Google Scholar]
- Elkhalifa, M.E.; Ashraf, M.; Ahmed, A.; Usman, A.; Hamdoon, A.A.; Elawad, M.A.; Almalki, M.G.; Mosa, O.F.; Niyazov, L.N.; Ayaz, M. Polyphenols and their nanoformulations as potential antibiofilm agents against multidrug-resistant pathogens. Fut. Microbiol. 2024, 19, 255–279. [Google Scholar] [CrossRef]
- Alvarez-Martinez, F.J.; Barrajon-Catalan, E.; Encinar, J.A.; Rodriguez-Diaz, J.C.; Micol, V. Antimicrobial capacity of plant polyphenols against gram-positive bacteria: A comprehensive review. Curr. Med. Chem. 2020, 27, 2576–2606. [Google Scholar] [CrossRef]
- Ryu, D.; Mouchiroud, L.; Andreux, P.A.; Katsyuba, E.; Moullan, N.; Nicolet-dit-Félix, A.A.; Williams, E.G.; Jha, P.; Lo Sasso, G.; Huzard, D. Urolithin A induces mitophagy and prolongs lifespan in C. elegans and increases muscle function in rodents. Nat. Med. 2016, 22, 879–888. [Google Scholar] [PubMed]
- Zhang, Q.; Zhang, W.; Yuan, X.; Peng, X.; Hu, G. Urolithin A in Central Nervous System Disorders: Therapeutic Applications and Challenges. Biomedicines 2025, 13, 1553. [Google Scholar] [CrossRef] [PubMed]
- Lavefve, L.; Howard, L.R.; Carbonero, F. Berry polyphenols metabolism and impact on human gut microbiota and health. Food Funct. 2020, 11, 45–65. [Google Scholar] [CrossRef]
- Zhang, M.; Cui, S.; Mao, B.; Zhang, Q.; Zhao, J.; Zhang, H.; Tang, X.; Chen, W. Ellagic acid and intestinal microflora metabolite urolithin A: A review on its sources, metabolic distribution, health benefits, and biotransformation. Crit. Rev. Food Sci. Nutr. 2023, 63, 6900–6922. [Google Scholar] [CrossRef]
- Del Rio, D.; Rodriguez-Mateos, A.; Spencer, J.P.; Tognolini, M.; Borges, G.; Crozier, A. Dietary (poly) phenolics in human health: Structures, bioavailability, and evidence of protective effects against chronic diseases. Antioxid. Redox Signal. 2013, 18, 1818–1892. [Google Scholar] [CrossRef]
- Zhou, M.; Pang, X.E. Polyphenols and miRNA interplay: A novel approach to combat apoptosis and inflammation in Alzheimer’s disease. Front. Aging Neurosci. 2025, 17, 1571563. [Google Scholar] [CrossRef]
- Mitrea, L.; Nemeş, S.-A.; Szabo, K.; Teleky, B.-E.; Vodnar, D.C. Guts imbalance imbalances the brain: A review of gut microbiota association with neurological and psychiatric disorders. Front. Med. 2022, 9, 813204. [Google Scholar] [CrossRef] [PubMed]
- Petersen, K.; Mansell, T.J. Unveiling the prebiotic potential of polyphenols in gut health and metabolism. Curr. Opin. Biotech. 2025, 95, 103338. [Google Scholar] [CrossRef]
- Nemzer, B.V.; Al-Taher, F.; Kalita, D.; Yashin, A.Y.; Yashin, Y.I. Health-improving effects of polyphenols on the human intestinal microbiota: A review. Int. J. Mol. Sci. 2025, 26, 1335. [Google Scholar] [CrossRef]
- Gonzalez-Sarrias, A.; Espin-Aguilar, J.C.; Romero-Reyes, S.; Puigcerver, J.; Alajarin, M.; Berna, J.; Selma, M.V.; Espin, J.C. Main determinants affecting the antiproliferative activity of stilbenes and their gut microbiota metabolites in colon cancer cells: Astructure– activity relationship study. Int. J. Mol. Sci. 2022, 23, 15102. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Yang, Y.; Wu, Z.; Weng, P. The modulatory effect of anthocyanins from purple sweet potato on human intestinal microbiota in vitro. J. Agric. Food Chem. 2016, 64, 2582–2590. [Google Scholar] [CrossRef]
- Godos, J.; Micek, A.; Caruso, G.; Carota, G.; Di Mauro, A.; Furnari, F.; Di Giorgio, J.; D’Agostino, M.; Leonardi, A.; Balzano, R.M. Anthocyanin metabolites from gut microbiota and cognitive health. J. Berry Res. 2025, 15, 239–248. [Google Scholar] [CrossRef]
- Mezhibovsky, E.; Wu, Y.; Bawagan, F.G.; Tveter, K.M.; Szeto, S.; Roopchand, D. Impact of grape polyphenols on Akkermansia muciniphila and the gut barrier. AIMS Microbiol. 2022, 8, 544. [Google Scholar] [CrossRef]
- Martinovic, J.; Ambrus, R.; Planinic, M.; Selo, G.; Klaric, A.-M.; Perkovic, G.; Bucic-Kojic, A. Microencapsulation of grape pomace extracts with alginate-based coatings by freeze-drying: Release kinetics and in vitro bioaccessibility assessment of phenolic compounds. Gels 2024, 10, 353. [Google Scholar] [CrossRef]
- Lamothe, S.; Azimy, N.; Bazinet, L.; Couillard, C.; Britten, M. Interaction of green tea polyphenols with dairy matrices in a simulated gastrointestinal environment. Food Funct. 2014, 5, 2621–2631. [Google Scholar] [CrossRef]
- Mulet-Cabero, A.-I.; Torres-Gonzalez, M.; Geurts, J.; Rosales, A.; Farhang, B.; Marmonier, C.; Ulleberg, E.K.; Hocking, E.; Neiderer, I.; Gandolfi, I. The dairy matrix: Its importance, definition, and current application in the context of nutrition and health. Nutrients 2024, 16, 2908. [Google Scholar] [CrossRef]
- Dobani, S.; Pourshahidi, L.K.; Ternan, N.G.; McDougall, G.J.; Pereira-Caro, G.; Bresciani, L.; Mena, P.; Almutairi, T.M.; Crozier, A.; Tuohy, K.M. A review on the effects of flavan-3-ols, their metabolites, and their dietary sources on gut barrier integrity. Food Funct. 2025, 16, 815–830. [Google Scholar] [CrossRef]
- Nazzaro, F.; Ombra, M.N.; Coppola, F.; De Giulio, B.; d’Acierno, A.; Coppola, R.; Fratianni, F. Antibacterial Activity and Prebiotic Properties of Six Types of Lamiaceae Honey. Antibiotics 2024, 13, 868. [Google Scholar] [CrossRef] [PubMed]
- Coppola, F.; Abdalrazeq, M.; Fratianni, F.; Ombra, M.N.; Testa, B.; Zengin, G.; Ayala Zavala, J.F.; Nazzaro, F. Rosaceae honey: Antimicrobial activity and prebiotic properties. Antibiotics 2025, 14, 298. [Google Scholar] [CrossRef] [PubMed]
- Snoussi, M.; Noumi, E.; Hajlaoui, H.; Bouslama, L.; Hamdi, A.; Saeed, M.; Alreshidi, M.; Adnan, M.; Al-Rashidi, A.; Aouadi, K.; et al. Phytochemical Profiling of Allium subhirsutum L. Aqueous Extract with Antioxidant, Antimicrobial, Antibiofilm, and Anti-Quorum Sensing Properties: In Vitro and In Silico Studies. Plants 2021, 11, 495. [Google Scholar] [CrossRef]
- Gibson, G.R.; Hutkins, R.; Sanders, M.E.; Prescott, S.L.; Reimer, R.A.; Salminen, S.J.; Scott, K.; Stanton, C.; Swanson, K.S.; Cani, P.D. Expert consensus document: The International Scientific Association for Probiotics and Prebiotics (ISAPP) consensus statement on the definition and scope of prebiotics. Nat. Rev. Gastr. Hepat. 2017, 14, 491–502. [Google Scholar] [CrossRef] [PubMed]
- Perez-Burillo, S.; Navajas-Porras, B.; Lopez-Maldonado, A.; Hinojosa-Nogueira, D.; Pastoriza, S.; Rufian-Henares, J.A. Green tea and its relation to human gut microbiome. Molecules 2021, 26, 3907. [Google Scholar] [CrossRef]
- WHO. Antimicrobial Resistance. 2025. Available online: https://www.who.int/news-room/fact-sheets/detail/antimicrobial-resistance (accessed on 25 September 2025).
- Helmy, Y.A.; Taha-Abdelaziz, K.; Hawwas, H.A.E.H.; Ghosh, S.; AlKafaas, S.S.; Moawad, M.M.; Saied, E.M.; Kassem, I.I.; Mawad, A.M. Antimicrobial Resistance and Recent Alternatives to Antibiotics for the Control of Bacterial Pathogens with an Emphasis on Foodborne Pathogens. Antibiotics 2023, 12, 274. [Google Scholar] [CrossRef]
- Friedman, N.D.; Temkin, E.; Carmeli, Y. The negative impact of antibiotic resistance. Clin. Microbiol. Infect. 2016, 22, 416–422. [Google Scholar] [CrossRef] [PubMed]
- Hailu, W.; Helmy, Y.A.; Carney-Knisely, G.; Kauffman, M.; Fraga, D.; Rajashekara, G. Prevalence and Antimicrobial Resistance Profiles of Foodborne Pathogens Isolated from Dairy Cattle and Poultry Manure Amended Farms in Northeastern Ohio, the United States. Antibiotics 2021, 10, 1450. [Google Scholar] [CrossRef]
- FDA. Summary Report on Antimicrobials Sold or Distributed for Use in Food-Producing Animals; Department of Health and Human Services: Washington, DC, USA, 2011.
- Murray, C.J.; Ikuta, K.S.; Sharara, F.; Swetschinski, L.; Aguilar, G.R.; Gray, A.; Han, C.; Bisignano, C.; Rao, P.; Wool, E.; et al. Global burden of bacterial antimicrobial resistance in 2019: A systematic analysis. Lancet 2022, 399, 629–655. [Google Scholar] [CrossRef]
- Scharff, R.L. Food Attribution and Economic Cost Estimates for Meat- and Poultry-Related Illnesses. J. Food Prot. 2020, 83, 959–967. [Google Scholar] [CrossRef]
- Bhunia, A.K. Introduction to foodborne pathogens. In Foodborne Microbial Pathogens: Mechanisms and Pathogenesis; Springer: New York, NY, USA, 2018; pp. 1–23. [Google Scholar]
- Coppola, F.; Fratianni, F.; Bianco, V.; Wang, Z.; Pellegrini, M.; Coppola, R.; Nazzaro, F. New Methodologies as Opportunities in the Study of Bacterial Biofilms, Including Food-Related Applications. Microorganisms 2025, 13, 1062. [Google Scholar] [CrossRef] [PubMed]
- Flemming, H.-C.; Wingender, J.; Szewzyk, U.; Steinberg, P.; Rice, S.A.; Kjelleberg, S. Biofilms: An emergent form of bacterial life. Nat. Rev. Microbiol. 2016, 14, 563–575. [Google Scholar] [CrossRef]
- Friedman, M. Antibacterial, antiviral, and antifungal properties of wines and winery byproducts in relation to their flavonoid content. J. Agric. Food Chem. 2014, 62, 6025–6042. [Google Scholar] [CrossRef]
- Daglia, M. Polyphenols as antimicrobial agents. Curr. Opin. Biotechnol. 2012, 23, 174–181. [Google Scholar] [CrossRef]
- Mandal, M.K.; Domb, A.J. Antimicrobial activities of natural bioactive polyphenols. Pharmaceutics 2024, 16, 718. [Google Scholar] [CrossRef]
- Hussain, Y.; Alam, W.; Ullah, H.; Dacrema, M.; Daglia, M.; Khan, H.; Arciola, C.R. Antimicrobial potential of curcumin: Therapeutic potential and challenges to clinical applications. Antibiotics 2022, 11, 322. [Google Scholar] [CrossRef]
- Friedman, M. Overview of antibacterial, antitoxin, antiviral, and antifungal activities of tea flavonoids and teas. Mol. Nutr. Food Res. 2007, 51, 116–134. [Google Scholar] [CrossRef]
- Lima, V.N.; Oliveira-Tintino, C.D.; Santos, E.S.; Morais, L.P.; Tintino, S.R.; Freitas, T.S.; Geraldo, Y.S.; Pereira, R.L.; Cruz, R.P.; Menezes, I.R. Antimicrobial and enhancement of the antibiotic activity by phenolic compounds: Gallic acid, caffeic acid and pyrogallol. Microbial Pathog. 2016, 99, 56–61. [Google Scholar] [CrossRef]
- Wu, M.; Luo, Q.; Nie, R.; Yang, X.; Tang, Z.; Chen, H. Potential implications of polyphenols on aging considering oxidative stress, inflammation, autophagy, and gut microbiota. Cri. Rev. Food Sci. Nutr. 2021, 61, 2175–2193. [Google Scholar] [CrossRef] [PubMed]
- Gimenez-Bastida, J.; Truchado, P.; Larrosa, M.; Espin, J.; Tomas-Barberán, F.; Allende, A.; Garcia-Conesa, M.T. Urolithins, ellagitannin metabolites produced by colon microbiota, inhibit quorum sensing in Yersinia enterocolitica: Phenotypic response and associated molecular changes. Food Chem. 2012, 132, 1465–1474. [Google Scholar] [CrossRef] [PubMed]
- Taiswa, A.; Andriolo, J.M.; Hailer, M.K.; Skinner, J.L. Electrospun controlled release anti-quorum sensing filter for biofouling prevention in MCE membranes. Separ. Purif. Technol. 2024, 332, 125874. [Google Scholar] [CrossRef]
- Fan, Y.; Pedersen, O. Gut microbiota in human metabolic health and disease. Nat. Rev. Microbiol. 2021, 19, 55–71. [Google Scholar] [CrossRef]
- Zhang, Y.-M.; Rock, C.O. Evaluation of epigallocatechin gallate and related plant polyphenols as inhibitors of the FabG and FabI reductases of bacterial type II fatty-acid synthase. J. Biol. Chem. 2004, 279, 30994–31001. [Google Scholar] [CrossRef] [PubMed]
- Gorniak, I.; Bartoszewski, R.; Króliczewski, J. Comprehensive review of antimicrobial activities of plant flavonoids. Phytochem. Rev. 2019, 18, 241–272. [Google Scholar] [CrossRef]
- Karas, D.; Ulrichova, J.; Valentova, K. Galloylation of polyphenols alters their biological activity. Food Chem. Toxicol. 2017, 105, 223–240. [Google Scholar] [CrossRef]
- Wang, R.; Peng, J.; Shi, X.; Cao, S.; Xu, Y.; Xiao, G.; Li, C. Change in membrane fluidity induced by polyphenols is highly dependent on the position and number of galloyl groups. Biochim. Biophys. Acta Biomembr. 2022, 1864, 184015. [Google Scholar] [CrossRef]
- Wang, Q.; Cao, J.; Yu, H.; Zhang, J.; Yuan, Y.; Shen, X.; Li, C. The effects of EGCG on the mechanical, bioactivities, cross-linking and release properties of gelatin film. Food Chem. 2019, 271, 204–210. [Google Scholar] [CrossRef] [PubMed]
- Cui, Y.; Oh, Y.; Lim, J.; Youn, M.; Lee, I.; Pak, H.; Park, W.; Jo, W.; Park, S. AFM study of the differential inhibitory effects of the green tea polyphenol (−)-epigallocatechin-3-gallate (EGCG) against Gram-positive and Gram-negative bacteria. Food Microbiol. 2012, 29, 80–87. [Google Scholar] [CrossRef] [PubMed]
- Matilla-Cuenca, L.; Toledo-Arana, A.; Valle, J. Anti-biofilm molecules targeting functional amyloids. Antibiotics 2021, 10, 795. [Google Scholar] [CrossRef]
- Matilla-Cuenca, L.; Gil, C.; Cuesta, S.; Rapun-Araiz, B.; Ziemyte, M.; Mira, A.; Lasa, I.; Valle, J. Antibiofilm activity of flavonoids on staphylococcal biofilms through targeting BAP amyloids. Sci. Rep. 2020, 10, 18968. [Google Scholar] [CrossRef] [PubMed]
- Slobodnikova, L.; Fialova, S.; Rendekova, K.; Kovac, J.; Mucaji, P. Antibiofilm activity of plant polyphenols. Molecules 2016, 21, 1717. [Google Scholar] [CrossRef]
- Sonnleitner, D.; Sommer, C.; Scheibel, T.; Lang, G. Approaches to inhibit biofilm formation applying natural and artificial silk-based materials. Mat. Sci. Eng. C 2021, 131, 112458. [Google Scholar] [CrossRef]
- Nassarawa, S.S.; Nayik, G.A.; Gupta, S.D.; Areche, F.O.; Jagdale, Y.D.; Ansari, M.J.; Hemeg, H.A.; Al-Farga, A.; Alotaibi, S.S. Chemical aspects of polyphenol-protein interactions and their antibacterial activity. Crit. Rev. Food Sci. Nutr. 2023, 63, 9482–9505. [Google Scholar] [CrossRef]
- De Rossi, L.; Rocchetti, G.; Lucini, L.; Rebecchi, A. Antimicrobial potential of polyphenols: Mechanisms of action and microbial responses— A narrative review. Antioxidants 2025, 14, 200. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Lam, A.T. Epigallocatechin gallate and gallic acid affect colonization of abiotic surfaces by oral bacteria. Arch. Oral Biol. 2020, 120, 104922. [Google Scholar] [CrossRef]
- Trentin, D.S.; Silva, D.B.; Frasson, A.P.; Rzhepishevska, O.; da Silva, M.V.; de, L.; Pulcini, E.; James, G.; Soares, G.V.; Tasca, T.; et al. Natural green coating inhibits adhesion of clinically important bacteria. Sci. Rep. 2015, 5, 8287. [Google Scholar] [CrossRef]
- Chen, X.; Sun, M.; Zhang, L.; Hu, Y.; Yang, Z.; Duan, S.; Xu, F.-J.; Jing, J. A one-step polyphenol-based functionalization strategy of dual-enhanced antibacterial and osteogenic surfaces. Chem. Engin. J. 2024, 490, 151792. [Google Scholar] [CrossRef]
- Bae, J.-Y.; Seo, Y.-H.; Oh, S.-W. Antibacterial activities of polyphenols against foodborne pathogens and their application as antibacterial agents. Food Sci. Biotechnol. 2022, 31, 985–997. [Google Scholar] [CrossRef]
- Woolard, K.J. Synthesis and Derivatization of Compounds for the Treatment of Salmonella enterica Infections; University of Notre Dame: Notre Dame, IN, USA, 2023. [Google Scholar]
- Lostroh, C.P.; Lee, C.A. The Salmonella pathogenicity island-1 type III secretion system. Microbes Infect. 2001, 3, 1281–1291. [Google Scholar] [CrossRef]
- Cheng, G.; Jian, S.; Li, W.; Yan, L.; Chen, T.; Cheng, T.; Liu, Z.; Ye, G.; Tang, H.; Zhang, L. Epigallocatechin gallate protects mice from Salmonella enterica ser. Typhimurium infection by modulating bacterial virulence through quorum sensing inhibition. Front. Cell. Infect. Microbiol. 2024, 14, 1432111. [Google Scholar] [CrossRef]
- Wong, H.S.; Maker, G.L.; Trengove, R.D.; O’ Handley, R.M. Gas chromatography-mass spectrometry-based metabolite profiling of Salmonella enterica serovar Typhimurium differentiates between biofilm and planktonic phenotypes. Appl. Environm. Microbiol. 2015, 81, 2660–2666. [Google Scholar] [CrossRef]
- Hamilton, S.; Bongaerts, R.J.; Mulholland, F.; Cochrane, B.; Porter, J.; Lucchini, S.; Lappin-Scott, H.M.; Hinton, J.C. The transcriptional programme of Salmonella enterica serovar Typhimurium reveals a key role for tryptophan metabolism in biofilms. Bmc Genomics 2009, 10, 599. [Google Scholar] [CrossRef] [PubMed]
- MacKenzie, K.D.; Palmer, M.B.; Köster, W.L.; White, A.P. Examining the link between biofilm formation and the ability of pathogenic Salmonella strains to colonize multiple host species. Front. Veterin. Sci. 2017, 4, 138. [Google Scholar] [CrossRef] [PubMed]
- Polat, G. Natural Compounds with Antibacterial Activity Against Cronobacter spp. in Powdered Infant. Nat. Compounds Food Safety Preserv. 2021, 20, 59596418. [Google Scholar] [CrossRef]
- Guan, N.; Shi, Y.; Tong, H.; Yang, Y.; Li, J.; Guo, D.; Wang, X.; Shan, Z.; Lu, X.; Shi, C. Inhibition of Cronobacter sakazakii biofilm formation and expression of virulence factors by coenzyme Q0. Foodborne Pathog. Dis. 2023, 20, 442–452. [Google Scholar] [CrossRef]
- Tian, L.; Wu, M.; Guo, W.; Li, H.; Gai, Z.; Gong, G. Evaluation of the membrane damage mechanism of chlorogenic acid against Yersinia enterocolitica and Enterobacter sakazakii and its application in the preservation of raw pork and skim milk. Molecules 2021, 26, 6748. [Google Scholar] [CrossRef] [PubMed]
- Vazquez-Armenta, F.J.; Alvarez-Armenta, A.; Sugich-Miranda, R.; Ayala-Zavala, F.; Morales-Ortega, A.; Arvizu-Flores, A.A.; Lopez-Zavala, A.A. Inhibition mechanism of lecithin-dependent hemolysin from Vibrio parahaemolyticus by flavonoids: An enzyme kinetic and structural approach. Catalysts 2025, 15, 257. [Google Scholar] [CrossRef]
- Faleye, O.S.; Lee, J.-H.; Lee, J. Selected flavonoids exhibit antibiofilm and antibacterial effects against Vibrio by disrupting membrane integrity, virulence and metabolic activities. Biofilm 2023, 6, 100165. [Google Scholar] [CrossRef] [PubMed]
- She, W.; Cheng, A.; Ye, W.; Zeng, P.; Wang, H.; Qian, P.-Y. Mode of action of antimicrobial agents albofungins in eradicating penicillin-and cephalosporin-resistant Vibrio parahaemolyticus biofilm. Microbiol. Spectr. 2023, 11, e01563-23. [Google Scholar] [CrossRef]
- Ali, I.A.; Neelakantan, P. Antibiofilm activity of phytochemicals against Enterococcus faecalis: A literature review. Phytother. Res. 2022, 36, 2824–2838. [Google Scholar] [CrossRef]
- Vazquez-Boland, J.A.; Kuhn, M.; Berche, P.; Chakraborty, T.; Dominguez-Bernal, G.; Goebel, W.; González-Zorn, B.; Wehland, J.; Kreft, J. Listeria pathogenesis and molecular virulence determinants. Clin. Microbiol. Rev. 2001, 14, 584–640. [Google Scholar] [CrossRef]
- Du, W.; Zhou, M.; Liu, Z.; Chen, Y.; Li, R. Inhibition effects of low concentrations of epigallocatechin gallate on the biofilm formation and hemolytic activity of Listeria monocytogenes. Food Control 2018, 85, 119–126. [Google Scholar] [CrossRef]
- Santos, C.A.; Lima, E.M.F.; Franco, B.D.G.d.M.; Pinto, U.M. Exploring phenolic compounds as quorum sensing inhibitors in foodborne bacteria. Front. Microbiol. 2021, 12, 735931. [Google Scholar] [CrossRef]
- Vazquez-Armenta, F.; Hernandez-Oñate, M.; Martinez-Tellez, M.; Lopez-Zavala, A.; Gonzalez-Aguilar, G.; Gutierrez-Pacheco, M.; Ayala-Zavala, J. Quercetin repressed the stress response factor (sigB) and virulence genes (prfA, actA, inlA, and inlC), lower the adhesion, and biofilm development of L. monocytogenes. Food Microbiol. 2020, 87, 103377. [Google Scholar] [CrossRef]
- Borges, A.; Saavedra, M.J.; Simoes, M. Insights on antimicrobial resistance, biofilms and the use of phytochemicals as new antimicrobial agents. Curr. Med. Chem. 2015, 22, 2590–2614. [Google Scholar] [CrossRef]
- Snoussi, M.; Lajimi, R.H.; Latif, S.; Hamadou, W.S.; Alreshidi, M.; Ashraf, S.A.; Patel, M.; Humaidi, J.R.; Anouar, E.H.; Kadri, A.; et al. Green synthesis and characterization of silver nanoparticles from Ducrosia flabellifolia Boiss. aqueous extract: Anti-quorum sensing screening and antimicrobial activities against ESKAPE pathogens. Cell. Mol. Biol. 2024, 70, 88–96. [Google Scholar] [CrossRef]
- Terzic, M.; Zengin, G.; Mocan, A.; Frumuzachi, O.; Cetiz, M.V.; Caprioli, G.; Acquaticci, L.; Angeloni, S.; Senkardes, I.; Saka, E. Connecting chemical and biological properties to identify new functional materials: A study on Trifolium nigrescens extracts. Ind. Crops Prod. 2025, 233, 121484. [Google Scholar] [CrossRef]
- Deryabin, D.; Galadzhieva, A.; Kosyan, D.; Duskaev, G. Plant-derived inhibitors of AHL-mediated quorum sensing in bacteria: Modes of action. Int. J. Mol. Sci. 2019, 20, 5588. [Google Scholar] [CrossRef]
- Truchado, P.; Larrosa, M.; Castro-Ibanez, I.; Allende, A. Plant food extracts and phytochemicals: Their role as quorum sensing inhibitors. Trends Food Sci. Technol. 2015, 43, 189–204. [Google Scholar] [CrossRef]
- de Llano, D.G.; Esteban-Fernández, A.; Sanchez-Patan, F.; Martín-Alvarez, P.J.; Moreno-Arribas, M.V.; Bartolomé, B. Anti-adhesive activity of cranberry phenolic compounds and their microbial-derived metabolites against uropathogenic Escherichia coli in bladder epithelial cell cultures. Int. J. Mol. Sci. 2015, 16, 12119–12130. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, D.; Sun, J.; Sun, Z.; Liu, F.; Du, L.; Wang, D. Synergistic antibiofilm effects of ultrasound and phenyllactic acid against Staphylococcus aureus and Salmonella enteritidis. Foods 2021, 10, 2171. [Google Scholar] [CrossRef] [PubMed]
- Alakomi, H.-L.; Puupponen-Pimiä, R.; Aura, A.-M.; Helander, I.M.; Nohynek, L.; Oksman-Caldentey, K.-M.; Saarela, M. Weakening of Salmonella with selected microbial metabolites of berry-derived phenolic compounds and organic acids. J. Agric. Food Chem. 2007, 55, 3905–3912. [Google Scholar] [CrossRef] [PubMed]
- Ballén, V.; Cepas, V.; Ratia, C.; Gabasa, Y.; Soto, S.M. Clinical Escherichia coli: From biofilm formation to new antibiofilm strategies. Microorganisms 2022, 10, 1103. [Google Scholar] [CrossRef]
- Ramata-Stunda, A.; Petriņa, Z.; Valkovska, V.; Borodušķis, M.; Gibnere, L.; Gurkovska, E.; Nikolajeva, V. Synergistic effect of polyphenol-rich complex of plant and green propolis extracts with antibiotics against respiratory infections causing bacteria. Antibiotics 2022, 11, 160. [Google Scholar] [CrossRef]
- Uddin Mahamud, A.S.; Nahar, S.; Ashrafudoulla, M.; Park, S.H.; Ha, S.-D. Insights into antibiofilm mechanisms of phytochemicals: Prospects in the food industry. Crit. Rev. Food Sci. Nutr. 2024, 64, 1736–1763. [Google Scholar] [CrossRef] [PubMed]
- Bhardwaj, D.; Tiwari, D.; Upadhye, V.J.; Ramniwas, S.; Rautela, I.; Ballal, S.; Kumar, S.; Bhat, M.; Sharma, S.; Kumar, M.R. Discovery of new natural phytocompounds: The modern tools to fight against traditional bacterial pathogens. Curr. Pharm. Biotechnol. 2024. [Google Scholar] [CrossRef]
- Chen, L.; Yu, K.; Chen, L.; Zheng, X.; Huang, N.; Lin, Y.; Jia, H.; Liao, W.; Cao, J.; Zhou, T. Synergistic activity and biofilm formation effect of colistin combined with PFK-158 against colistin-resistant gram-negative bacteria. Infect. Drug. Resist. 2021, 14, 2143–2154. [Google Scholar] [CrossRef]
- Colletti, A.; Sangiorgio, L.; Martelli, A.; Testai, L.; Cicero, A.F.; Cravotto, G. Highly active cranberry’s polyphenolic fraction: New advances in processing and clinical applications. Nutrients 2021, 13, 2546. [Google Scholar] [CrossRef]
- Tomas-Barberan, F.A.; Gonzalez-Sarrias, A.; Garcia-Villalba, R.; Nunez-Sanchez, M.A.; Selma, M.V.; Garcia-Conesa, M.T.; Espin, J.C. Urolithins, the rescue of “old” metabolites to understand a “new” concept: Metabotypes as a nexus among phenolic metabolism, microbiota dysbiosis, and host health status. Mol. Nutr. Food Res. 2017, 61, 1500901. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, S.; Erickson, D.; Chua, M.J.; Collins, J.; Jala, V.R. The microbial metabolite urolithin A reduces Clostridioides difficile toxin expression and toxin-induced epithelial damage. Msystems 2024, 9, e01255-23. [Google Scholar] [CrossRef]
- Nandi, P.; Kanthal, J.; Hansda, S.; Ghosh, S. Neurophysiological Implications of Food Safety: Food Born Pathogens and Its Neurological Impact. In Physiological Perspectives on Food Safety: Exploring the Intersection of Health and Nutrition; Sarkar, T., Hamad, A., Chatterjee, A., Eds.; Springer: Cham, Switzerland, 2025. [Google Scholar] [CrossRef]
- Marano, G.; Mazza, M.; Lisci, F.M.; Ciliberto, M.; Traversi, G.; Kotzalidis, G.D.; Berardis, D.D.; Laterza, L.; Sani, G.; Gasbarrini, A.; et al. The microbiota–gut–brain- axis: Psychoneuroimmunological insights. Nutrients 2023, 15, 1496. [Google Scholar] [CrossRef]
- Mhanna, A.; Martini, N.; Hmaydoosh, G.; Hamwi, G.; Jarjanazi, M.; Zaifah, G.; Kazzazo, R.; Mohamad, A.H.; Alshehabi, Z. The correlation between gut microbiota and both neurotransmitters and mental disorders: A narrative review. Medicine 2024, 103, e37114. [Google Scholar] [CrossRef] [PubMed]
- Aljeradat, B.; Kumar, D.; Abdulmuizz, S.; Kundu, M.; Almealawy, Y.F.; Batarseh, D.R.; Atallah, O.; Ennabe, M.; Alsarafandi, M.; Alan, A.; et al. Neuromodulation and the gut–brain-axis: Therapeutic mechanisms and implications for gastrointestinal and neurological disorders. Pathophysiology 2024, 31, 244–268. [Google Scholar] [CrossRef]
- Chaudhry, T.S.; Senapati, S.G.; Gadam, S.; Mannam, H.P.S.S.; Voruganti, H.V.; Abbasi, Z.; Abhinav, T.; Challa, A.B.; Pallipamu, N.; Bheemisetty, N.; et al. The impact of microbiota on the gut–brain-axis: Examining the complex interplay and implications. J. Clin. Med. 2023, 12, 5231. [Google Scholar] [CrossRef]
- Bhatt, A.P.; Redinbo, M.R.; Bultman, S.J. The role of the microbiome in cancer development and therapy. CA A Cancer J. Clin. 2017, 67, 326–344. [Google Scholar] [CrossRef] [PubMed]
- Hamon, M.A.; Cossart, P. Histone modifications and chromatin remodeling during bacterial infections. Cell Host Micr. 2008, 4, 100–109. [Google Scholar] [CrossRef]
- Warren, A.; Nyavor, Y.; Zarabian, N.; Mahoney, A.; Frame, L.A. The microbiota-gut-brain-immune interface in the pathogenesis of neuroinflammatory diseases: A narrative review of the emerging literature. Front. Immunol. 2024, 15, 1365673. [Google Scholar] [CrossRef] [PubMed]
- Nakhal, M.M.; Yassin, L.K.; Alyaqoubi, R.; Saeed, S.; Alderei, A.; Alhammadi, A.; Alshehhi, M.; Almehairbi, A.; Al Houqani, S.; BaniYas, S.; et al. The microbiota–gut–brain-axis and neurological disorders: A comprehensive review. Life 2024, 14, 1234. [Google Scholar] [CrossRef]
- Shu, Z.; Dan, D.; Ming, X.; Wei, C.; Huan, D. Direct and indirect effects of pathogenic bacteria on the integrity of intestinal barrier. Therapeutic Adv. Gastroenterol. 2023, 16, 17562848231176427. [Google Scholar] [CrossRef]
- Camilleri, M. Leaky gut: Mechanisms, measurement and clinical implications in humans. Gut 2019, 68, 1516–1526. [Google Scholar] [CrossRef]
- Haroon, E.; Miller, A.H.; Sanacora, G. Inflammation, glutamate, and glia: A trio of trouble in mood disorders. Neuropsychopharmacology 2017, 42, 193–215. [Google Scholar] [CrossRef]
- Boyd, R.J.; Avramopoulos, D.; Jantzie, L.L.; McCallion, A.S. Neuroinflammation represents a common theme amongst genetic and environmental risk factors for Alzheimer and Parkinson diseases. J. Neuroinflammation 2022, 19, 223. [Google Scholar] [CrossRef] [PubMed]
- Disson, O.; Lecuit, M. Targeting of the central nervous system by Listeria monocytogenes. Virulence 2012, 3, 213–221. [Google Scholar] [CrossRef]
- Taha, A.R. Central nervous system infection. In Highly Infectious Diseases in Critical Care: A Comprehensive Clinical Guide; Springer International Publishing: Cham, Switzerland, 2020; pp. 147–174. [Google Scholar]
- Liu, D. Clostridium botulinum and associated neurotoxins. In Molecular Medical Microbiology; Academic Press: Cambridge, MA, USA, 2024; pp. 933–944. [Google Scholar] [CrossRef]
- Zhan, C.Y. E.coli pathogenesis: From commensal to pathogenic strains. Mol. Microbiol. Res. 2025, 15, 18–27. [Google Scholar]
- Goldstein, J.; Nuñez-Goluboay, K.; Pinto, A. Therapeutic strategies to protect the central nervous system against Shiga toxin from enterohemorrhagic Escherichia coli. Curr. Neuropharmacol. 2021, 19, 24–44. [Google Scholar] [CrossRef] [PubMed]
- Jiang, T.; Zhu, K.; Kang, G.; Wu, G.; Tan, Y. Neuroinflammation in neurodegenerative disorders: Activation of microglia by microbial infection. AIMS Molecular Science 2025, 12, 198–215. [Google Scholar] [CrossRef]
- De Chiara, S.; De Simone Carone, L.; Cirella, R.; Andretta, E.; Silipo, A.; Molinaro, A.; Mercogliano, M.; Di Lorenzo, F. Beyond the toll-like receptor 4. Structure-dependent lipopolysaccharide recognition systems: How far are we? Chem. Med. Chem. 2025, 20, e202400780. [Google Scholar] [CrossRef]
- Li, L.; Acioglu, C.; Heary, R.F.; Elkabes, S. Role of astroglial toll-like receptors (TLRs) in central nervous system infections, injury and neurodegenerative diseases. Brain Behav. Immun. 2021, 91, 740–755. [Google Scholar]
- Cassidy, B.R.; Zhang, M.; Drevets, D.A. Neuroimmune Consequences of L. monocytogenes Infection. In In Vitro and In Vivo Models to Study Infections of the Central Nervous System; Iovino, F., Ed.; Humana: New York, NY, USA, 2025; pp. 35–49. [Google Scholar] [CrossRef]
- Monash, A.; Tam, J.; Rosen, O.; Soreq, H. Botulinum neurotoxins: History, mechanism, and applications. A narrative review. J. Neurochem. 2025, 169, e70187. [Google Scholar] [CrossRef]
- Trisal, A.; Singh, I.; Garg, G.; Jorwal, K.; Singh, A.K. Gut–brain-axis and brain health: Modulating neuroinflammation, cognitive decline, and neurodegeneration. 3 Biotech 2025, 15, 25. [Google Scholar] [CrossRef] [PubMed]
- Gouri, H.; Bhalla, G. Neurotoxic chemistry: Unraveling the chemical mechanisms connecting environmental toxin exposure to neurological disorders. J. Integr. Sci. Technol. 2025, 13, 1064. [Google Scholar] [CrossRef]
- Rajasekaran, R.; Saahithya, R. Elements of Reproduction and Reproductive Diseases of Goats; Tanmoy, R., Ed.; Wiley online: Hoboken, NJ, USA, 2025; pp. 463–472. [Google Scholar]
- Clauss, H.E.; Lorber, B. Central nervous system infection with Listeria monocytogenes. Curr. Infect. Dis. Rep. 2008, 10, 300–306. [Google Scholar] [CrossRef]
- Lee, M.S.; Tesh, V.L. Roles of Shiga toxins in immunopathology. Toxins 2019, 11, 212. [Google Scholar] [CrossRef]
- Vida, C.; Gonzalez, M.E.; De la Fuente, M. Increase of oxidation and inflammation in nervous and immune systems with aging and anxiety. Curr. Pharm. Des. 2014, 20, 4656–4678. [Google Scholar] [CrossRef] [PubMed]
- Abdol Samat, H.N.; Razali, N.N.; Mahadzir, H.; Tengku Muhammad, T.S.; Ling, K.H.; Mansor, N.I.; Abidin, S.Z. The Interplay of Inflammation and Gut-Microbiota Dysbiosis in Alzheimer’s Disease: Mechanisms and Therapeutic Potential. Int. J. Mol. Sci. 2025, 26, 8905. [Google Scholar] [CrossRef]
- Yang, K.; Chen, J.; Wang, T.; Zhang, Y. Pathogenesis of sepsis-associated encephalopathy: More than blood–brain barrier dysfunction. Mol. Biol. Rep. 2022, 49, 10091–10099. [Google Scholar] [CrossRef] [PubMed]
- Tran, V.T.A.; Zhu, X.; Jamsranjav, A.; Lee, L.P.; Cho, H. Escherichia coli K1-colibactin meningitis induces microglial NLRP3/IL-18 exacerbating H3K4me3-synucleinopathy in human inflammatory gut-brain-axis. Commun. Biol. 2025, 8, 382. [Google Scholar] [CrossRef]
- Muenkel, M.; Keskin, E.; Balmes, A.; Schäffer, T.E.; Romer, F.; Lebtig, M.; Kretschmer, D.; Wright, K.; Loskill, P.; Mostowy, S.; et al. Listeria-Infected Macrophages Promote Biomechanical Alterations in Endothelial Cell Monolayers for Transmigration; Cell Press: Cambridge, MA, USA, 2025. [Google Scholar]
- Wortel, I.M.; Kim, S.; Liu, A.Y.; Ibarra, E.C.; Miller, M.J. Listeria motility increases the efficiency of epithelial invasion during intestinal infection. PLoS Pathogens 2022, 18, e1011028. [Google Scholar]
- Quan, Y.; Wang, Y.; Gao, S.; Yuan, S.; Song, S.; Liu, B.; Wang, Y. Breaking the fortress: A mechanistic review of meningitis-causing bacteria breaching tactics in blood brain barrier. Cell Commun. Signal. 2025, 23, 235. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Liu, C.C.; Zheng, H.; Huang, T.Y. Amyloid, tau, pathogen infection and antimicrobial protection in Alzheimer’s disease–conformist, nonconformist, and realistic prospects for AD pathogenesis. Transl. Neurod. 2018, 7, 34. [Google Scholar]
- Blake, S.; Harding, T.; Baroni, L.; Harding, M.; Blake, C.; Piboolnurak, P.; Grant, W. Reducing dietary lipopolysaccharides to slow progression of cognitive impairment and Alzheimer’s disease. J. Brain Sci. 2024, 7, 10–18488. [Google Scholar] [CrossRef]
- Brown, G.C.; Camacho, M.; Williams-Gray, C.H. The endotoxin hypothesis of Parkinson ’s disease. Mov. Dis. 2023, 38, 1143–1155. [Google Scholar] [CrossRef]
- Brooks, P.T.; Brakel, K.A.; Bell, J.A.; Bejcek, C.E.; Gilpin, T.; Brudvig, J.M.; Mansfield, L.S. Transplanted human fecal microbiota enhanced Guillain Barré syndrome autoantibody responses after Campylobacter jejuni infection in C57BL/6 mice. Microbiome 2017, 5, 92. [Google Scholar] [CrossRef]
- Hansda, S.; Das, H. Unraveling the Bone–Brain Communication Network. Biology 2025, 14, 1279. [Google Scholar] [CrossRef] [PubMed]
- Sweeney, M.D.; Zhao, Z.; Montagne, A.; Nelson, A.R.; Zlokovic, B.V. Blood-brain barrier: From physiology to disease and back. Physiol. Rev. 2018, 99, 21–78. [Google Scholar] [CrossRef] [PubMed]
- Van De Haar, H.J.; Burgmans, S.; Jansen, J.F.; Van Osch, M.J.; Van Buchem, M.A.; Muller, M.; Hofman, P.A.; Verhey, F.R.; Backes, W.H. Blood-brain barrier leakage in patients with early Alzheimer disease. Radiology 2016, 281, 527–535. [Google Scholar] [CrossRef]
- Dominguez-Lopez, I.; Lopez-Yerena, A.; Vallverdu-Queralt, A.; Pallas, M.; Lamuela-Raventos, R.M.; Perez, M. From the gut to the brain: The long journey of phenolic compounds with neurocognitive effects. Nutr. Rev. 2025, 83, e533–e546. [Google Scholar] [CrossRef]
- Gong, Q.Y.; Cai, L.; Jing, Y.; Wang, W.; Yang, D.X.; Chen, S.W.; Tian, H.L. Urolithin A alleviates blood-brain barrier disruption and attenuates neuronal apoptosis following traumatic brain injury in mice. Neu. Regen. Res. 2022, 17, 2007–2013. [Google Scholar]
- Cecarini, V.; Cuccioloni, M.; Zheng, Y.; Bonfili, L.; Gong, C.; Angeletti, M.; Mena, P.; Del Rio, D.; Eleuteri, A.M. Flavan-3-ol microbial metabolites modulate proteolysis in neuronal cells reducing amyloid- beta (1-42) levels. Mol. Nutr. Food Res. 2021, 65, 2100380. [Google Scholar] [CrossRef]
- Gonzalez de Llano, D.; Roldan, M.; Parro, L.; Bartolome, B.; Moreno-Arribas, M.V. Activity of microbial-derived phenolic acids and their conjugates against LPS-induced damage in neuroblastoma cells and macrophages. Metabolites 2023, 13, 108. [Google Scholar] [CrossRef] [PubMed]
- Ruotolo, R.; Minato, I.; La Vitola, P.; Artioli, L.; Curti, C.; Franceschi, V.; Brindani, N.; Amidani, D.; Colombo, L.; Salmona, M. Flavonoid-derived human phenyl–γ-valerolactone metabolites selectively detoxify amyloid-β oligomers and prevent memory impairment in a mouse model of Alzheimer’ s disease. Mol. Nutr. Food Res. 2020, 64, 1900890. [Google Scholar] [CrossRef] [PubMed]
- Melrose, J. The potential of flavonoids and flavonoid metabolites in the treatment of neurodegenerative pathology in disorders of cognitive decline. Antioxidants 2023, 12, 663. [Google Scholar] [CrossRef] [PubMed]
- Holland, T.M.; Agarwal, P.; Wang, Y.; Leurgans, S.E.; Bennett, D.A.; Booth, S.L.; Morris, M.C. Dietary flavonols and risk of Alzheimer dementia. Neurology 2020, 94, e1749–e1756. [Google Scholar] [CrossRef]
- Sekikawa, A.; Wharton, W.; Murray-Krezan, C.; Wu, M.; Chang, Y.; Snitz, B.E.; Coccari, M.; Yang, S.; Love, M.L.; Cusick, D. ACE trial design: Equol targeting estrogen receptor-β in vascular and cognitive aging. Alzheimer’s Dement. 2025, 1, e70144. [Google Scholar] [CrossRef]
- Fox, S.H.; Cardoso, F. Unmet Needs in Parkinson’ s Disease. Movem. Dis. Clin Pract. 2023, 10 (Suppl. S2), S47. [Google Scholar]
- Farez, M.F.; Fiol, M.P.; Gaitán, M.I.; Quintana, F.J.; Correale, J. Sodium intake is associated with increased disease activity in multiple sclerosis. J. Neurol. Neurosurg. Psych. 2015, 86, 26–31. [Google Scholar] [CrossRef] [PubMed]
- Iadecola, C. The pathobiology of vascular dementia. Neuron 2013, 80, 844–866. [Google Scholar] [CrossRef]
- Valls-Pedret, C.; Sala-Vila, A.; Serra-Mir, M.; Corella, D.; de la Torre, R.; Martínez-González, M.Á.; Martínez-Lapiscina, E.H.; Fitó, M.; Pérez-Heras, A.; Salas-Salvadó, J. Mediterranean diet and age-related cognitive decline: A randomized clinical trial. JAMA Internal Med. 2015, 175, 1094–1103. [Google Scholar] [CrossRef] [PubMed]
- Martín-Peláez, S.; Mosele, J.I.; Pizarro, N.; Farras, M.; De la Torre, R.; Subirana, I.; Pérez-Cano, F.J.; Castaner, O.; Solà, R.; Fernandez-Castillejo, S. Effect of virgin olive oil and thyme phenolic compounds on blood lipid profile: Implications of human gut microbiota. Eur. J. Nutr. 2017, 56, 119–131. [Google Scholar] [CrossRef]
- Habtemariam, S. Anti-inflammatory therapeutic mechanisms of natural products: Insight from rosemary diterpenes, carnosic acid and carnosol. Biomedicines 2023, 11, 545. [Google Scholar] [CrossRef]
- Kaur, D.; Singh, S. Isoquercetin neuroprotective molecular targets in Parkinson’s disease: Recent highlights and future perspectives. Lett. Drug Des. Discov. 2024, 21, 3639–3647. [Google Scholar] [CrossRef]
- Jalouli, M.; Rahman, M.A.; Biswas, P.; Rahman, H.; Harrath, A.H.; Lee, I.-S.; Kang, S.; Choi, J.; Park, M.N.; Kim, B. Targeting natural antioxidant polyphenols to protect neuroinflammation and neurodegenerative diseases: A comprehensive review. Front. Pharmacol. 2025, 16, 1492517. [Google Scholar] [CrossRef]
- Spagnuolo, C.; Moccia, S.; Russo, G.L. Anti-inflammatory effects of flavonoids in neurodegenerative disorders. Eur. J. Med. Chem. 2018, 153, 105–115. [Google Scholar] [CrossRef]
- Bucciantini, M.; Leri, M.; Nardiello, P.; Casamenti, F.; Stefani, M. Olive polyphenols: Antioxidant and anti-inflammatory properties. Antioxidants 2021, 10, 1044. [Google Scholar] [CrossRef]
- Andreux, P.A.; Blanco-Bose, W.; Ryu, D.; Burdet, F.; Ibberson, M.; Aebischer, P.; Auwerx, J.; Singh, A.; Rinsch, C. The mitophagy activator urolithin A is safe and induces a molecular signature of improved mitochondrial and cellular health in humans. Nature Metab. 2019, 1, 595–603. [Google Scholar] [CrossRef]
- Ju, T.; Zhang, Y.; Liu, L.; Zhao, X.; Li, X.; Liu, C.; Sun, S.; Wu, L. The role of gut microbiota–mitochondria crosstalk in neurodegeneration: Underlying mechanisms and potential therapies. Neural Regen. Res. 2025, 10, 4103. [Google Scholar] [CrossRef] [PubMed]
- Fang, E.F.; Hou, Y.; Palikaras, K.; Adriaanse, B.A.; Kerr, J.S.; Yang, B.; Lautrup, S.; Hasan-Olive, M.M.; Caponio, D.; Dan, X. Mitophagy inhibits amyloid-β and tau pathology and reverses cognitive deficits in models of Alzheimer’ s disease. Nature neurosci. 2019, 22, 401–412. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wang, K.; Yan, J.; Zhou, Q.; Wang, X. Recent progress in research on mechanisms of action of natural products against Alzheimer’s disease: Dietary plant polyphenols. Int. J. Mol. Sci. 2022, 23, 13886. [Google Scholar] [CrossRef]
- Zhong, W.; Gong, J.; Su, Q.; Farag, M.A.; Simal-Gandara, J.; Wang, H.; Cao, H. Dietary polyphenols ameliorate inflammatory bowel diseases: Advances and future perspectives to maximize their nutraceutical applications. Phytochem. Rev. 2025, 24, 1227–1259. [Google Scholar] [CrossRef]
- Al Olayan, E.M.; Aloufi, A.S.; AlAmri, O.D.; El-Habit, O.H.; Moneim, A.E.A. Protocatechuic acid mitigates cadmium-induced neurotoxicity in rats: Role of oxidative stress, inflammation and apoptosis. Sci. Total Environ. 2020, 723, 137969. [Google Scholar] [CrossRef]
- Gong, Z.; Huang, J.; Xu, B.; Ou, Z.; Zhang, L.; Lin, X.; Ye, X.; Kong, X.; Long, D.; Sun, X. Urolithin A attenuates memory impairment and neuroinflammation in APP/PS1 mice. J. Neuroinflam. 2019, 16, 62. [Google Scholar] [CrossRef]
- Ray, S.K.; Mukherjee, S. Evolving interplay between dietary polyphenols and gut microbiota—an emerging importance in healthcare. Front. Nutr. 2021, 8, 634944. [Google Scholar] [CrossRef]
- Conte, R.; Calarco, A.; Napoletano, A.; Valentino, A.; Margarucci, S.; Di Cristo, F.; Di Salle, A.; Peluso, G. Polyphenols nanoencapsulation for therapeutic applications. J. Biomol. Res. Ther. 2016, 5, 139. [Google Scholar]
- Kim, H.J.; Li, H.; Collins, J.J.; Ingber, D.E. Contributions of microbiome and mechanical deformation to intestinal bacterial overgrowth and inflammation in a human gut-on-a-chip. Proc. Nat. Acad. Sci. USA 2016, 113, E7–E15. [Google Scholar] [CrossRef] [PubMed]




| Polyphenol Class | Representative Compounds | Main Dietary Sources | Key Features |
|---|---|---|---|
| Phenolic acids | gallic acid, protocatechuic acid, caffeic acid, ferulic acid | berries, coffee, tea, whole grains, olives | Widely distributed in plants; precursors of many microbial metabolites [11] |
| Flavonoids | quercetin, catechins (EGCG), naringenin, hesperetin, anthocyanins | fruits (apples, berries, citrus), vegetables, tea, cocoa, red wine | Largest subclass; diverse structures and bioactivities [12] |
| Stilbenes | resveratrol, piceatannol | grapes, red wine, peanuts, berries | Known for their antioxidant and neuroprotective effects [13] |
| Lignans | secoisolariciresinol, matairesinol, enterolactone | flaxseed, sesame, whole grains, and vegetables | Converted by gut microbiota into enterolignans [14] |
| Tannins (hydrolyzable & condensed) | ellagitannins, proanthocyanidins | Nuts, berries, pomegranate, tea, wine | High molecular weight; precursors of urolithins and valerolactones [15] |
| Flavonoid Subclass | Representative Compounds | Main Dietary Sources | Key Biological Activities | Bioavailability and Metabolism |
|---|---|---|---|---|
| Flavonols | Quercetin, Kaempferol | Onions, kale, apples, berries | Potent antioxidant and anti-inflammatory effects | Mostly occur as glycosides; poorly absorbed in the small intestine but extensively metabolized by gut microbiota into bioactive phenolic acids. |
| Flavones | Luteolin, Apigenin | Parsley, celery, chamomile | Anti-cancer and neuroprotective properties | Undergo microbial deconjugation and transformation into more minor phenolic metabolites with enhanced bioactivity. |
| Flavanones | Naringenin, Hesperetin | Citrus fruits (oranges, grapefruits) | Vascular protection, antioxidant, and anti-inflammatory effects | Microbial metabolism yields phenylpropionic and phenylacetic acids, which support cardiovascular health. |
| Flavanols (Catechins) | Epicatechin, Epigallocatechin gallate (EGCG) | Tea (especially green tea), cocoa, grapes | Modulation of gut microbiota; cardiovascular and metabolic benefits | Converted into valerolactones and hydroxyphenylvaleric acids by colonic microbiota, and exhibit improved absorption and stability. |
| Anthocyanins | Cyanidin, Delphinidin | Berries, grapes, red cabbage, eggplants | Anti-inflammatory, anti-diabetic, neuroprotective activities | Rapidly degraded by gut microbiota to produce phenolic acids with preserved bioactivity. |
| Isoflavones | Genistein, Daidzein | Soybeans, legumes | Estrogenic, anti-osteoporotic, and cardioprotective effects | Act as phytoestrogens; metabolized by intestinal bacteria (e.g., into equol) with enhanced bioavailability and selective estrogen receptor modulation. |
| Factor Influencing Polyphenol Content | Description | Biological Implication |
|---|---|---|
| Processing | Drying, thermal treatment, fermentation, and mechanical processing can degrade, tianstorm or release polyphenols from plant | Alters structure and bioactivity, affects colonic availability |
| Ripeness and harvest timing | Levels of anthocyanins, flavan-3-ols, and flavonols vary significantly with ripening, with optimum harvest being matrix-dependent | Modulates precursor availability for colonic bioconversion |
| Agricultural and environmental conditions | Organic practices; UV exposure, altitude, and water availability con modify, polyphenol biosynthesis via stress responses | Affects content and profile of phenctic compounds in crops |
| Polyphenol Class | Microbial Transformation Pathways | Key Enzymes | Representative Metabolites |
|---|---|---|---|
| Flavan-3-ols | Ring fission, dehydroxylation, and decarboxylation | Reductases, dehydroxylases, esterases | 5-(3′,4′-Dihydroxyphenyl)-γ-valerolactone; Hydroxyphenylpropionic acids [46,47,48,49] |
| Ellagitannins | Hydrolysis, lactonization | Tannase, decarboxylases | Urolithins (A, B, C, D) [44,50] |
| Flavonols | Deglycosylation, dehydroxylation | β-Glucosidases, reductases | Phenylacetic acids, phenylpropionic acids [51,52,53] |
| Anthocyanins | Deglycosylation, ring cleavage | β-Glucosidases, esterases | Protocatechuic acid, gallic acid [21,53,54] |
| Stilbenes | Hydrogenation, dehydroxylation | Reductases, dehydroxylases | Dihydroresveratrol, lunularin [54] |
| Lignans | Demethylation, dehydroxylation, dehydrogenation | β-Glucosidases, dehydrogenases | Enterodiol, enterolactone [55,56]. |
| Tannins (general hydrolysable) | Hydrolysis, microbial fermentation | Esterases, decarboxylases | Gallic acid, pyrogallol, catechol derivatives [57] |
| Microbial Metabolites | Main Microbial Genera | Functional Outcomes |
|---|---|---|
| Urolithins A, B, C, D | Gordonibacter, Ellagibacter, Akkermansia | Anti-inflammatory, anti-biofilm, mitochondrial biogenesis [37,62,63,64] |
| γ-Valerolactones, hydroxyvaleric acids | Clostridium, Eubacterium, Blautia, Flavonifractor plautii, Eggerthella, Lactobacillus | BBB modulation, QS inhibition, redox regulation [33,65,66,67] |
| Protocatechuic acid, hippuric acid, phloroglucinol | Bifidobacterium, Lactobacillus, Bacteroides | Antioxidant, barrier protection, microbial modulation [68,69,70] |
| Dihydroresveratrol, lunularin | Eggerthella lenta, Slackia equolifaciens, Adlercreutzia | Anti-amyloidogenic, estrogenic modulation, neuroprotective, antifungal [43,54] |
| Hydroxyphenylpropionic acids, benzoic acids | Faecalibacterium, Roseburia, Anaerostipes | SCFA co-production, TLR modulation, colonocyte health [71,72,73,74] |
| Equol, O-desmethylangolensin | Slackia, Adlercreutzia, Eggerthella | Estrogen receptor modulation, antioxidant, neuroprotection [75,76,77] |
| Enterodiol, enterolactone | Bacteroides, Ruminococcus, Clostridium, Eggerthella | Antiproliferative, estrogenic/anti-estrogenic, cardioprotective, antioxidant, cardioprotective [71,78,79,80] |
| 3,4-dihydroxyphenylacetic acid, phenylacetic acid | Eubacterium, Lactobacillus, Bacteroides | Anti-inflammatory, immune modulation, antioxidant, intestinal protection [52,81,82,83] |
| 8-prenyl-naringenin | Eubacterium limosum | Estrogen modulation, antioxidant activity [84,85] |
| Tetrahydrocurcumin, dihydrocaffeic acid | Escherichia coli, Blautia, Clostridium | Anti-inflammatory, immune modulation, antioxidant [59,86] |
| Caffeic acid, ferulic acid | Bifidobacterium, Lactobacillus, Eubacterium | Glycemic modulation, antioxidant, liver protection [87,88,89] |
| 3-(4-hydroxyphenyl) propionic acid | Clostridium, Eubacterium, Bacteroides | Anti-inflammatory, lipid modulation, cardiovascular protection [44,86] |
| Gallic acid, ellagic acid | Lactobacillus, Bifidobacterium, Streptococcus | Antimicrobial, immune modulation, gut protection [45,90] |
| Phenylpropionic acid, phenylacetic acid | Bacteroides, Clostridium, Eubacterium | Antioxidant, microbiota modulation, cardiovascular protection [52,91,92] |
| 3,4-dihydroxyphenylacetic acid, protocatechuic acid | Bacteroides, Clostridium, Eubacterium | Anti-inflammatory, immune modulation, intestinal protection [21,48,83,90] |
| Aspect | Mechanism | Examples |
|---|---|---|
| Microbial metabolism of polyphenols | Microbial enzymes degrade polyphenols into more minor, bioactive metabolites | β-glucosidases, esterases, reductases; production of urolithins, valerolactones, hydroxyphenylacetic acids. [46,48] |
| Microbiota modulation by polyphenols | Selective growth promotion of beneficial microbes and suppression of pathogens | ↑ Akkermansia muciniphila, Faecalibacterium prausnitzii; ↓ Clostridium spp., Enterobacteriaceae [52,60,61,101,102,103,104] |
| Functional gene enrichment | Polyphenol intake increases the abundance of microbial genes involved in polyphenol catabolism. | Tannase, phenolic acid decarboxylase genes [45,104] |
| Feedback amplification of biotransformation | Polyphenol-induced taxa enhance further degradation of polyphenols into more minor metabolites. | Increased production of urolithins and hydroxycinnamic acid derivatives with repeated intake [21,46] |
| Class | Mechanism | Anti-Biofilm Outcomes |
|---|---|---|
| Urolithins (A/B) | QS interference; antivirulence at sub-MIC | ↓ AHLs, ↓ motility, ↓ biofilm maturation; reduced toxin expression (e.g., C. difficile) [134,184,185] |
| PVLs & hydroxyphenylvaleric acids | Anti-adhesive; QS attenuation | ↓ adhesion to bladder cells; ↓ initial attachment; putative repression of virulence in enterics [42] |
| DOPAC, 3,4-DHPPA, 3-HPA | Anti-adhesive; antibiotic sensitization | ↓ adhesion (UPEC, Salmonella); ↑ susceptibility to novobiocin; virulence attenuation [81,177] |
| Polyphenol metabolites (general) | Membrane & macromolecule disruption | Membrane depolarization; ROS-mediated damage; cell lysis (strain-dependent) [137,138,139,140,142] |
| EGCG and galloylated flavonoids | Anti-amyloid (biofilm matrix); membrane effects | Off-pathway oligomers; weakened matrix; ↓ biomass; ↑ antibiotic susceptibility [104,144] |
| Surface-active metabolites/coatings | Physical interference with adhesion/EPS | ↑ electrostatic repulsion; ↑ hydrophilicity; ↓ initial attachment [145] |
| Combinations (polyphenols-derived metabolites + antibiotics) | Synergy/sensitization | ↓ MIC/MBC; restored activity vs. resistant biofilms; ↓ selective pressure [170,179,180] |
| Pathogen | Polyphenols | Readouts |
|---|---|---|
| Uropathogenic E. coli (UPEC), E. coli O157 | PVLs; DOPAC; phenolic acids | ↓ adherence to T24 cells; dose-dependent anti-adhesion [49,94,152] |
| S. aureus/MRSA | EGCG, tannic acid; phenolics | ↓ biofilm maturation; ↑ IsaA; ↑ antibiotic efficacy [94,144]. |
| S. enterica | Catechin-derived metabolites (PVLs, HPVAs) (inference); EGCG | ↓ biofilm; ↓ virulence; synergy with ciprofloxacin (in vivo) [153,155] |
| C. sakazakii | Coenzyme Q0; chlorogenic acid | ↓ adhesion/motility; biofilm disruption [161] |
| L. monocytogenes | Quercetin; EGCG; general phenolics | ↓ adhesion/invasion; ↓ hemolysis; ↓ biofilm [167,168,169] |
| General AMR context | polyphenols-derived metabolites + antibiotics | Enhanced efficacy vs. biofilms; ↓ resistance pressure [179,180,183] |
| Bacterium | Effect on CNS/Neurodegenerative |
|---|---|
| E. coli (EHEC, K1) | Neuroinflammation, oxidative stress, and neuronal injury. Contributes to Alzheimer’s (amyloid-β accumulation) and Parkinson’s (α-synuclein aggregation) [186,202,203,204,213,214,215,217,221,223,226] |
| S. enterica | Cognitive decline and neurodegeneration due to chronic inflammation. May exacerbate Alzheimer’s- or Parkinson-like pathology through endotoxin exposure and α-synuclein aggregation [205,206,215,216,218,219,223] |
| L. monocytogenes | Causes meningitis, meningoencephalitis, encephalitis. Leads to chronic neuroinflammation, neuronal degeneration, and BBB disruption [199,207,211,218,219] |
| C. botulinum | Neurotoxicity, neuromuscular paralysis, and possible contribution to chronic neurodegenerative conditions (by altering gut–brain axis). [208,212] |
| Streptococcus suis | Induces bacterial meningitis and neuroinflammation [220] |
| C. jejuni | Associated with Guillain–Barré Syndrome (GBS) and potentially with multiple sclerosis (MS)-like pathology [224] |
| Metabolite/Class | Mechanism of Action |
|---|---|
| Urolithin A (UA) | Inhibition of amyloid aggregation; synaptic protection [252] |
| Phenyl-γ-valerolactones (PVLs) | Proteostasis modulation; anti-amyloid oligomer detoxification [228,232] |
| Urolithin A (UA) | Mitophagy activation & mitochondrial biogenesis; BBB support [95,229,246] |
| Microbial phenolic acids (3,4-dihydroxyphenylacetic acid; protocatechuic; dihydrocaffeic; conjugates) | Anti-inflammatory & antioxidant actions [231] |
| Phenylacetic & phenylpropionic acid derivatives | Neuroinflammation modulation; microglial polarization [240,250] |
| DOPAC & Protocatechuic acid | Antioxidant/redox modulation [251] |
| PVLs & phenolic acids (vascular axis) | Endothelial/BBB support relevant to VCI [231,238] |
| Equol (microbiome derived ERβ agonist) | Vascular–cognitive aging; metabotype-aware translation [235] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nazzaro, F.; Coppola, F.; Fratianni, F.; Abdalrazeq, M.; Ombra, M.N.; De Giulio, B.; Coppola, R.; Zengin, G. Polyphenols Bioactive Metabolites, and Their Anti-Biofilm and Neuroprotective Potential. Foods 2025, 14, 3976. https://doi.org/10.3390/foods14223976
Nazzaro F, Coppola F, Fratianni F, Abdalrazeq M, Ombra MN, De Giulio B, Coppola R, Zengin G. Polyphenols Bioactive Metabolites, and Their Anti-Biofilm and Neuroprotective Potential. Foods. 2025; 14(22):3976. https://doi.org/10.3390/foods14223976
Chicago/Turabian StyleNazzaro, Filomena, Francesca Coppola, Florinda Fratianni, Manar Abdalrazeq, Maria Neve Ombra, Beatrice De Giulio, Raffaele Coppola, and Gokhan Zengin. 2025. "Polyphenols Bioactive Metabolites, and Their Anti-Biofilm and Neuroprotective Potential" Foods 14, no. 22: 3976. https://doi.org/10.3390/foods14223976
APA StyleNazzaro, F., Coppola, F., Fratianni, F., Abdalrazeq, M., Ombra, M. N., De Giulio, B., Coppola, R., & Zengin, G. (2025). Polyphenols Bioactive Metabolites, and Their Anti-Biofilm and Neuroprotective Potential. Foods, 14(22), 3976. https://doi.org/10.3390/foods14223976










