Formation and Characterization of Bifunctional Nanoparticles Fabricated from Insoluble Rice Peptide Aggregate: Effect of Enzymes
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Chemicals
2.2. Preparation of Insoluble Rice Peptide Aggregates (IRPAs)
2.3. Rice Peptide Nanoparticles (RPNs) Preparation
2.4. Particle Size and Zeta Potential
2.5. Scanning Electron Microscopy (SEM)
2.6. Transmission Electron Microscope (TEM)
2.7. Contact Angle
2.8. Inner Interactive Forces of RPNs
2.9. Amino Acid Analysis
2.10. DPPH Radical Scavenging Activity
2.11. Iron (Fe2+) Chelating Activity
2.12. Preparation of High Internal Phase Emulsion (HIPE)
2.13. Confocal Laser Scanning Microscopy (CLSM)
2.14. Statistical Analysis
3. Results
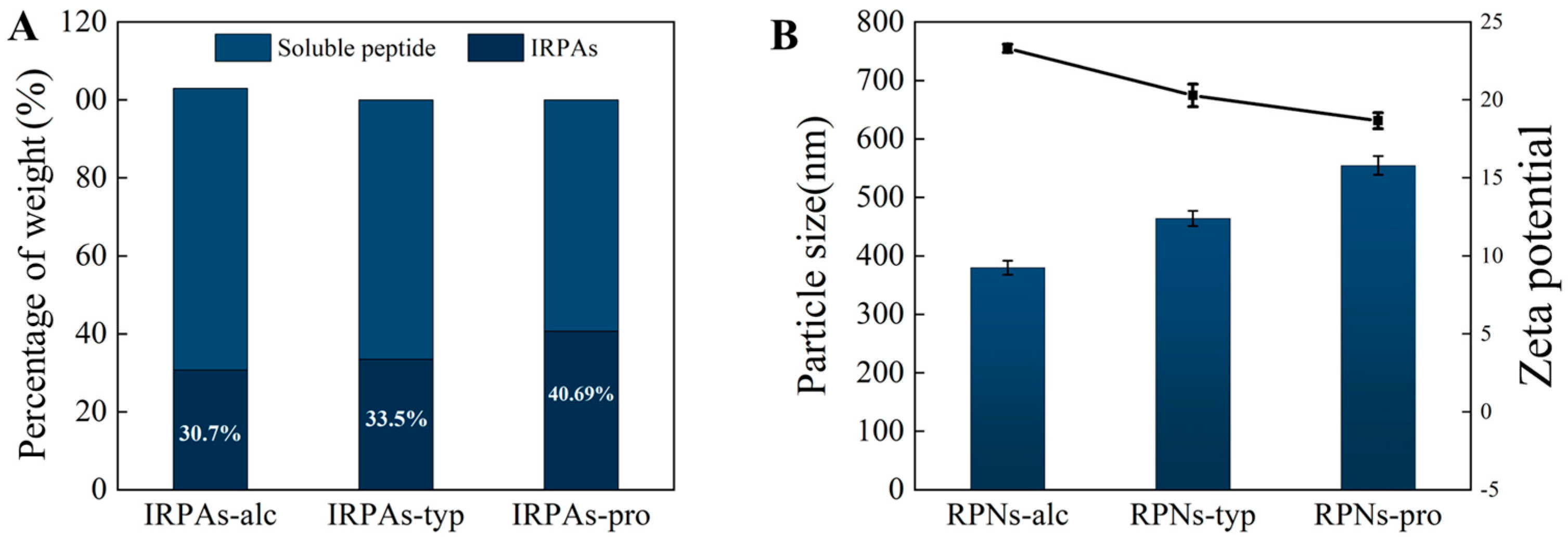
3.1. Yield of the Insoluble Rice Peptide Aggregates (IRPAs)
3.2. Size and Morphological Properties of RPNs
3.3. Three-Phase Contact Angle of RPNs
3.4. Intra-Particle Interactive Forces of RPNs
3.5. Amino Acid Composition of RPNs
3.6. Antioxidant Activity of RPNs
3.7. Characterization of HIPEs Stabilized by RPNs
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Li, T.; Wang, L.; Chen, Z.; Sun, D.; Li, Y. Electron beam irradiation induced aggregation behaviour, structural and functional properties changes of rice proteins and hydrolysates. Food Hydrocoll. 2019, 97, 105192. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, L.; Chen, Z.; Li, Y.; Luo, X.; Li, Y. Effect of high energy electron beam on proteolysis and antioxidant activity of rice proteins. Food Funct. 2020, 11, 871–882. [Google Scholar] [CrossRef]
- Cruz-Casas, D.E.; Aguilar, C.N.; Ascacio-Valdés, J.A.; Rodríguez-Herrera, R.; Chávez-González, M.L.; Flores-Gallegos, A.C. Enzymatic hydrolysis and microbial fermentation: The most favorable biotechnological methods for the release of bioactive peptides. Food Chem. Mol. Sci. 2021, 3, 100047. [Google Scholar] [CrossRef]
- Franck, M.; Perreault, V.; Suwal, S.; Marciniak, A.; Bazinet, L.; Doyen, A. High hydrostatic pressure-assisted enzymatic hydrolysis improved protein digestion of flaxseed protein isolate and generation of peptides with antioxidant activity. Food Res. Int. 2019, 115, 467–473. [Google Scholar] [CrossRef]
- Tang, T.; Filippino, K.C.; Liu, Z.; Mulholland, M.R.; Lee, C. Peptide hydrolysis and uptake of peptide hydrolysis products in the James River estuary and lower Chesapeake Bay. Mar. Chem. 2017, 197, 52–63. [Google Scholar] [CrossRef]
- de Souza Rocha, T.; Hernandez, L.M.R.; Mojica, L.; Johnson, M.H.; Chang, Y.K.; González de Mejía, E. Germination of Phaseolus vulgaris and alcalase hydrolysis of its proteins produced bioactive peptides capable of improving markers related to type-2 diabetes in vitro. Food Res. Int. 2015, 76, 150–159. [Google Scholar] [CrossRef]
- Zhang, X.; Zuo, Z.; Yu, P.; Li, T.; Guang, M.; Chen, Z.; Wang, L. Rice peptide nanoparticle as a bifunctional food-grade Pickering stabilizer prepared by ultrasonication: Structural characteristics, antioxidant activity, and emulsifying properties. Food Chem. 2021, 343, 128545. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Wang, L.; Wang, R.; Luo, X.; Li, Y.; Chen, Z. Protective effects of rice dreg protein hydrolysates against hydrogen peroxide-induced oxidative stress in HepG-2 cells. Food Funct. 2016, 7, 1429–1437. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Luo, Q.; Hong, H.; Liu, H.; Luo, Y. Novel antioxidant and ACE inhibitory peptide identified from Arthrospira platensis protein and stability against thermal/pH treatments and simulated gastrointestinal digestion. Food Res. Int. 2021, 139, 109908. [Google Scholar] [CrossRef]
- Li, T.; Lu, X.-M.; Zhang, M.-R.; Hu, K.; Li, Z. Peptide-based nanomaterials: Self-assembly, properties and applications. Bioact. Mater. 2021, 11, 268–282. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Zhang, Y.; Zhang, J.; Xiong, Y.; Peng, S.; McClements, D.J.; Zou, L.; Liang, R.; Liu, W. Utilization of protein nanoparticles to improve the dispersibility, stability, and functionality of a natural pigment: Norbixin. Food Hydrocoll. 2022, 124, 107329. [Google Scholar] [CrossRef]
- Basu, A.; Kundu, S.; Basu, C.; Ghosh, S.K.; Sur, R.; Mukherjee, A. Biopolymer nanoparticle surface chemistry dictates the nature and extent of protein hard corona. J. Mol. Liq. 2019, 282, 169–176. [Google Scholar] [CrossRef]
- Salem, A.; Ramadan, A.R.; Shoeib, T. Entrapment of β-carotene and zinc in whey protein nanoparticles using the pH cycle method: Evidence of sustained release delivery in intestinal and gastric fluids. Food Biosci. 2018, 26, 161–168. [Google Scholar] [CrossRef]
- Raeeszadeh-Sarmazdeh, M.; Hartzell, E.; Price, J.V.; Chen, W. Protein nanoparticles as multifunctional biocatalysts and health assessment sensors. Curr. Opin. Chem. Eng. 2016, 13, 109–118. [Google Scholar] [CrossRef]
- Du, Z.; Li, Q.; Li, J.; Su, E.; Liu, X.; Wan, Z.; Yang, X. Self-Assembled Egg Yolk Peptide Micellar Nanoparticles as a Versatile Emulsifier for Food-Grade Oil-in-Water Pickering Nanoemulsions. J. Agric. Food Chem. 2019, 67, 11728–11740. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhou, F.; Shen, P.; Zhao, Q.; Zhao, M. Influence of thermal treatment on oil-water interfacial properties and emulsion stabilization prepared by sono-assembled soy peptide nanoparticles. Food Hydrocoll. 2020, 103, 105646. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhao, M.; Ning, Z.; Yu, S.; Tang, N.; Zhou, F. Development of a Sono-Assembled, Bifunctional Soy Peptide Nanoparticle for Cellular Delivery of Hydrophobic Active Cargoes. J. Agric. Food Chem. 2018, 66, 4208–4218. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhou, F.; Zhao, M.; Lin, L.; Ning, Z.; Sun, B. Soy peptide nanoparticles by ultrasound-induced self-assembly of large peptide aggregates and their role on emulsion stability. Food Hydrocoll. 2018, 74, 62–71. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhou, F.; Zhao, M.; Ning, Z.; Sun-Waterhouse, D.; Sun, B. Soy peptide aggregates formed during hydrolysis reduced protein extraction without decreasing their nutritional value. Food Funct. 2017, 8, 4384–4395. [Google Scholar] [CrossRef] [PubMed]
- Creusot, N.; Gruppen, H. Protein-peptide interactions in mixtures of whey peptides and whey proteins. J. Agric. Food Chem. 2007, 55, 2474–2481. [Google Scholar] [CrossRef]
- Morales, R.; Martinez, K.D.; Pizones Ruiz-Henestrosa, V.M.; Pilosof, A.M.R. Modification of foaming properties of soy protein isolate by high ultrasound intensity: Particle size effect. Ultrason. Sonochemistry 2015, 26, 48–55. [Google Scholar] [CrossRef]
- Adinarayana, K.; Ellaiah, P.; Prasad, D.S. Purification and partial characterization of thermostable serine alkaline protease from a newly isolatedBacillus subtilis PE-11. AAPS Pharmscitech 2003, 4, 440–448. [Google Scholar] [CrossRef] [PubMed]
- Ohshima, Y.; Suzuki, Y.; Nakatani, A.; Nohara, D. Refolding of Fully Reduced Bovine Pancreatic Trypsin. J. Biosci. Bioeng. 2008, 106, 345–349. [Google Scholar] [CrossRef]
- Shen, P.; Zhou, F.; Zhang, Y.; Yuan, D.; Zhao, Q.; Zhao, M. Formation and characterization of soy protein nanoparticles by controlled partial enzymatic hydrolysis. Food Hydrocoll. 2020, 105, 105844. [Google Scholar] [CrossRef]
- Pardeike, J.; Müller, R.H. Nanosuspensions: A promising formulation for the new phospholipase A2 inhibitor PX-18. Int. J. Pharm. 2010, 391, 322–329. [Google Scholar] [CrossRef] [PubMed]
- Gezimati, J.; Creamer, L.K.; Singh, H. Heat-Induced Interactions and Gelation of Mixtures of β-Lactoglobulin and α-Lactalbumin. J. Agric. Food Chem. 1997, 45, 1130–1136. [Google Scholar] [CrossRef]
- Kuipers, B.J.H.; Gruppen, H. Identification of Strong Aggregating Regions in Soy Glycinin upon Enzymatic Hydrolysis. J. Agric. Food Chem. 2008, 56, 3818–3827. [Google Scholar] [CrossRef]
- Rajabzadeh, M.; Pourashouri, P.; Shabanpour, B.; Alishahi, A. Amino acid composition, antioxidant and functional properties of protein hydrolysates from the roe of rainbow trout (Oncorhynchus mykiss). Int. J. Food Sci. Technol. 2018, 53, 313–319. [Google Scholar] [CrossRef]
- Weiss, J.; Gulseren, I.; Kjartansson, G. Physicochemical Effects of High-Intensity Ultrasonication on Food Proteins and Carbohydrates. In Nonthermal Processing Technologies for Food; Blackwell Publishing, Ltd.: Oxford, UK, 2010; pp. 109–134. [Google Scholar]





| Amino Acid | RP | RPNs-alc | RPNs-typ | RPN-pro |
|---|---|---|---|---|
| asp | 9.62 ± 0.17 a | 10.64 ± 0.12 c | 10.72 ± 0.1 c | 10.03 ± 0.12 b |
| glu | 18.45 ± 0.21 d | 17.27 ± 0.54 c | 16.54 ± 0.28 b | 14.98 ± 0.25 a |
| ser | 4.25 ± 0.03 c | 3.34 ± 0.11 a | 3.94 ± 0.05 b | 4.29 ± 0.06 c |
| his | 2.54 ± 0.08 a | 2.78 ± 0.09 b | 2.94 ± 0.1 c | 2.7 ± 0.09 b |
| gly | 3.68 ± 0.07 a | 4.34 ± 0.04 b | 4.46 ± 0.07 b | 5.68 ± 0.02 c |
| thr | 4.31 ± 0.03 d | 2.9 ± 0.04 a | 3.03 ± 0.04 b | 3.31 ± 0.1 c |
| arg | 9.29 ± 0.05 c | 9.42 ± 0.05 d | 8.98 ± 0.08 b | 7.76 ± 0.08 a |
| tyr | 4.75 ± 0.06 c | 3.11 ± 0.01 a | 3.79 ± 0.09 b | 5.06 ± 0.07 d |
| ala | 5.46 ± 0.04 b | 5.5 ± 0.09 b | 5.5 ± 0.07 b | 5.14 ± 0.04 a |
| cys-s | 0.39 ± 0.1 a | 1.88 ± 0.08 c | 1.32 ± 0.09 b | 0.58 ± 0.08 a |
| val | 6.69 ± 0.02 a | 8.91 ± 0.02 b | 8.79 ± 0.05 b | 8.53 ± 0.07 b |
| met | 1.89 ± 0.07 c | 1.07 ± 0.07 a | 1.62 ± 0.1 b | 0.98 ± 0.12 a |
| phe | 5.62 ± 0.02 a | 6.61 ± 0.06 c | 6.5 ± 0.04 c | 6.03 ± 0.01 b |
| ile | 4.81 ± 0.01 a | 5.59 ± 0.12 b | 5.12 ± 0.07 b | 6.33 ± 0.01 c |
| leu | 8.06 ± 0.08 a | 10.92 ± 0.11 c | 9.98 ± 0.04 b | 9.16 ± 0.04 b |
| lys | 3.62 ± 0.05 b | 3.34 ± 0.08 a | 3.39 ± 0.07 a | 5.62 ± 0.04 c |
| pro | 6.56 ± 0.02 c | 4.27 ± 0.02 a | 4.09 ± 0.05 a | 4.83 ± 0.04 b |
| THAA | 32.93 ± 0.61 a | 40.48 ± 0.78 d | 38.83 ± 0. 72 c | 36.75 ± 0.69 b |
| TNEAA/TEAA | 60.13 ± 0.88 a | 70.49 ± 0.79 b | 69.74 ± 0.89 b | 73.11 ± 0.92 c |
| TAAA | 64.29 ± 0.85 b | 68.04 ± 0.93 c | 65.6 ± 0.97 b | 61.32 ± 0.82 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, X.; Ma, S.; Li, T.; Wang, L. Formation and Characterization of Bifunctional Nanoparticles Fabricated from Insoluble Rice Peptide Aggregate: Effect of Enzymes. Foods 2025, 14, 3974. https://doi.org/10.3390/foods14223974
Zhang X, Ma S, Li T, Wang L. Formation and Characterization of Bifunctional Nanoparticles Fabricated from Insoluble Rice Peptide Aggregate: Effect of Enzymes. Foods. 2025; 14(22):3974. https://doi.org/10.3390/foods14223974
Chicago/Turabian StyleZhang, Xinxia, Shengze Ma, Ting Li, and Li Wang. 2025. "Formation and Characterization of Bifunctional Nanoparticles Fabricated from Insoluble Rice Peptide Aggregate: Effect of Enzymes" Foods 14, no. 22: 3974. https://doi.org/10.3390/foods14223974
APA StyleZhang, X., Ma, S., Li, T., & Wang, L. (2025). Formation and Characterization of Bifunctional Nanoparticles Fabricated from Insoluble Rice Peptide Aggregate: Effect of Enzymes. Foods, 14(22), 3974. https://doi.org/10.3390/foods14223974







