Developing Healthier Meat Products: Application of Natural Polyphenols to Reduce Hazardous Compounds During High Temperature Processing and Digestion
Abstract
1. Introduction
2. Hazardous Compounds Produced in Meat Products
2.1. Heterocyclic Aromatic Amines
2.2. Polycyclic Aromatic Hydrocarbons
2.3. N-Nitrosamines
3. Toxic Effects of Carcinogens Generated in Meat Products
4. Controlling the Formation of Hazardous Compounds in Meat Products by Polyphenols
4.1. Influence of Polyphenols on HAAs in Meat Products
| Hazardous Compounds | Polyphenols | Meat Type | Cooking or Processing Parameters | Effects | Reference | ||
|---|---|---|---|---|---|---|---|
| Sources or Names | Dosage | Application Mode | |||||
| HAAs | Polyphenol-rich India session ale and white ale | A mixture consisting of 1 g oregano, 1 g parsley, 4 g mustard, 2 g salt, 8 g pepper, 1 g garlic, 25 g fresh onions, 25 mL olive oil, and 15 mL vinegar was uniformly incorporated into the unfiltered beer marinade. | Marinated the beef and moose steaks with 600 mL of each beer-based marinade at 4 °C for 12 h. | Moose and beef | Grilled at 200–250 °C for 25 min. | India session ale and wheat ale-based marination significantly reduced IQ, MeIQx, MeIQ, PhIP, Harman, and Norharman content in grilled moose and beef. | [8] |
| Different spices (bay leaf, star anise, red chili) and phenolic compounds (quercetin, kaempferol, capsaicin). | Different spices (1%, 2%, 3%) and phenolic compounds (0.01%, 0.02%, 0.03%). | Different spices or phenolic compounds were individually mixed with fresh beef. | Roasted beef patties | Roasted for 10 min on each side at 200 °C. | 3% red chili and 0.03% capsaicin reduced the total HAAs content by 57.09% and 68.79%, respectively. | [41] | |
| Green tea (GT), TP, EGCG | 0.05%, 0.25% and 0.50% (w/w) | GT, TP, and EGCG powders were separately added to the ground pork uniformly. | Roasted pork patties | Roasted for 30 min (each side for 15 min) at 240 °C. | GT (0.05%, and 0.25%) and EGCG (0.05%) showed inhibition rates for MeIQx, 4,8-DiMeIQx, and PhIP ranging from 4.70 to 8.32%, 6.15–16.19%, and 5.69–24.69%, respectively. | [44] | |
| Chlorogenic acid, epicatechin, rutin, quercetin, and quinic acid | 0.025, 0.125 and 0.625 mmol | Individually mixed with the lamb meat patties uniformly. | Charcoal-roasted lamb | Charcoal roasted at 550–600 °C for 10 min. | Chlorogenic acid and epicatechin significantly inhibited the formation of IQx, 8-MeIQx, Norharman, Harman, and PhIP. | [45] | |
| Zanthoxylum bungeanum Maxim. leaf (ZML) extract, chlorogenic acid, hyperoside, and quercitrin | For ZML extract, 0.015, 0.030 and 0.045% (w/w) chlorogenic acid (5 μg/g, 10 μg/g, 15 μg/g), hyperoside (15 μg/g, 30 μg/g, 45 μg/g), quercitrin (20 μg/g, 40 μg/g, 60 μg/g) | Mixed with the ground beef individually. | Roasted beef patties | Roasted for 10 min on each side at 225 °C in an electric oven. | ZML extract, hyperoside, and quercitrin dose-dependently inhibited the formation of PhIP. Specifically, ZML extract significantly reduced the formation of PhIP by 40–78.02%. | [46] | |
| Quercetin, naringenin, epicatechin, luteolin, genistein, gallic acid, resveratrol, aesculetin, and phloretin. | 0.1 mmol | Individually mixed with meat slurries per phenolic compound and prepared into round patties. | Roasted lamb patties | Roasted in a 250–300 °C electric oven for 10 min. | Among these phenolic compounds, chalcone, coumarin, and stilbene compounds had higher inhibition rates, of 69.59%, 70.41%, and 72.30%, respectively. Flavonoids inhibited HAA production by 44.05–64.87%. | [49] | |
| Tea polyphenol (TP) powder | 0.03%, 0.05%, 0.1%, 0.3% and 0.5% (w/w) | TP powder at different ratios was added into the ground meat for 4 and 6 h at 4 °C. | Grilled mutton patties | Grilled for 20 min (each side for 10 min) at 220 °C and 250 °C. | MeIQx, PhIP, and 4,8-DiMeIQx at 220 °C were reduced by 16.7%, 64.7%, and 31.1%, respectively. | [50] | |
| Apple peel polyphenol extract | 0.1%, 0.15% and 0.3% (w/w) | Mixed or applied to the surface of beef patties | Pan-fried beef patties | Fried on each side for 10 min at 223 °C. | When applied to the surface of beef patties, the extract reduced total HAA formation by 52–71%, while when mixed internally, it inhibited HAAs by 32–45%. | [51] | |
| Isorhamnetin, hispidulin, cirsimaritin, and quercetin | Isorhamnetin (6 μg/g, 12 μg/g, 18 μg/g), hispidulin (3 μg/g, 6 μg/g, 9 μg/g), cirsimaritin and quercetin (1.5 μg/g, 3 μg/g, 4.5 μg/g) | Individually added into the minced meat. | Roast lamb patties | Roasted in a preheated electric oven at 220 ± 2 °C for 20 min. | Isorhamnetin and hispidulin demonstrated dose-dependent inhibition of IQ and MeIQ formation, while quercetin showed the strongest activity, with a 65.97% suppression of IQ. | [52] | |
| Kaempferol, naringenin, and quercetin | 0.25%, 0.5%, 0.75% and 1% | Individually added to the patty. | Roasted pork patties | Roasted in an electric oven at 225 °C for 10 min per side. | Naringenin exhibited the strongest inhibitory effect on PhIP formation compared to kaempiferol and quercetin. | [53] | |
| Citrus peel extract | 1% | Added to the patty. | Grilled pork meat patties | The patties were cooked at a temperature of 225 °C for 10 min on each side. | The extract from choline chloride-based DES significantly reduced the formation of free PhIP. MeIQx, 7,8-DiMeIQx, AαC, and norharmane, with levels of PhIP, MeIQx, and AαC decreasing by 49.2–68.3%, 34.7–53.2%, and 56.6–77.4%, respectively. | [54] | |
| Avocado peel extract (APE) | 0.5% and 1% | Inclusion in the burgers. | Burgers | Samples were pan-fried for 6 min, with one turn at 3 min. The internal temperature reached 75 °C and the pans surface was 180/200 °C. | APE incorporation dose-dependently reduced HAAs. PhIP inhibition was lower at 0.5% than at 1% APE (67.18% vs. 88.44%), while AαC was reduced by 72.50% and 86.63% at these respective concentrations. | [55] | |
| Apigenin, luteolin, kaempferol, quercetin, genistein, naringenin, phlorizin, and EGCG | 0.2 mM | Mixed with the ground beef individually. | Roast beef patties | The beef patties were roasted in an electric oven for 20 min (10 min per side) at 230 °C. | Phlorizin, EGCG, and quercetin effectively reduced both total HAAs and PhIP contents, with inhibition rates of 63.76% and 60.08% for phlorizin, 78.56% and 77.45% for EGCG, and 53.74% and 67.29% for quercetin. | [56] | |
| PAHs | Heineken, Tsing Tao, Corona, Snow and Harbin beer | Marinating at a ratio of 1:1 (w/v, g/mL) | Chicken wings were marinated with different beers individually for 4 h at 4 °C. | Charcoal-grilled chicken wings | Samples were grilled 20 cm above 220 °C coals for 8 min (2 min/flip cycle), achieving 75 °C core temperature. | PAH8 generation was significantly inhibited by Heineken and Tsing Tao beer marinade, with inhibition rates of 66.92% and 31.77%, respectively. | [57] |
| (−)-Epicatechin | 0.2 mM/L, 1 mM/L, 5 mM/L | The beef was treated through pressure-assisted immersion using epicatechin. | Roasted beef meat cubes | Beef cubes were charcoal-roasted at 500–600 °C and a distance of 10 cm, turning every minute. After 12 min, the core temperature reached 85 ± 2.5 °C. | (−)-Epicatechin at 0.2 mM/L resulted in reduced PAH contents. | [58] | |
| Apple polyphenol (AP) | 0.2% | The pork pieces were marinated in the prepared AP solution 1:1 (g/mL) at 4 °C for 4 h. | Barbecued pork | The meat was grilled once the charcoal surface reached 200 °C, and turned continuously until the core temperature of the meat reached 75 °C. | 0.2% AP significantly inhibited PAH formation, with inhibition rates of 100% for Bap, 52.68% for PAH4, 53.10% for PAH8, and 37.36% for PAH16. | [59] | |
| Grape seed extract (GSE), proanthocyanidins B2 and isorhamnetin | 0.01% and 0.03% GSS, 0.001% and 0.003% (w/w) of proanthocyanidin B2 and isorhamnetin | Individually incorporated into the meat during the marination process. | Bacon | The pork slices were baked at 220 °C for 6 min. | Grape seed polyphenols significantly reduced total BaP levels in bacon. At 0.01% and 0.03%, GSE decreased BaP by 57.56% and 51.16%, respectively. Proanthocyanidin B2 (0.001% and 0.003%) inhibited BaP by 49.47% and 65.43%, while isorhamnetin at these concentrations reduced it by 39.89% and 52.66%. | [60] | |
| Red grape pomace (RGP) | 0.5%, 1%, and 3% (w/w) | The RGP was added to the burgers. | Barbecued pork burgers. | Briquettes: >300 °C (20 min preheat); Burgers: 0.22 m2 grill surface, 8–10 cm height, 7.5 min/side rotation, core temperature 98–101 °C. | The RGP did not significantly affect the PAH content. | [61] | |
| Pine needle extract (PNE) from Cedrus deodara | Marinade contains PNE of 0.025%, 0.05%, 0.1%, 0.2% (w/w) | All samples were marinated at 4 °C for 4 days and turned over once a day. | Smoked bacon | The marinated samples were smoked on a wire rack in a cylindrical smoker using applewood at 80 ± 5 °C for 2 h. | PNE reduced total PAH16 content in a dose-dependent manner. After PNE marinade, PAH4 was undetectable. | [62] | |
| Chlorogenic acid | 25 mmol/L | Minced mutton was supplemented with 2 mL of chlorogenic acid solution per 20 ± 0.1 g of meat. | Roasted mutton patties | The patties were roasted at 180–200 °C smokeless electric oven for 10 min (5 min on each side) until the surface and center temperature of the patties reached 145 °C and 72 °C, respectively. | Chlorogenic acid significantly reduced Chr, BbF, BaP, and PAH4 content compared with control group. | [63] | |
| NAs | TP, EGCG, and their palmitic acid-modified derivatives palmitoyl-TP (pTP) and palmitoyl-EGCG (pEGCG) | 0.05% (w/w) | Mixed with the sausage ingredients individually. | Chinese sausages | Lean pork and pork back fat at a 4:1 ratio were mixed with ingredients. Then, the mixtures were stuffed into hog casings after curing, and subject to fermentation and drying. | TP, EGCG, pTP and pEGCG significantly inhibited the accumulation of NDMA in sausages, with a bioactivity order of EGCG > TP > pEGCG > pTP. | [29] |
| Gallic acid | 0.05% (w/w) | Incorporated into meat matrices. | Chinese fermented sausages | Lean pork and pork back fat at a 4:1 ratio were mixed with salt before adding NaNO2 and GA alone or together. After curing, the samples were stuffed, fermented and dried. | GA at 0.05% (w/w) effectively inhibited the formation of NDMA. | [64] | |
| Prunus mume polyphenol (PMP) | 0.3, 0.6 and 0.9 g/kg | Mixed with sausage ingredients. | Cantonese sausage | Sausages with the addition of NaNO2 at 150 mg/kg meat as NIT group, and added with NaNO2+PMP as the PMP group. The obtained meat batter was stuffed into cellulose casings, ligated, and dried at 50 °C for 36 h until the moisture reached 18–20%. | PMP at 0.6 and 0.9 g/kg effectively reduced the NDPA and total NAs contents in sausages after 21-day storage. | [65] | |
| TP, AP, and cinnamon polyphenol (CP) | 100, 300, and 500 mg/kg | Pork belly pieces were marinated in the brines containing polyphenols of different concentrations for 20 h at 4 °C. | Dry-fried bacon | The bellies were heat-dried at 50 °C for 1 h with 10% relative humidity before being subjected to smoking at 55 °C for 3 h with 50% relative humidity in a smoking chamber. | TP and CP at high concentrations (500 mg/kg) significantly inhibited the content of NMPhA by 38.87% and 23.09%, respectively. | [66] | |
| Barberry extract (BE) | 200, 300 and 400 mg/kg | Added to the meat mixture. | Fermented sausages | The minced beef meat and fat were mixed with curing agents, spices, and starter culture. Then, the mixture was subject to fermentation and drying. | BE incorporation significantly reduced NPIP and NPYR levels, particularly at higher concentrations (300 mg/kg and 400 mg/kg). | [67] | |
| Rosemary extract, GSE, and green tea polyphenol (GTP) | 0.1, 0.2, 0.3, 0.4, and 0.5 µg/g | Individually added into the prepared sausage mixture. | Western-style smoked sausage | The extracts were individually added to the prepared sausage mix. Then, the mixture was cured at 4 °C for 48 h, steamed at 80 °C for 50 min, and smoked at 75 °C for 45 min. | The extracts dose-dependently inhibited the formation of NDMA, NDPA, and NMOR in sausages. | [68] | |
| Catechin liposomes (CTL) | 600 mg/kg | CT/CTL was evenly smeared on the samples and ripened at 22 °C at 75–80% relative humidity for 6 days. | Traditional Chinese bacon | Pork was cured at 4 °C for 48 h, baked at 60 °C for another 2 days, and surface-smeared with 600 mg/kg CT/CTL for 6 days. | The CTL achieved 40.45% NA reduction vs. 15.13% in CT-treated groups at storage terminus (49 days). | [69] | |
| TP | 300 mg/kg | TP was added to the sausage formulations. | Cured sausage | The fresh meat batter (85%) and backfat batter (15%) were mixed together before adding TP as the control group, 150 mg/kg NaNO2 as the NaNO2 group, and TP+NaNO2 as the TP group. | TP significantly reduced the content of total N-nitrosamines in cured sausages than treated only with NaNO2. | [70] | |
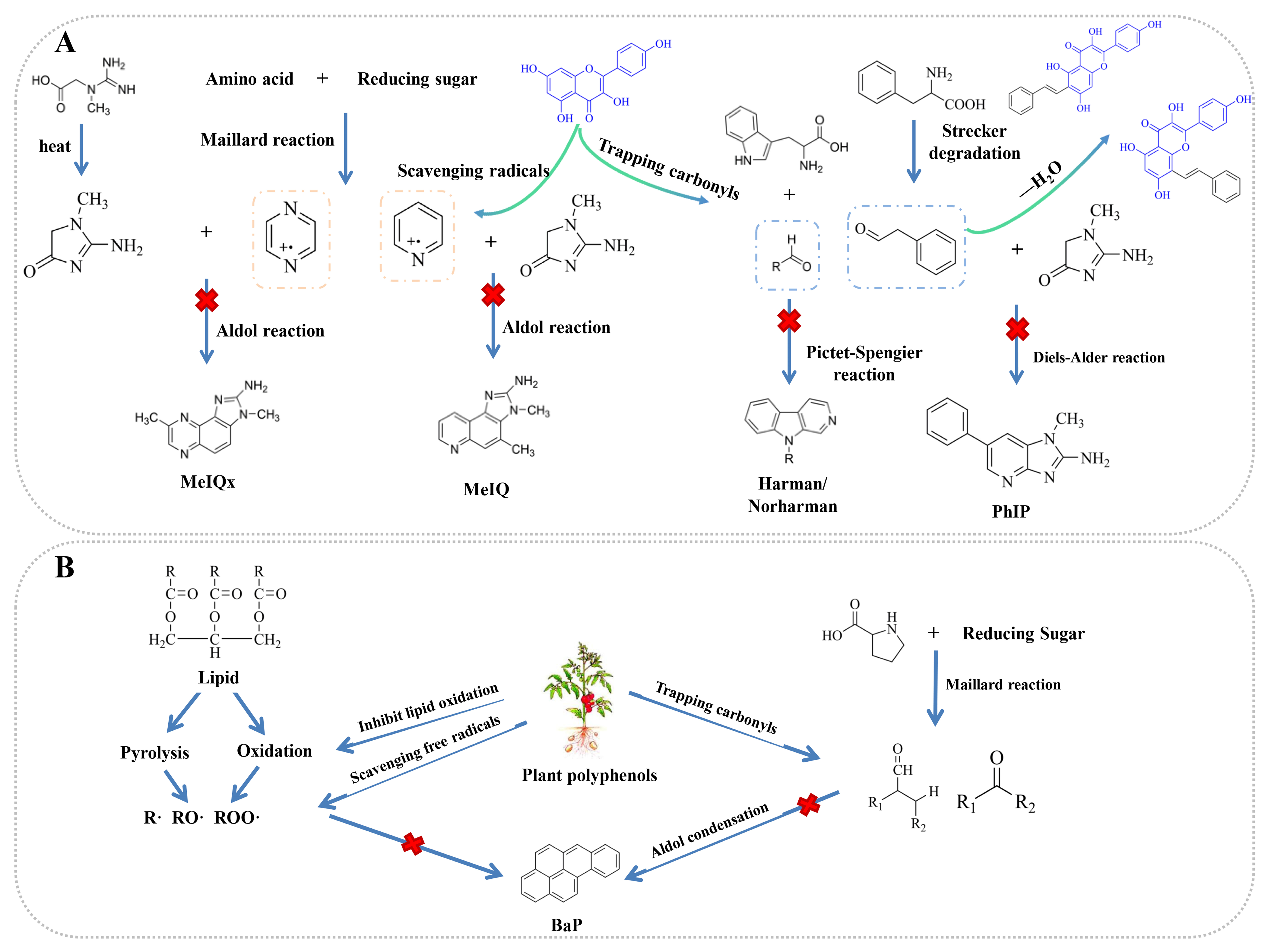
4.2. Inhibition of PAH Formation by Polyphenols
4.3. Inhibition of Polyphenols Against the Formation of NAs
5. Modulation of Toxicant Formation by Polyphenols During Gastrointestinal Transit of Meat Products
5.1. Impact of Polyphenols on Lipid Oxidation During Meat Digestion
5.2. Influences of Polyphenols on Nitrosation During Meat Digestion
6. Limitations and Future Perspectives
7. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| HAAs | Heterocyclic aromatic amines |
| IQ | 2-amino-3-methylimidazo[4,5-f]quinolone |
| IQx | 2-amino-3-methylimidazo[4,5-f]quinoxaline |
| IFP | 2-amino-1,6-dimethyl-furo[3,2-e]imidazo[4,5-b]pyridine |
| PhIP | 2-amino-1-methyl-6-phenyl-imidazo[4,5-b]pyridine |
| MeIQ | 2-amino-3,4-dimethylimidazo[4,5-f]quinoline |
| MeIQx | 2-amino-3,8-dimethylimidazo[4,5-f]quinoxaline |
| 4,8-DiMeIQx | 2-amino-3,4,8-trimethylimidazo[4,5-f]quinoxaline |
| 7,8-DiMeIQx | 2-amino-3,7,8-trimethylimidazo[4,5-f]quinoxaline |
| PAHs | Polycyclic aromatic hydrocarbons |
| BaP | benzo[a]pyrene |
| BaA | benz[a]anthracene |
| BbF | benzo[b]fluoranthene |
| Chr | chrysene |
| BkF | benzo[k]gluoranthene |
| BgP | benzo[g, h, i]perylene |
| DahA | dibenzo[a. h]anthracene |
| IP | indeno[1,2,3-c,d]pyrene |
| Pyr | pyrene |
| Phe | phenanthrene |
| Fla | fluoranthene |
| NAs | N-nitrosamines |
| NDMA | N-nitrosodimethylamine |
| NDEA | N-nitrosodiethylamine |
| NPIP | N-nitrospiperidine |
| NPYR | N-nitrosopyrolidine |
| MDA | Malondialdehyde |
| ARPs | Amadori rearrangement products |
| EGCG | (−)-epigallocatechin-3-O-gallate |
| TP | Tea polyphenol |
| GSE | Grape seed extract |
References
- Xiong, K.; Li, M.M.; Chen, Y.Q.; Hu, Y.M.; Jin, W. Formation and reduction of toxic compounds derived from the Maillard reaction during the thermal processing of different food matrices. J. Food Prot. 2024, 87, 100338. [Google Scholar] [CrossRef]
- Zheng, C.; Pettinger, M.; Gowda, G.A.N.; Lampe, J.W.; Raftery, D.; Tinker, L.F.; Huang, Y.; Navarro, S.L.; O’Brien, D.M.; Snetselaar, L.; et al. Biomarker-calibrated red and combined red and processed meat intakes with chronic disease risk in a cohort of postmenopausal women. J. Nutr. 2022, 152, 1711–1720. [Google Scholar] [CrossRef] [PubMed]
- Flores, M.; Mora, L.; Reig, M.; Toldrá, F. Risk assessment of chemical substances of safety concern generated in processed meats. Food Sci. Hum. Wellness 2019, 8, 244–251. [Google Scholar] [CrossRef]
- Efenberger-Szmechtyk, M.; Nowak, A.; Czyzowska, A. Plant extracts rich in polyphenols: Antibacterial agents and natural preservatives for meat and meat products. Crit. Rev. Food Sci. Nutr. 2021, 61, 149–178. [Google Scholar] [CrossRef]
- Calderón-Oliver, M.; López-Hernández, L.H. Food vegetable and fruit waste used in meat products. Food Rev. Int. 2020, 38, 628–654. [Google Scholar] [CrossRef]
- Hadidi, M.; Orellana-Palacios, J.C.; Aghababaei, F.; Gonzalez-Serrano, D.J.; Moreno, A.; Lorenzo, J.M. Plant by-product antioxidants: Control of protein-lipid oxidation in meat and meat products. LWT 2022, 169, 114003. [Google Scholar] [CrossRef]
- Bi, T.; Tian, Y.; Zhou, D.; Wang, X.; Jiang, H. Green tea marinades can reduce formaldehyde of pan-fried pork via Mannich reaction mechanism. LWT 2024, 197, 115886. [Google Scholar] [CrossRef]
- Manful, C.F.; Vidal, N.P.; Pham, T.H.; Nadeem, M.; Wheeler, E.; Hamilton, M.C.; Doody, K.M.; Thomas, R.H. Unfiltered beer based marinades reduced exposure to carcinogens and suppressed conjugated fatty acid oxidation in grilled meats. Food Control 2020, 111, 107040. [Google Scholar] [CrossRef]
- Chen, N.; Xu, X.; Yang, X.; Hu, X.; Chen, F.; Zhu, Y. Polyphenols as reactive carbonyl substances regulators: A comprehensive review of thermal processing hazards mitigation. Food Res. Int. 2025, 200, 115515. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Li, X.; Cao, Q.; Feng, Y.; Guo, J.; Guo, M.; Cai, J.; Liu, G.; Zhao, Y.; Qing, M. Carcinogens in meat products: Formation mechanism and mitigation strategy. Food Rev. Int. 2025, 1–32. [Google Scholar] [CrossRef]
- Xie, Y.; Geng, Y.; Yao, J.; Ji, J.; Chen, F.; Xiao, J.; Hu, X.; Ma, L. N-Nitrosamines in processed meats: Exposure, formation and mitigation strategies. J. Agric. Food Res. 2023, 13, 100645. [Google Scholar] [CrossRef]
- Keuleyan, E.; Bonifacie, A.; Gatellier, P.; Ferreira, C.; Blinet, S.; Promeyrat, A.; Nassy, G.; Santé-Lhoutellier, V.; Théron, L. Design of an in vitro model to screen the chemical reactivity induced by polyphenols and vitamins during digestion: An application to processed meat. Foods 2021, 10, 2230. [Google Scholar] [CrossRef]
- Zhao, L.; Wei, J.; Zhao, H.; Zhu, B.; Zhang, B. Detoxification of cancerogenic compounds by lactic acid bacteria strains. Crit. Rev. Food Sci. Nutr. 2018, 58, 2727–2742. [Google Scholar]
- Angénieux, M.; Bouville, A.; Mercier, F.; Laleuw, A.; Chevolleau, S.; Debrauwer, L.; Scislowski, V.; Meurillon, M. Quantification of process-induced toxicants in bovine meats cooked according to the usual preparation and cooking practices in France. Food Control 2025, 176, 111374. [Google Scholar] [CrossRef]
- Xu, Y.; Li, H.; Liang, J.; Ma, J.; Yang, J.; Zhao, X.; Zhao, W.; Bai, W.; Zeng, X.; Dong, H. High-throughput quantification of eighteen heterocyclic aromatic amines in roasted and pan-fried meat on the basis of high performance liquid chromatography-quadrupole-orbitrap high resolution mass spectrometry. Food Chem. 2021, 361, 130147. [Google Scholar] [CrossRef]
- Shi, H.; Gao, R.; Liu, H.; Wang, Z.; Zhang, C.; Zhang, D. Qualitative and quantitative assessment of key aroma compounds, advanced glycation end products and heterocyclic amines in different varieties of commercially roasted meat products. Food Chem. 2024, 436, 137742. [Google Scholar] [CrossRef]
- Neves, T.M.; da Cunha, D.T.; de Rosso, V.V.; Domene, S.M.A. Effects of seasoning on the formation of heterocyclic amines and polycyclic aromatic hydrocarbons in meats: A meta-analysis. Compr. Rev. Food Sci. Food Saf. 2021, 20, 526–541. [Google Scholar] [CrossRef]
- Lv, X.; Zhang, H.; Lin, Q.; Xie, R.; Chen, B.H.; Lai, Y.W.; Teng, H.; Chen, L.; Cao, H. Formation mechanism of polycyclic aromatic hydrocarbons in grilled beef and the mitigative effect of flavonoids. EFood 2024, 5, e143. [Google Scholar] [CrossRef]
- Alexander, J.; Benford, D.; Cockburn, A.; Cravedi, J.P.; Dogliotti, E.; Di Domenico, A.; Fernández-Cruz, M.L.; Fink-Gremmels, J.; Fürst, P.; Galli, C.; et al. Polycyclic aromatic hydrocarbons in food scientific opinion of the panel on contaminants in food chain. EFSA J. 2008, 724, 1–114. [Google Scholar]
- Kafouris, D.; Koukkidou, A.; Christou, E.; Hadjigeorgiou, M.; Yiannopoulos, S. Determination of polycyclic aromatic hydrocarbons in traditionally smoked meat products and charcoal grilled meat in Cyprus. Meat Sci. 2020, 164, 108088. [Google Scholar] [CrossRef] [PubMed]
- Onopiuk, A.; Kołodziejczak, K.; Marcinkowska-Lesiak, M.; Poltorak, A. Determination of polycyclic aromatic hydrocarbons using different extraction methods and HPLC-FLD detection in smoked and grilled meat products. Food Chem. 2022, 373, 131506. [Google Scholar] [CrossRef]
- Oz, E. The presence of polycyclic aromatic hydrocarbons and heterocyclic aromatic amines in barbecued meatballs formulated with different animal fats. Food Chem. 2021, 352, 129378. [Google Scholar] [CrossRef]
- Badyda, A.J.; Rogula-Kozłowska, W.; Majewski, G.; Bralewska, K.; Widziewicz-Rzońca, K.; Piekarska, B.; Rogulski, M.; Bihałowicz, J.S. Inhalation risk to PAHs and BTEX during barbecuing: The role of fuel/food type and route of exposure. J. Hazard. Mater. 2022, 440, 129635. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Z.; Xu, Y.; Huang, T.; Yu, Y.; Bassey, A.P.; Huang, M. The contamination, formation, determination and control of polycyclic aromatic hydrocarbons in meat products. Food Control 2022, 141, 109194. [Google Scholar] [CrossRef]
- Ravanbakhsh, M.; Yousefi, H.; Lak, E.; Ansari, M.J.; Suksatan, W.; Qasim, Q.A.; Asban, P.; Kianizadeh, M.; Mohammadi, M.J. Effect of polycyclic aromatic hydrocarbons (PAHs) on respiratory diseases and the risk factors related to cancer. Polycycl. Aromat. Comp. 2023, 43, 8371–8387. [Google Scholar] [CrossRef]
- Rot, T.; Kovačević, D.; Habschied, K.; Mastanjević, K. N-Nitrosamines in meat Products: Formation, detection and regulatory challenges. Processes 2025, 13, 1555. [Google Scholar] [CrossRef]
- Niklas, A.A.; Herrmann, S.S.; Pedersen, M.; Jakobsen, M.; Duedahl-Olesen, L. The occurrence of volatile and non-volatile N-nitrosamines in cured meat products from the Danish market. Food Chem. 2022, 378, 132046. [Google Scholar] [CrossRef]
- Lu, J.; Li, M.; Huang, Y.; Xie, J.; Shen, M.; Xie, M. A comprehensive review of advanced glycosylation end products and N-nitrosamines in thermally processed meat products. Food Control 2022, 131, 108449. [Google Scholar] [CrossRef]
- Zhou, Q.; Mo, M.; Wang, A.; Tang, B.; He, Q. Changes in N-nitrosamines, residual nNitrites, lipid oxidation, biogenic amines, and microbiota in Chinese sausages following treatment with tea polyphenols and their palmitic acid-modified derivatives. J. Food Prot. 2023, 86, 100072. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Y.; Li, P.; Zhou, Y.; Ma, F.; Chen, C. Effect of inoculating Lactobacillus pentosus R3 on N-nitrosamines and bacterial communities in dry fermented sausages. Food Control 2018, 87, 126–134. [Google Scholar] [CrossRef]
- Jee, S.C.; Kim, M.; Kim, K.S.; Kim, H.S.; Sung, J.S. Protective effects of myricetin on benzo[a]pyrene-induced 8-hydroxy-2′-deoxyguanosine and BPDE-DNA adduct. Antioxidants 2020, 9, 446. [Google Scholar] [CrossRef]
- Singh, R.; Arlt, V.M.; Henderson, C.J.; Phillips, D.H.; Farmer, P.B.; da Costa, G.G. Detection and quantitation of N-(deoxyguanosin-8-yl)-2-amino-1-methyl-6-phenylimidazo[4,5-b]pyridine adducts in DNA using online column-switching liquid chromatography tandem mass spectrometry. J. Chromatogr. B 2010, 878, 2155–2162. [Google Scholar] [CrossRef]
- Konorev, D.; Yao, L.; Turesky, R.J. Multi-DNA adduct and abasic site quantitation in vivo by nano-liquid chromatography/high-resolution orbitrap tandem mass spectrometry: Methodology for biomonitoring colorectal DNA damage. Chem. Res. Toxicol. 2022, 35, 1519–1532. [Google Scholar] [CrossRef]
- Farvid, M.S.; Sidahmed, E.; Spence, N.D.; Mante Angua, K.; Rosner, B.A.; Barnett, J.B. Consumption of red meat and processed meat and cancer incidence: A systematic review and meta-analysis of prospective studies. Eur. J. Epidemiol. 2021, 36, 937–951. [Google Scholar] [CrossRef]
- Warensjo Lemming, E.; Byberg, L.; Hoijer, J.; Baron, J.A.; Wolk, A.; Michaelsson, K. Meat consumption and the risk of hip fracture in women and men: Two prospective Swedish cohort studies. Eur. J. Nutr. 2024, 63, 1819–1833. [Google Scholar] [CrossRef]
- Myers, J.N.; Harris, K.L.; Rekhadevi, P.V.; Pratap, S.; Ramesh, A. Benzo(a)pyrene-induced cytotoxicity, cell proliferation, DNA damage, and altered gene expression profiles in HT-29 human colon cancer cells. Cell Biol. Toxicol. 2021, 37, 891–913. [Google Scholar] [CrossRef] [PubMed]
- Yang, D.; He, Z.; Wang, Z.; Fang, Q.; Oz, F.; Chen, J.; Zeng, M. Processing stage-guided effects of spices on the formation and accumulation of heterocyclic amines in smoked and cooked sausages. Food Biosci. 2022, 47, 101776. [Google Scholar] [CrossRef]
- Dong, H.; Ye, H.; Bai, W.; Zeng, X.; Wu, Q. A comprehensive review of structure-activity relationships and effect mechanisms of polyphenols on heterocyclic aromatic amines formation in thermal-processed food. Compr. Rev. Food Sci. Food Saf. 2024, 23, e70032. [Google Scholar] [CrossRef] [PubMed]
- Cao, H.; Chen, B.H.; Inbaraj, B.S.; Chen, L.; Alvarez-Rivera, G.; Cifuentes, A.; Zhang, N.; Yang, D.J.; Simal-Gandara, J.; Wang, M.; et al. Preventive potential and mechanism of dietary polyphenols on the formation of heterocyclic aromatic amines. Food Frontiers 2020, 1, 134–151. [Google Scholar] [CrossRef]
- Han, Z.; Zhu, M.; Wan, X.; Zhai, X.; Ho, C.T.; Zhang, L. Food polyphenols and Maillard reaction: Regulation effect and chemical mechanism. Crit. Rev. Food Sci. Nutr. 2024, 64, 4904–4920. [Google Scholar] [CrossRef]
- Zhou, Y.; Ma, Y.; Ma, Z.; Ma, Q.; Li, Z.; Wang, S. Theoretical exploration of the phenolic compounds’ inhibition mechanism of heterocyclic aromatic amines in roasted beef patties by density functional theory. Food Res. Int. 2024, 186, 114394. [Google Scholar] [CrossRef]
- Zhao, Y.; Fan, D.; Zheng, Z.P.; Li, E.T.S.; Chen, F.; Cheng, K.W.; Wang, M. 8-C-(E-phenylethenyl)quercetin from onion/beef soup induces autophagic cell death in colon cancer cells through ERK activation. Molec. Nutr. Food Res. 2017, 61, 1600437. [Google Scholar] [CrossRef]
- Zheng, Z.P.; Yan, Y.; Xia, J.; Zhang, S.; Wang, M.; Chen, J.; Xu, Y. A phenylacetaldehyde–flavonoid adduct, 8-C-(E-phenylethenyl)norartocarpetin, exhibits intrinsic apoptosis and MAPK pathwaysrelated anticancer potential on HepG2, SMMC-7721 and QGY-7703. Food Chem. 2016, 197, 1085–1092. [Google Scholar] [CrossRef]
- Deng, Z.; Zhang, Q.; Long, P.; Wen, M.; Han, Z.; Granato, D.; Qi, J.; Zhang, L.; Zhu, M. Effects of green tea and its polyphenols on the formation of heterocyclic aromatic amines, antioxidant capacity, and quality characteristics of roasted pork patties. Appl. Food Res. 2024, 4, 100606. [Google Scholar] [CrossRef]
- Ding, X.; Zhang, D.; Liu, H.; Wang, Z.; Hui, T. Chlorogenic acid and epicatechin: An efficient inhibitor of heterocyclic amines in charcoal roasted lamb meats. Food Chem. 2022, 368, 130865. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Ren, X.; Bao, Y.; Zhu, Y.; Zhang, Y.; Li, J.; Peng, Z. Inhibitory effects of hyperoside and quercitrin from Zanthoxylum bungeanum Maxim. leaf on 2-amino-1-methyl-6-phenylimidazo [4,5-b] pyridine formation by trapping phenylacetaldehyde. Eur. Food Res. Technol. 2022, 248, 25–34. [Google Scholar] [CrossRef]
- Xu, Y.; Huang, T.; Huang, Y.; Huang, M. Effect of mulberry leaf (Morus alba L.) extract on the quality and formation of heterocyclic amines in braised muscovy duck. Food Control 2024, 156, 110137. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhou, Y. Formation of heterocyclic aromatic amines in spiced pork shoulder: Effects of heat treatment parameters and number of soup cycles. J. Food Compos. Anal. 2022, 107, 104382. [Google Scholar] [CrossRef]
- Yang, X.; Blecker, C.; Liu, H.; Zhang, D.; Wang, Z. Effect of plant polyphenols with different m-hydroxy and o-hydroxy groups on the inhibition of heterocyclic amines formation in roasted meat. Food Control 2023, 153, 109960. [Google Scholar] [CrossRef]
- Sha, L.; Liu, S. Effect of tea polyphenols on the inhibition of heterocyclic aromatic amines in grilled mutton patties. J. Food Process. Preserv. 2022, 46, e16811. [Google Scholar] [CrossRef]
- Sabally, K.; Sleno, L.; Jauffrit, J.; Iskandar, M.M.; Kubow, S. Inhibitory effects of apple peel polyphenol extract on the formation of heterocyclic amines in pan fried beef patties. Meat Sci. 2016, 117, 57–62. [Google Scholar] [CrossRef]
- Ren, X.; Xie, B.; Li, M.; Kang, S.; Wang, W.; Yu, Q.; Zhang, Y.; Xing, L. Dual-action mechanism of Tamarix polyphenols in inhibiting IQ and MeIQ: Synergistic scavenging of free radicals and trapping of reactive carbonyls. Food Chem. 2025, 493, 145824. [Google Scholar] [CrossRef]
- Dong, H.; Chen, Q.; Xu, Y.; Li, C.; Bai, W.; Zeng, X.; Wu, Q.; Xu, H.; Deng, J. Effect and mechanism of polyphenols containing m-dihydroxyl structure on 2-amino-1-methyl-6-phenylimidazole [4, 5-b] pyridine (PhIP) formation in chemical models and roast pork patties. Food Chem. X 2024, 23, 101672. [Google Scholar] [CrossRef]
- Xu, Y.; Li, G.; Mo, L.; Li, M.; Luo, J.; Shen, Q.; Quan, W. Citrus peel extracts: Effective inhibitors of heterocyclic amines and advanced glycation end products in grilled pork meat patties. Foods 2024, 13, 114. [Google Scholar] [CrossRef]
- Trujillo-Mayol, I.; Sobral, M.M.C.; Viegas, O.; Cunha, S.C.; Alarcón-Enos, J.; Pinho, O.; Ferreira, I.M.P.L.V.O. Incorporation of avocado peel extract to reduce cooking-induced hazards in beef and soy burgers: A clean label ingredient. Food Res. Int. 2021, 147, 110434. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Q.; Zhang, S.; Wang, M.; Chen, J.; Zheng, Z.P. Inhibitory effects of selected dietary flavonoids on the formation of total heterocyclic amines and 2-amino-1-methyl-6-phenylimidazo[4,5-b] pyridine (PhIP) in roast beef patties and in chemical models. Food Funct. 2016, 7, 1057. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Xie, Y.; Wang, H.; Bai, Y.; Dai, C.; Li, C.; Xu, X.; Zhou, G. Phenolic compounds in beer inhibit formation of polycyclic aromatic hydrocarbons from charcoal-grilled chicken wings. Food Chem. 2019, 294, 578–586. [Google Scholar] [CrossRef]
- Hui, T.; Fang, Z.; Hamid, N.; Ma, Q.; Cai, K. Effect of variable pressure-assisted immersion process using (−)-epicatechin on the color, flavor, and polycyclic aromatic hydrocarbons content in roasted beef meat. LWT 2023, 178, 114602. [Google Scholar] [CrossRef]
- Cao, J.; Yang, L.; Ye, B.; Chai, Y.; Liu, L. Effect of apple polyphenol and three antioxidants on the formation of polycyclic aromatic hydrocarbon in barbecued pork. Polycycl. Aromat. Comp. 2022, 43, 6076–6087. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, Y.; Fang, H.; Wang, Y.; Cai, K.; Zhou, H.; Xu, B. Phenolic compounds in grape seed extract inhibiting the formation of benzo[a]pyrene in baked bacon related to scavenging free radicals and attenuating Maillard reaction. LWT 2023, 188, 115391. [Google Scholar] [CrossRef]
- Petrón, M.J.; Martín-Mateos, M.J.; Sánchez-Ordóñez, M.; Godoy, B.; Ramírez-Bernabé, M.R. Antioxidant and quality effects of red grape pomace in barbecued pork burgers: Implications for PAH formation. Antioxidants 2025, 14, 832. [Google Scholar] [CrossRef] [PubMed]
- Xu, Q.D.; Zhou, Z.Q.; Jing, Z.; He, Q.; Sun, Q.; Zeng, W.C. Pine needle extract from Cedrus deodara: Potential applications on hazardous chemicals and quality of smoked bacon and its mechanism. Food Control 2021, 130, 108368. [Google Scholar] [CrossRef]
- Yang, X.; Sun, X.; Zhang, D.; Wang, Z.; Gao, X. Effect of chlorogenic acid and cold plasma synergistic treatment on flavor, heterocyclic amines and polycyclic aromatic hydrocarbons content of roasted mutton patties. LWT 2025, 231, 118356. [Google Scholar] [CrossRef]
- Zhou, Q.; Jiang, L.; Zhu, J.; Lu, Y.; He, Q. The metabolic regulation mechanism of gallic acid on biogenic amines and nitrosamines in reduced-nitrite Chinese fermented sausages: A perspective of metabolomics and metagenomics. Food Chem. 2024, 456, 139900. [Google Scholar] [CrossRef]
- Cheng, J.; Chen, P.; Liu, X.; Tang, D.; Yang, H.; Zhu, M. Oxidation and nitrosation in Cantonese sausage during processing and storage-The impact of adding Prunus mume polyphenol. Food Control 2025, 167, 110789. [Google Scholar] [CrossRef]
- Deng, S.; Shi, S.; Xia, X. Effect of plant polyphenols on the physicochemical properties, residual nitrites, and N-nitrosamine formation in dry-fried bacon. Meat Sci. 2022, 191, 108872. [Google Scholar] [CrossRef]
- Kavuşan, H.S.; Serdaroğlu, M. Exploring the impact of barberry extract and grilling on oxidative and nitrosative reactions in fermented sausages: Insights into lipid-protein oxidation, nitrosamine, and 3-nitrotyrosine as a potential biomarker. Meat Sci. 2025, 226, 109830. [Google Scholar] [CrossRef]
- Zhou, Y.; Wang, Q.; Wang, S. Effects of rosemary extract, grape seed extract and green tea polyphenol on the formation of N-nitrosamines and quality of western-style smoked sausage. J. Food Process. Preserv. 2020, 44, e14459. [Google Scholar] [CrossRef]
- Wu, J.; Guan, R.; Huang, H.; Liu, Z.; Shenc, H.; Xia, Q. Effect of catechin liposomes on the nitrosamines and quality of traditional Chinese bacon. Food Funct. 2019, 10, 625. [Google Scholar] [CrossRef]
- Gao, X.; Xia, L.; Fan, Y.; Jin, C.; Xiong, G.; Hao, X.; Fu, L.; Lian, W. Evaluation of coloration, nitrite residue and antioxidant capacity of theaflavins, tea polyphenols in cured sausage. Meat Sci. 2022, 192, 108877. [Google Scholar] [CrossRef] [PubMed]
- Timón, M.L.; Manzano, R.; Martín-Mateos, M.J.; Sánchez-Ordóñez, M.; Godoy, B.; Ramírez-Bernabé, M.R. Reduction of polycyclic aromatic hydrocarbons in pork burgers using high-pressure processed white grape pomace. LWT 2025, 218, 117492. [Google Scholar] [CrossRef]
- Ma, Y.; Lin, J.; Li, M.; Zhu, Y.; Zhao, L.; Liang, D.; Cho, D.H.; Zhao, G. Effect of lignin on the formation of polycyclic aromatic hydrocarbons in smoked and grilled meat products. Int. J. Biol. Macromol. 2024, 261, 129574. [Google Scholar] [CrossRef]
- Lu, J.; Zhang, Y.; Zhou, H.; Cai, K.; Xu, B. A review of hazards in meat products: Multiple pathways, hazards and mitigation of polycyclic aromatic hydrocarbons. Food Chem. 2024, 445, 138718. [Google Scholar] [CrossRef] [PubMed]
- Cordeiro, T.; Viegas, O.; Silva, M.; Martins, Z.E.; Fernandes, I.; Ferreira, I.; Pinho, O.; Mateus, N.; Calhau, C. Inhibitory effect of vinegars on the formation of polycyclic aromatic hydrocarbons in charcoal-grilled pork. Meat Sci. 2020, 167, 108083. [Google Scholar] [CrossRef] [PubMed]
- Schaefer, S.V.; do Amaral, A.M.P.; Cherobin, A.K.; Monteiro, L.K.; Morandin, G.C.; Fischer, C.; Cavalheiro, D.; Sehn, G.A.R. Japanese grape (Hovenia dulcis) powder as an antioxidant agent in Bologna sausages. J. Sci. Food Agric. 2022, 102, 6255–6262. [Google Scholar] [CrossRef] [PubMed]
- Deng, S.; Jin, J.; He, Q. Inhibitory effect of epigallocatechin gallate, epigallocatechin, and gallic acid on the formation of N-nitrosodiethylamine in vitro. J. Food Sci. 2019, 84, 2159–2164. [Google Scholar] [CrossRef]
- Wang, Y.; Li, F.; Zhuang, H.; Chen, X.; Li, L.; Qiao, W.; Zhang, J. Effects of plant polyphenols and α-tocopherol on lipid oxidation, residual nitrites, biogenic amines, and N-nitrosamines formation during ripening and storage of dry-cured bacon. LWT 2015, 60, 199–206. [Google Scholar] [CrossRef]
- Nitta, Y.; Yasukata, F.; Kitamoto, N.; Ito, M.; Sakaue, M.; Kikuzaki, H.; Ueno, H. Inhibition of Morganella morganii histidine decarboxylase activity and histamine accumulation in mackerel muscle derived from Filipendula ulumaria extracts. J. Food Prot. 2016, 79, 463–467. [Google Scholar] [CrossRef]
- Xu, Z.; Chang, J.; Zhou, J.; Shi, Y.; Chen, H.; Han, L.; Tu, M.; Li, T. Characterization and mechanism of tea polyphenols inhibiting biogenic amine accumulation in marinated spanish mackerel. Foods 2023, 12, 2347. [Google Scholar] [CrossRef]
- Hernandez-Macias, S.; Martin-Garcia, A.; Ferrer-Bustins, N.; Comas-Baste, O.; Riu-Aumatell, M.; Lopez-Tamames, E.; Jofre, A.; Latorre-Moratalla, M.L.; Bover-Cid, S.; Vidal-Carou, M.C. Inhibition of biogenic amines formation in fermented foods by the addition of Cava Lees. Front. Microbiol. 2021, 12, 818565. [Google Scholar] [CrossRef]
- Sirvins, C.; Goupy, P.; Promeyrat, A.; Dufour, C. C-Nitrosation, C-nitration, and coupling of flavonoids with N-acetyltryptophan limit this amine N-nitrosation in a simulated cured and cooked meat. J. Agric. Food Chem. 2024, 72, 4777–4787. [Google Scholar] [CrossRef]
- Martini, S.; Cattivelli, A.; Conte, A.; Tagliazucchi, D. Black, green, and pink pepper affect differently lipid oxidation during cooking and in vitro digestion of meat. Food Chem. 2021, 350, 129246. [Google Scholar] [CrossRef]
- Ariz-Hernandez, I.; Schulz, P.; Garayoa, R.; Ansorena, D.; Astiasaran, I. Beef- and pork-based dishes from catering services: Composition and in vitro digestion effects on digestibility and lipid oxidation. Foods 2025, 14, 789. [Google Scholar] [CrossRef]
- Han, S.; Wang, Y.; Fang, Z.; Zhang, Y.; Zeng, W.; Karrar, E.; Zhang, H.; Jin, Q.; Wu, G.; Wang, X. Effect of olive polyphenols on lipid oxidation of high-fat beef during digestion. Food Res. Int. 2022, 161, 111843. [Google Scholar] [CrossRef]
- Li, J.; Zhang, H.; Yang, X.; Zhu, L.; Wu, G.; Qi, X.; Zhang, H.; Wang, Y.; Chen, X. Effect of fiber-bound polyphenols from highland barley on lipid oxidation products of cooked pork during in vitro gastrointestinal digestion. J. Sci. Food Agric. 2023, 103, 5070–5076. [Google Scholar] [CrossRef]
- Van Hecke, T.; Ho, P.L.; Goethals, S.; De Smet, S. The potential of herbs and spices to reduce lipid oxidation during heating and gastrointestinal digestion of a beef product. Food Res. Int. 2017, 102, 785–792. [Google Scholar] [CrossRef]
- Martini, S.; Cavalchi, M.; Conte, A.; Tagliazucchi, D. The paradoxical effect of extra-virgin olive oil on oxidative phenomena during in vitro co-digestion with meat. Food Res. Int. 2018, 109, 82–90. [Google Scholar] [CrossRef] [PubMed]
- Kuffa, M.; Priesbe, T.J.; Krueger, C.G.; Reed, J.D.; Richards, M.P. Ability of dietary antioxidants to affect lipid oxidation of cooked turkey meat in a simulated stomach and blood lipids after a meal. J. Funct. Foods 2009, 1, 208–216. [Google Scholar] [CrossRef]
- Van Hecke, T.; Wouters, A.; Rombouts, C.; Izzati, T.; Berardo, A.; Vossen, E.; Claeys, E.; Van Camp, J.; Raes, K.; Vanhaecke, L.; et al. Reducing compounds equivocally influence oxidation during digestion of a high-fat beef product, which promotes cytotoxicity in colorectal carcinoma cell lines. J. Agric. Food Chem. 2016, 64, 1600–1609. [Google Scholar] [CrossRef]
- Schwarz, K.; Frankel, E.N.; German, J.B. Partition behaviour of antioxidative phenolic compounds in heterophasic systems. Lipid/Fett 1996, 98, 115–121. [Google Scholar] [CrossRef]
- Bonifacie, A.; Aubry, L.; Sayd, T.; Bourillon, S.; Duval, A.; Kombolo, M.; Nassy, G.; Promeyrat, A.; Santé-Lhoutellier, V.; Théron, L. Chemical effects of nitrite reduction during digestion of cured cooked and recooked meat on nitrosation, nitrosylation and oxidation. Food Res. Int. 2024, 195, 114969. [Google Scholar] [CrossRef]
- Ren, S.; Hu, H.; Zhu, X.; Wang, S.; Zhao, W.; Xie, D.; Xi, J.; Liu, K. Inhibitory effects and reactions of gallic acid, catechin, and procyanidin B2 with nitrosation under stomach simulating conditions. Food Funct. 2024, 15, 3130. [Google Scholar] [CrossRef] [PubMed]
- Herraiz, T.; Galisteo, J. Nitrosative deamination of 2′-deoxyguanosine and DNA by nitrite, and antinitrosating activity of β-carboline alkaloids and antioxidants. Food Chem. Toxicol. 2018, 112, 282–289. [Google Scholar] [CrossRef]
- Sirvins, C.; Goupy, P.; Promeyrat, A.; Ginies, C.; Dufour, C. Deciphering key structural elements in the ability of phenolic compounds and plant extracts to alleviate secondary amine N-nitrosation and lipid oxidation in a cured meat model. Food Res. Int. 2025, 221, 117387. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Sun, Y.; Zhang, Q.; Capanoglu, E.; Xiao, J.; Zeng, K. Dietary fiber-phenolic interactions: Recent advances in formed conjugates and complexes and interaction-driven functional property changes. Trends Food Sci. Technol. 2025, 163, 105165. [Google Scholar] [CrossRef]
- Yang, X.; Zhang, D.; Blecker, C.; Zhang, C.; Sun, X.; Wang, Z. Effect of interaction between resveratrol and myofibrillar protein on the production of bound heterocyclic amines. Food Biosci. 2024, 59, 104166. [Google Scholar] [CrossRef]
- Wen, W.; Li, S.; Wang, J. The effects of tea polyphenol on chicken protein digestion and the mechanism under thermal processing. Foods 2023, 12, 2905. [Google Scholar] [CrossRef]
- Liu, G.; Li, Z.; Li, Z.; Hao, C.; Liu, Y. Molecular dynamics simulation and in vitro digestion to examine the impact of theaflavin on the digestibility and structural properties of myosin. Int. J. Biol. Macromol. 2023, 247, 125836. [Google Scholar] [CrossRef]
- Gasaly, N.; Gotteland, M. Interference of dietary polyphenols with potentially toxic amino acid metabolites derived from the colonic microbiota. Amino Acids 2022, 54, 311–324. [Google Scholar] [CrossRef]
- Chang, Y.; Zhang, B.; Jia, Y.; Shi, X.; Lu, Y.; Wei, F.; Zhang, Y.; Lv, H.; Dong, L.; Zhang, Y.; et al. Effects of dietary polyphenols addition and oxygen concentration on digestion and absorption of fat in roast beef patties. Food Chem. 2025, 483, 144280. [Google Scholar] [CrossRef]
- Chen, H.; Zhang, F.; Yang, Y.X.; Meng, X.H.; Ding, X.Q.; Jian, T.Y.; Niu, G.T.; Tong, B.; Gai, Y.N.; Lü, H.; et al. Effect of chitosan edible coating containing anthocyanins and tea polyphenols on cold storage of chilled pork. Front. Nutr. 2025, 12, 1546618. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Cheng, J.R.; Liu, X.M.; Zhu, M.J. Effect of microencapsulated process on stability of mulberry polyphenol and oxidation property of dried minced pork slices during heat processing and storage. LWT 2019, 100, 62–68. [Google Scholar] [CrossRef]
- Pajuelo, A.; Folgado, C.; Moreno, C.; Trujillo-Echeverría, L.; Perez-Palacios, T. Microcapsules of grape peel extract as antioxidant vehicle: Characterization, bioaccessibility and addition influence in meat model systems. Appl. Food Res. 2025, 5, 101191. [Google Scholar] [CrossRef]
- Oyom, W.; Awuku, R.B.; Bi, Y.; Tahergorabi, R. Mitigating toxic compounds in deep-fried meat: The antioxidant potential of edible coatings. Food Bioprocess Technol. 2025, 18, 2270–2295. [Google Scholar] [CrossRef]
- Wang, Z.; Ng, K.; Warner, R.D.; Stockmann, R.; Fang, Z. Effect of incorporation strategies of sesamol and chitosan nanoparticle-methylcellulose edible coating on the polycyclic aromatic hydrocarbon levels in deep-fried meatballs. Food Res. Int. 2025, 208, 116210. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jin, D.-X.; Jin, Y.-X. Developing Healthier Meat Products: Application of Natural Polyphenols to Reduce Hazardous Compounds During High Temperature Processing and Digestion. Foods 2025, 14, 3952. https://doi.org/10.3390/foods14223952
Jin D-X, Jin Y-X. Developing Healthier Meat Products: Application of Natural Polyphenols to Reduce Hazardous Compounds During High Temperature Processing and Digestion. Foods. 2025; 14(22):3952. https://doi.org/10.3390/foods14223952
Chicago/Turabian StyleJin, Du-Xin, and Yu-Xuan Jin. 2025. "Developing Healthier Meat Products: Application of Natural Polyphenols to Reduce Hazardous Compounds During High Temperature Processing and Digestion" Foods 14, no. 22: 3952. https://doi.org/10.3390/foods14223952
APA StyleJin, D.-X., & Jin, Y.-X. (2025). Developing Healthier Meat Products: Application of Natural Polyphenols to Reduce Hazardous Compounds During High Temperature Processing and Digestion. Foods, 14(22), 3952. https://doi.org/10.3390/foods14223952





