Bioactive Flavonoids from Paulownia tomentosa Flowers: Extraction Optimization and α-Glucosidase Inhibitory Kinetics
Abstract
1. Introduction
2. M aterials and Methods
2.1. Chemicals and Materials
2.1.1. Sample Pretreatment
2.1.2. Materials and Reagents
2.2. Optimization of UA-Cellulase Extraction for PFF
2.2.1. Rutin Standard Curve Production
2.2.2. Determination of Flavonoid Content in Extracts
2.2.3. One-Factor and Response Surface Optimization Experiments
2.3. Optimization of UA-ATPE for PFF
2.3.1. Preparation of Double Aqueous Phase Diagram
2.3.2. Determination of Flavonoid Content in Extracts
2.3.3. One-Factor and Response Surface Optimization Experiments
2.4. Optimization of PFF Purification
2.4.1. Pretreatment of Macroporous Resins
2.4.2. Screening of Macroporous Resins
2.4.3. Determination of Leakage Curve, Washing Volume, and Elution Profile
2.4.4. One-Factor Experiments
2.4.5. Purification Effect of Macroporous Resin
2.5. UV–Vis and FT-IR Spectroscopy
2.6. Scanning Electron Microscopy (SEM) Analysis
2.7. UPLC-MS/MS Analysis
2.8. Inhibition of α-Glucosidase by PFF and Flavonoid Monomer Substances
2.9. Reversibility of Inhibition of α-Glucosidase by PFF and Luteolin 7-O-Glucuronide
2.10. Type of Inhibition of α-Glucosidase by PFF and Luteolin 7-O-Glucuronide
2.11. Statistical Analysis
3. Results and Discussion
3.1. Optimization of UA-Cellulase Extraction for PFF
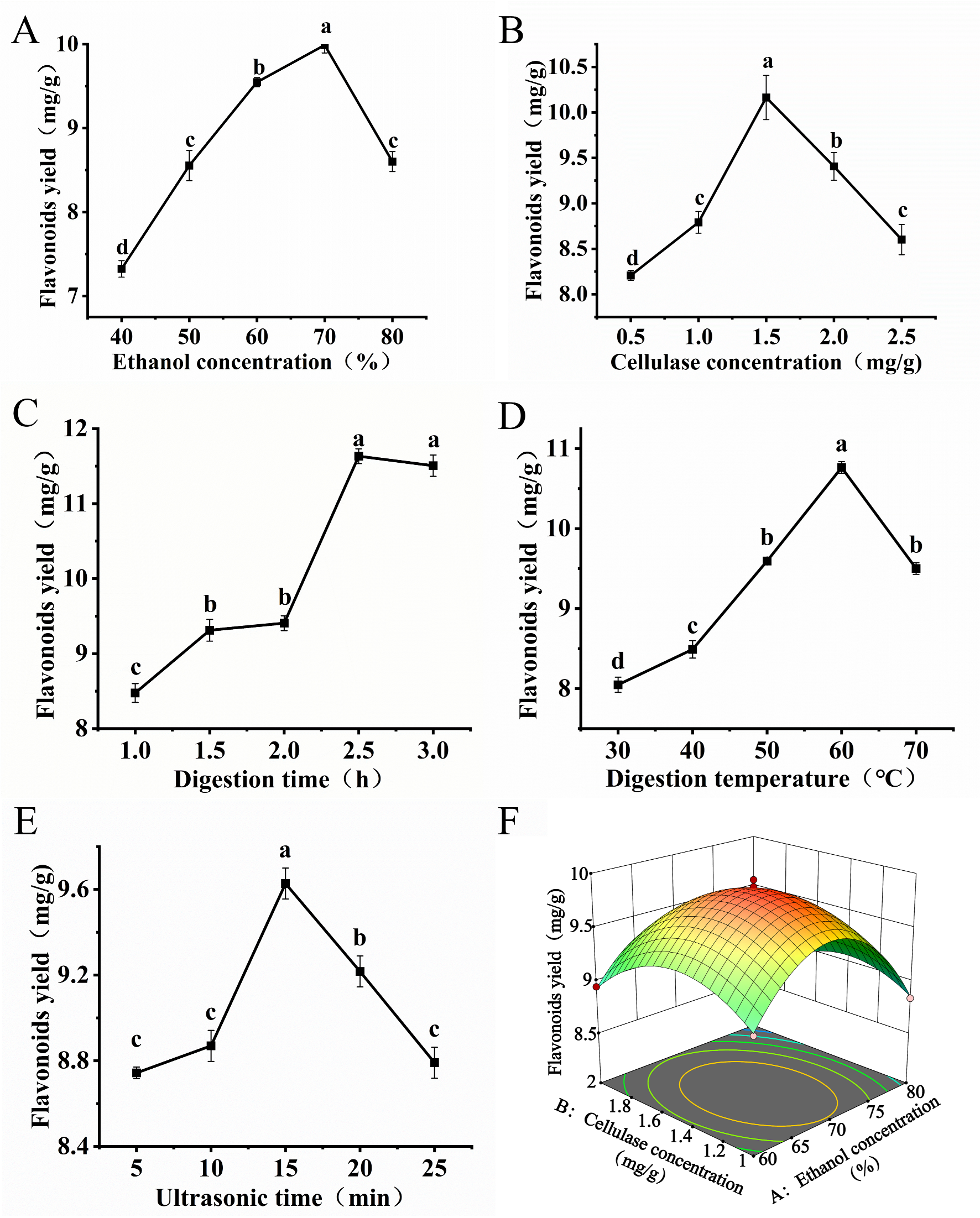
3.1.1. Single-Factor Experiments
3.1.2. Response Surface Optimization Experiments
3.1.3. Model Validation
3.2. Optimization of UA-ATPE for PFF
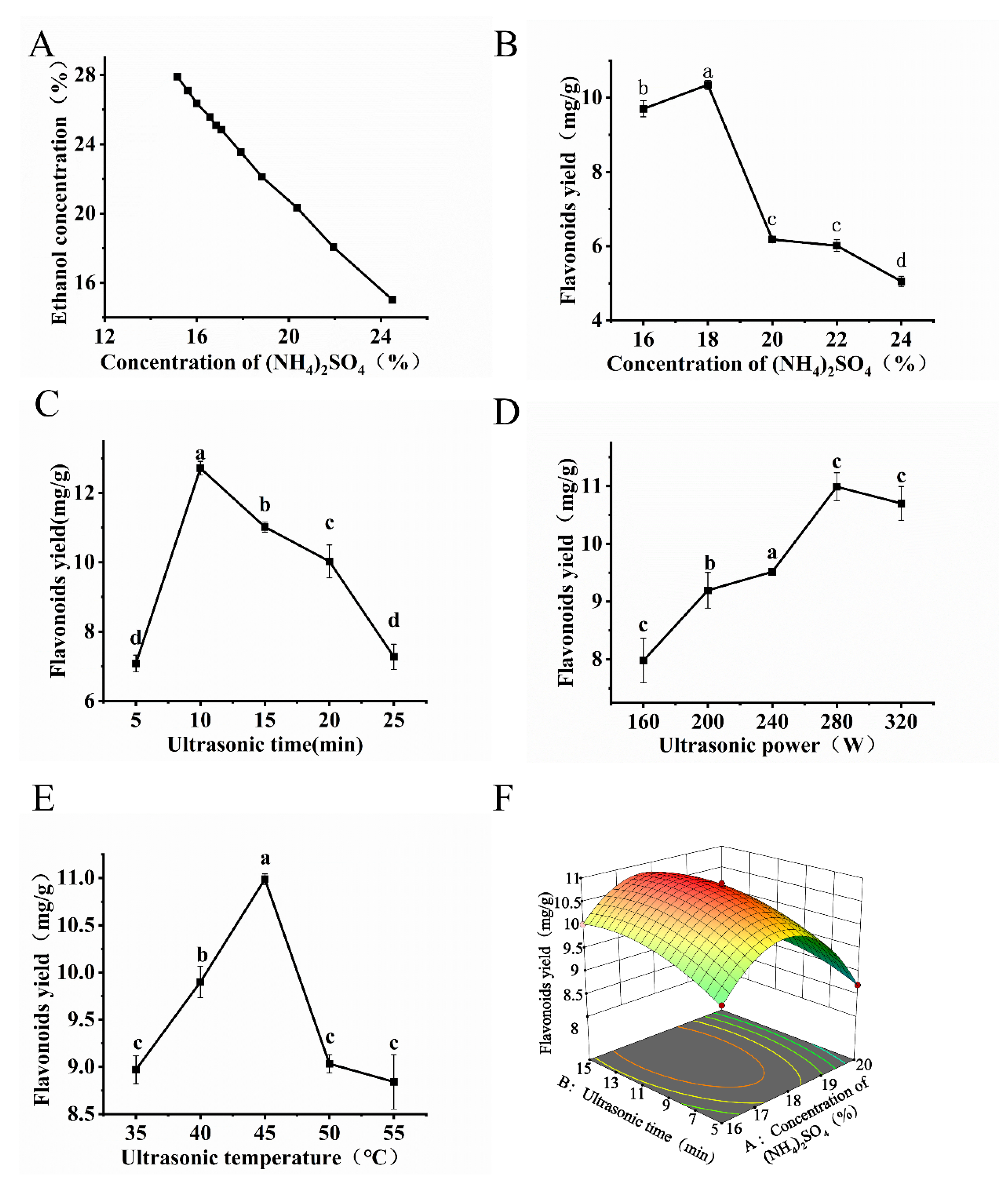
3.2.1. Phase Diagram and Single-Factor Experiments
3.2.2. Response Surface Optimization Experiments
3.2.3. Model Validation
3.3. Optimization of PFF Purification Using Macroporous Resins
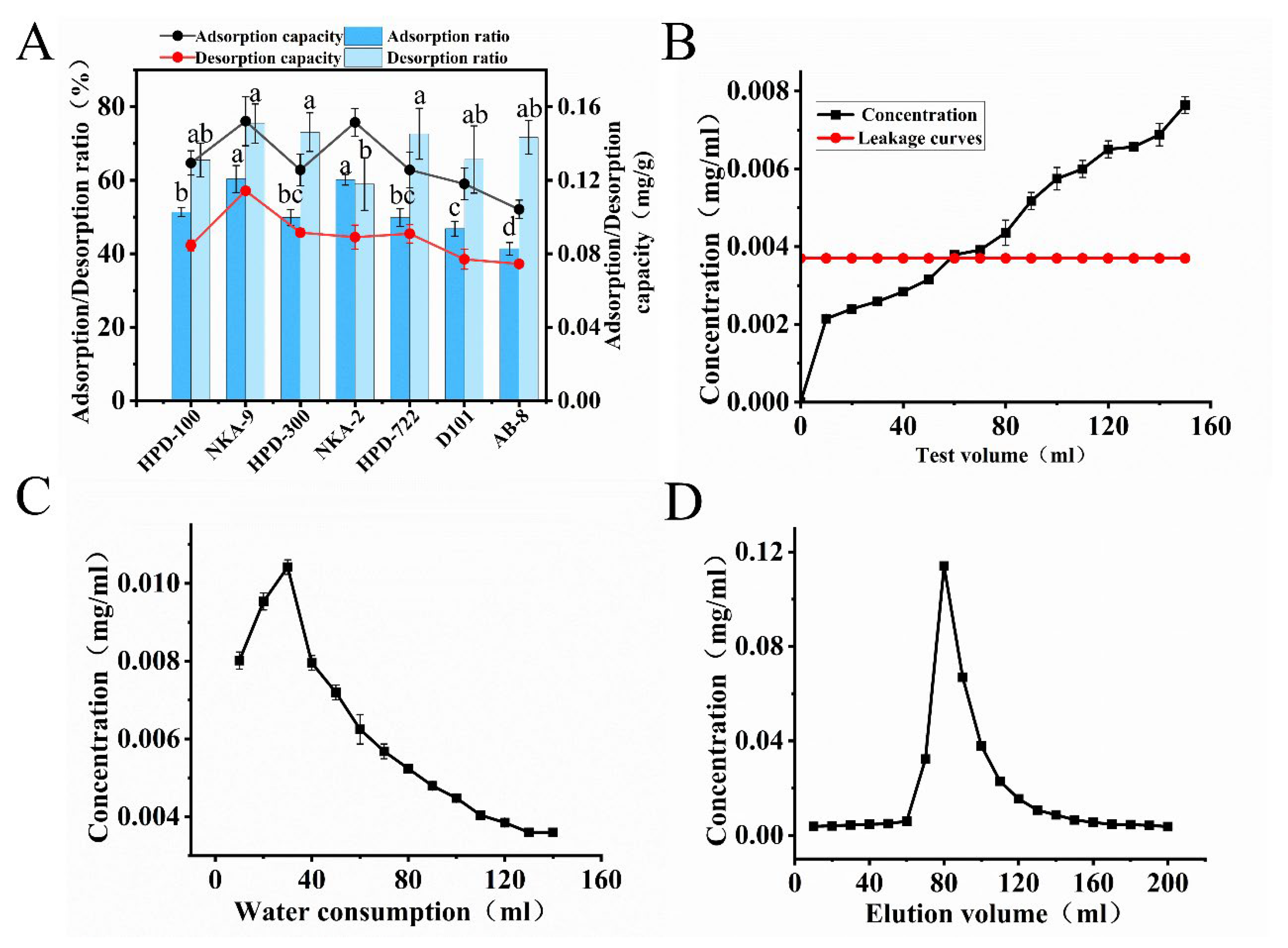
3.3.1. Screening of Macroporous Resins
3.3.2. Determination of the Leakage Point, Washing Volume, and Elution Profile
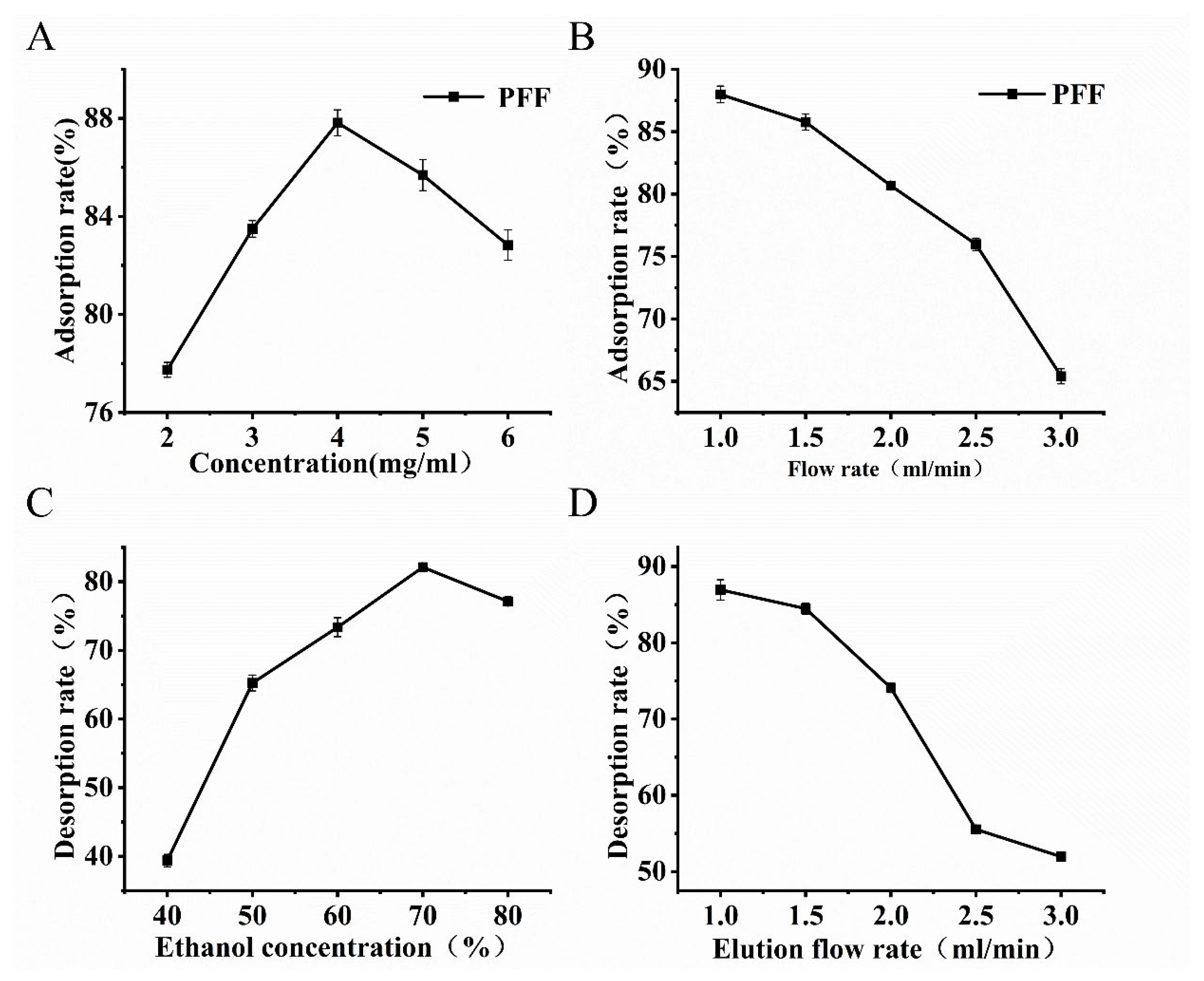
3.3.3. One-Factor Experiments
3.3.4. Purification Effect of Macroporous Resin
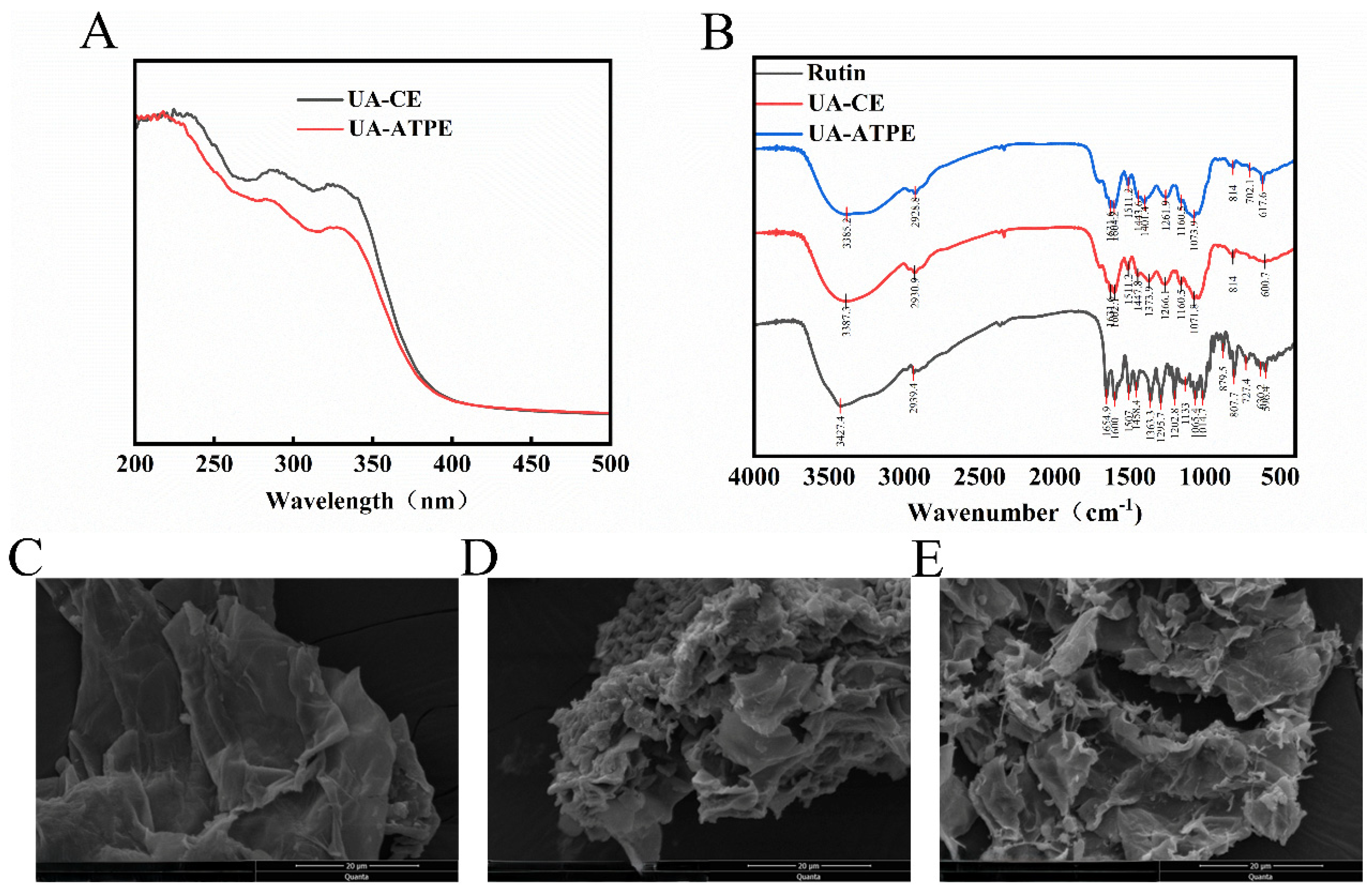
3.4. UV–Vis and FT-IR Characterization
3.5. SEM Analysis
3.6. UPLC-MS/MS Analysis
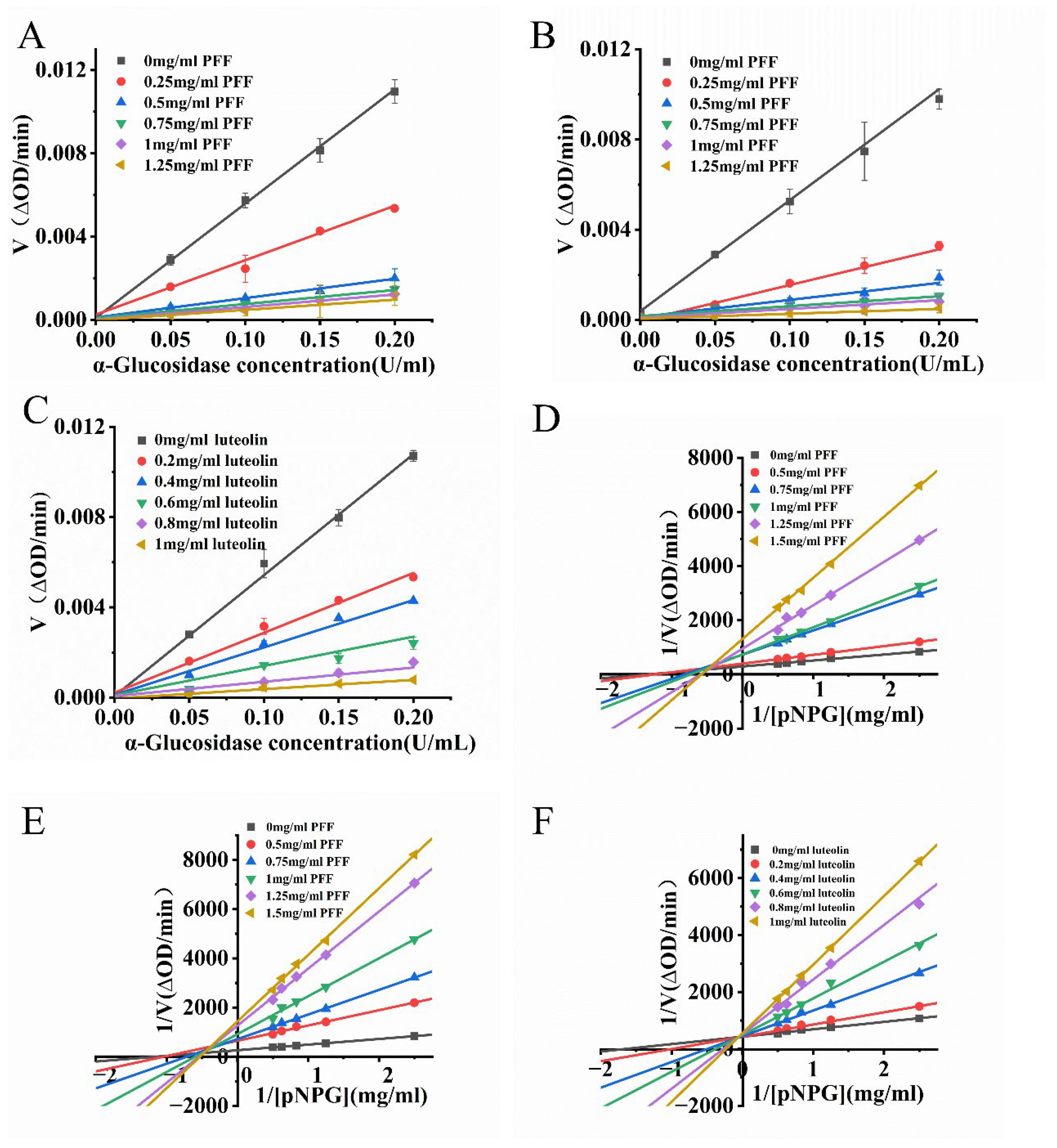
3.7. Inhibition of α-Glucosidase by PFF and Flavonoid Monomer Substances
3.8. Reversibility of Inhibition of α-Glucosidase by PFF and Luteolin 7-O-Glucuronide
3.9. Type of Inhibition of α-Glucosidase by PFF and Luteolin 7-O-Glucuronide
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Berg, E.C.; Zarnoch, S.J.; McNab, W.H. Survivorship, attained diameter, height and volume of three Paulownia species after 9 years in the southern Appalachians, USA. J. For. Res. 2019, 31, 2181–2191. [Google Scholar] [CrossRef]
- Guo, N.; Zhai, X.-Q.; Fan, G.-Q. Chemical composition, health benefits and future prospects of Paulownia flowers: A review. Food Chem. 2023, 412, 135496. [Google Scholar] [CrossRef]
- Wang, Q.; Meng, X.; Zhu, L.; Xu, Y.; Cui, W.; He, X.; Wei, K.; Zhu, R. A polysaccharide found in Paulownia fortunei flowers can enhance cellular and humoral immunity in chickens. Int. J. Biol. Macromol. 2019, 130, 213–219. [Google Scholar] [CrossRef]
- Yang, H.; Li, B.; Fan, G.; Zhai, X.; Song, W.; Tang, X. A comprehensive study on the bioactive flavonoids in Paulownia flowers: Uncovering metabolic pathways, effective components, and regulatory genes for industrial applications. Ind. Crops Prod. 2024, 222, 119786. [Google Scholar] [CrossRef]
- Liu, C.; Ma, J.; Sun, J.; Cheng, C.; Feng, Z.; Jiang, H.; Yang, W. Flavonoid-Rich Extract of Paulownia fortunei Flowers Attenuates Diet-Induced Hyperlipidemia, Hepatic Steatosis and Insulin Resistance in Obesity Mice by AMPK Pathway. Nutrients 2017, 9, 959. [Google Scholar] [CrossRef]
- Wen, C.; Zhang, J.; Zhang, H.; Dzah, C.S.; Zandile, M.; Duan, Y.; Ma, H.; Luo, X. Advances in ultrasound assisted extraction of bioactive compounds from cash crops—A review. Ultrason. Sonochem. 2018, 48, 538–549. [Google Scholar] [CrossRef] [PubMed]
- Rostami, H.; Gharibzahedi, S.M.T. Cellulase-assisted extraction of polysaccharides from Malva sylvestris: Process optimization and potential functionalities. Int. J. Biol. Macromol. 2017, 101, 196–206. [Google Scholar] [CrossRef] [PubMed]
- Iqbal, M.; Tao, Y.; Xie, S.; Zhu, Y.; Chen, D.; Wang, X.; Huang, L.; Peng, D.; Sattar, A.; Shabbir, M.A.B.; et al. Aqueous two-phase system (ATPS): An overview and advances in its applications. Biol. Proced. Online 2016, 18, 18. [Google Scholar] [CrossRef]
- Yang, Y.; Liang, Q.; Zhang, B.; Zhang, J.; Fan, L.; Kang, J.; Lin, Y.; Huang, Y.; Tan, T.-C.; Ho, L.-H. Adsorption and desorption characteristics of flavonoids from white tea using macroporous adsorption resin. J. Chromatogr. A 2024, 1715, 464621. [Google Scholar] [CrossRef] [PubMed]
- Peng, X.; Zhang, G.; Liao, Y.; Gong, D. Inhibitory kinetics and mechanism of kaempferol on α-glucosidase. Food Chem. 2016, 190, 207–215. [Google Scholar] [CrossRef]
- Zhang, Z.; Luo, A.; Zhong, K.; Huang, Y.; Gao, Y.; Zhang, J.; Gao, H.; Xu, Z.; Gao, X. α-Glucosidase inhibitory activity by the flower buds of Lonicera japonica Thunb. J. Funct. Foods 2013, 5, 1253–1259. [Google Scholar] [CrossRef]
- Jiang, Z.; Shi, D.; Tu, Y.; Tian, J.; Zhang, W.; Xing, B.; Wang, J.; Liu, S.; Lou, J.; Gustafsson, J.-Å.; et al. Human Proislet Peptide Promotes Pancreatic Progenitor Cells to Ameliorate Diabetes Through FOXO1/Menin-Mediated Epigenetic Regulation. Diabetes 2018, 67, 1345–1355. [Google Scholar] [CrossRef]
- Ma, Y.-Y.; Zhao, D.-G.; Zhou, A.-Y.; Zhang, Y.; Du, Z.; Zhang, K. α-Glucosidase Inhibition and Antihyperglycemic Activity of Phenolics from the Flowers of Edgeworthia gardneri. J. Agric. Food Chem. 2015, 63, 8162–8169. [Google Scholar] [CrossRef]
- Hayat, S.; Miana, G.A.; Kanwal, M.; Ahsan, Z.; Tariq, M.J. Determination of Total Flavonoid Content and Phenolic Content, Antioxidant Assay, and Antiepileptic Activity of Achillea millefolium Extract. Nat. Prod. Commun. 2025, 20, 1934578X251319221. [Google Scholar] [CrossRef]
- Nainegali, B.S.; Iyyaswami, R.; Belur, P.D. Partitioning of bio-active compounds from rinds of Garcinia indica using aqueous two-phase system: Process evaluation and optimization. Sep. Purif. Technol. 2020, 253, 117520. [Google Scholar] [CrossRef]
- Zheng, P.-F.; Xiong, Z.; Liao, C.-Y.; Zhang, X.; Feng, M.; Wu, X.-Z.; Lin, J.; Lei, L.-S.; Zhang, Y.-C.; Wang, S.-H.; et al. In vitro and in silico studies of bis (indol-3-yl) methane derivatives as potential α-glucosidase and α-amylase inhibitors. J. Enzym. Inhib. Med. Chem. 2021, 36, 1938–1951. [Google Scholar] [CrossRef]
- Ni, M.; Pan, J.; Hu, X.; Gong, D.; Zhang, G. Inhibitory effect of corosolic acid on α-glucosidase: Kinetics, interaction mechanism, and molecular simulation. J. Sci. Food Agric. 2019, 99, 5881–5889. [Google Scholar] [CrossRef]
- Liao, J.; Guo, Z.; Yu, G. Process intensification and kinetic studies of ultrasound-assisted extraction of flavonoids from peanut shells. Ultrason. Sonochem. 2021, 76, 105661. [Google Scholar] [CrossRef] [PubMed]
- Yin, H.; Zhang, Y.; Hu, T.; Li, W.; Deng, Y.; Wang, X.; Tang, H.; Zhao, L.; Yan, G. Optimization of Cellulase-Assisted Extraction of Total Flavonoids from Equisetum via Response Surface Methodology Based on Antioxidant Activity. Processes 2023, 11, 1978. [Google Scholar] [CrossRef]
- Tian, J.; Muhammad, S.; Chen, A.; Chen, P.; Wang, J.; Yang, C.; Yuan, H.; Wang, Z. An experimental study exploring the influencing factors for ultrasonic-assisted extraction of flavonoid compounds from leaves of Amorpha fruticosa L. J. For. Res. 2019, 30, 1735–1741. [Google Scholar] [CrossRef]
- Wen, Y.; Zeng, X.; Dai, H.; Liu, B. Optimization of ultrasonic assisted extraction and biological activity of total flavonoids from Ligusticum chuanxiong Hort. using response surface methodology. Biomass Convers. Biorefinery 2023, 14, 17101–17113. [Google Scholar] [CrossRef]
- Wang, Y.; Han, J.; Xu, X.; Hu, S.; Yan, Y. Partition behavior and partition mechanism of antibiotics in ethanol/2-propanol–ammonium sulfate aqueous two-phase systems. Sep. Purif. Technol. 2010, 75, 352–357. [Google Scholar] [CrossRef]
- Sun, X.; Zhang, Y.; Zhu, X.; Korir, N.K.; Tao, R.; Wang, C.; Fang, J. Advances in identification and validation of plant microRNAs and their target genes. Physiol. Plant. 2014, 152, 203–218. [Google Scholar] [CrossRef]
- Zhu, J.; Kou, X.; Wu, C.; Fan, G.; Li, T.; Dou, J.; Shen, D. Enhanced extraction of bioactive natural products using ultrasound-assisted aqueous two-phase system: Application to flavonoids extraction from jujube peels. Food Chem. 2022, 395, 133530. [Google Scholar] [CrossRef] [PubMed]
- Hou, M.; Hu, W.; Xiu, Z.; Shi, Y.; Hao, K.; Cao, D.; Guan, Y.; Yin, H. Efficient enrichment of total flavonoids from Pteris ensiformis Burm. extracts by macroporous adsorption resins and in vitro evaluation of antioxidant and antiproliferative activities. J. Chromatogr. B 2020, 1138, 121960. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, Y.; Cheng, J.; Zhao, J.; Shi, R.; He, L.; Li, Q.; Chen, Y. Efficient purification of flavonoids from bamboo shoot residues of Phyllostachys edulis by macroporous resin and their hypoglycemic activity. Food Chem. X 2022, 16, 100505. [Google Scholar] [CrossRef]
- Huang, P.; Zhang, Q.; Pan, H.; Luan, L.; Liu, X.; Wu, Y. Optimization of integrated extraction-adsorption process for the extraction and purification of total flavonoids from Scutellariae barbatae herba. Sep. Purif. Technol. 2017, 175, 203–212. [Google Scholar] [CrossRef]
- Delgado, J.A.; Uguina, M.A.; Sotelo, J.L.; Águeda, V.I.; García, A.; Roldán, A. Separation of ethanol–water liquid mixtures by adsorption on silicalite. Chem. Eng. J. 2012, 180, 137–144. [Google Scholar] [CrossRef]
- Tošović, J.; Marković, S. Reproduction and interpretation of the UV–vis spectra of some flavonoids. Chem. Pap. 2017, 71, 543–552. [Google Scholar] [CrossRef]
- Wiercigroch, E.; Szafraniec, E.; Czamara, K.; Pacia, M.Z.; Majzner, K.; Kochan, K.; Kaczor, A.; Baranska, M.; Malek, K. Raman and infrared spectroscopy of carbohydrates: A review. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2017, 185, 317–335. [Google Scholar] [CrossRef]
- Radhalakshmi, V.; Raman, M.; Joy, M.R. Physico-chemical characterization of betel leaf and its effect on oxidative stability and shelf-life of coconut oil. Food Biosci. 2023, 52, 102343. [Google Scholar] [CrossRef]
- Dip, G.; Aggarwal, P.; Kapoor, A.; Grover, S.; Kaur, S. Recovery of high-value components from Bauhinia variegata leaves using ultrasound-microwave-assisted extraction technique. Biomass Bioenergy 2025, 195, 107709. [Google Scholar] [CrossRef]
- Reche, C.; Rosselló, C.; Dalmau, E.; Eim, V.; Simal, S. Quantification of microstructural changes in artichoke by-products by image analysis after high-power ultrasound-assisted extraction of bioactive compounds. LWT 2022, 171, 114127. [Google Scholar] [CrossRef]
- Chemat, F.; Rombaut, N.; Sicaire, A.-G.; Meullemiestre, A.; Fabiano-Tixier, A.-S.; Abert-Vian, M. Ultrasound assisted extraction of food and natural products. Mechanisms, techniques, combinations, protocols and applications. A review. Ultrason. Sonochem. 2017, 34, 540–560. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Liu, Y.; Shi, Y.-P. Determination of flavonoids in the flowers of Paulownia tomentosa by high-performance liquid chromatography. J. Anal. Chem. 2009, 64, 282–288. [Google Scholar] [CrossRef]
- Dai, B.; Hu, Z.; Li, H.; Yan, C.; Zhang, L. Simultaneous determination of six flavonoids from Paulownia tomentosa flower extract in rat plasma by LC–MS/MS and its application to a pharmacokinetic study. J. Chromatogr. B 2015, 978–979, 54–61. [Google Scholar] [CrossRef]
- Zhang, P.-F.; Li, C. Flavones from flowers of Paulownia fortunei. Zhongguo Zhong Yao Za Zhi Zhongguo Zhongyao Zazhi China J. Chin. Mater. Med. 2008, 33, 2629–2632. [Google Scholar]
- Hogan, S.; Zhang, L.; Li, J.; Sun, S.; Canning, C.; Zhou, K. Antioxidant rich grape pomace extract suppresses postprandial hyperglycemia in diabetic mice by specifically inhibiting alpha-glucosidase. Nutr. Metab. 2010, 7, 71. [Google Scholar] [CrossRef]
- Li, K.; Yao, F.; Xue, Q.; Fan, H.; Yang, L.; Li, X.; Sun, L.; Liu, Y. Inhibitory effects against α-glucosidase and α-amylase of the flavonoids-rich extract from Scutellaria baicalensis shoots and interpretation of structure–activity relationship of its eight flavonoids by a refined assign-score method. Chem. Cent. J. 2018, 12, 82. [Google Scholar] [CrossRef]
- Hamed, Y.S.; Abdin, M.; Rayan, A.M.; Akhtar, H.M.S.; Zeng, X. Synergistic inhibition of isolated flavonoids from Moringa oleifera leaf on α-glucosidase activity. LWT 2021, 141, 111081. [Google Scholar] [CrossRef]
- Dlamini, B.S.; Chen, C.-R.; Kuo, Y.-H.; Chen, Y.-K.; Hsu, J.-L.; Chang, C.-I. Bio-Assay Guided Isolation of Flavonoids from Scutellaria barbata D. Don and Their Mechanism of α-Glucosidase Inhibition. Pharm. Chem. J. 2022, 56, 683–691. [Google Scholar] [CrossRef]
- Mo, Q.-G.; Gao, Z.; Wei-Dong, Z.; Lan-Lan, G.; Wang, Y.-W. Coumaroyl and feruloyl flavonoid glycosides from the male flowers of Ginkgo biloba L. and their inhibitory activity against α-glucosidase. Nat. Prod. Res. 2022, 36, 4365–4372. [Google Scholar] [CrossRef] [PubMed]
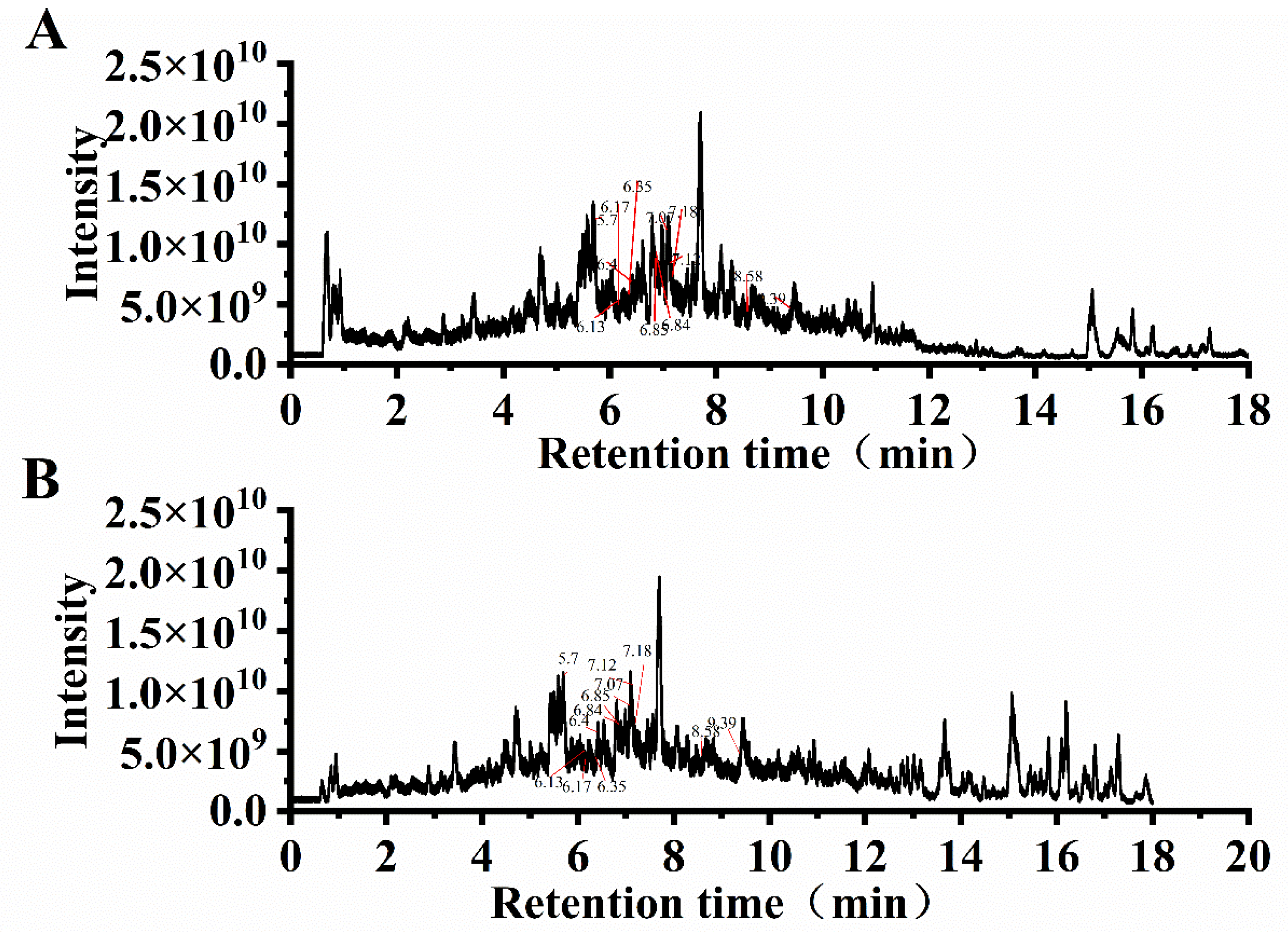

| No. | Name | Class | UA-CE Content (ng/mg) | UA-APTE Content (ng/mg) | m/z | Retention Time (min) |
|---|---|---|---|---|---|---|
| 1 | Isorhamnetin-3-O-glucoside | Flavonols | 31.26 | 19.15 | 479.12 | 7.18 |
| 2 | Robinetin | Flavonols | 17.91 | 9.32 | 303.05 | 6.17 |
| 3 | Rutin hydrate | Flavonols | 40.42 | 16.01 | 611.16 | 6.35 |
| 4 | Rutin | Flavonols | 40.42 | 16.01 | 611.16 | 6.35 |
| 5 | Astragalin | Flavonols | 55.64 | 41.94 | 449.11 | 7.12 |
| 6 | Quercitrin | Flavonols | 56.03 | 42.23 | 449.11 | 7.07 |
| 7 | Isoquercetin | Flavonols | 71.06 | 34.81 | 465.10 | 6.4 |
| 8 | Hyperoside | Flavonols | 71.15 | 34.85 | 465.10 | 6.4 |
| 9 | Cynaroside | Flavones | 87.73 | 58.69 | 449.11 | 6.13 |
| 10 | Apigenin 7-glucoside | Flavones | 122.11 | 126.23 | 433.11 | 6.84 |
| 11 | Luteolin | Flavones | 255.45 | 219.70 | 287.06 | 8.58 |
| 12 | Apigenin | Flavones | 266.23 | 490.91 | 271.06 | 9.39 |
| 13 | Luteolin 7-O-glucuronide | Flavones | 284.44 | 269.59 | 463.09 | 6.13 |
| 14 | Scutellarin | Flavones | 291.46 | 276.24 | 463.09 | 6.17 |
| 15 | Kaempferol 3-O-sophoroside | Flavonols | 363.58 | 53.586 | 611.16 | 5.7 |
| 16 | Apigenin-7-glucuronide | Flavones | 777.98 | 767.82 | 447.09 | 6.85 |
| 17 | Ombuin | Flavones | 15.73 | 34.87 | 331.08 | 11.06 |
| 18 | Diosmetin | Flavones | 13.29 | 31.12 | 301.07 | 9.51 |
| 19 | 3′,4′,7-Trimethoxyquercetin | Flavonols | 2.43 | 31.07 | 345.10 | 12.04 |
| 20 | Chrysoeriol | Flavones | 13.24 | 31.00 | 301.07 | 9.47 |
| 21 | Hispidulin | Flavones | 13.23 | 30.97 | 301.07 | 9.45 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiang, F.; Yang, H.; Zhai, X.; Zhao, Z.; Fan, G. Bioactive Flavonoids from Paulownia tomentosa Flowers: Extraction Optimization and α-Glucosidase Inhibitory Kinetics. Foods 2025, 14, 3941. https://doi.org/10.3390/foods14223941
Jiang F, Yang H, Zhai X, Zhao Z, Fan G. Bioactive Flavonoids from Paulownia tomentosa Flowers: Extraction Optimization and α-Glucosidase Inhibitory Kinetics. Foods. 2025; 14(22):3941. https://doi.org/10.3390/foods14223941
Chicago/Turabian StyleJiang, Fu, Haibo Yang, Xiaoqiao Zhai, Zhenli Zhao, and Guoqiang Fan. 2025. "Bioactive Flavonoids from Paulownia tomentosa Flowers: Extraction Optimization and α-Glucosidase Inhibitory Kinetics" Foods 14, no. 22: 3941. https://doi.org/10.3390/foods14223941
APA StyleJiang, F., Yang, H., Zhai, X., Zhao, Z., & Fan, G. (2025). Bioactive Flavonoids from Paulownia tomentosa Flowers: Extraction Optimization and α-Glucosidase Inhibitory Kinetics. Foods, 14(22), 3941. https://doi.org/10.3390/foods14223941




