Chemical Mechanisms Underlying Sweetness Enhancement During Processing of Rehmanniae Radix: Carbohydrate Hydrolysis, Degradation of Bitter Compounds, and Interaction with Taste Receptors
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.1.1. Samples
2.1.2. Reagents
2.2. Sensory Analysis
2.3. Electronic Tongue Analysis
2.4. Chemical Quantification Methods
2.4.1. Carbohydrate Analysis
2.4.2. Characteristic Spectrum and Marker Compound Analysis
2.5. Molecular Docking Methodology
2.6. Data Analysis
3. Results and Discussion
3.1. Sensory Taste Differences Between RRR and RRP
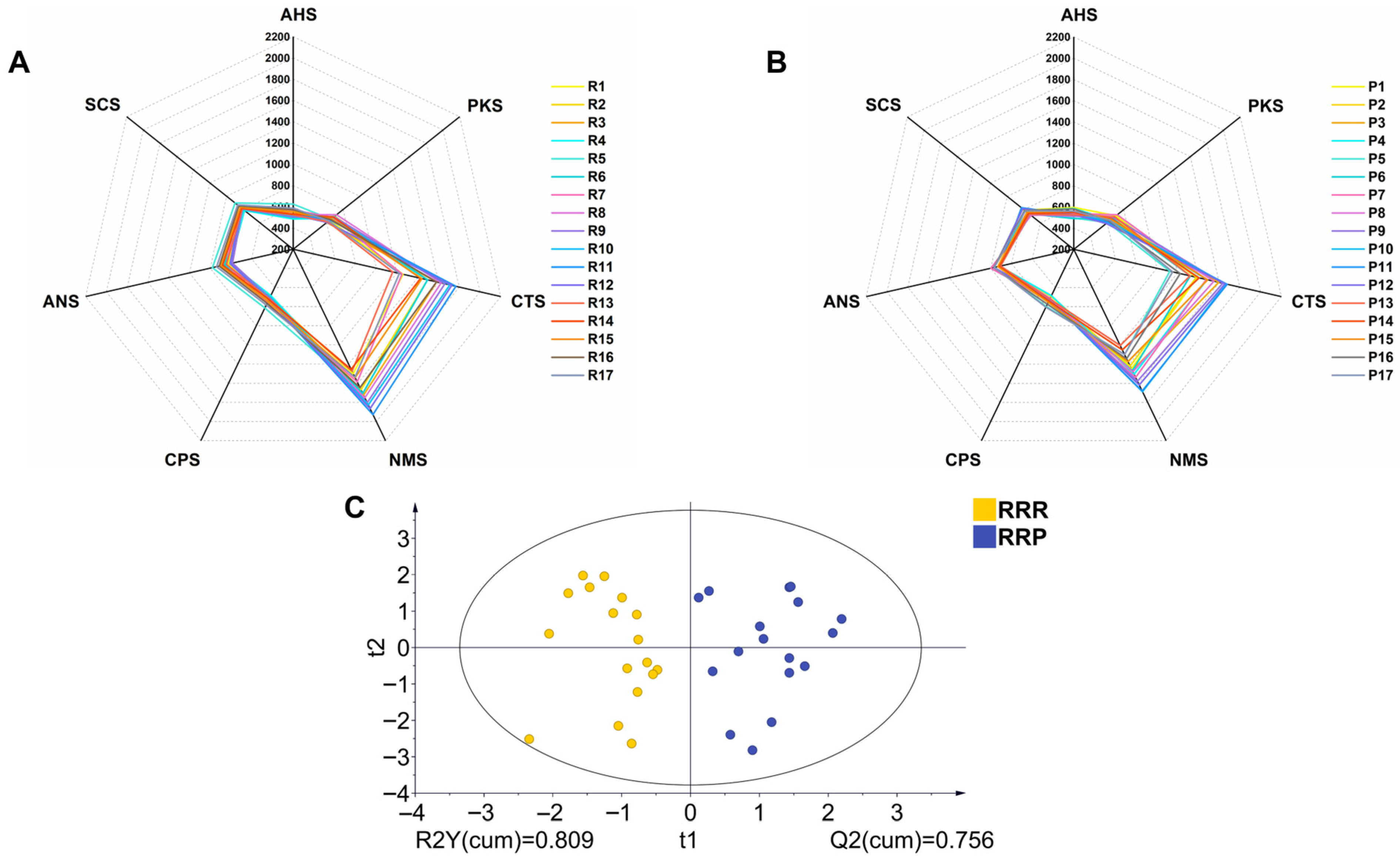
3.2. Electronic Tongue Taste Differences Between RRR and RRP
3.3. Chemical Analysis
3.3.1. Carbohydrate Compounds
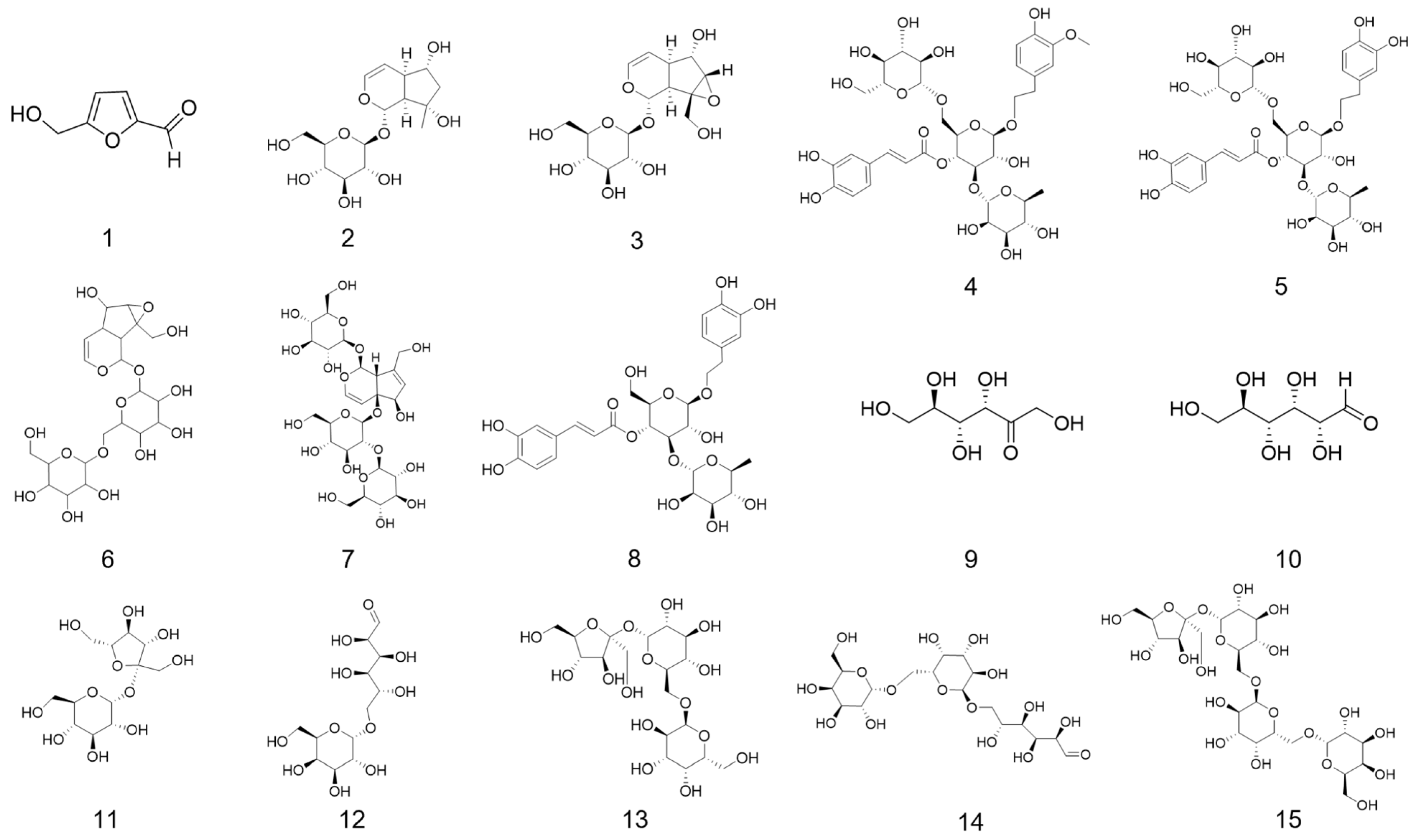
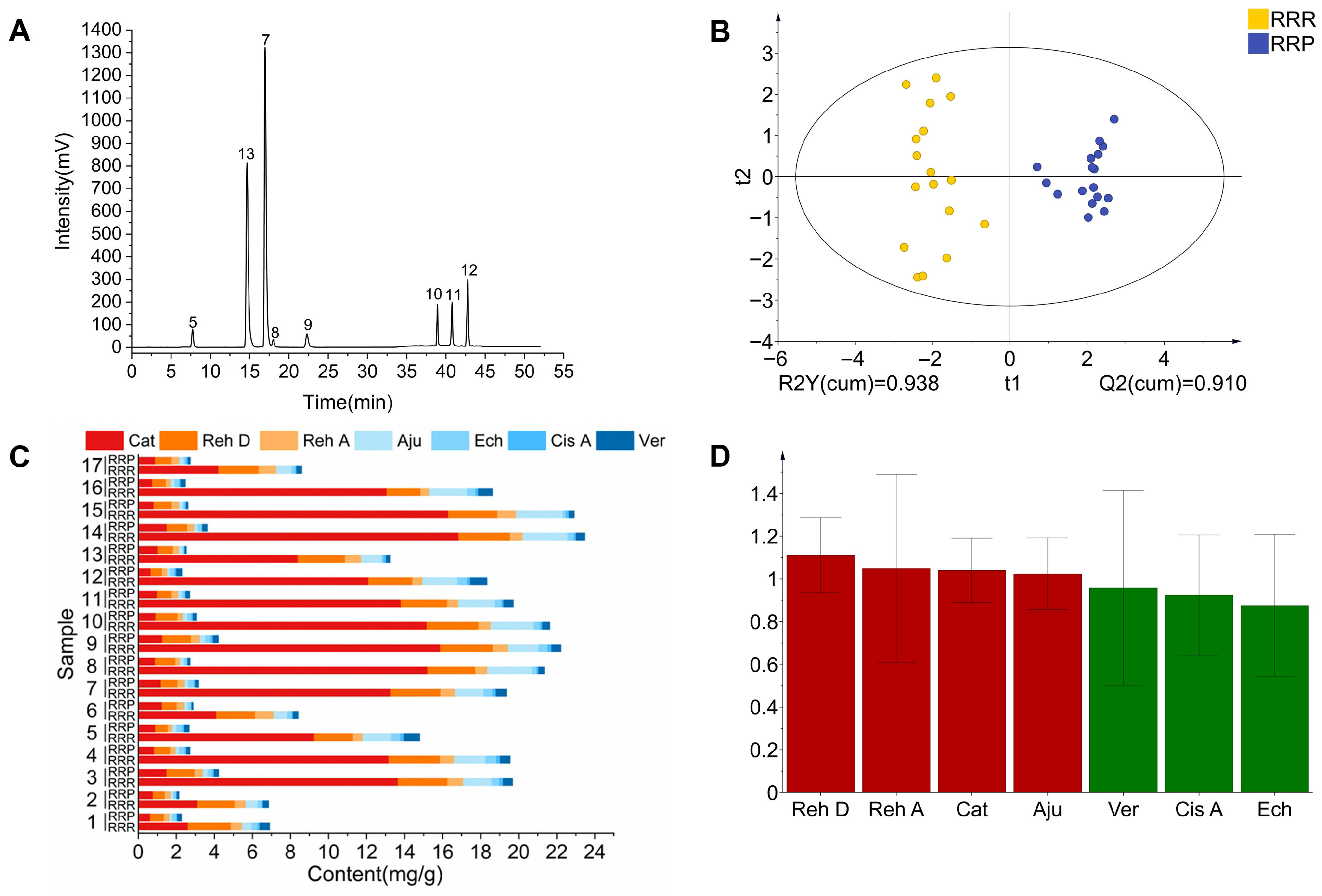
3.3.2. Characteristic Spectrum and Quantification of Marker Compounds
3.4. Molecular Docking
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- National Health Commission; State Administration for Market Regulation. Announcement on Rehmannia Radix and Other 4 Substances Traditionally Used as Both Food and Chinese Medicinal Materials (No. 4 of 2024) [EB/OL]. Available online: https://www.shiguai.gov.cn/ylws/20764.html (accessed on 17 September 2025).
- National Pharmacopoeia Commission. Chinese Pharmacopoeia, 2020th ed.; China Pharmaceutical Science and Technology Press: Beijing, China, 2020.
- Yoshida, R.; Ninomiya, Y. Taste information derived from T1R-expressing taste cells in mice. Biochem. J. 2016, 473, 525–536. [Google Scholar] [CrossRef]
- Khan, A.S.; Hichami, A.; Khan, N.A. Taste perception and its effects on oral nutritional supplements in younger life phases. Curr. Opin. Clin. Nutr. Metab. Care 2018, 21, 411–415. [Google Scholar] [CrossRef]
- Zopun, M.; Liszt, K.I.; Stoeger, V.; Behrens, M.; Redel, U.; Ley, J.P.; Hans, J.; Somoza, V. Human Sweet Receptor T1R3 is Functional in Human Gastric Parietal Tumor Cells (HGT-1) and Modulates Cyclamate and Acesulfame K-Induced Mechanisms of Gastric Acid Secretion. J. Agric. Food Chem. 2018, 66, 4842–4852. [Google Scholar] [CrossRef]
- Zhang, Z.Y.; Zhang, L.B.; Lyu, J.L. Research progress on the main chemical constituents and pharmacological actions of Rehmannia glutinosa. J. Xinxiang Med. Univ. 2024, 41, 979–986. [Google Scholar]
- Feng, W.S. Research Progress on Chemical Composition and Pharmacological Effects of Dihuang (Rehmannia glutinosa Libosch.). Acta Chin. Med. 2025, 40, 2035–2055. [Google Scholar] [CrossRef]
- Yao, X.M.; Guan, H.B. Research Progress on Chemical Components and Pharmacological Effects of Rehmanniae Radix. Inf. Tradit. Chin. Med. 2025, 42, 84–89. [Google Scholar] [CrossRef]
- Li, Y.D.; Zhang, Y.; Ma, X.; He, Y.; Liu, B.; Jin, J.; Heng, J.Y.; Yan, W.J.; Wang, F. Research progress on structural characteristics and biological activities of functional sugars from Rehmannia glutinosa. Food Sci. 2025, 1–28. Available online: https://link.cnki.net/urlid/11.2206.TS.20250918.0930.016 (accessed on 20 September 2025).
- Liu, T.; Ren, Q.; Wang, S.; Gao, J.; Shen, C.; Zhang, S.; Wang, Y.; Guan, F. Chemical Modification of Polysaccharides: A Review of Synthetic Approaches, Biological Activity and the Structure-Activity Relationship. Molecules 2024, 28, 6073. [Google Scholar] [CrossRef] [PubMed]
- Feng, Z.Y.; Zhang, W.; Li, Y.F.; Duan, H.H.; Li, Y.Y.; Xie, C.X.; Zhang, J.; Lei, J.W. Variation of sugars and cyclic enol ether terpene glycosides based on different forms of fresh Radix et Rhizoma Dioscorea. Chin. Arch. Tradit. Chin. Med. 2025, 1–14. Available online: https://link.cnki.net/urlid/21.1546.r.20250811.1007.002 (accessed on 20 September 2025).
- Ma, L.Z.; Shen, J.H. Study on the Change of Intracorporeal Reduction Sugar in Rehmanniae Radix Praeparata with Steamed for Nine Times and Shined for Nine Times. J. Liaoning Univ. Tradit. Chin. Med. 2018, 20, 59–62. [Google Scholar] [CrossRef]
- Qi, Y.T.; Zhang, M.; Liu, J.X.; Meng, S.; Ren, J.G. Modern Research Progress and Q-marker Prediction Analysis of Traditional Chinese Medicine Dihuang (Rehmannia glutinosa Radix). Chin. Arch. Tradit. Chin. Med. 2023, 41, 176–184. [Google Scholar] [CrossRef]
- Chen, Q.Y.; Lei, X.; Gao, Y.Z.; Xu, J.; Wang, L.F.; Yang, M.; Wang, X.P.; Wang, F. Quality Evaluation in Processing of Rehmanniae Radix Praeparata Processed with Amomi Fructus and Citri Reticulatae Pericarpium Based on “Color as Paint, Sweetas Maltose. Chin. J. Exp. Tradit. Med. Formulae 2022, 28, 154–162. [Google Scholar] [CrossRef]
- Wang, D.; Kuang, D.N.; Liu, R.Y.; Zhang, Z.J.; Hou, T.Y.; Li, H. Formation of DDMP, HMF and Furfural in Caramelization and Maillard Reaction. Sci. Technol. Food Ind. 2022, 43, 100–107. [Google Scholar] [CrossRef]
- Tang, X.H.; Feng, S.Y.; Liu, Y.W.; Zhu, W.H.; Bu, Y.; Li, J.N.; Liu, C.K.; Li, X.P. Identification, characterization and molecular docking study of umami peptides from Spanish mackerel head enzymatic hydrolysate and Maillard reaction products. Int. J. Biol. Macromol. 2025, 304 Pt 1, 140876. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.H.; Tian, Y.; Chou, M.; Han, X.; Ma, Y.H.; Han, L.; Zhang, D.K. Combined anti-bitterness strategy for extremely bitter characteristics of Andrographis Herba decoction and mechanism. Chin. J. Chin. Mater. Med. 2022, 47, 5424–5433. [Google Scholar] [CrossRef]
- Ge, G.B.; Wu, X.Y.; Wang, Y.J.; Hu, J.L.; Tang, B.; Wu, F. Research on bitter taste and efficacy of Ginkgo biloba extract based on UPLC-Q-TOF-MSE integrated with molecular docking. Chin. J. Chin. Mater. Med. 2024, 49, 3784–3795. [Google Scholar] [CrossRef]
- GB/T 13662-2018; Huangjiu. Standardization Administration of China: Beijing, China, 2018.
- GB/T 16291.1-2012; Sensory Analysis—General Guidance for the Selection, Training and Monitoring of Assessors—Part 1: Selected Assessors. Standardization Administration of China: Beijing, China, 2012.
- GB/T 10220-2012; Sensory Analysis—Methodology—General Guidance. Standardization Administration of China: Beijing, China, 2012.
- GH/T 1408-2022; Sensory Evaluation Method for Fresh-Eating Apples. Ministry of Agriculture and Rural Affairs of the People’s Republic of China: Beijing, China, 2022.
- DB42/T 2253-2024; Operating Procedures for Sensory Evaluation of Eating Quality of High-Quality Rice. Hubei Provincial Market Supervision Administration: Wuhan, China, 2024.
- T/HBLS 0015-2023; Sensory Evaluation Method for Cooking Quality of Whole-Grain Black Rice. Jingchu Grain and Oil Society (HBLS): Hubei, China, 2023.
- GB/T 39625-2020; Sensory Analysis—Methodology—General Guidance for Establishing a Sensory Profile. Standardization Administration of China: Beijing, China, 2020.
- Tian, H.X.; Li, Y.H.; Lu, Y.R.; Zhang, Q.L.; Wang, Z.J.; Li, S.S.; Zhou, Y.Q.; Zhang, Q.; Xiao, J.H. A cooking and eating quality evaluating system for whole grain black rice. Mol. Breed. 2024, 45, 7. [Google Scholar] [CrossRef]
- Jiang, Y.; Luan, Y.G.; Huang, R.; He, X.J.; Wang, X.; Tan, P. Comparative Analysis of Characters and Components of Wine-stewed and Steamed Rehmanniae Radix with Different Processing Degrees. Mod. Chin. Med. 2023, 25, 1957–1965. [Google Scholar] [CrossRef]
- Dai, M.R.; Li, C.; Li, R.R.; Lin, L.M.; Shen, C.X.; Zhang, Y.X.; Feng, W.H.; Wang, Z.M. Qualitative and Quantitative Analysis of Rehmanniae Radix and Its Decoction Pieces Based on Sugar Spectrum. Chin. J. Exp. Tradit. Med. Formulae 2024, 30, 157–163. [Google Scholar] [CrossRef]
- Xia, M.Y.; Cao, J.N.; Wang, Y.Y.; Meng, Y.; Chi, Y.M.; Pan, J.H. Evaluate the quality of wine-prepared Rehmannia glutinosa based on content determination and fingerprint. J. Chin. Med. Mater. 2021, 44, 1110–1114. [Google Scholar] [CrossRef]
- Martins, S.I.F.S.; Jongen, W.M.F.; Van Boekel, M.A.J.S. A review of Maillard reaction in food and implications to kinetic modelling. Trends Food Sci. Technol. 2000, 11, 364–373. [Google Scholar] [CrossRef]
- Wang, H.; Li, X.; Wang, W.J.; Zhang, S.Y.; He, J.; Zhao, G.Z. Cross modal perception interaction of flavor substances in soy sauce on umami and salt taste. China Brew. 2024, 43, 125–130. [Google Scholar] [CrossRef]
- Keast, R.S.J.; Breslin, P.A.S. An overview of binary taste–taste interactions. Food Qual. Prefer. 2003, 14, 111–124. [Google Scholar] [CrossRef]
- Xia, Y.X.; Chen, J.; Wang, Z.Y.; Wang, J.; Wu, J.X.; Zhong, F. A Systematic Review of the Interactions between Sweetness and Other Basic Tastes. J. Chin. Inst. Food Sci. Technol. 2024, 24, 12–30. [Google Scholar] [CrossRef]
- Chen, J.P.; Zhang, K.X.; Liu, Y.; Gai, X.H.; Ren, T.; Liu, S.X.; Tian, C.W. Research progress on chemical constituents and pharmacological actions of Rehmannia glutinosa. Chin. Tradit. Herb. Drugs 2021, 52, 1772–1784. [Google Scholar] [CrossRef]
- Sun, Y.X.; Wang, S.Y.; Chen, R.; Liu, H.X.; Liu, Q.; Wang, M.; Gou, M.L.; Han, T.Y.; Liu, D.Y. Recent Advances in the Mechanism of Sweetness Signaling and the Factors Influencing It. Food Sci. 2024, 45, 325–334. [Google Scholar]
- Ferreira, M.; Riul, A.J.; Wohnrath, K.; Fonseca, F.J.; Oliveira, O.N.J.; Mattoso, L.H. High-performance taste sensor made from Langmuir-Blodgett films of conducting polymers and a ruthenium complex. Anal. Chem. 2003, 75, 953–955. [Google Scholar] [CrossRef]
- Kanaga Raj, D.R.; Gonçalves, M.H.; De Medeiros, A.C.; Bolini, H.M.A.; Riul, A.J.; Barbin, D.F. Impedimetric multi-sensor system with gold and silver nanoparticles applied for basic taste assessment compared with human threshold method sensory analysis. Food Chem. 2025, 472, 142859. [Google Scholar] [CrossRef]
- Zhong, L. Research on Character and Chemical Composition Change in the Nine Process of Rehmanniae. Master’s Thesis, Chengdu University of Traditional Chinese Medicine, Chengdu, China, 2015. [Google Scholar]
- Xue, C. Study on the Formation Mechanism of “Black as Lacquer, Sweet as Candy” in the Processed Radix Rehmanniae from the Reactions of “Cross-Linking Color—Maillard” and “Sugar Hydrolysis”. Master’s Thesis, Tianjin University of Traditional Chinese Medicine, Tianjin, China, 2021. Available online: https://link.cnki.net/doi/10.27368/d.cnki.gtzyy.2021.000055 (accessed on 17 April 2025).
- Tan, V.W.K.; Wee, M.S.M.; Tomic, O.; Forde, C.G. Temporal sweetness and side tastes profiles of 16 sweeteners using temporal check-all-that-apply (TCATA). Food Res. Int. 2019, 121, 39–47. [Google Scholar] [CrossRef]
- Deng, J.L.; Tang, M.J.; Su, X.X.; Ye, Y.T.; Wei, J.Y.; Chen, Z.X.; Qin, Y.M. Rapid Kinetic Interactions of Sugar and Sugar Alcohol with Sweet Taste Receptors on Live Cells Using Stopped-Flow Spectroscopy. J. Agric. Food Chem. 2023, 71, 14731–14741. [Google Scholar] [CrossRef]
- Zhou, G.; Peng, C.; Liu, X.S.; Chang, F.; Xiao, Y.Z.; Liu, J.J.; Fang, Z.M. Identification and Immobilization of an Invertase With High Specific Activity and Sucrose Tolerance Ability of Gongronella sp. w5 for High Fructose Syrup Preparation. Front. Microbiol. 2020, 11, 633. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.M.; Chen, J.B.; Zheng, M.F.; Zhang, L.L.; Ji, H.F.; Cao, H.J.; Dai, F.X.; Wang, L. Precursors and formation pathways of furfural in sugarcane juice during thermal treatment. Food Chem. 2023, 402, 134318. [Google Scholar] [CrossRef]
- Wang, K.L.; Yu, B.K.; Zhao, H.F.; Liu, Y.X.; Wu, C.Y.; Zhang, Y.H.; Mu, Z.S. Preparation and characterization of microcapsules for tuna oil by maillard reaction products of whey protein isolate and Arabic gum via complex coacervation. Food Chem. 2025, 475, 143269. [Google Scholar] [CrossRef] [PubMed]
- Cui, Y.Q.; Zhang, H.W.; Wang, X.S.; Shi, W.Y.; Bao, Y.Z. Establishment of a high performance liquid chromatographic method for the determination of 5-HMF and analysis of 5-HMF content between functional sugar and Radix Codonopsis Pilosulae, Radix Polygonui Multifloriand Radix Rehmanniae Preparata from different origins. J. Tradit. Chin. Vet. Med. 2024, 43, 13–17. [Google Scholar] [CrossRef]
- Gao, T.; Sheng, T.; Zhang, T.; Han, H. Characterization of picroside II metabolites in rats by ultra-high-performance liquid chromatography combined with electrospray ionization quadrupole time-of-flight tandem mass spectrometry. J. Pharm. Biomed. Ana. 2016, 128, 352–359. [Google Scholar] [CrossRef]
- Fan, M.C.; Fan, Y.H.; Huang, W.P.; Wang, L.; Li, Y.; Qian, H.F.; Zhang, H.; Qi, X.G. Tentative characterization of precursor compounds and co-factors of pigment formation in production of ‘wu mi’ from Vaccinium bracteatum Thunb. Leaves. Food Chem. 2018, 262, 199–205. [Google Scholar] [CrossRef]
- Wang, F.; Chi, J.; Guo, H.; Wang, J.; Wang, P.; Li, Y.X.; Wang, Z.M.; Dai, L.P. Revealing the effects and mechanism of wine processing on Corni Fructus using chemical characterization integrated with multi-dimensional analyses. J. Chromatogr. A 2024, 1730, 465100. [Google Scholar] [CrossRef]
- Yu, H.H.; Yoshimitsu, M.; Xu, X.Y.; Zheng, Y.M.; Li, L.Y. Investigate optimum conditions and determinate changes of β-glucosidase activity in Scrophularia root under different drying conditions. Chin. J. Tradit. Chin. Med. 2017, 42, 274–279. [Google Scholar] [CrossRef]
- Chen, G.; Sui, X.Y.; Liu, T.T.; Wang, H.Y.; Zhang, J.; Sun, J.K.; Xu, T. Application of cellulase treatment in ionic liquid based enzyme-assisted extraction in combine with in-situ hydrolysis process for obtaining genipin from Eucommia ulmoides Olive barks. J. Chromatogr. A 2018, 1569, 26–35. [Google Scholar] [CrossRef]
- Du, K.; Gao, X.F.; Wang, F.; Wang, P.Y.; Qin, X.M. Research progress on quality and efficacy evaluation of Rehmanniae RadixPraeparata based on pharmacodynamic material basis. Chin. Tradit. Herb. Drugs 2019, 50, 1477–1484. [Google Scholar] [CrossRef]
- Antoniadi, L.; Bartnik, M.; Angelis, A.; Wawruszak, A.; Halabalaki, M.; Kukula-Koch, W.; Skaltsounis, L.A. Gentiopicroside-An Insight into Its Pharmacological Significance and Future Perspectives. Cells 2023, 13, 70. [Google Scholar] [CrossRef]
- Chéron, J.B.; Soohoo, A.; Wang, Y.; Golebiowski, J.; Antonczak, S.; Jiang, P.; Fiorucci, S. Conserved Residues Control the T1R3-Specific Allosteric Signaling Pathway of the Mammalian Sweet-Taste Receptor. Chem. Senses 2019, 44, 303–310. [Google Scholar] [CrossRef]
- Mayank; Jaitak, V. Interaction model of steviol glycosides from Stevia rebaudiana (Bertoni) with sweet taste receptors: A computational approach. Phytochemistry 2015, 116, 12–20. [Google Scholar] [CrossRef] [PubMed]
- Han, P.; Keast, R.S.J.; Roura, E. Salivary leptin and TAS1R2/TAS1R3 polymorphisms are related to sweet taste sensitivity and carbohydrate intake from a buffet meal in healthy young adults. Br. J. Nutr. 2017, 118, 763–770. [Google Scholar] [CrossRef]
- Ben Shoshan-Galeczki, Y.; Niv, M.Y. Structure-based screening for discovery of sweet compounds. Food Chem. 2020, 315, 126286. [Google Scholar] [CrossRef] [PubMed]
- Smith, N.J.; Grant, J.N.; Moon, J.I.; So, S.S.; Finch, A.M. Critically evaluating sweet taste receptor expression and signaling through a molecular pharmacology lens. FEBS J. 2021, 288, 2660–2672. [Google Scholar] [CrossRef] [PubMed]
- Juhász, R.; Penksza, P.; Sipos, L. Effect of xylo-oligosaccharides (XOS) addition on technological and sensory attributes of cookies. Food Sci. Nutr. 2022, 8, 5452–5460. [Google Scholar] [CrossRef]









| Batches | Place of Origin | Batches | Place of Origin |
|---|---|---|---|
| S1 | Qu Village, Dahongqiao Township, Wuzhi County, Jiaozuo City, Henan Province | S10 | Jiabu Fourth Village, Dafeng Town, Wuzhi County, Jiaozuo City, Henan Province |
| S2 | Xitao Village, Xitao Town, Wuzhi County, Jiaozuo City, Henan Province | S11 | Chenxinzhuang Village, Zhaobao Town, Wen County, Jiaozuo City, Henan Province |
| S3 | Xitao Village, Xitao Town, Wuzhi County, Jiaozuo City, Henan Province | S12 | Mafenglin Village, Wude Town, Wen County, Jiaozuo City, Henan Province |
| S4 | Jiabu Fourth Village, Dafeng Town, Wuzhi County, Jiaozuo City, Henan Province | S13 | Beibaofeng Village, Wude Town, Wen County, Jiaozuo City, Henan Province |
| S5 | Yijing Village, Xiguo Town, Mengzhou City, Jiaozuo City, Henan Province | S14 | Beibaofeng Village, Wude Town, Wen County, Jiaozuo City, Henan Province |
| S6 | Xitao Village, Xitao Town, Wuzhi County, Jiaozuo City, Henan Province | S15 | Hengshan Village, Huagong Town, Mengzhou City, Jiaozuo City, Henan Province |
| S7 | Jiabu Third Village, Dafeng Town, Wuzhi County, Jiaozuo City, Henan Province | S16 | Dangsongma Village, Gudan Town, Mengzhou City, Jiaozuo City, Henan Province |
| S8 | Jiabu Fourth Village, Dafeng Town, Wuzhi County, Jiaozuo City, Henan Province | S17 | Dangsongma Village, Gudan Town, Mengzhou City, Jiaozuo City, Henan Province |
| S9 | Qu Village, Dahongqiao Township, Wuzhi County, Jiaozuo City, Henan Province |
| Characters | Intensity Scale for Sensory | ||||||
|---|---|---|---|---|---|---|---|
| Negative | Reference | Positive | |||||
| Strong | Moderate | Slight | Slight | Moderate | Strong | ||
| −3 | −2 | −1 | 0 | 1 | 2 | 3 | |
| sweet | |||||||
| sour | |||||||
| bitter | |||||||
| salty | |||||||
| umami | |||||||
| aftertaste | |||||||
| others | |||||||
| Batches | Fru % | Glu % | Suc % | Mel % | Raf % | Mnt % | Sta % |
|---|---|---|---|---|---|---|---|
| R1 | 4.10 | 3.70 | 11.83 | 2.05 | 8.80 | 8.75 | 42.48 |
| R2 | 3.38 | 3.73 | 12.20 | 2.40 | 9.85 | 7.33 | 40.92 |
| R3 | 1.58 | 2.13 | 15.63 | 1.00 | 8.95 | 3.23 | 44.32 |
| R4 | 1.43 | 1.65 | 15.95 | 1.00 | 9.15 | 2.83 | 44.49 |
| R5 | 1.90 | 2.40 | 15.35 | 1.63 | 10.58 | 4.65 | 42.56 |
| R6 | 4.55 | 4.58 | 11.60 | 3.08 | 9.50 | 8.98 | 39.58 |
| R7 | 1.98 | 2.10 | 17.48 | 1.08 | 9.85 | 2.90 | 43.68 |
| R8 | 1.50 | 1.75 | 15.93 | 1.20 | 11.35 | 2.55 | 45.32 |
| R9 | 1.33 | 1.88 | 12.83 | 0.98 | 8.05 | 2.60 | 47.17 |
| R10 | 1.08 | 1.45 | 11.70 | 1.19 | 10.05 | 1.95 | 50.20 |
| R11 | 1.25 | 1.58 | 12.53 | 1.00 | 9.68 | 2.10 | 48.74 |
| R12 | 1.40 | 1.43 | 11.65 | 0.95 | 8.85 | 2.08 | 48.37 |
| R13 | 1.90 | 2.15 | 12.60 | 0.78 | 8.65 | 2.60 | 46.31 |
| R14 | 1.08 | 1.58 | 12.48 | 0.87 | 9.58 | 1.85 | 48.86 |
| R15 | 1.43 | 1.68 | 12.03 | 0.90 | 9.93 | 2.08 | 48.23 |
| R16 | 1.33 | 1.53 | 12.33 | 0.95 | 8.93 | 1.98 | 48.89 |
| R17 | 3.45 | 3.30 | 10.85 | 1.80 | 8.35 | 5.65 | 38.75 |
| P1 | 15.70 | 11.03 | \ | 6.45 | \ | 38.63 | 0.91 |
| P2 | 17.13 | 13.25 | \ | 7.08 | \ | 32.48 | 0.95 |
| P3 | 19.33 | 12.43 | \ | 5.43 | \ | 41.23 | 2.66 |
| P4 | 19.18 | 12.53 | \ | 7.65 | \ | 37.15 | 0.84 |
| P5 | 15.58 | 15.28 | \ | 6.93 | \ | 29.28 | 1.36 |
| P6 | 18.20 | 12.03 | \ | 5.63 | \ | 36.90 | 1.70 |
| P7 | 19.35 | 13.43 | \ | 6.83 | \ | 29.25 | 0.87 |
| P8 | 20.55 | 12.48 | \ | 6.83 | \ | 39.15 | 1.86 |
| P9 | 17.20 | 10.10 | \ | 4.63 | \ | 35.25 | 1.81 |
| P10 | 17.65 | 8.58 | \ | 5.43 | \ | 37.33 | 2.37 |
| P11 | 17.65 | 9.18 | \ | 5.15 | \ | 32.60 | 1.30 |
| P12 | 18.60 | 9.88 | \ | 5.38 | \ | 34.25 | 1.00 |
| P13 | 17.05 | 10.23 | \ | 5.08 | \ | 25.60 | 1.07 |
| P14 | 19.43 | 10.75 | \ | 5.75 | \ | 33.63 | 2.08 |
| P15 | 18.20 | 8.88 | \ | 5.53 | \ | 35.98 | 1.05 |
| P16 | 17.98 | 8.68 | \ | 4.90 | \ | 34.10 | 0.91 |
| P17 | 17.70 | 11.18 | \ | 6.03 | \ | 29.60 | 0.95 |
| Δavg | 15.99 | 8.90 | −13.23 | 4.58 | −9.42 | 30.49 | −43.83 |
| Batches | Cat mg/g | Reh D mg/g | Reh A mg/g | Aju mg/g | Ech mg/g | Cis A mg/g | Ver mg/g |
|---|---|---|---|---|---|---|---|
| R1 | 2.621 | 2.262 | 0.581 | 0.522 | 0.298 | 0.118 | 0.503 |
| R2 | 3.125 | 1.955 | 0.585 | 0.628 | 0.205 | 0.069 | 0.283 |
| R3 | 13.664 | 2.601 | 0.828 | 1.491 | 0.427 | 0.178 | 0.488 |
| R4 | 13.170 | 2.703 | 0.743 | 1.633 | 0.585 | 0.197 | 0.525 |
| R5 | 4.227 | 2.125 | 0.915 | 0.802 | 0.198 | 0.061 | 0.268 |
| R6 | 9.252 | 2.055 | 0.503 | 1.501 | 0.477 | 0.191 | 0.817 |
| R7 | 4.115 | 2.039 | 0.977 | 0.717 | 0.246 | 0.052 | 0.266 |
| R8 | 13.270 | 2.638 | 0.757 | 1.498 | 0.474 | 0.170 | 0.547 |
| R9 | 15.214 | 2.509 | 0.641 | 2.347 | 0.245 | 0.098 | 0.288 |
| R10 | 15.884 | 2.767 | 0.805 | 1.591 | 0.489 | 0.199 | 0.483 |
| R11 | 15.174 | 2.722 | 0.650 | 2.265 | 0.321 | 0.125 | 0.383 |
| R12 | 13.818 | 2.434 | 0.591 | 1.922 | 0.346 | 0.104 | 0.510 |
| R13 | 12.103 | 2.318 | 0.531 | 1.829 | 0.507 | 0.175 | 0.873 |
| R14 | 8.399 | 2.477 | 0.847 | 1.079 | 0.104 | 0.133 | 0.197 |
| R15 | 16.848 | 2.707 | 0.664 | 2.365 | 0.340 | 0.107 | 0.450 |
| R16 | 16.305 | 2.583 | 0.985 | 2.456 | 0.177 | 0.149 | 0.264 |
| R17 | 13.072 | 1.767 | 0.479 | 1.981 | 0.440 | 0.148 | 0.733 |
| P1 | 0.637 | 0.728 | 0.289 | 0.146 | 0.206 | 0.057 | 0.223 |
| P2 | 0.776 | 0.636 | 0.303 | 0.154 | 0.135 | 0.034 | 0.103 |
| P3 | 1.500 | 1.481 | 0.434 | 0.260 | 0.236 | 0.085 | 0.230 |
| P4 | 0.846 | 0.847 | 0.291 | 0.183 | 0.304 | 0.068 | 0.184 |
| P5 | 0.895 | 0.876 | 0.404 | 0.213 | 0.136 | 0.093 | 0.121 |
| P6 | 0.906 | 0.665 | 0.237 | 0.219 | 0.301 | 0.100 | 0.254 |
| P7 | 1.251 | 0.782 | 0.408 | 0.197 | 0.159 | 0.020 | 0.069 |
| P8 | 1.201 | 0.884 | 0.383 | 0.170 | 0.298 | 0.074 | 0.166 |
| P9 | 0.899 | 1.065 | 0.246 | 0.232 | 0.119 | 0.045 | 0.121 |
| P10 | 1.268 | 1.518 | 0.475 | 0.277 | 0.298 | 0.102 | 0.275 |
| P11 | 0.927 | 1.149 | 0.286 | 0.240 | 0.197 | 0.083 | 0.172 |
| P12 | 0.993 | 0.781 | 0.336 | 0.193 | 0.152 | 0.050 | 0.210 |
| P13 | 0.665 | 0.601 | 0.267 | 0.156 | 0.222 | 0.092 | 0.296 |
| P14 | 1.025 | 0.817 | 0.316 | 0.186 | 0.055 | 0.053 | 0.078 |
| P15 | 1.505 | 1.077 | 0.378 | 0.182 | 0.198 | 0.044 | 0.247 |
| P16 | 0.817 | 0.956 | 0.392 | 0.180 | 0.113 | 0.072 | 0.086 |
| P17 | 0.748 | 0.723 | 0.258 | 0.208 | 0.241 | 0.062 | 0.241 |
| Δavg | −88.15 | −61.96 | −52.17 | −84.68 | −41.77 | −46.94 | −60.33 |
| Receptor Name | Model | ERRAT Value | C-Score | Percentage of Amino Acids in Allowed Regions (%) |
|---|---|---|---|---|
| hT1R2 | I | 89.19 | 0.19 | 98.8 |
| II | 86.10 | 0.02 | 97.6 | |
| III | 81.42 | −0.83 | 98.1 | |
| IV | 82.13 | −2.04 | 98.0 | |
| V | 80.34 | −2.28 | 98.5 | |
| hT1R3 | I | 85.56 | −0.16 | 98.6 |
| II | 88.11 | −0.68 | 97.5 | |
| III | 87.33 | −1.99 | 97.5 | |
| IV | 84.32 | −0.93 | 98.6 | |
| V | 82.82 | −3.07 | 97.5 | |
| hT2R4 | I | 99.66 | 0.40 | 98.2 |
| II | 92.78 | −2.30 | 99.6 | |
| III | 98.63 | −2.35 | 98.6 | |
| IV | 94.85 | −4.20 | 98.9 | |
| V | 97.94 | −4.58 | 99.6 | |
| hT2R14 | I | 96.76 | −0.63 | 99.7 |
| II | 96.76 | −1.02 | 99.3 | |
| III | 98.63 | −1.45 | 99.7 | |
| IV | 96.44 | −3.26 | 99.0 | |
| V | 92.56 | −3.04 | 97.3 |
| Receptor Name | TM-Score | RMSD |
|---|---|---|
| hT1R2 | 0.72 ± 0.11 | 8.4 ± 4.5 |
| hT1R3 | 0.69 ± 0.12 | 8.9 ± 4.6 |
| hT2R4 | 0.77 ± 0.10 | 5.3 ± 3.4 |
| hT2R14 | 0.63 ± 0.13 | 7.7 ± 4.3 |
| Compound Name | AR2 | AR3 | As | AR4 | AR14 | Ab |
|---|---|---|---|---|---|---|
| 5-HMF | −5.1 | −4.9 | −10.0 | −4.7 | −4.9 | −9.6 |
| Aju | −8.1 | −7.8 | −15.9 | −6.6 | −6.7 | −13.3 |
| Cat | −7.8 | −8.3 | −16.1 | −6.8 | −6.6 | −13.4 |
| Cis A | −8.9 | −10.8 | −19.7 | −7.3 | −8.7 | −16.0 |
| Ech | −9.1 | −9.7 | −18.8 | −8.0 | −9.1 | −17.1 |
| Reh A | −9.8 | −10.3 | −20.1 | −7.5 | −8.1 | −15.6 |
| Reh D | −6.2 | −9.3 | −15.5 | −6.0 | −6.8 | −12.8 |
| Ver | −10.7 | −9.5 | −20.2 | −6.9 | −9.3 | −16.2 |
| Fru | −4.9 | −5.5 | −10.4 | −5.2 | −4.7 | −9.9 |
| Glu | −4.8 | −5.3 | −10.1 | −4.9 | −4.6 | −9.5 |
| Suc | −6.8 | −7.1 | −13.9 | −5.4 | −6.6 | −12.0 |
| Mel | −6.7 | −7.2 | −13.9 | −5.9 | −6.5 | −12.4 |
| Raf | −7.7 | −7.8 | −15.5 | −5.9 | −6.9 | −12.8 |
| Mnt | −7.6 | −8.0 | −15.6 | −5.8 | −6.8 | −12.6 |
| Sta | −9.5 | −10.0 | −19.5 | −6.5 | −6.8 | −13.3 |
| Compound Name | hT1R2 (Binding Residues: Distance, Å) | hT1R3 (Binding Residues: Distance, Å) | Compound Name | hT1R2 (Binding Residues: Distance, Å) | hT1R3 (Binding Residues: Distance, Å) |
|---|---|---|---|---|---|
| 5-HMF | ASP-213:3.1; SER-144:2.1; ILE-167:2.0; TYR-103:2.7 | SER-182:2.1; PHE-183:2.4 | Fru | HIS-311:2.4; GLN-459:2.1,2.5; ASN-460:2.2; ASP-169:2.2; ARG-378:2.5,2.5 | GLN-193:2.8; GLY-168:2.4,2.5; GLU-301:2.4 |
| Aju | ASP-142:2.5; SER-303:2.6; SER-165:2.2; ILE-306:2.5 | HIS-145:2.5; GIU-301:2.3,2.5; GLN-389:2.3 | Glu | HIS-311:2.7; ASN-460:2.4,2.5, 2.6; ARG-378:2.5,2.6 | ALA-302:2.2; SER-306:2.4,2.8; THR-305:2.0,2.1; HIS-388:2.1 |
| Cat | GIU-302:2.3; ILE-306:2.8 | ASP-307:2.8; SER-306:2.4; THR-305:2.3; HIS-388:2.7 | Suc | SER-303:2.0,2.3; ILE-306:2.0 | HIS-278:2.3 |
| Cis A | TYR-62:2.6; ASN-312:2.2; VAL-64:2.2; CLN-355:2.3, 2.4; SER-356:2.5, 1.8; ASN-374:2.2; SER-372:2.4 | GLU-45:2.4; SER-170:2.7; TRP-72:2.0; HIS-388:2.6; ASN-386:2.3,2.4 | Mel | SER-144:2.5; ASP-142:2.4,3.5; SER-165:2.1; ILE-306:2.0 | HIS-145:2.1,2.3; ASN-68:2.0; ASP-307:2.2; THR-305:2.6; HIS-388:2.4 |
| Ech | ASP-278:2.3, 2.4; ILE-167:2.6,2.9; SER-165:2.9; GLU-302:2.4 | ASP-307:2.1,2.6; THR-305:2.0; GLU-45:2.1; SER-67:2.3; TRP-72:1.8 | Raf | ASP-142:2.6, 2.8; SER-165:2.4; ILE-306:2.2; VAL-384:2.8 | SER-146:1.9; GLY-44:2.3; VAL-277:2.6; ALA-302:2.2; ASN-68:1.8 |
| Reh A | SER-40:2.4; ILE-306:2.4; GLU-302:2.2; ARG-378:2.4, 2.5,2.6 | GLY-44:2.2; VAL-277:2.8; HIS-145:2.7; ASN-68:2.5,2.7; THR-305:1.8,2.4; ASN-386:1.9,2.2 | Mnt | SER-144:2.4; ASP-142:2.7 | SER-147:2.5; SER-104:2.5; SER-146:2.2; PRO-42:2.6; SER-66:2.5; THR-305:2.2,2.4; HIS-388:2.7 |
| Reh D | ARG-217:1.9; GLU-145:2.3; ASP-213:1.9; SER-212:2.2; THR-280:2.1 | HIS-278:2.1; SER-147:2.2; HIS-145:1.9,2.4; SER-306:2.7; HIS-388:2.6 | Sta | SER-144:2.6; ASP-142:2.3; ILE-67:1.9; SER-303:2.5; GLU-302:2.4; ILE-306:2.3; GLY-381:2.1 | SER-104:2.1; SER-146:1.9,2.6; TYR-218:2.8; HIS-145:1.9,2.2; GLN-389:2.2,2.6; ASN-68:2.0; HIS-388:2.2,2.4, 2.6; ASP-307:2.5 |
| Ver | SER-165:2.1; ASN-70:2.3,2.5 | ASN-68:2.0,2.5; ASN-386:2.8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zu, W.; Wang, J.; Wang, J.; Wang, H.; Song, L.; Li, Y.; Chi, H.; She, G.; Du, H. Chemical Mechanisms Underlying Sweetness Enhancement During Processing of Rehmanniae Radix: Carbohydrate Hydrolysis, Degradation of Bitter Compounds, and Interaction with Taste Receptors. Foods 2025, 14, 3932. https://doi.org/10.3390/foods14223932
Zu W, Wang J, Wang J, Wang H, Song L, Li Y, Chi H, She G, Du H. Chemical Mechanisms Underlying Sweetness Enhancement During Processing of Rehmanniae Radix: Carbohydrate Hydrolysis, Degradation of Bitter Compounds, and Interaction with Taste Receptors. Foods. 2025; 14(22):3932. https://doi.org/10.3390/foods14223932
Chicago/Turabian StyleZu, Wenting, Jiasheng Wang, Jing Wang, Hongyue Wang, Liangliang Song, Yichen Li, Hongshuang Chi, Gaimei She, and Hong Du. 2025. "Chemical Mechanisms Underlying Sweetness Enhancement During Processing of Rehmanniae Radix: Carbohydrate Hydrolysis, Degradation of Bitter Compounds, and Interaction with Taste Receptors" Foods 14, no. 22: 3932. https://doi.org/10.3390/foods14223932
APA StyleZu, W., Wang, J., Wang, J., Wang, H., Song, L., Li, Y., Chi, H., She, G., & Du, H. (2025). Chemical Mechanisms Underlying Sweetness Enhancement During Processing of Rehmanniae Radix: Carbohydrate Hydrolysis, Degradation of Bitter Compounds, and Interaction with Taste Receptors. Foods, 14(22), 3932. https://doi.org/10.3390/foods14223932







