Effect of Simulated Gastrointestinal Digestion on the Phenolic Composition and Bioactivity of Cymbopogon flexuosus Extracts
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Plant Material
2.3. In Vitro Simulated Gastrointestinal Digestion
2.4. The Determination Total Phenolic (TPC) and Flavonoid (TFC) Content
2.5. UHPLC Q-ToF MS Analysis and Bioaccessibility
2.6. Biological Activity of Control and Digested Samples
2.6.1. Antioxidant Assays
2.6.2. Antidiabetic Assays
2.6.3. Cytotoxicity Assay
2.7. Statistical Analysis
3. Results and Discussion
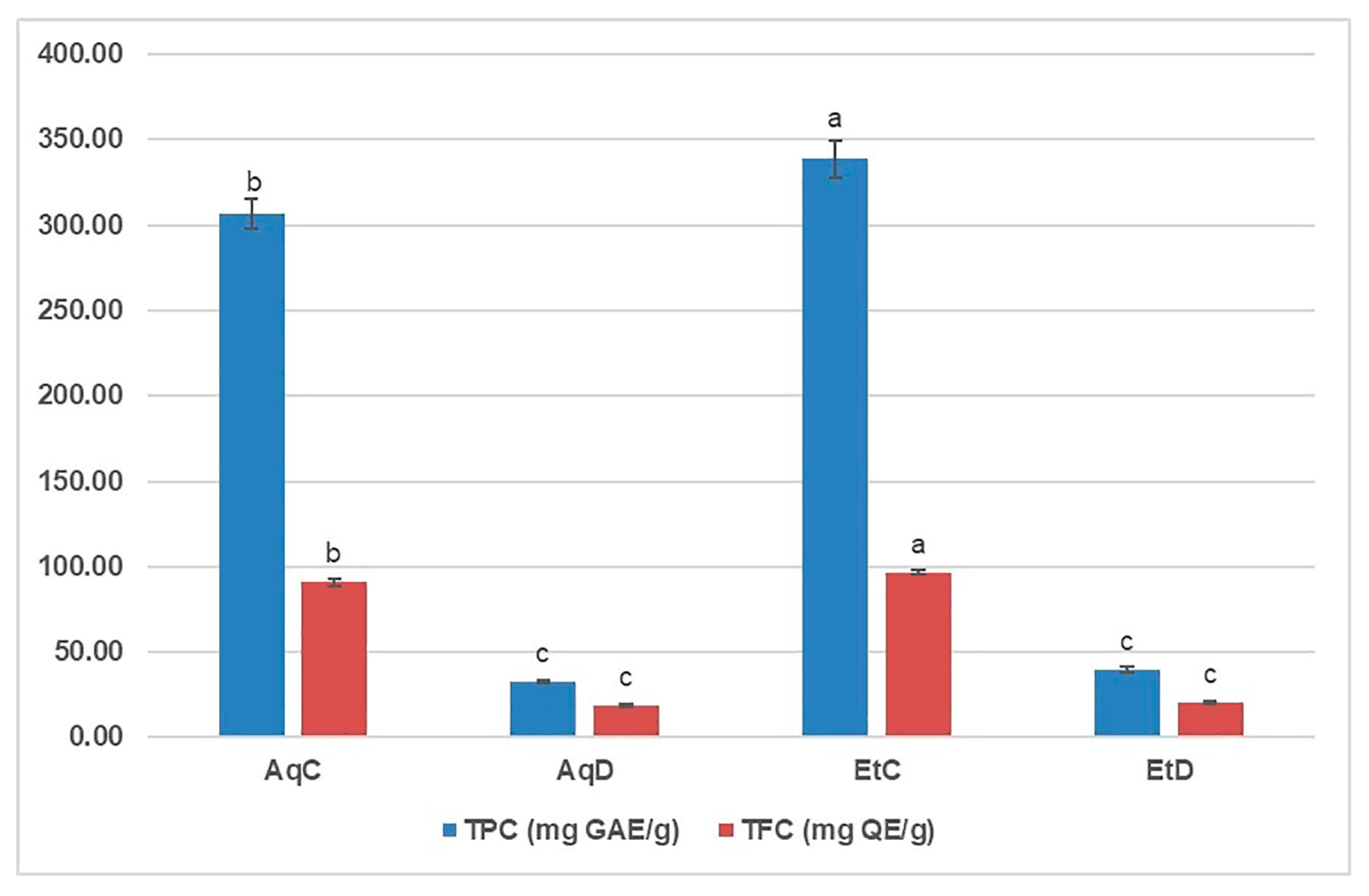
3.1. Total Phenolic (TPC) and Flavonoid (TFC) Content
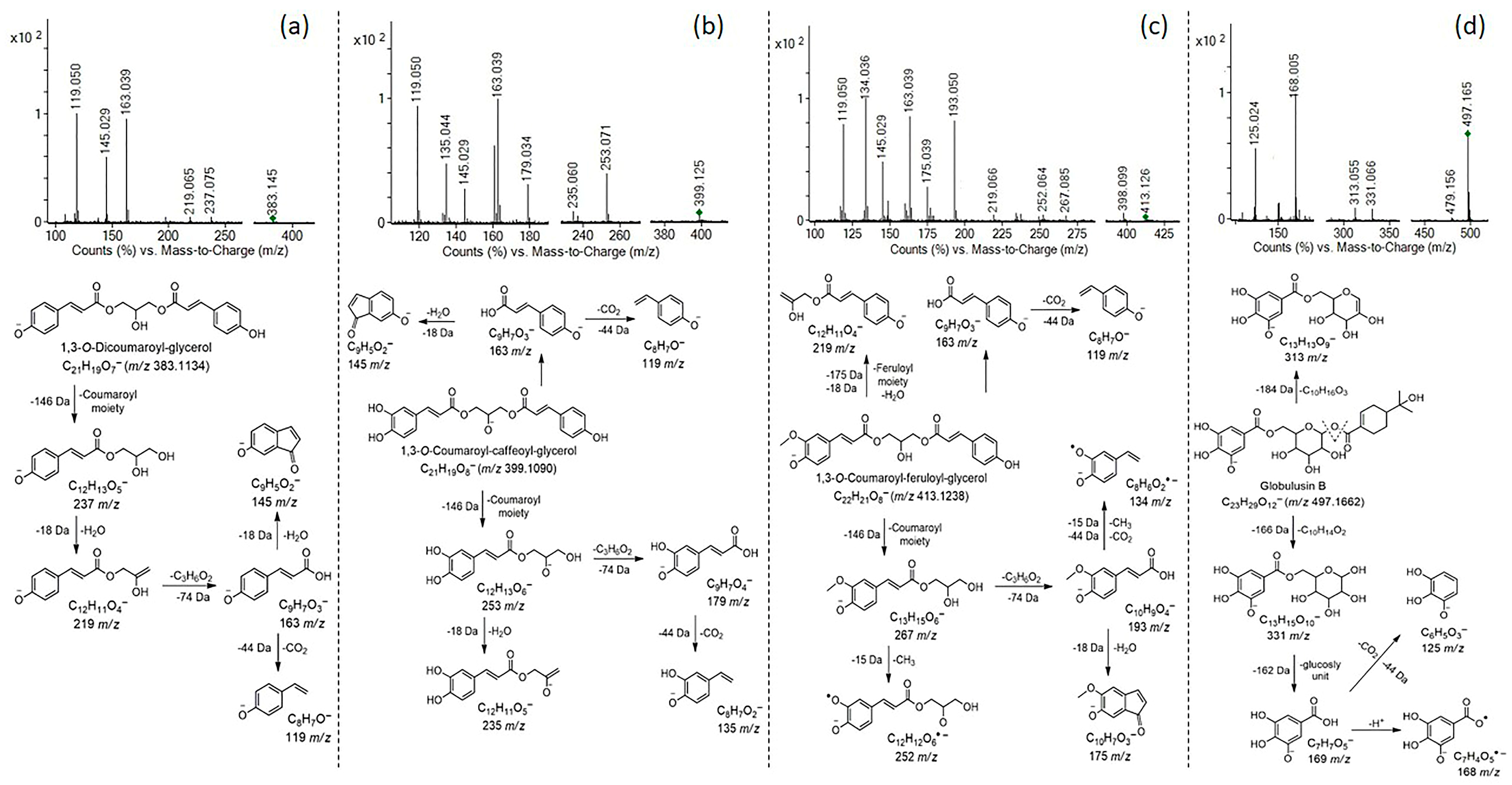
3.2. UHPLC Q-ToF MS Identification and Characterization of Phenolic Compounds in Control and Digested Extracts
3.3. Bioaccessibility of Phenolic Compounds After In Vitro GID of C. flexuosus Leaves
3.4. Antioxidant Properties of Control and Digested C. flexuosus Extracts
3.5. Antidiabetic Activity of Control and Digested C. flexuosus Extracts
| Concentration (mg/mL) | Value Ratio D:C | ||
|---|---|---|---|
| Extract | Ethanolic | Aqueous | |
| TPC (mg GAE/g) | 5 | 1:8.5 | 1:9.5 |
| TFC (mg QE/g) | 5 | 1:4.7 | 1:4.9 |
| DPPH (% of inhibition) | 2.5 | 1:4.0 | 1:4.2 |
| 5 | 1:3.6 | 1:3.9 | |
| 10 | 1:2.6 | 1:3.8 | |
| TRP (µM AAE/g) | 5 | 1:17.3 | 1:29.9 |
| 10 | 1:12.9 | 1:20.7 | |
| β-carotene bleaching (% of inhibition) | 2.5 | 1:3.7 | 1:3.8 |
| 5 | 1:6.0 | 1:2.7 | |
| 10 | 1:3.6 | 1:4.1 | |
| α-glucosidase (% of inhibition) | 0.15625 | 1:1.3 | 1:0.8 |
| 0.3125 | 1:1.1 | 1:0.9 | |
| 0.625 | 1:0.8 | 1:0.8 | |
| 1.25 | 1:0.9 | 1:0.8 | |
| 2.5 | 1:0.8 | 1:0.9 | |
| 5 | 1:0.9 | 1:0.7 | |
| 10 | 1:1 | 1:0.7 | |
| α-amylase (% of inhibition) | 10 | 1:3.2 | 1:3.0 |
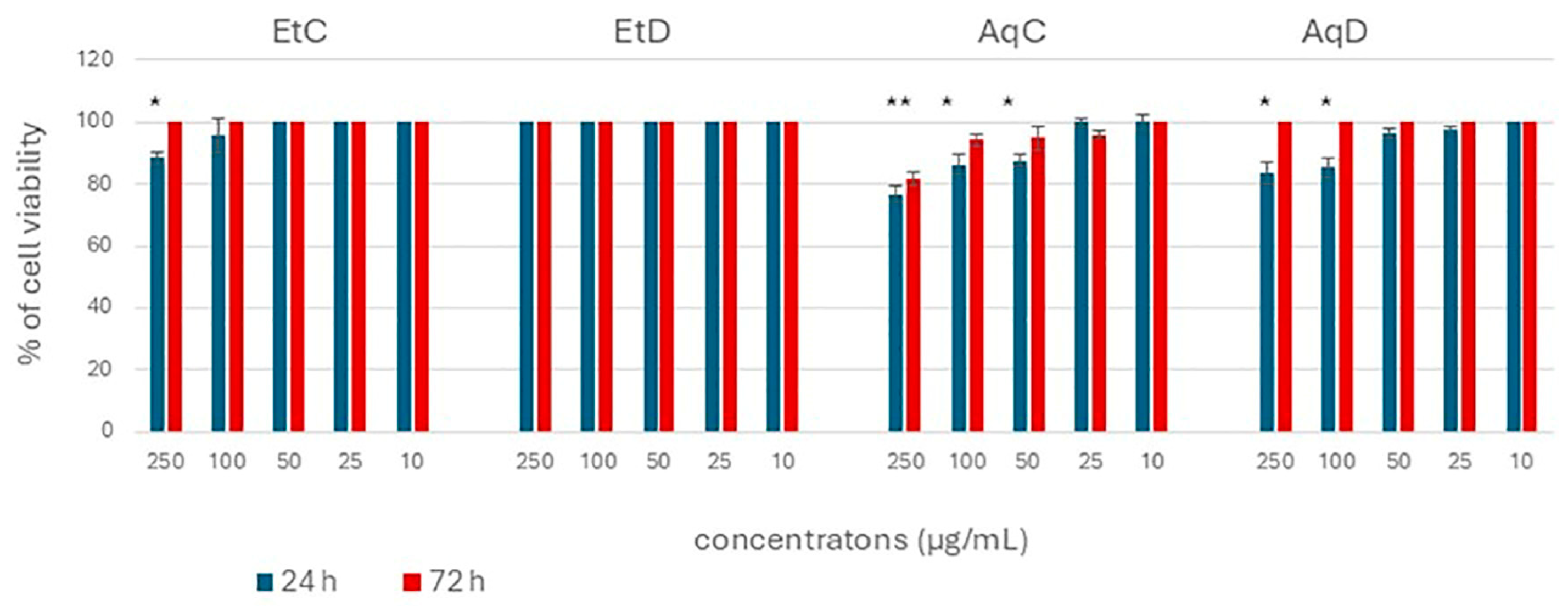
3.6. Cytotoxic Activity of Control and Digested C. flexuosus Extract
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sharma, P.R.; Mondhe, D.M.; Muthiah, S.; Pal, H.C.; Shahi, A.K.; Saxena, A.K.; Qazi, G.N. Anticancer activity of an essential oil from Cymbopogon flexuosus. Chem. Biol. Interact. 2009, 179, 160–168. [Google Scholar] [CrossRef]
- Ganjewala, D.E.; Gupta, A.K. Lemongrass (Cymbopogon flexuosus Steud.) Wats essential oil: Overview and biological activities. Recent. Prog. Med. Plants 2013, 37, 235–271. [Google Scholar]
- Tibenda, J.J.; Yi, Q.; Wang, X.; Zhao, Q. Review of phytomedicine, phytochemistry, ethnopharmacology, toxicology, and pharmacological activities of Cymbopogon genus. Front. Pharmacol. 2022, 13, 997918. [Google Scholar]
- Wahyuni, D.K.; Kharisma, V.D.; Murtadlo, A.A.A.; Rahmawati, C.T.; Syukriya, A.J.; Prasongsuk, S.; Subramaniam, S.; Wibowo, A.T.; Purnobasuki, H. The antioxidant and antimicrobial activity of ethanolic extract in roots, stems, and leaves of three commercial Cymbopogon species. BMC Complement. Med. Ther. 2024, 24, 272. [Google Scholar] [PubMed]
- Skaria, B.P.; Joy, P.P.; Mathew, G.; Mathew, S.; Joseph, A. Lemongrass. In Handbook of Herbs and Spices, 2nd ed.; Peter, K.V., Ed.; Woodhead Publishing: Cambridge, UK, 2012; Volume 2, pp. 348–370. [Google Scholar]
- Lonkar, P.B.; Chavan, U.D.; Pawar, V.D.; Bansode, V.V.; Amarowicz, R. Studies on preparation and preservation of lemongrass (Cymbopogon flexuosus (Steud) Wats) powder for tea. Emir. J. Food Agric. 2013, 25, 585–592. [Google Scholar] [CrossRef]
- Nwauche, K.T.; Berezi, E.P.; Okari, K.A. Phytochemical profiling of Cymbopogon flexuosus plant leaves. Int. J. Sci. Res. Arch. 2024, 13, 3239–3247. [Google Scholar] [CrossRef]
- Adukwu, E.C.; Bowles, M.; Edwards-Jones, V.; Bone, H. Antimicrobial activity, cytotoxicity and chemical analysis of lemongrass essential oil (Cymbopogon flexuosus) and pure citral. Appl. Microbiol. Biotechnol. 2016, 100, 9619–9627. [Google Scholar] [CrossRef]
- Caballero-Gallardo, K.; Quintero-Rincón, P.; Stashenko, E.E.; Olivero-Verbel, J. Photoprotective agents obtained from aromatic plants grown in Colombia: Total phenolic content, antioxidant activity, and assessment of cytotoxic potential in cancer cell lines of Cymbopogon flexuosus L. and Tagetes lucida Cav. essential oils. Plants 2022, 11, 1693. [Google Scholar] [CrossRef]
- Júnior, A.S.S.; Aidar, F.J.; Silva, L.A.S.; Silva, T.d.B.; de Almeida, S.F.M.; Teles, D.C.S.; Junior, W.d.L.; Schimieguel, D.M.; de Souza, D.A.; Nascimento, A.C.S.; et al. Influence of lemongrass essential oil (Cymbopogon flexuosus) supplementation on diabetes in rat model. Life 2024, 14, 336. [Google Scholar] [CrossRef]
- Sharma, R.; Pokhrel, G.; Bhattarai, M.; Roy, K. Anti-diabetic activity of aqueous extract of Cymbopogon flexuosus in high fat diet induced obese guinea pigs. Int. J. Pharm. Res. Appl. 2021, 6, 346–351. [Google Scholar]
- Nomier, Y.; Asaad, G.F.; Alshahrani, S.; Safhi, S.; Medrba, L.; Alharthi, N.; Rehman, Z.; Alhazmi, H.; Sanobar, S. Antidepressant and anxiolytic profiles of Cymbopogon flexuosus ethanolic extract in chronic unpredictable mild stress induced in rats. Biomed. Pharmacol. J. 2021, 14, 175–185. [Google Scholar] [CrossRef]
- Le, Q.U.; Lay, H.L.; Wu, M.C. The isolation, structural characterization, and anticancer activity from the aerial parts of Cymbopogon flexuosus. J. Food Biochem. 2019, 43, e12718. [Google Scholar] [CrossRef]
- Bellesia, A.; Verzelloni, E.; Tagliazucchi, D. Pomegranate ellagitannins inhibit α-glucosidase activity in vitro and reduce starch digestibility under simulated gastro-intestinal conditions. Int. J. Food Sci. Nutr. 2015, 66, 85–92. [Google Scholar] [CrossRef] [PubMed]
- Diab, F.; Khalil, M.; Lupidi, G.; Zbeeb, H.; Salis, A.; Damonte, G.; Bramucci, M.; Portincasa, P.; Vergani, L. Influence of simulated in vitro gastrointestinal digestion on the phenolic profile, antioxidant, and biological activity of Thymbra spicata L. extracts. Antioxidants 2022, 11, 1778. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.L.; Wang, Z.T.; Yang, D.M.; Li, Z.; Wu, Q.L.; Guo, X.; Shang, Y.F.; Thakur, K.; Wei, Z.J. Effect of processing and simulated digestion on phenolics, antioxidant and hypoglycemic potential of winter jujube. LWT 2024, 211, 116944. [Google Scholar] [CrossRef]
- Kasipandi, M.; Manikandan, A.; Sreeja, P.S.; Suman, T.; Saikumar, S.; Dhivya, S.; Parimelazhagan, T. Effects of in vitro simulated gastrointestinal digestion on the antioxidant, α-glucosidase and α-amylase inhibitory activities of water-soluble polysaccharides from Opilia amentacea Roxb fruit. LWT 2019, 111, 774–781. [Google Scholar] [CrossRef]
- Singleton, V.L.; Rossi, J.A. Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Am. J. Enol. Vitic. 1965, 16, 144–158. [Google Scholar] [CrossRef]
- Park, Y.K.; Koo, M.H.; Ikegaki, M.; Contado, J.O.S.E. Comparison of the flavonoid aglycone contents of Apis mellifera propolis from various regions of Brazil. Arq. Biol. Tecnol. 1997, 40, 97–106. [Google Scholar]
- Milinčić, D.D.; Vidović, B.B.; Gašić, U.M.; Milenković, M.; Kostić, A.Ž.; Stanojević, S.P.; Ilić, T.; Pešić, M.B. A systematic UHPLC Q-ToF MS approach for the characterization of bioactive compounds from freeze-dried red goji berries (L. barbarum L.) grown in Serbia: Phenolic compounds and phenylamides. Food Chem. 2024, 456, 140044. [Google Scholar] [CrossRef]
- Aly, O.; Mekky, R.; Pereira, F.; Diab, Y.; Tammam, M.; El-Demerdash, A. Deciphering the potential of Cymbopogon citratus (DC.) Stapf as an anti-obesity agent: Phytochemical profiling, in vivo evaluations and molecular docking studies. Food Funct. 2024, 15, 12146–12168. [Google Scholar] [CrossRef]
- Lan, W.; Rencoret, J.; Lu, F.; Karlen, S.D.; Smith, B.G.; Harris, P.J.; Del Río, J.C.; Ralph, J. Tricin-lignins: Occurrence and quantitation of tricin in relation to phylogeny. Plant J. 2016, 88, 1046–1057. [Google Scholar] [CrossRef] [PubMed]
- Boulekbache-Makhlouf, L.; Meudec, E.; Mazauric, J.P.; Madani, K.; Cheynier, V. Qualitative and semi-quantitative analysis of phenolics in Eucalyptus globulus leaves by high-performance liquid chromatography coupled with diode array detection and electrospray ionisation mass spectrometry. Phytochem. Anal. 2013, 24, 162–170. [Google Scholar] [CrossRef] [PubMed]
- Bylka, W.; Frański, R.; Stobiecki, M. Differentiation between isomeric acacetin-6-C-(6″-O-malonyl)glucoside and acacetin-8-C-(6″-O-malonyl)glucoside by using low-energy CID mass spectra. J. Mass. Spectrom. 2002, 37, 648–650. [Google Scholar] [CrossRef]
- Costa, G.; Ferreira, J.P.; Vitorino, C.; Pina, M.E.; Sousa, J.J.; Figueiredo, I.V.; Batista, M.T. Polyphenols from Cymbopogon citratus leaves as topical anti-inflammatory agents. J. Ethnopharmacol. 2016, 178, 222–228. [Google Scholar] [CrossRef] [PubMed]
- Costa, G.; Nunes, F.; Vitorino, C.; Sousa, J.; Figueiredo, I.; Batista, M. Validation of a RP-HPLC method for quantitation of phenolic compounds in three different extracts from Cymbopogon citratus. Res. J. Med. Plant 2015, 9, 331–339. [Google Scholar] [CrossRef]
- Du, X.; Zhang, M.; Wang, S.; Li, J.; Zhang, J.; Liu, D. Ethnopharmacology, chemical composition and functions of Cymbopogon citratus. Chin. Herb. Med. 2024, 16, 358–374. [Google Scholar] [CrossRef]
- Shao, S.Y.; Ting, Y.; Wang, J.; Sun, J.; Guo, X.F. Characterization and identification of the major flavonoids in Phyllostachys edulis leaf extract by UPLC–QTOF–MS/MS. Acta Chromatogr. 2020, 32, 228–237. [Google Scholar] [CrossRef]
- Singh, A.; Kumar, S.; Bajpai, V.; Reddy, T.J.; Rameshkumar, K.B.; Kumar, B. Structural characterization of flavonoid C-and O-glycosides in an extract of Adhatoda vasica leaves by liquid chromatography with quadrupole time-of-flight mass spectrometry. Rapid Commun. Mass. Spectrom. 2015, 29, 1095–1106. [Google Scholar] [CrossRef]
- Blois, M.S. Antioxidant determinations by the use of a stable free radical. Nature 1958, 181, 1199–1200. [Google Scholar] [CrossRef]
- Tusevski, O.; Kostovska, A.; Iloska, A.; Trajkovska, L.; Simic, S.G. Phenolic production and antioxidant properties of some Macedonian medicinal plants. Cent. Eur. J. Biol. 2014, 9, 888–900. [Google Scholar] [CrossRef]
- Dapkevicius, A.; Venskutonis, R.; van Beek, T.A.; Linssen, J.P. Antioxidant activity of extracts obtained by different isolation procedures from some aromatic herbs grown in Lithuania. J. Sci. Food Agric. 1998, 77, 140–146. [Google Scholar] [CrossRef]
- Zengin, G.; Uysal, A.; Gunes, E.; Aktumsek, A. Survey of phytochemical composition and biological effects of three extracts from a wild plant (Cotoneaster nummularia Fisch. et Mey.): A potential source for functional food ingredients and drug formulations. PLoS ONE 2014, 9, e113527. [Google Scholar] [CrossRef] [PubMed]
- Wan, L.S.; Min, Q.X.; Wang, Y.L.; Yue, Y.D.; Chen, J.C. Xanthone glycoside constituents of Swertia kouitchensis with α-glucosidase inhibitory activity. J. Nat. Prod. 2013, 76, 1248–1253. [Google Scholar] [CrossRef] [PubMed]
- Milutinović, M.; Čurović, D.; Nikodijević, D.; Vukajlović, F.; Predojević, D.; Marković, S.; Pešić, S. The silk of Plodia interpunctella as a potential biomaterial and its cytotoxic effect on cancer cells. Anim. Biotechnol. 2020, 30, 195–202. [Google Scholar] [CrossRef]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]
- Hammer, O.; Harper, D.T.; Ryan, P.D. PAST: Paleontological statistics software package for education and data analysis. Palaeontol. Electron. 2001, 4, 1–9. [Google Scholar]
- Muala, W.C.B.; Desobgo, Z.S.C.; Jong, N.E. Optimization of extraction conditions of phenolic compounds from Cymbopogon citratus and evaluation of phenolics and aroma profiles of extract. Heliyon 2021, 7, e06744. [Google Scholar] [CrossRef]
- Colombo, R.; Yariwake, J.; McCullagh, M. Study of C- and O-glycosylflavones in sugarcane extracts using liquid chromatography—Exact mass measurement mass spectrometry. Artic. J. Braz. Chem. Soc. 2008, 19, 483–490. [Google Scholar] [CrossRef]
- El-Seedi, H.R.; Taher, E.A.; Sheikh, B.Y.; Anjum, S.; Saeed, A.; AlAjmi, M.F.; Moustafa, M.S.; Al-Mousawi, S.M.; Farag, M.A.; Hegazy, M.-E.F.; et al. Chapter 8—Hydroxycinnamic Acids: Natural Sources, Biosynthesis, Possible Biological Activities, and Roles in Islamic Medicine. In Studies in Natural Products Chemistry; Attaur, R., Ed.; Elsevier: Amsterdam, The Netherlands, 2018; Volume 55, pp. 269–292. [Google Scholar]
- Cheynier, V.; Comte, G.; Davies, K.M.; Lattanzio, V.; Martens, S. Plant phenolics: Recent advances on their biosynthesis, genetics, and ecophysiology. Plant Physiol. Biochem. 2013, 72, 1–20. [Google Scholar] [CrossRef]
- Eudes, A.; Dutta, T.; Deng, K.; Jacquet, N.; Sinha, A.; Benites, V.T.; Baidoo, E.E.K.; Richel, A.; Sattler, S.E.; Northen, T.R.; et al. SbCOMT (Bmr12) is involved in the biosynthesis of tricin-lignin in sorghum. PLoS ONE 2017, 12, e0178160. [Google Scholar] [CrossRef]
- Pešić, M.B.; Milinčić, D.D.; Kostić, A.Ž.; Stanisavljević, N.S.; Vukotić, G.N.; Kojić, M.O.; Gašić, U.M.; Barać, M.B.; Stanojević, S.P.; Popović, D.A.; et al. In vitro digestion of meat-and cereal-based food matrix enriched with grape extracts: How are polyphenol composition, bioaccessibility and antioxidant activity affected? Food Chem. 2019, 284, 28–44. [Google Scholar] [CrossRef] [PubMed]
- Wojtunik-Kulesza, K.; Oniszczuk, A.; Oniszczuk, T.; Combrzyński, M.; Nowakowska, D.; Matwijczuk, A. Influence of in vitro digestion on composition, bioaccessibility and antioxidant activity of food polyphenols—A non-systematic review. Nutrients 2020, 12, 1401. [Google Scholar] [CrossRef] [PubMed]
- Lai, X.; Li, X.; Chen, J.; Liu, X.; Pan, P.; Zhou, Y.; Zhao, G. Advances in flavonoid glycosylation: Chemical and biological basis, mechanisms, physicochemical properties, and applications in the food industry. Trends Food Sci. Technol. 2025, 12, 105296. [Google Scholar] [CrossRef]
- Cattivelli, A.; Zannini, M.; De Angeli, M.; D’Arca, D.; Minischetti, V.; Conte, A.; Tagliazucchi, D. Bioaccessibility of flavones, flavanones, and flavonols from vegetable foods and beverages. Biology 2024, 13, 1081. [Google Scholar] [CrossRef]
- Shen, W.; Hu, X.; Niu, Y.; Lu, Y.; Wang, B.; Wang, H. Bioaccessibility and absorption of flavonoid C-glycosides from Abrus mollis using simulated digestion, Caco-2 cell, and in situ single-pass perfusion models. Planta Med. 2021, 87, 570–580. [Google Scholar] [CrossRef]
- de Armas-Ricard, M.; Ruiz-Reyes, E.; Ramírez-Rodríguez, O. Caffeates and caffeamides: Synthetic methodologies and their antioxidant properties. Int. J. Med. Chem. 2019, 2019, 2592609. [Google Scholar] [CrossRef]
- Islam, S.; Adam, Z.; Akanda, J.H. Quinic and caffeic acids derivatives: Affecting antioxidant capacities and phenolics contents of certain therapeutic and specialty crops employing water and ethanolic extracts. Food Chem. Adv. 2024, 4, 100693. [Google Scholar] [CrossRef]
- Pei, K.; Ou, J.; Huang, J.; Ou, S. p-Coumaric acid and its conjugates: Dietary sources, pharmacokinetic properties and biological activities. J. Sci. Food Agric. 2016, 96, 2952–2962. [Google Scholar] [CrossRef]
- Lee, Y.H.; So, B.H.; Lee, K.S.; Kuk, M.U.; Park, J.H.; Yoon, J.H.; Lee, Y.J.; Kim, D.Y.; Kim, M.S.; Kwon, H.W.; et al. Identification of cellular isoschaftoside-mediated anti-senescence mechanism in RAC2 and LINC00294. Molecules 2024, 29, 4182. [Google Scholar] [CrossRef]
- Zaripova, M.; Abdullaev, I.; Bogbekov, A.; Gayibov, U.; Omonturdiev, S.; Makhmudov, R.; Ergashev, N.; Jabbarova, G.; Gayibova, S.; Aripov, T. In vitro and in silico studies of Gnaphalium U. extract: Inhibition of α-amylase and α-glucosidase as a potential strategy for metabolic syndrome regulation. Trends Sci. 2025, 22, 10098. [Google Scholar] [CrossRef]
- de Oliveira, A.P.; Coppede, J.S.; Bertoni, B.W.; Crotti, A.E.; França, S.C.; Pereira, A.M.S.; Taleb-Contini, S.H. Costus spiralis (Jacq.) Roscoe: A novel source of flavones with α-glycosidase inhibitory activity. Chem. Biodivers. 2018, 15, e1700421. [Google Scholar] [CrossRef] [PubMed]
- Dwivedi, M. Phytochemical characterization and biological evaluation of lemongrass (Cymbopogon citratus) extracts: A systematic experimental study. Int. J. Pharm. Chem. Anal. 2024, 11, 253–259. [Google Scholar] [CrossRef]
- Santoso, F.; Winarno, J.; Gunawan-Puteri, M. Application of lemongrass (Cymbopogon citratus) as a functional food ingredient with alpha-glucosidase inhibitory activity. In Proceedings of the 4th International Conference on Food, Agriculture and Natural Resources (FANRes 2018); Advances in Engineering Research; Atlantis Press: Dordrecht, The Netherlands, 2018; pp. 204–208. [Google Scholar]
- Gaonkar, R.; Shiralgi, Y.; Lakkappa, D.B.; Hegde, G. Essential oil from Cymbopogon flexuosus as the potential inhibitor for HSP90. Toxicol. Rep. 2018, 5, 489–496. [Google Scholar] [CrossRef] [PubMed]
- Alam, M.; Ahmed, S.; Elasbali, A.M.; Adnan, M.; Alam, S.; Hassan, M.I.; Pasupuleti, V.R. Therapeutic implications of caffeic acid in cancer and neurological diseases. Front. Oncol. 2022, 12, 860508. [Google Scholar] [CrossRef]
- Cortez, N.; Villegas, C.; Burgos, V.; Cabrera-Pardo, J.R.; Ortiz, L.; González-Chavarría, I.; Nchiozem-Ngnitedem, V.-A.; Paz, C. Adjuvant properties of caffeic acid in cancer treatment. Int. J. Mol. Sci. 2024, 25, 7631. [Google Scholar] [CrossRef]
- Lodise, O.; Patil, K.; Karshenboym, I.; Prombo, S.; Chukwueke, C.; Pai, S.B. Inhibition of prostate cancer cells by 4,5-Dicaffeoylquinic acid through cell cycle arrest. Prostate Cancer 2019, 2019, 4520645. [Google Scholar] [CrossRef]
- Indy Tamayose, C.; Dos Santos, E.A.; Roque, N.; Costa-Lotufo, L.V.; Pena Ferreira, M.J. Caffeoylquinic acids: Separation method, antiradical properties and cytotoxicity. Chem. Biodivers. 2019, 16, e1900093. [Google Scholar] [CrossRef]
- Laurindo, L.F.; Pomini, K.T.; de Lima, E.P.; Laurindo, L.F.; Rodrigues, V.D.; da Silva Camarinha Oliveira, J.; Araújo, A.C.; Guiguer, E.L.; Rici, R.E.G.; Maria, D.A.; et al. Isoorientin: Unveiling the hidden flavonoid’s promise in combating cancer development and progression—A comprehensive review. Life Sci. 2025, 360, 123280. [Google Scholar] [CrossRef]
- Li, J.; Zhong, X.; Zhao, Y.; Shen, J.; Xiao, Z.; Pilapong, C. Acacetin inhibited non-small-cell lung cancer (NSCLC) cell growth via upregulating miR-34a in vitro and in vivo. Sci. Rep. 2024, 14, 2348. [Google Scholar] [CrossRef]
- Weerapreeyakul, N.; Nonpunya, A.; Barusrux, S.; Thitimetharoch, T.; Sripanidkulchai, B. Evaluation of the Anticancer Potential of Six Herbs against a Hepatoma Cell Line. Chin. Med. 2012, 7, 15. [Google Scholar] [CrossRef]

| Simulated Digestion Fluid | SSF | SGF | SIF |
|---|---|---|---|
| pH | 7 | 3 | 7 |
| Potassium chloride (0.5 M) | 15.1 | 6.9 | 6.6 |
| Potassium dihydrogen phosphate (0.5 M) | 3.7 | 0.9 | 0.8 |
| Sodium hydrogen carbonate (1 M) | 6.8 | 12.5 | 42.5 |
| Sodium chloride (1.5 M) | - | 11.8 | 9.6 |
| Magnesium chloride hexahydrate (0.15 M) | 0.5 | 0.4 | 1.1 |
| Sodium carbonate (0.5 M) | 0.06 | 0.5 | - |
| No | RT | Tentatively Identified Compounds | Formulas | Calculated Mass | m/z Exact Mass | mDa | MS Fragments (Main Fragment, %) | Presence of Compounds in Extracts | Ref. ** |
|---|---|---|---|---|---|---|---|---|---|
| Phenolicacids | |||||||||
| Hydroxybenzoic acid and derivatives | |||||||||
| 1 | 4.24 | Hydroxybenzoic acid | C7H5O3− | 137.0239 | 137.0240 | 0.13 | 108.0212(100), 109.0264 | EtC, AqC, AqD, EtD | [27] |
| 2 | 2.42 | Dihydroxybenzoic acid is. I (Protocatehuic acid) * | C7H5O4− | 153.0188 | 153.0191 | 0.32 | 108.0212(100), 109.0287 | EtC, AqC, AqD, EtD | Std.; [38] |
| 3 | 5.80 | Dihydroxybenzoic acid is. II (Gentisic acid) * | C7H5O4− | 153.0188 | 153.0191 | 0.32 | 109.0288(100), 108.0213 | EtC, AqC, AqD, EtD | Std.; [38] |
| 4 | 2.51 | Dihydroxybenzoic acid hexoside is. I | C13H15O9− | 315.0716 | 315.0722 | 0.59 | 108.0212(100), 109.0285, 152.0106, 153.0176 | EtC, AqC, AqD, EtD | - |
| 5 | 3.00 | Dihydroxybenzoic acid hexoside is. II | C13H15O9− | 315.0716 | 315.0722 | 0.59 | 109.0288(100), 153.0181, 108.0212, 152.0104 | EtC, AqC, AqD, EtD | - |
| 6 | 8.84 | Globulusin B | C23H29O12− | 497.1659 | 497.1662 | 0.3 | 168.0055(100), 497.1652, 125.0239, 169.0118, 124.0161, 313.0552, 331.0655, 479.1556 | EtC, AqC, AqD, EtD | - |
| Hydroxycinnamic acid and derivatives | |||||||||
| 7 | 7.28 | Coumaric acid * | C9H7O3− | 163.0395 | 163.0397 | 0.18 | 119.0498(100), 120.0532, 117.0343 | EtC, AqC, AqD, EtD | Std.; [25] |
| 8 | 6.67 | Caffeic acid * | C9H7O4− | 179.0344 | 179.0346 | 0.17 | 135.0443(100), 134.0367, 107.0497 | EtC, AqC | Std.; [38] |
| 9 | 9.70 | Ethyl caffeate | C11H11O4− | 207.0657 | 207.0660 | 0.27 | 133.0290(100), 135.0444, 134.0358, 161.0236, 179.0346 | EtC, EtD | - |
| 10 | 6.37 | Coumaric acid hexoside | C15H17O8− | 325.0923 | 325.0921 | −0.24 | 119.0497(100), 163.0384 | EtC, AqC, AqD, EtD | - |
| 11 | 7.58 | Ferulic acid hexoside | C16H19O9− | 355.1029 | 355.1026 | −0.31 | 193.0497(100), 134.0367, 161.0235, 133.0275, 178.0233 | AqD, EtD | - |
| 12 | 6.93 | Coumaroylquinic acid | C16H17O8− | 337.0923 | 337.0925 | 0.16 | 191.0549(100), 119.0497, 163.0388, 111.0442, 173.0445, 127.0396, 155.0337, 145.0283 | EtC, AqC, AqD, EtD | - |
| 13 | 3.72 | Caffeoylquinic acid is. I | C16H17O9− | 353.0873 | 353.0870 | −0.26 | 191.055(100), 135.0444, 179.0338, 173.0444, 161.0230, 155.0343, 127.0393, 111.0444 | EtC, AqC | [25,26] |
| 14 | 4.78 | Caffeoylquinic acid is. II | C16H17O9− | 353.0873 | 353.0870 | −0.26 | 191.0551(100), 135.0444, 179.0339, 173.0447, 161.0234, 155.0338, 127.0394, 111.0446 | EtC, AqC | [25,26] |
| 15 | 6.27 | Caffeoylquinic acid is. III (Chlorogenic acid) * | C16H17O9− | 353.0873 | 353.0870 | −0.26 | 191.0551(100), 135.0444, 127.0395, 161.0235, 173.0448 | EtC, AqC, AqD, EtD | Std.; [25,26] |
| 16 | 8.37 | Dicaffeoylquinic acid | C25H23O12− | 515.119 | 515.1193 | 0.35 | 179.0340(100), 173.0448, 191.055, 353.0864, 135.0445, 111.0088, 161.024, 155.0337, 323.0554 | EtC, AqC, AqD | - |
| 17 | 10.46 | 1,3-O-Dicoumaroyl-glycerol | C21H19O7− | 383.1131 | 383.1134 | 0.32 | 119.0497(100), 163.0393, 145.0290, 197.0811, 219.0645, 237.0751, 297.0402, 311.0552 | EtC, EtD | [21] |
| 18 | 9.82 | 1,3-O-Coumaroyl-caffeoyl-glycerol | C21H19O8− | 399.108 | 399.1090 | 1.01 | 163.0392(100), 119.0496, 135.0444, 145.0289, 179.0339, 253.0707, 235.0600, 217.1065, 297.0731, 327.0949 | EtC, EtD | [21] |
| 19 | 10.56 | 1,3-O-Coumaroyl-feruloyl-glycerol | C22H21O8− | 413.1236 | 413.1238 | 0.16 | 134.0365(100), 163.0391, 119.0497, 145.0290, 193.0498, 175.0395, 219.0662, 234.0522, 267.0849, 337.0340, 398.0992 | EtC | [21] |
| Flavonoids | |||||||||
| Flavone aglycones and derivatives (O-glycosides and flavonolignans) | |||||||||
| 20 | 9.51 | Luteolin * | C15H9O6− | 285.0399 | 285.0399 | −0.01 | 133.0290(100), 151.0030, 285.0393, 107.0137, 121.0292, 175.0391, 199.0389, 217.0493, 241.0491, 257.0433, 267.0287 | EtC, AqC, AqD, EtD | Std.; [27] |
| 21 | 10.25 | Tricin | C17H13O7− | 329.0661 | 329.0663 | 0.17 | 299.0187(100), 271.0238, 227.0337, 314.0417, 161.0236, 203.0337, 185.0234, 285.0398 | EtC, AqC, AqD, EtD | - |
| 22 | 8.59 | Tricin 7-O-hexoside or Tricin 5-O-hexoside | C23H25O12+ | 493.1346 | 493.1353 | 0.7 | 331.0811(100), 332.0843, 315.0493, 316.0569, 270.052 | EtC, AqC, AqD, EtD | [21] |
| 23 | 10.45 | Tricin 4′-O-(erythro-β-guaiacyl-glyceryl) ether | C27H25O11− | 525.1397 | 525.1402 | 0.51 | 165.0549(100), 329.0654, 314.0417, 150.0312, 299.0190, 195.0652, 180.0414, 285.0376, 271.0230, 477.1168 | EtC, AqC, EtD | [21] |
| 24 | 8.91 | Tricin 4′-O-(erythro-β-guaiacyl-glyceryl) ether-7-O-hexoside | C33H35O16− | 687.1925 | 687.193 | 0.49 | 329.0649(100), 195.0656, 165.0545, 150.0314, 314.0410, 299.0195, 525.1384 | EtC, AqC, AqD, EtD | - |
| Flavone-C-glycosides (Subgroups of Flavone) | |||||||||
| 25 | 7.88 | Isovitexin (Apigenin-6-C-glucoside) * | C21H21O10+ | 433.1135 | 433.1143 | 0.83 | 283.0597(100), 313.0704, 271.0598, 295.0622, 323.0912, 337.0708, 349.0705, 361.0706, 379.0812, 397.0918, 415.1026 | EtC, AqC, AqD, EtD | Std. |
| 26 | 7.32 | Isoschaftoside or Schaftoside (Apigenin-6,8-C-pentoside hexoside) | C26H29O14+ | 565.1557 | 565.1563 | 0.57 | 379.0813(100), 325.0700, 409.0911, 295.0598, 337.0700, 355.0809, 445.0999, 475.1034, 457.1037, 451.1031, 493.1121, 511.1230 | EtC, AqC, AqD, EtD | [21,25] |
| 27 | 7.47 | Isoorientin (Luteolin-6-C-glucoside) | C21H21O11+ | 449.1084 | 449.1099 | 1.51 | 299.0548(100), 329.0654, 287.0548, 325.0698, 339.0856, 353.0652, 365.0661, 377.0659, 395.0766, 413.0868, 431.0975 | EtC, AqC, AqD, EtD | [25,27] |
| 28 | 7.19 | Isocarlinoside or Carlinoside (Luteolin-6,8-C-pentoside hexoside) | C26H29O15+ | 581.1506 | 581.1514 | 0.75 | 395.0761(100), 425.0868, 341.0652, 443.0961, 407.0761, 413.0864, 353.0656, 491.0989, 461.0949, 527.1186, 509.1075, 449.0871, 467.0975 | EtC, AqC | [21,25] |
| 29 | 7.00 | Isoorientin 4′-O-glucoside | C27H31O16+ | 611.1612 | 611.1624 | 1.19 | 329.0652(100), 299.0547, 353.0654, 311.0541, 287.0547, 383.0757, 395.0761, 413.0868, 431.0970, 449.1063 | EtC, AqC | - |
| 30 | 8.05 | Isoscoparin (Chrysoeriol-6-C-glucoside) | C22H23O11+ | 463.124 | 463.1249 | 0.86 | 313.0710(100), 343.0815, 367.0813, 325.0721, 339.0848, 353.1013, 367.0813, 379.0810, 391.0810, 409.0921, 427.1025, 445.1121 | EtC, AqC, EtD | [27] |
| 31 | 8.05 | Acacetin-6-C-(6”-O-malonyl)glucoside | C25H23O13− | 531.1139 | 531.1146 | 0.73 | 339.0497(100), 191.0551, 229.0129, 295.0569, 327.0508, 159.0448, 199.0968, 357.0602, 487.127 | EtC, AqC | - |
| Other compounds | |||||||||
| 32 | 0.61 | Quinic acid | C7H11O6− | 191.0556 | 191.0555 | −0.06 | 109.0287(100), 127.0388, 191.0545, 171.0279, 137.0224, 173.0457 | EtC, AqC, AqD, EtD | [21] |
| No. | Tentatively Identified Compounds | Bioaccessibility (Recovery) | ||
|---|---|---|---|---|
| R1, % | R2, % | PRC, % | ||
| Hydroxybenzoic acid and derivatives | ||||
| 1 | Hydroxybenzoic acid | 16.07 | 6.52 | 8.89 |
| 2 | Dihydroxybenzoic acid is. I (Protocatehuic acid) | 2.02 | 2.20 | 2.69 |
| 3 | Dihydroxybenzoic acid is. II (Gentisic acid) | 4.24 | 4.07 | 15.05 |
| 4 | Dihydroxybenzoic acid hexoside is. I | 12.03 | 14.76 | 24.42 |
| 5 | Dihydroxybenzoic acid hexoside is. II | 7.87 | 10.67 | 26.50 |
| 6 | Globulusin B | 2.13 | 1.95 | 4.59 |
| Hydroxycinnamic acid and derivatives | ||||
| 7 | Coumaric acid | 10.12 | 9.02 | 205.20 |
| 8 | Caffeic acid | 0 | 0 | 0 |
| 9 | Ethyl caffeate | - | 0 | 3.24 |
| 10 | Coumaric acid hexoside | 100.81 | 113.97 | 134.32 |
| 11 | Ferulic acid hexoside | - | - | - |
| 12 | Coumaroylquinic acid | 46.05 | 63.24 | 43.81 |
| 13 | Caffeoylquinic acid is. I | 0 | 0 | 0 |
| 14 | Caffeoylquinic acid is. II | 0 | 0 | 0 |
| 15 | Caffeoylquinic acid is. III (Chlorogenic acid) | 0.73 | 0.75 | 0.57 |
| 16 | Dicaffeoylquinic acid | 19.43 | 8.12 | 0 |
| 17 | 1,3-O-Dicoumaroyl-glycerol | - | 0 | 4.96 |
| 18 | 1,3-O-Coumaroyl-caffeoyl-glycerol | - | 0 | 4.95 |
| 19 | 1,3-O-Coumaroyl-feruloyl-glycerol | - | 0 | 0 |
| Flavone aglycones and derivatives | ||||
| 20 | Luteolin | 4.29 | 0.65 | 2.78 |
| 21 | Tricin | 4.07 | 0.61 | 13.76 |
| 22 | Tricin 7-O-hexoside or Tricin 5-O-hexoside | 68.81 | 24.84 | 77.38 |
| 23 | Tricin 4′-O-(erythro-β-guaiacyl-glyceryl)ether | 0 | 0 | 12.24 |
| 24 | Tricin 4′-O-(erythro-β-guaiacyl-glyceryl)ether-7-O-hexoside | 86.91 | 39.44 | 120.56 |
| Flavone-C-glycosides (Subgroups of Flavone) | ||||
| 25 | Isovitexin (Apigenin-6-C-glucoside) | 2.97 | 1.34 | 12.27 |
| 26 | Isoschaftoside or Schaftoside (Apigenin-6,8-C-pentoside hexoside) | 110.11 | 100.84 | 114.64 |
| 27 | Isoorientin (Luteolin-6-C-glucoside) | 0.48 | 0.28 | 0.56 |
| 28 | Isocarlinoside or Carlinoside (Luteolin-6,8-C-pentoside hexoside) | 0 | 0 | 0 |
| 29 | Isoorientin 4′-O-glucoside | 0 | 0 | 0 |
| 30 | Isoscoparin (Chrysoeriol-6-C-glucoside) | 0 | 0 | 5.12 |
| 31 | Acacetin-6-C-(6″-O-malonyl)glucoside | 0 | 0 | 0 |
| Other compounds | ||||
| 32 | Quinic acid | 20.98 | 16.66 | 18.91 |
| Samples | Concentration [mg/mL] | DPPH Assay [% of Inhibition] | TRP Assay [µM AAE/g] | β-Carotene Bleaching Assay [% of Inhibition] |
|---|---|---|---|---|
| Extracts | ||||
| AqC | 0.15625 | 12.07 ± 0.43 d | 3.67 ± 0.50 c | na |
| 0.3125 | 16.69 ± 0.43 d | 6.90 ± 0.50 c | na | |
| 0.625 | 23.29 ± 1.96 d | 12.76 ± 1.95 d | 11.71 ± 1.50 b | |
| 1.25 | 32.09 ± 1.53 d | 22.71 ± 1.86 d | 17.06 ± 1.82 b | |
| 2.5 | 42.75 ± 2.12 d | 41.67 ± 1.53 c | 21.03 ± 1.82 b | |
| 5 | 59.45 ± 1.54 d | 66.90 ± 2.42 d | 26.59 ± 1.82 b | |
| 10 | 87.03 ± 0.59 ab | 111.81 ± 3.77 c | 56.15 ± 2.48 b | |
| IC50 (mg/mL) | 4.30 ± 0.10 | - | 7.46 ± 0.32 | |
| AqD | 0.15625 | na | na | na |
| 0.3125 | na | na | na | |
| 0.625 | na | na | na | |
| 1.25 | na | na | na | |
| 2.5 | 10.27 ± 1.38 e | na | 5.56 ± 0.91 c | |
| 5 | 15.23 ± 1.34 e | 2.24 ± 0.81 e | 9.72 ± 0.91 c | |
| 10 | 23.01 ± 1.92 d | 5.71 ± 0.29 d | 13.69 ± 1.57 d | |
| IC50 (mg/mL) | nd | - | nd | |
| EtC | 0.15625 | 29.61 ± 0.94 c | 5.19 ± 0.22 c | na |
| 0.3125 | 36.66 ± 1.57 c | 9.33 ± 0.44 c | na | |
| 0.625 | 44.05 ± 1.27 c | 17.48 ± 0.93 c | 5.75 ± 0.69 b | |
| 1.25 | 48.45 ± 1.36 c | 27.62 ± 0.68 c | 8.53 ± 1.50 c | |
| 2.5 | 55.61 ± 2.14 c | 42.33 ± 1.79 c | 14.68 ± 1.50 b | |
| 5 | 69.04 ± 0.90 c | 75.00 ± 2.02 c | 21.03 ± 2.08 b | |
| 10 | 88.16 ± 2.15 ab | 127.10 ± 2.27 b | 37.50 ± 2.15 c | |
| IC50 (mg/mL) | 2.23 ± 0.11 | - | 13.75 ± 0.88 | |
| EtD | 0.15625 | na | na | na |
| 0.3125 | na | na | na | |
| 0.625 | na | na | na | |
| 1.25 | na | na | na | |
| 2.5 | 13.99 ± 0.93 e | na | 3.97 ± 0.91 c | |
| 5 | 19.01 ± 0.93 f | 4.33 ± 0.30 e | 5.16 ± 0.91 c | |
| 10 | 33.39 ± 1.44 c | 9.86 ± 0.62 d | 10.32 ± 1.24 d | |
| IC50 (mg/mL) | nd | - | nd | |
| Positive controls | ||||
| BHT | 1 | 84.10 ± 0.15 b | 138.86 ± 1.51 a | >100 |
| Ascorbic acid | 1 | 88.26 ± 0.10 a | 129.71 ± 1.51 b | 98.08 ± 6.17 a |
| Samples | Concentration [mg/mL] | α-Glucosidase [% of Inhibition] | α-Amylase [% of Inhibition] |
|---|---|---|---|
| Extracts | |||
| AqC | 0.15625 | 10.25 ± 0.42 c | na |
| 0.3125 | 13.99 ± 1.20 c | na | |
| 0.625 | 20.90 ± 0.67 c | na | |
| 1.25 | 24.87 ± 0.91 d | na | |
| 2.5 | 33.76 ± 0.97 c | na | |
| 5 | 41.05 ± 1.03 e | 4.52 ± 0.97 b | |
| 10 | 58.89 ± 2.20 c | 8.23 ± 0.84 c | |
| IC50 (mg/mL) | 7.44 ± 0.31 | nd | |
| AqD | 0.15625 | 13.14 ± 0.66 c | na |
| 0.3125 | 15.51 ± 0.69 c | na | |
| 0.625 | 25.52 ± 0.51 b | na | |
| 1.25 | 31.41 ± 0.98 bc | na | |
| 2.5 | 39.53 ± 1.32 b | na | |
| 5 | 55.15 ± 0.99 b | na | |
| 10 | 79.15 ± 3.39 b | 2.64 ± 0.18 d | |
| IC50 (mg/mL) | 4.87 ± 0.22 | nd | |
| EtC | 0.15625 | 15.93 ± 0.34 b | na |
| 0.3125 | 20.31 ± 1.16 b | na | |
| 0.625 | 21.21 ± 1.06 c | na | |
| 1.25 | 28.95 ± 1.04 c | na | |
| 2.5 | 35.47 ± 0.97 c | na | |
| 5 | 47.50 ± 1.04 d | 5.80 ± 0.68 b | |
| 10 | 63.56 ± 2.06 c | 10.09 ± 0.74 b | |
| IC50 (mg/mL) | 6.42 ± 0.22 | nd | |
| EtD | 0.15625 | 11.86 ± 0.95 cd | na |
| 0.3125 | 18.42 ± 0.36 b | na | |
| 0.625 | 26.27 ± 1.20 b | na | |
| 1.25 | 33.37 ± 1.18 b | na | |
| 2.5 | 42.04 ± 1.52 b | na | |
| 5 | 51.31 ± 0.62 c | na | |
| 10 | 76.93 ± 1.79 b | 3.20 ± 0.37 d | |
| IC50 (mg/mL) | 4.99 ± 0.11 | nd | |
| Positive control | |||
| Acarbose | 1 | 87.74 ± 0.25 a | 82.99 ± 1.18 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alimpić Aradski, A.; Milinčić, D.D.; Pešić, M.B.; Milutinović, M.; Kuraya, E.; Touyama, A.; Bukvički, D. Effect of Simulated Gastrointestinal Digestion on the Phenolic Composition and Bioactivity of Cymbopogon flexuosus Extracts. Foods 2025, 14, 3868. https://doi.org/10.3390/foods14223868
Alimpić Aradski A, Milinčić DD, Pešić MB, Milutinović M, Kuraya E, Touyama A, Bukvički D. Effect of Simulated Gastrointestinal Digestion on the Phenolic Composition and Bioactivity of Cymbopogon flexuosus Extracts. Foods. 2025; 14(22):3868. https://doi.org/10.3390/foods14223868
Chicago/Turabian StyleAlimpić Aradski, Ana, Danijel D. Milinčić, Mirjana B. Pešić, Milena Milutinović, Eisuke Kuraya, Akiko Touyama, and Danka Bukvički. 2025. "Effect of Simulated Gastrointestinal Digestion on the Phenolic Composition and Bioactivity of Cymbopogon flexuosus Extracts" Foods 14, no. 22: 3868. https://doi.org/10.3390/foods14223868
APA StyleAlimpić Aradski, A., Milinčić, D. D., Pešić, M. B., Milutinović, M., Kuraya, E., Touyama, A., & Bukvički, D. (2025). Effect of Simulated Gastrointestinal Digestion on the Phenolic Composition and Bioactivity of Cymbopogon flexuosus Extracts. Foods, 14(22), 3868. https://doi.org/10.3390/foods14223868










