Comparison of the Volatile Components of Apocynum venetum Honey from Different Production Areas in Xinjiang
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
2.2. Equipment and Instruments
2.3. Test Method
2.3.1. Determination of Volatile Components in A. venetum Honey
2.3.2. Method Validation
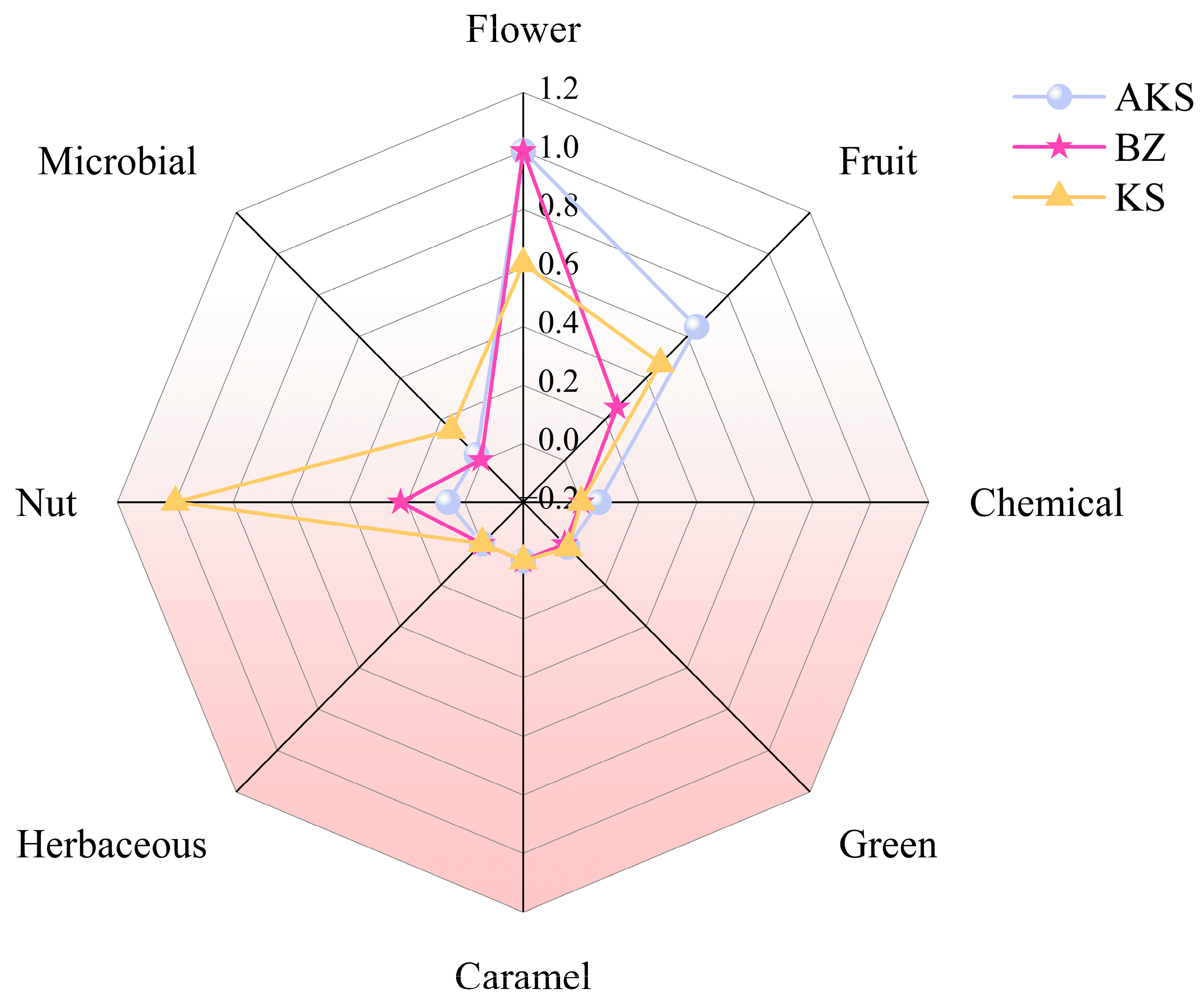
2.3.3. Aroma Analysis of A. venetum Honey
2.3.4. Data Processing
3. Results and Analysis
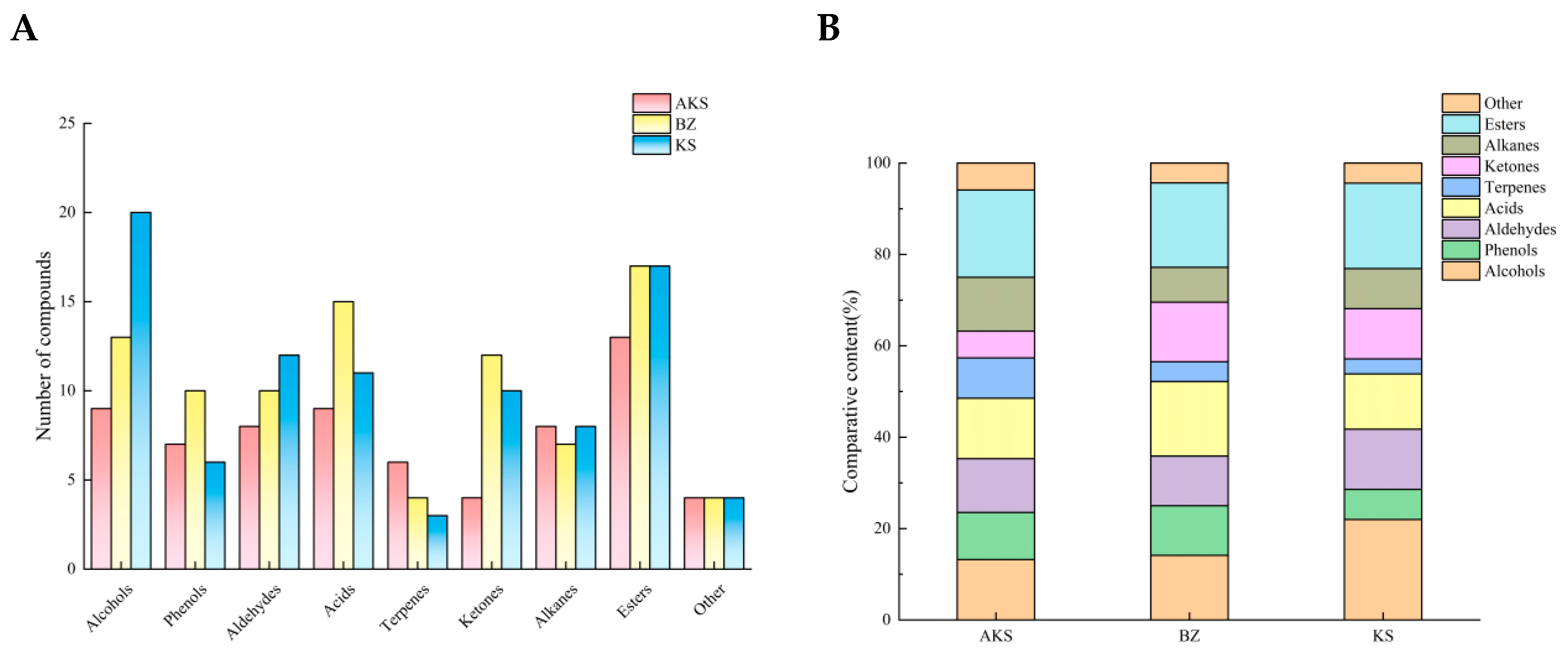
3.1. Analysis of the Volatile Components of A. venetum Honey
3.2. Difference Analysis of Volatile Components in A. venetum Honey
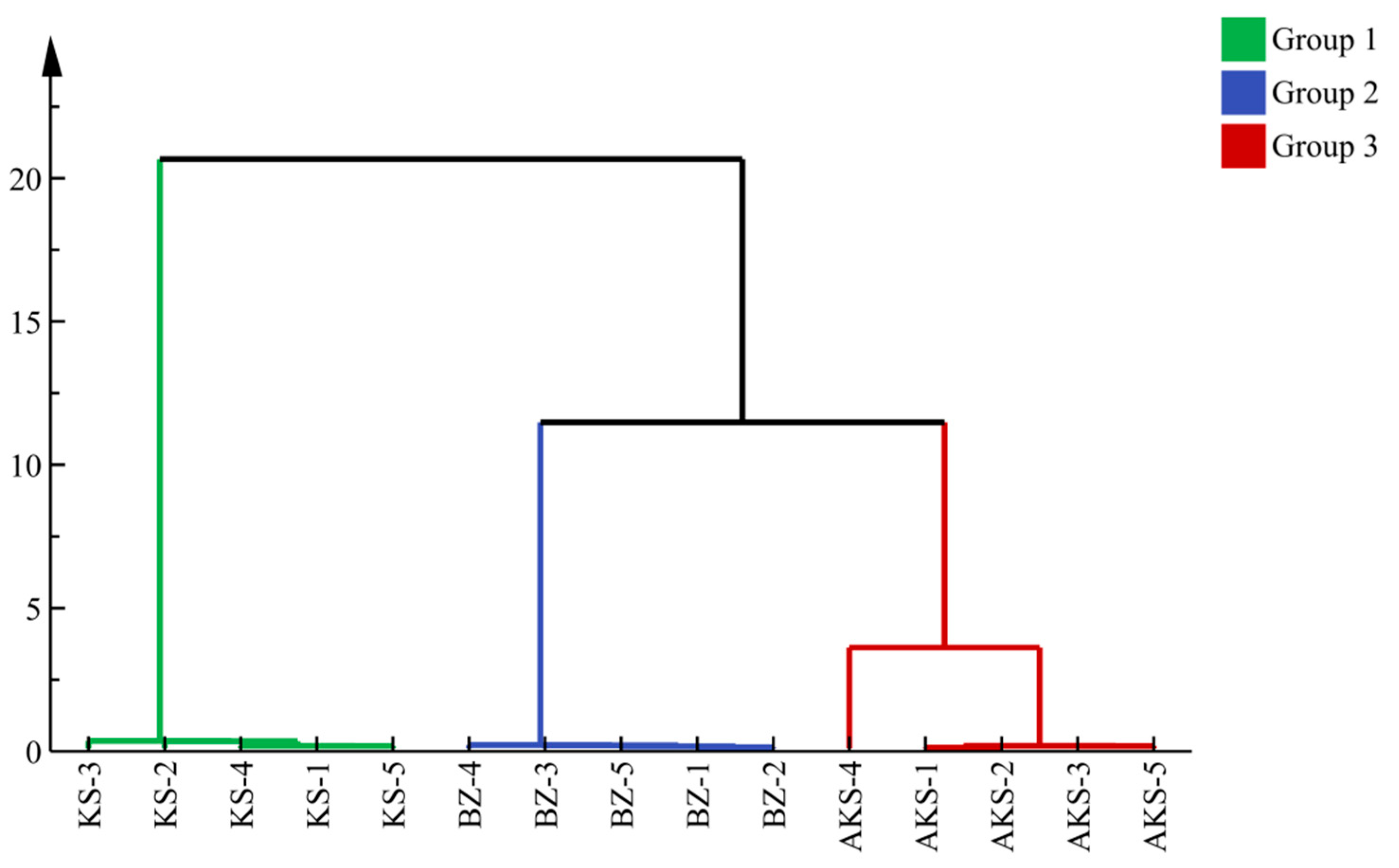
3.2.1. Systematic Cluster Analysis
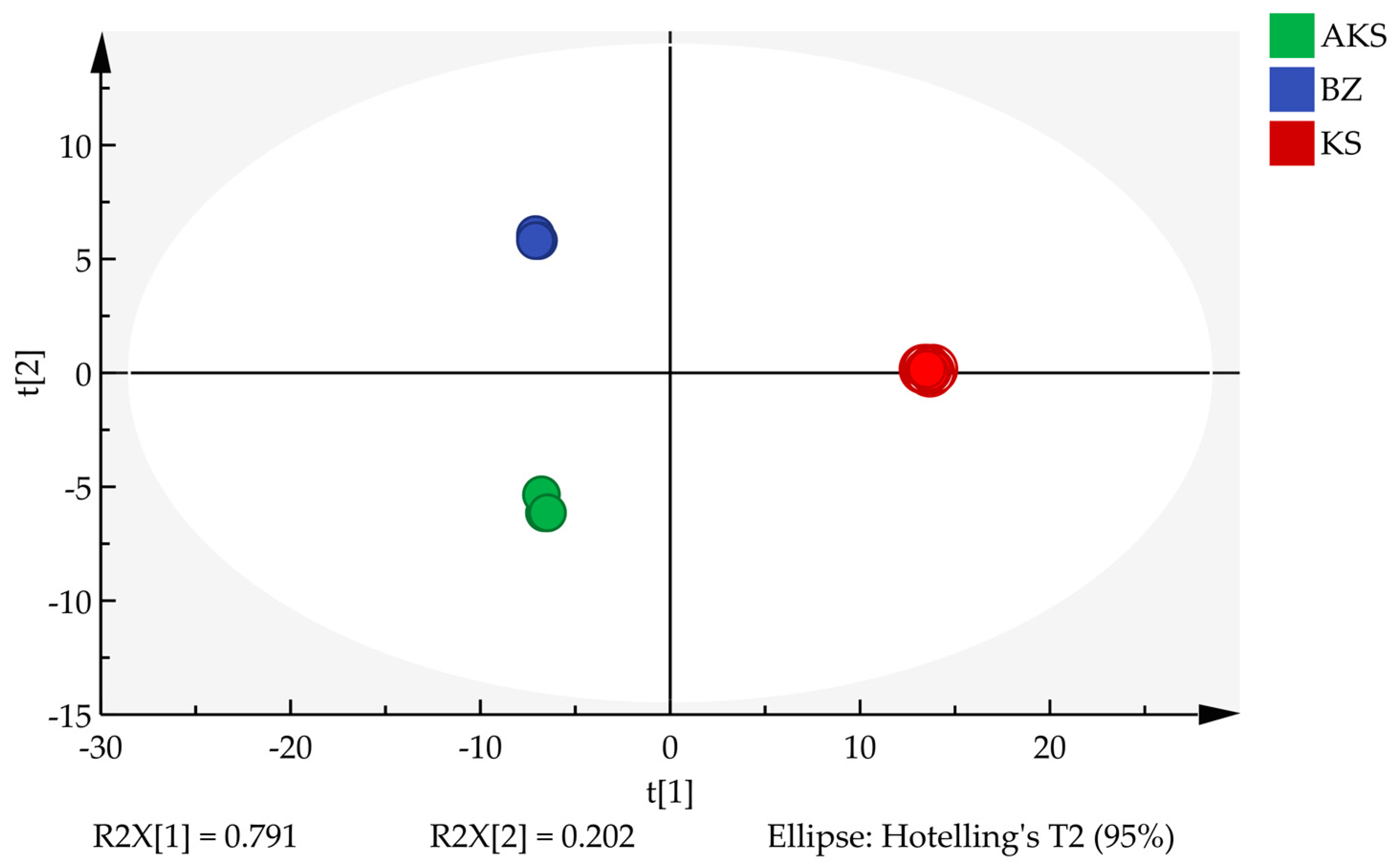
3.2.2. PCA
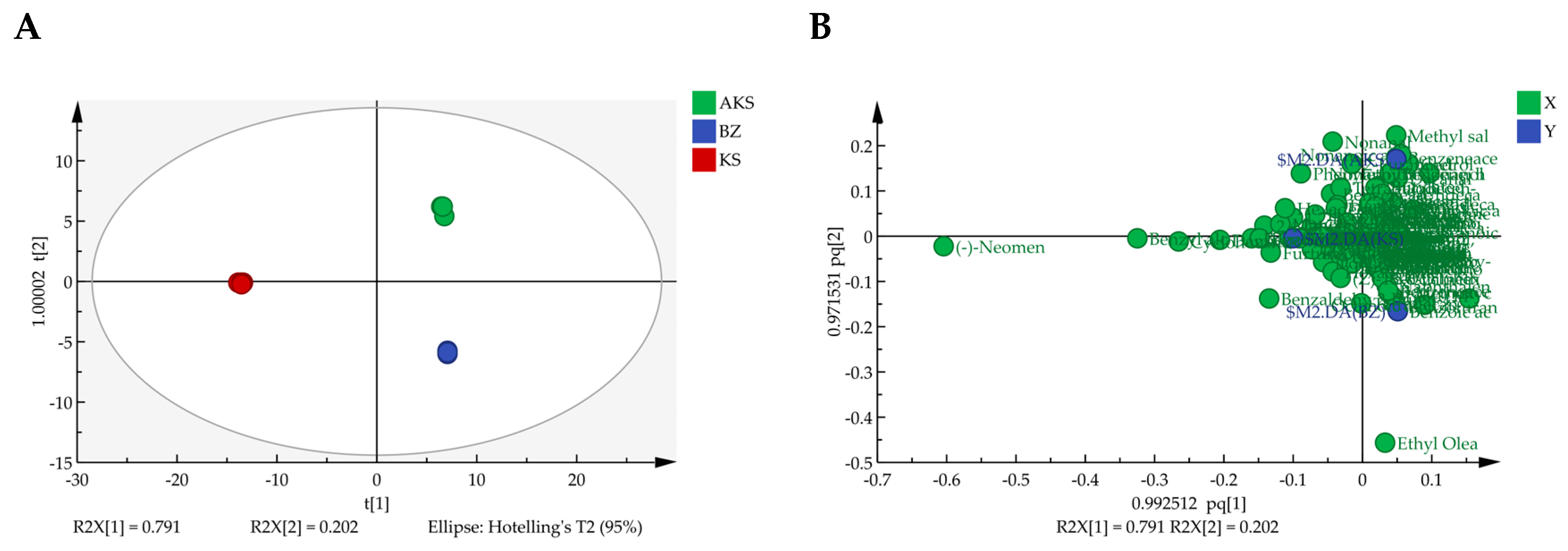
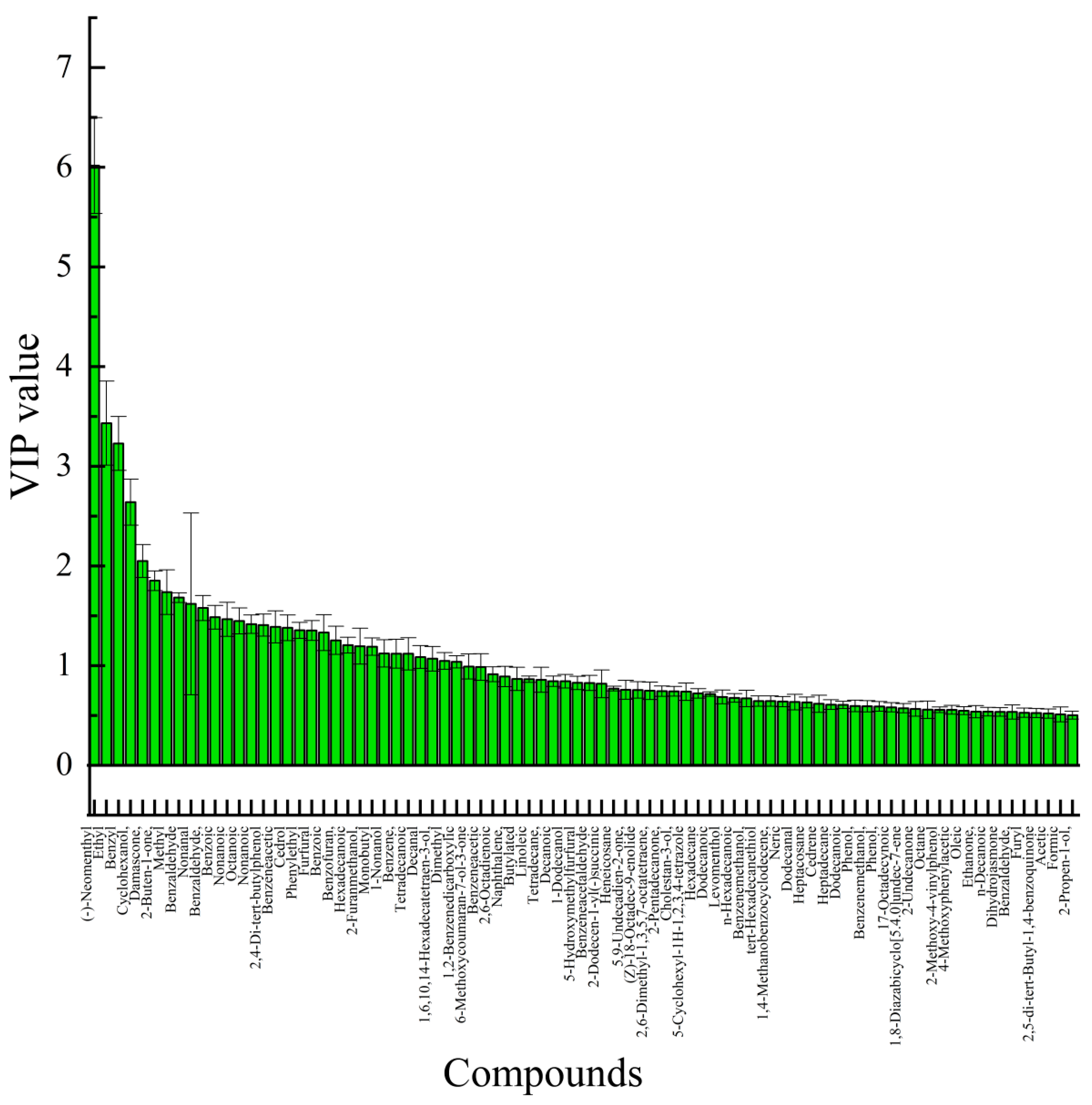
3.2.3. OPLS-DA
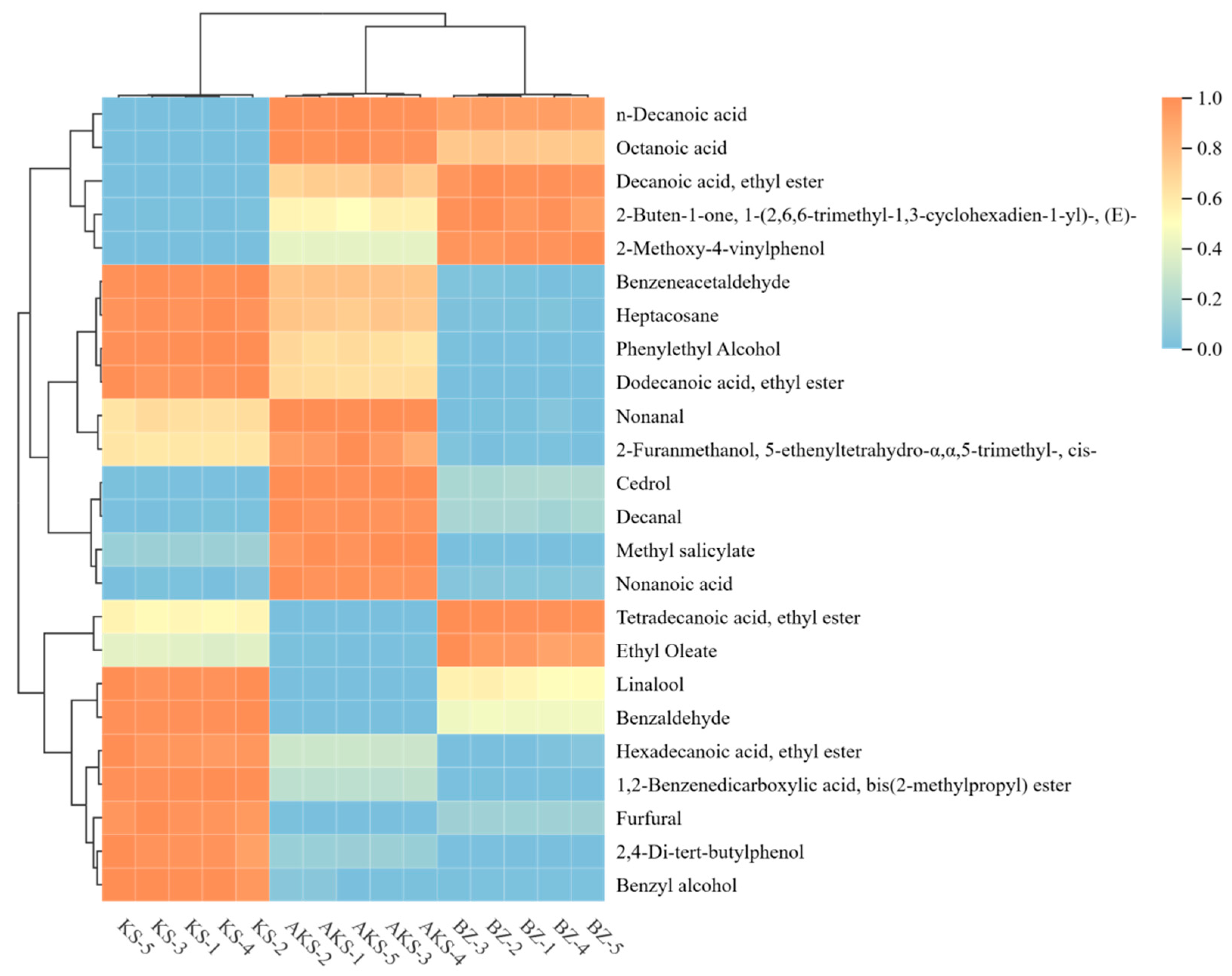
3.2.4. Clustering Heatmap Analysis
3.3. Identification of Aroma Active Substances in A. venetum Honey
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhao, X.; Hu, L.; Guo, H. Research Progress on Pharmacological Effects of Apocynum Venetum. Jiangxi Tradit. Chin. Med. 2014, 45, 76–78. [Google Scholar]
- Gao, X.; Li, X.; Zhang, C.; Bai, C. Scopoletin: A review of its pharmacology, pharmacokinetics, and toxicity. Front. Pharmacol. 2024, 15, 1268464. [Google Scholar] [CrossRef]
- Wang, L.; Wei, T.; Zheng, L.; Jiang, F.; Ma, W.; Lu, M.; Wu, X.; An, H. Recent Advances on Main Active Ingredients, Pharmacological Activities of Rosa roxbughii and Its Development and Utilization. Foods 2023, 12, 1051. [Google Scholar] [CrossRef]
- Aga, M.B.; Sharma, V.; Dar, A.H.; Dash, K.K.; Singh, A.; Shams, R.; Khan, S.A. Comprehensive review on functional and nutraceutical properties of honey. eFood 2023, 4, e71. [Google Scholar] [CrossRef]
- Zaid, S.S.M.; Ruslee, S.S.; Mokhtar, M.H. Protective Roles of Honey in Reproductive Health: A Review. Molecules 2021, 26, 3322. [Google Scholar] [CrossRef]
- Chang, D.; Wu, F.; Yang, Y.; Zhang, J.; Ma, T.; Guo, S.; Zhao, H.; Cao, W. Effect of honey-aloe paste on alleviating loperamide-induced constipation, in relation to regulation on gastrointestinal function and intestinal microenvironment. Food Res. Int. 2025, 219, 117006. [Google Scholar] [CrossRef]
- Ounjaijean, S.; Chaipoot, S.; Phongphisutthinant, R.; Kanthakat, G.; Taya, S.; Pathomrungsiyounggul, P.; Wiriyacharee, P.; Boonyapranai, K. Honey-Conjugated Honeybee Brood Biopeptides Improve Gastrointestinal Stability, Antioxidant Capacity, and Alleviate Diet-Induced Metabolic Syndrome in a Rat Model. Foods 2025, 14, 2907. [Google Scholar] [CrossRef]
- Wang, J.; Liu, H.; Ma, Y.; An, R.; Sun, T. Analysis of fatty acids in Apocynum venetum honey by gas chromatography-mass spectrometry. J. Food Saf. Qual. Insp. 2016, 7, 779–783. [Google Scholar]
- Zhang, J.; He, Y. Xinjiang Apocynum honey source plants and their beekeeping use. Bee Mag. 2016, 36, 29–30. [Google Scholar]
- Quirantes-Piné, R.; Sanna, G.; Mara, A.; Borrás-Linares, I.; Mainente, F.; Picó, Y.; Zoccatelli, G.; Lozano-Sánchez, J.; Ciulu, M. Mass Spectrometry Characterization of Honeydew Honey: A Critical Review. Foods 2024, 13, 2229. [Google Scholar] [CrossRef] [PubMed]
- Machado, A.M.; Miguel, M.G.; Vilas-Boas, M.; Figueiredo, A.C. Honey Volatiles as a Fingerprint for Botanical Origin—A Review on their Occurrence on Monofloral Honeys. Molecules 2020, 25, 374. [Google Scholar] [CrossRef]
- Silva, P.; Freitas, J.; Nunes, F.M.; Câmara, J.S. Effect of processing and storage on the volatile profile of sugarcane honey: A four-year study. Food Chem. 2021, 365, 130457. [Google Scholar] [CrossRef]
- Cucu, A.; Baci, G.; Moise, A.R.; Dezsi, Ş.; Marc, B.D.; Stângaciu, Ş.; Dezmirean, D.S. Towards a Better Understanding of Nutritional and Therapeutic Effects of Honey and Their Applications in Apitherapy. Appl. Sci. 2021, 11, 4190. [Google Scholar] [CrossRef]
- Wang, Y.; Huang, Y.; Cheng, N.; Zhao, H.; Zhang, Y.; Liu, C.; He, L.; Ma, T.; Li, Y.; Cao, W. Identification of Volatile Markers during Early Zygosaccharomyces rouxii Contamination in Mature and Immature Jujube Honey. Foods 2023, 12, 2730. [Google Scholar] [CrossRef]
- Gialouris, P.L.P.; Koulis, G.A.; Nastou, E.S.; Dasenaki, M.E.; Maragou, N.C.; Thomaidis, N.S. Development and validation of a high-throughput headspace solid-phase microextraction gas chromatography-mass spectrometry methodology for target and suspect determination of honey volatiles. Heliyon 2023, 9, e21311. [Google Scholar] [CrossRef] [PubMed]
- de Lima Morais Da Silva, P.; de Lima, L.S.; Caetano, Í.K.; Torres, Y.R. Comparative analysis of the volatile composition of honeys from Brazilian stingless bees by static headspace GC–MS. Food Res. Int. 2017, 102, 536–543. [Google Scholar] [CrossRef]
- Yang, Z.; Song, X.; Zhang, S.; Zhao, H.; Zhou, C.; Ma, Q.; Liang, L.; Zhang, B. Comparative analysis of flavor profiles in Chinese hybrid hazelnut (Corylus heterophylla × Corylus avellana) under different roasting conditions using HS-SPME-GC–MS and UPLC-MS/MS. Food Chem. 2025, 493, 145779. [Google Scholar] [CrossRef]
- Karabagias, I.K.; Badeka, A.; Kontakos, S.; Karabournioti, S.; Kontominas, M.G. Characterization and classification of Thymus capitatus (L.) honey according to geographical origin based on volatile compounds, physicochemical parameters and chemometrics. Food Res. Int. 2014, 55, 363–372. [Google Scholar] [CrossRef]
- Yang, X.; Shi, Z.; Zhou, S.; Li, H.; Hu, W.; Yi, Z.; Du, M. Analysis of volatile components in gallnut honey from different producing areas in Chongqing. Food Ferment. Ind. 2025, 51, 249–258. [Google Scholar]
- Zhu, M.; Sun, J.; Zhao, H.; Wu, F.; Xue, X.; Wu, L.; Cao, W. Volatile compounds of five types of unifloral honey in Northwest China: Correlation with aroma and floral origin based on HS-SPME/GC–MS combined with chemometrics. Food Chem. 2022, 384, 132461. [Google Scholar] [PubMed]
- Zhang, J.; Ying, X.; Ye, J.; Deng, S.; Benjakul, S.; Ma, L. Lipidomics analysis of lipid composition and differences across different tissues in golden pomfret (Trachinotus ovatus): Exploring the potential of utilizing aquatic by-products in processing. Food Sci. Technol. 2025, 228, 118130. [Google Scholar] [CrossRef]
- Carabetta, S.; Di Sanzo, R.; Fuda, S.; Muscolo, A.; Russo, M. A Predictive Model to Correlate Amino Acids and Aromatic Compounds in Calabrian Honeys. Foods 2023, 12, 3284. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Gu, Y.; Zhang, C.; Fei, T.; Ma, L.; Wu, R.; Tao, L.; Tian, Z.; Feng, T. A study of different pre-treatment methods for cigarettes and their aroma differences. bioRxiv 2025. [Google Scholar] [CrossRef]
- Xu, Y.; Luo, Y.; Zhong, C.; He, Y.; Wu, Y.H.; Yi, X.; Chen, Y. Study on flavor quality characteristics of Sichuan Xiaoqu liquor from different producing areas. Chin. Brew. 2024, 43, 45–53. [Google Scholar]
- Schmidberger, P.C.; Schieberle, P. Changes in the Key Aroma Compounds of Raw Shiitake Mushrooms (Lentinula edodes) Induced by Pan-Frying as Well as by Rehydration of Dry Mushrooms. J. Agric. Food. Chem. 2020, 68, 4493–4506. [Google Scholar] [CrossRef]
- Li, H.; Lang, Y.; Liu, Z.; Song, M.; Jiang, A.; Li, N.; Chen, L. Dynamic variation in the aroma characteristics of Rhus chinensis honey at different stages after capping. Food Chem. 2024, 449, 139226. [Google Scholar] [CrossRef]
- Castro-Vázquez, L.; Díaz-Maroto, M.C.; Pérez-Coello, M.S. Aroma composition and new chemical markers of Spanish citrus honeys. Food Chem. 2007, 103, 601–606. [Google Scholar] [CrossRef]
- Wang, X.; Yan, S.; Zhao, W.; Wu, L.; Tian, W.; Xue, X. Comprehensive study of volatile compounds of rare Leucosceptrum canum Smith honey: Aroma profiling and characteristic compound screening via GC–MS and GC–MS/MS. Food Res. Int. 2023, 169, 112799. [Google Scholar] [CrossRef]
- Li, H.; Liu, Z.; Song, M.; Jiang, A.; Lang, Y.; Chen, L. Aromatic profiles and enantiomeric distributions of volatile compounds during the ripening of Dendropanax dentiger honey. Food Res. Int. 2024, 175, 113677. [Google Scholar] [CrossRef]
- Vicente, J.; Calderón, F.; Santos, A.; Marquina, D.; Benito, S. High Potential of Pichia kluyveri and Other Pichia Species in Wine Technology. Int. J. Mol. Sci. 2021, 22, 1196. [Google Scholar] [CrossRef]
- Chai, R.; An, C.; Liu, T.; Sun, Y.; Wu, G.; Duan, C. Analysis of key aroma components in ‘Shuanghong’ dry red wine based on SAFE-GC-O-MS_Chai Ruixue. Food Sci. 2022, 4, 175–182. [Google Scholar]
- Browne, E.; Kavanagh, S.; Devery, S. The In Vitro Antioxidant and Immunomodulatory Effects of the Irish Monofloral Ivy and Heather Honey Varieties. Int. J. Mol. Sci. 2025, 26, 3625. [Google Scholar] [CrossRef]
- Angioi, R.; Morrin, A.; White, B. Advantages of a Multifaceted Characterization of Honey, Illustrated with Irish Honey Marketed as Heather Honey. Acs Food Sci. Technol. 2024, 4, 606–616. [Google Scholar] [CrossRef]
- Kang, M.J.; Kim, K.; Kim, K.; Morrill, A.G.; Jung, C.; Sun, S.; Lee, D.; Suh, J.H.; Sung, J. Metabolomic analysis reveals linkage between chemical composition and sensory quality of different floral honey samples. Food Res. Int. 2023, 173, 113454. [Google Scholar] [CrossRef] [PubMed]
- Nataraj, N.; Hussain, M.; Ibrahim, M.; Hausmann, A.E.; Rao, S.; Kaur, S.; Khazir, J.; Mir, B.A.; Olsson, S.B. Effect of Altitude on Volatile Organic and Phenolic Compounds of Artemisia brevifolia Wall ex Dc. From the Western Himalayas. Front. Ecol. Evol. 2022, 10, 864728. [Google Scholar] [CrossRef]
- Descamps, C.; Quinet, M.; Jacquemart, A. Climate Change-Induced Stress Reduce Quantity and Alter Composition of Nectar and Pollen From a Bee-Pollinated Species (Borago officinalis, Boraginaceae). Front. Plant Sci. 2021, 12, 755843. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Yang, D.; Huo, J.; Wu, R. Spatial-temporal evolution law of urban-rural development level and its coordination degree in Tarim Basin in recent 10 years. J. Univ. Chin. Acad. Sci. 2020, 37, 63–73. [Google Scholar]
- Chen, Y. Analysis of river ice characteristics in Bazhou. Groundwater 2016, 38, 152–153. [Google Scholar]
- Ozcan Sinir, G.; Copur, O.U.; Barringer, S.A. Botanical and geographical origin of Turkish honeys by selected-ion flow-tube mass spectrometry and chemometrics. J. Sci. Food. Agric. 2020, 100, 2198–2207. [Google Scholar] [CrossRef]
- Starowicz, M.; Hanus, P.; Lamparski, G.; Sawicki, T. Characterizing the Volatile and Sensory Profiles, and Sugar Content of Beeswax, Beebread, Bee Pollen, and Honey. Molecules 2021, 26, 3410. [Google Scholar] [CrossRef]
- Uckun, O.; Selli, S. Characterization of key aroma compounds in a representative aromatic extracts from citrus and astragalus honeys based on aroma extract dilution analyses. J. Food Meas. Charact. 2017, 11, 512–522. [Google Scholar] [CrossRef]
- Pascoal, A.; Anjos, O.; Feás, X.; Oliveira, J.M.; Estevinho, L.M. Impact of fining agents on the volatile composition of sparkling mead. J. Inst. Brew. 2019, 125, 125–133. [Google Scholar] [CrossRef]
- Jiang, A.; Li, H.; Liu, Z.; Song, M.; Li, N.; Chen, L. Analysis of volatile components and key aroma components of Toddalia asiatica honey. Food Sci. 2024, 45, 175–185. [Google Scholar]
- Medici, S.; Sarlo, E.; Sánchez Pascua, G.; Garcia De La Rosa, S.; Casales, M.R.; Fuselli, S. Characterization of Argentine honeys based on odour, colour and flavour attributes by descriprive sensory analysis. Columella J. Agric. Environ. Sci. 2023, 10, 37–48. [Google Scholar] [CrossRef]
- Avîrvarei, A.; Pop, C.R.; Mudura, E.; Ranga, F.; Hegheș, S.; Gal, E.; Zhao, H.; Fărcaș, A.C.; Chiș, M.S.; Coldea, T.E. Contribution of Saccharomyces and Non-Saccharomyces Yeasts on the Volatile and Phenolic Profiles of Rosehip Mead. Antioxidants 2023, 12, 1457. [Google Scholar] [CrossRef]
- Karabagias, I.K. Headspace volatile compounds fluctuations in honeydew honey during storage at in-house conditions. Eur. Food Res. Technol. 2022, 248, 715–726. [Google Scholar] [CrossRef]
- Song, B.; Wang, W.; Liu, R.; Cai, J.; Jiang, Y.; Tang, X.; Wu, H.; Ao, H.; Chen, L. Geographic Differentiation of Essential Oil from Rhizome of Cultivated Atractylodes lancea by Using GC-MS and Chemical Pattern Recognition Analysis. Molecules 2023, 28, 2216. [Google Scholar] [CrossRef]
- Kaldeli, A.; Zakidou, P.; Paraskevopoulou, A. Volatilomics as a tool to ascertain food adulteration, authenticity, and origin. Compr. Rev. Food. Sci. Food Saf. 2024, 23, e13387. [Google Scholar] [CrossRef]
- Kortesniemi, M.; Rosenvald, S.; Laaksonen, O.; Vanag, A.; Ollikka, T.; Vene, K.; Yang, B. Sensory and chemical profiles of Finnish honeys of different botanical origins and consumer preferences. Food Chem. 2018, 246, 351–359. [Google Scholar] [CrossRef] [PubMed]


| ID | Compound | Aroma Description | AKS | BZ | KS | |||
|---|---|---|---|---|---|---|---|---|
| OAV | OCR% | OAV | OCR% | OAV | OCR% | |||
| 1 | 1-Hexanol, 2-ethyl- | Sweet and flower | <1 | 0 | <1 | 0 | - | - |
| 2 | Phenylethyl alcohol | Rosy and flower | 2.708 | 0.007 | <1 | 0 | 3.757 | 0.032 |
| 3 | Benzyl alcohol | Flower | <1 | 0 | <1 | 0 | 22.131 | 0.189 |
| 4 | 1-Nonanol | Citrus | <1 | 0 | - | - | - | - |
| 5 | Cedrol | Violet, woody, fruity | 61.960 | 0.150 | 20.060 | 0.041 | 10.640 | 0.091 |
| 6 | Linalool | Flower | - | 0.000 | 10.400 | 0.021 | 19.100 | 0.163 |
| 7 | 1-Dodecanol | Citrus | - | 0.000 | - | - | 741.500 | 6.341 |
| 8 | 2,4-Di-tert-butylphenol | Almondy | 1345.000 | 3.260 | 8440 | 1.727 | 5176.000 | 44.265 |
| 9 | (3R,3aR,3bR,4S,7R,7aR)-4-isopropyl-3,7-dimethyloctahydro-1H-cyclopenta[1,3]cyclopropa[1,2]benzen-3-ol | Fruity | 7000 | 16.964 | - | - | - | - |
| 10 | 2-Methoxy-4-vinylphenol | Flower | 223.000 | 0.540 | 546.000 | 1.117 | - | - |
| 11 | 1-Heptatriacotanol | Flower | 0.000 | 1.840 | 0.004 | - | - | |
| 12 | Ethanol, 2-(2-butoxyethoxy)-, acetate | Herbaceous | 22.400 | 0.054 | 26.400 | 0.054 | - | - |
| 13 | Benzaldehyde | Almondy | <1 | 0.000 | 1.084 | 0.002 | 2.343 | 0.020 |
| 14 | Decanal | Mandarin orange | 6.139 | 0.015 | 2.517 | 0.005 | 1.825 | 0.016 |
| 15 | Dodecanal | Mandarin orange | 906.000 | 2.196 | - | - | - | - |
| 16 | Benzeneacetaldehyde | Flower, honey, and green | 2.178 | 0.005 | <1 | 0 | 2.535 | 0.022 |
| 17 | Octanal | Mandarin orange, fat, and green | 1.900 | 0.005 | 1.757 | 0.004 | 1.086 | 0.009 |
| 18 | Nonanal | Fat, mandarin orange, and rosy | 41.664 | 0.101 | 9.364 | 0.019 | 36.064 | 0.308 |
| 19 | Furfural | Bread and almondy | 2.647 | 0.006 | 4.380 | 0.009 | 15.873 | 0.136 |
| 20 | phenylacetic acid | Honey | 217.000 | 0.526 | - | - | - | - |
| 21 | Nonanoic acid | Fat and cheese | 14.033 | 0.034 | 6.027 | 0.012 | 5.717 | 0.049 |
| 22 | Octanoic acid | Cheese | 8.840 | 0.021 | 6.700 | 0.014 | - | - |
| 23 | n-Decanoic acid | Fat | 3.105 | 0.008 | 2.900 | 0.006 | - | - |
| 24 | α-Pinene | Pinewood, resin, and mandarin orange | - | 0.000 | 128.000 | 0.262 | - | - |
| 25 | 2(3H)-furanone, 5-heptyldihydro- | Flower | - | 0.000 | - | - | 15.200 | 0.130 |
| 26 | Cyclohexanone | Peppermint | 3.433 | 0.008 | 3.380 | 0.007 | 3.387 | 0.029 |
| 27 | 2-Buten-1-one, 1-(2,6,6-trimethyl-1,3-cyclohexadien-1-yl)-, (E)- | Flower, honey, and apple | 22,235.000 | 53.886 | 37,370 | 76.453 | 3005.000 | 25.699 |
| 28 | Benzene, hexamethyl- | Drug-like | 1406.000 | 3.407 | - | - | - | - |
| 29 | Octane | Petrol fumes | 1.760 | 0.004 | - | - | <1 | 0 |
| 30 | Heptacosane | Rotten smell | 619.000 | 1.500 | 176.000 | 0.360 | 764.000 | 6.534 |
| 31 | Phenylacetic acid, ethyl ester | Flower | - | 0.000 | <1 | 0 | - | - |
| 32 | Octanoic acid, ethyl ester | Fruity | <1 | 0.000 | <1 | 0 | - | - |
| 33 | Dodecanoic acid, ethyl ester | Fruity | 397.000 | 0.962 | - | - | 598.000 | 5.114 |
| 34 | Decanoic acid, ethyl ester | Fruity | 5865.000 | 14.214 | 7840 | 16.039 | - | - |
| 35 | Octadecanoic acid, ethyl ester | Fruity | 132.000 | 0.320 | 131.000 | 0.268 | 176.000 | 1.505 |
| 36 | Ethyl oleate | Fruity | 346.200 | 0.839 | 1689.900 | 3.457 | 882.200 | 7.545 |
| 37 | Methyl salicylate | Dongqing oil and peppermint | 398.700 | 0.966 | 57.900 | 0.118 | 97.900 | 0.837 |
| 38 | Acetic acid, phenylmethyl ester | Flower | - | - | - | - | 113.000 | 0.966 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, N.; Lv, J.; Zhang, R.; Sun, B.; Du, N.; Li, Y. Comparison of the Volatile Components of Apocynum venetum Honey from Different Production Areas in Xinjiang. Foods 2025, 14, 3860. https://doi.org/10.3390/foods14223860
Zhang N, Lv J, Zhang R, Sun B, Du N, Li Y. Comparison of the Volatile Components of Apocynum venetum Honey from Different Production Areas in Xinjiang. Foods. 2025; 14(22):3860. https://doi.org/10.3390/foods14223860
Chicago/Turabian StyleZhang, Na, Jingjing Lv, Ruili Zhang, Beibei Sun, Ning Du, and Yawen Li. 2025. "Comparison of the Volatile Components of Apocynum venetum Honey from Different Production Areas in Xinjiang" Foods 14, no. 22: 3860. https://doi.org/10.3390/foods14223860
APA StyleZhang, N., Lv, J., Zhang, R., Sun, B., Du, N., & Li, Y. (2025). Comparison of the Volatile Components of Apocynum venetum Honey from Different Production Areas in Xinjiang. Foods, 14(22), 3860. https://doi.org/10.3390/foods14223860





