A Synergistic Approach Combining Stable Carbon Isotope Ratio Analysis and Melissopalynology for the Authentication of Honey from Thailand
Abstract
1. Introduction
2. Materials and Methods
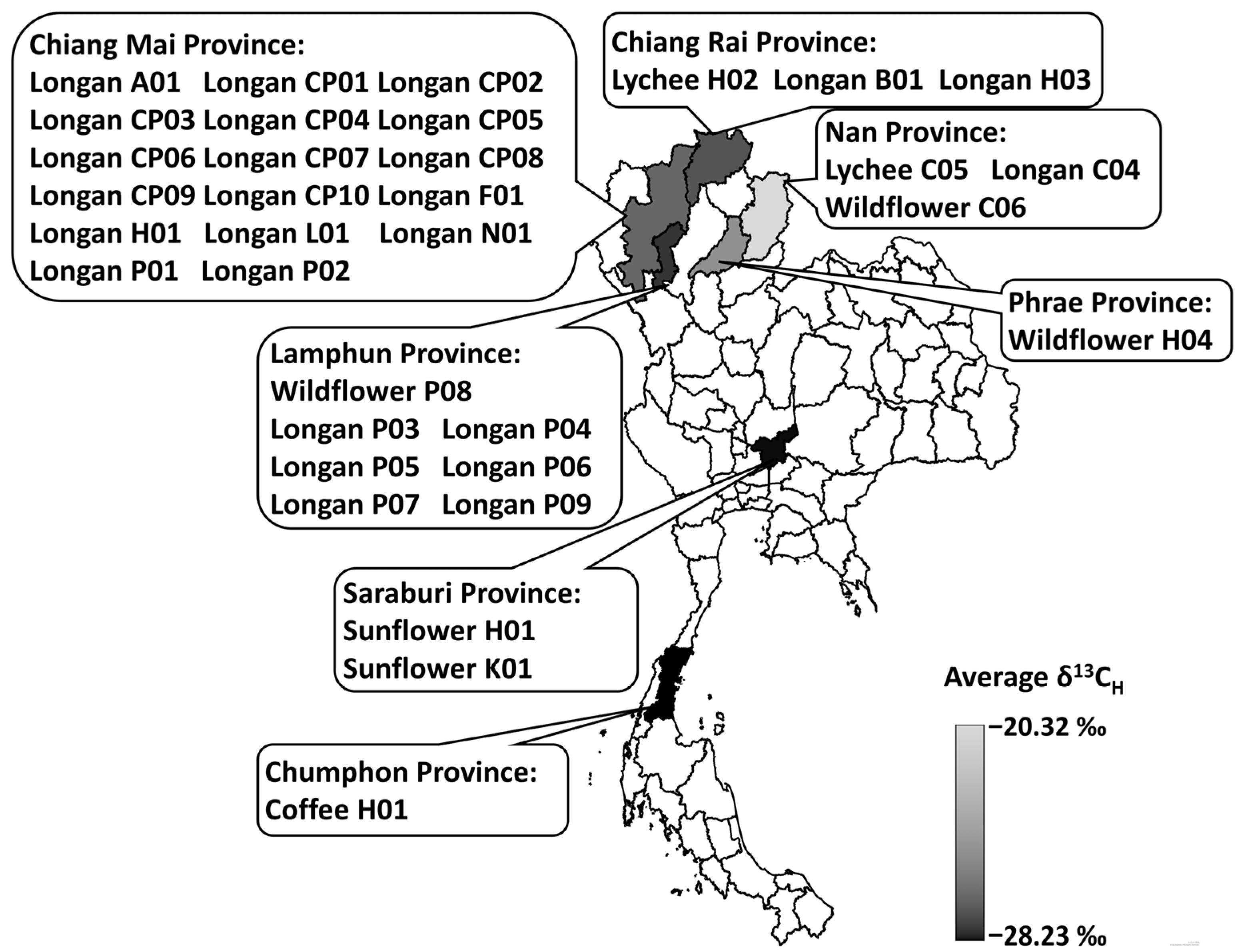
2.1. Honey Samples
2.2. Adulterants
2.3. Sugar Profile Determination
2.4. Stable Carbon Isotope Analysis
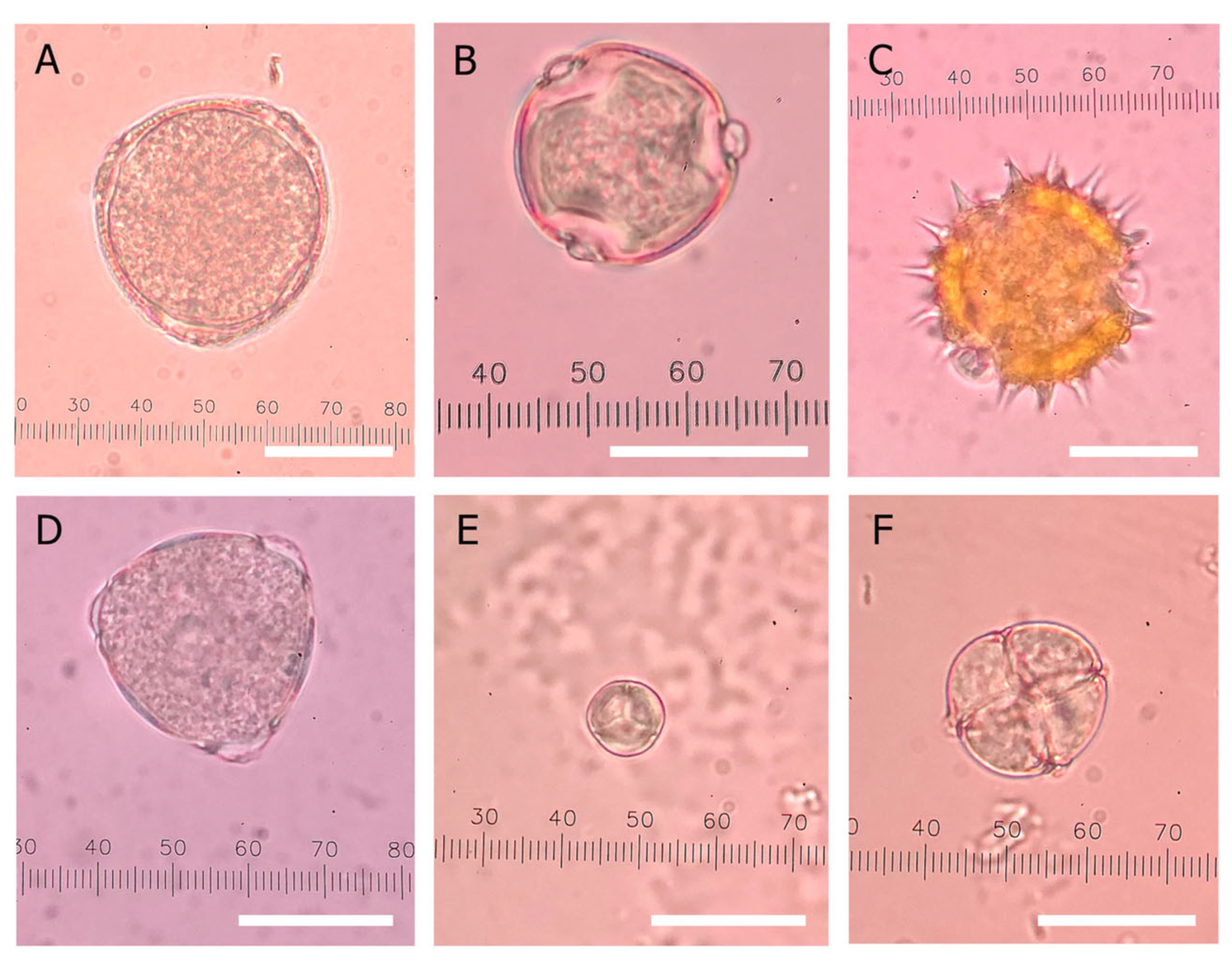
2.5. Melissopalynology
2.6. Statistical Analysis
3. Results and Discussion
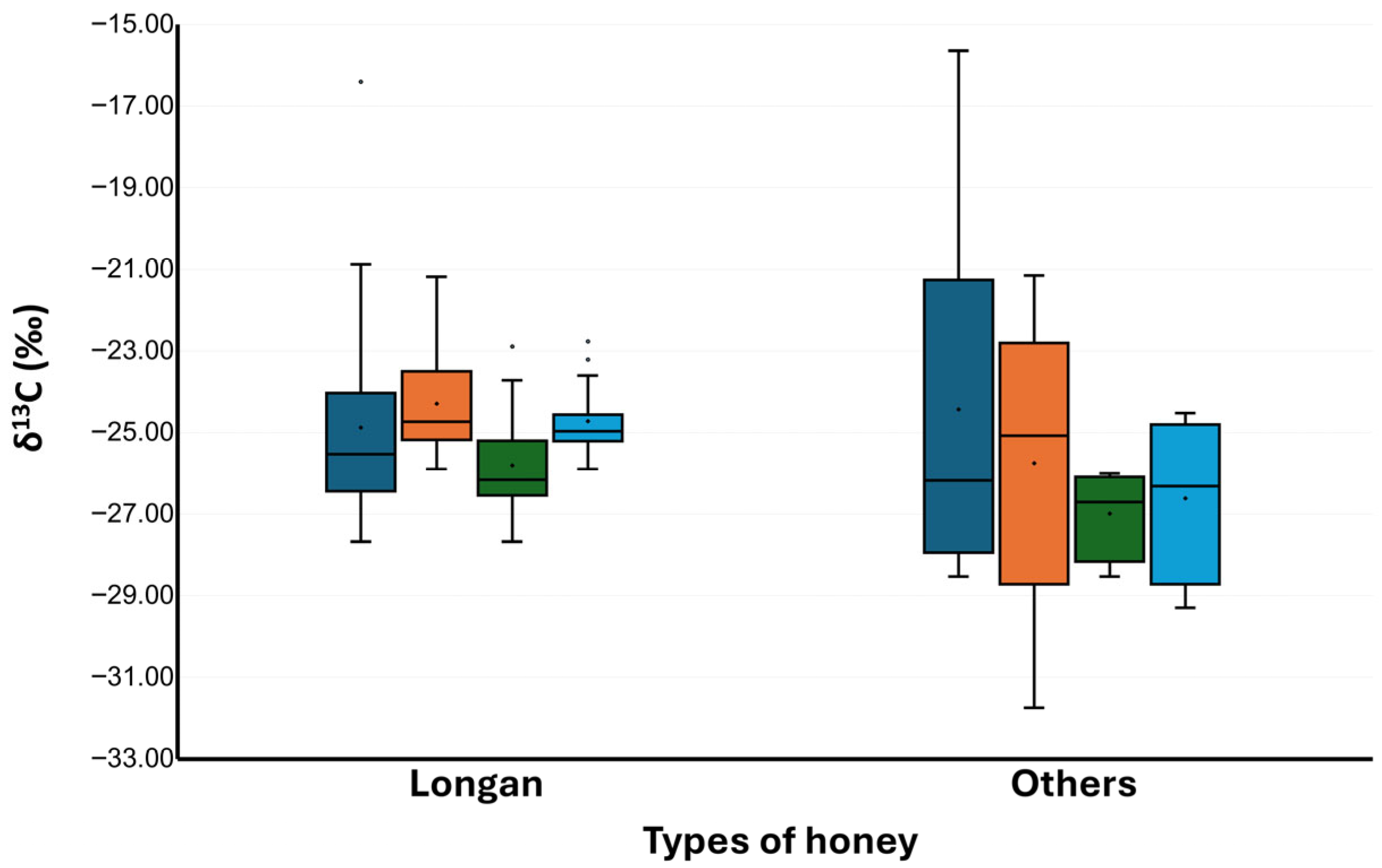
3.1. δ13C of Authentic Honey
3.2. Comparison Between CM-CRDS and EA-IRMS for δ13C Measurements
3.3. Deliberate Adulteration of Authentic Honey
3.3.1. δ13C of Adulterants
3.3.2. Sugar Profile of Authentic Honey
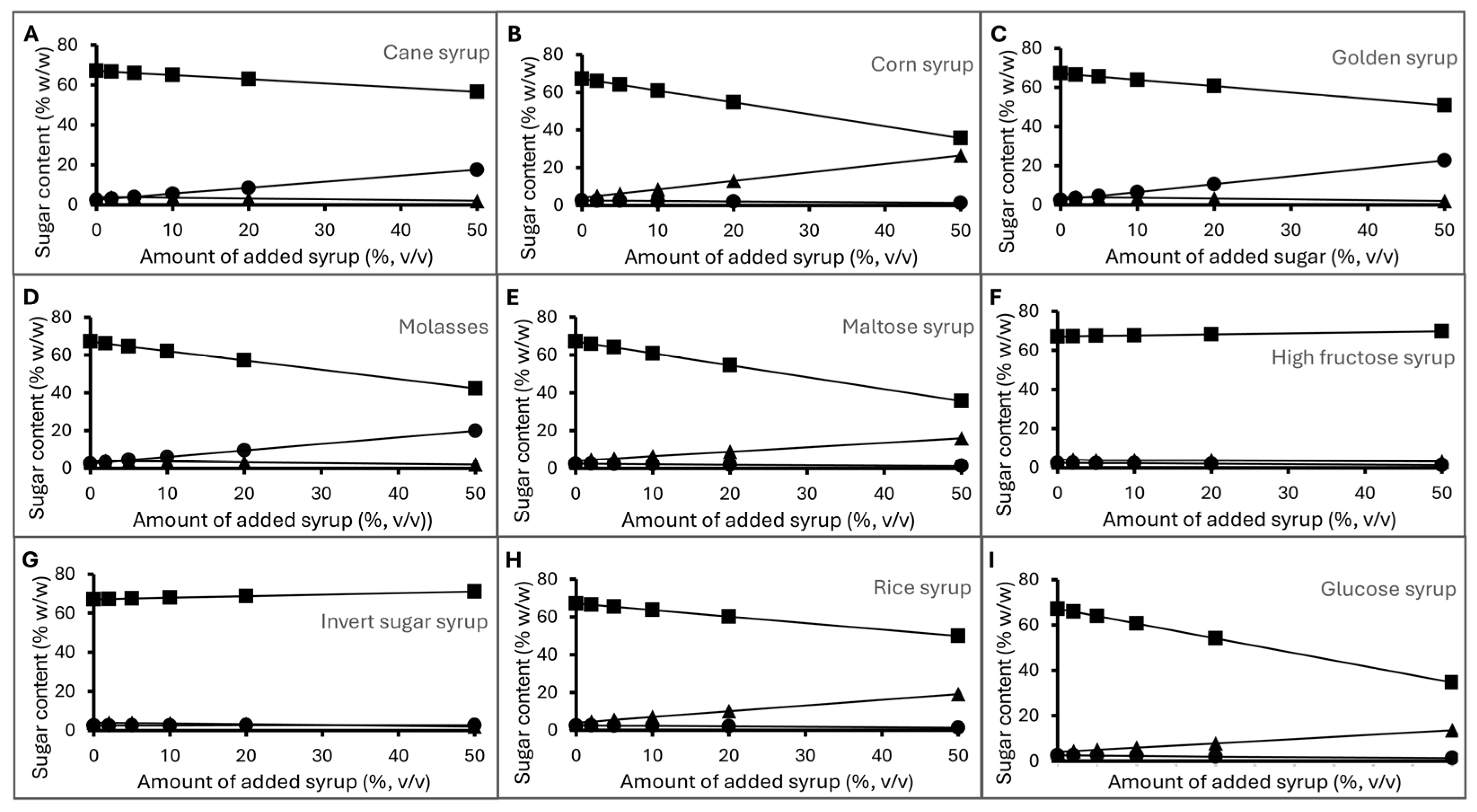
3.3.3. Sugar Levels of Deliberately Adulterated Honey
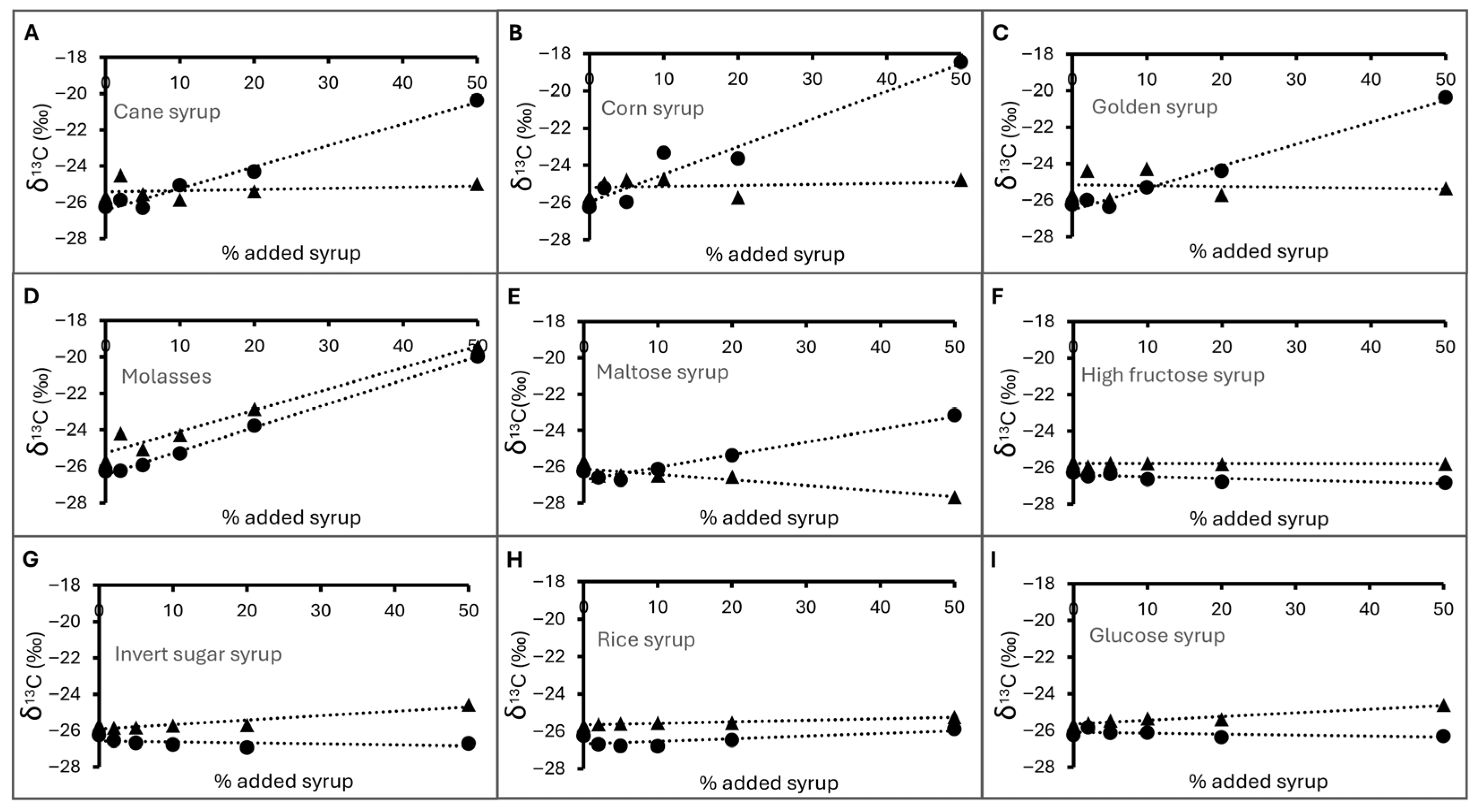
3.3.4. δ13C of Deliberately Adulterated Honey
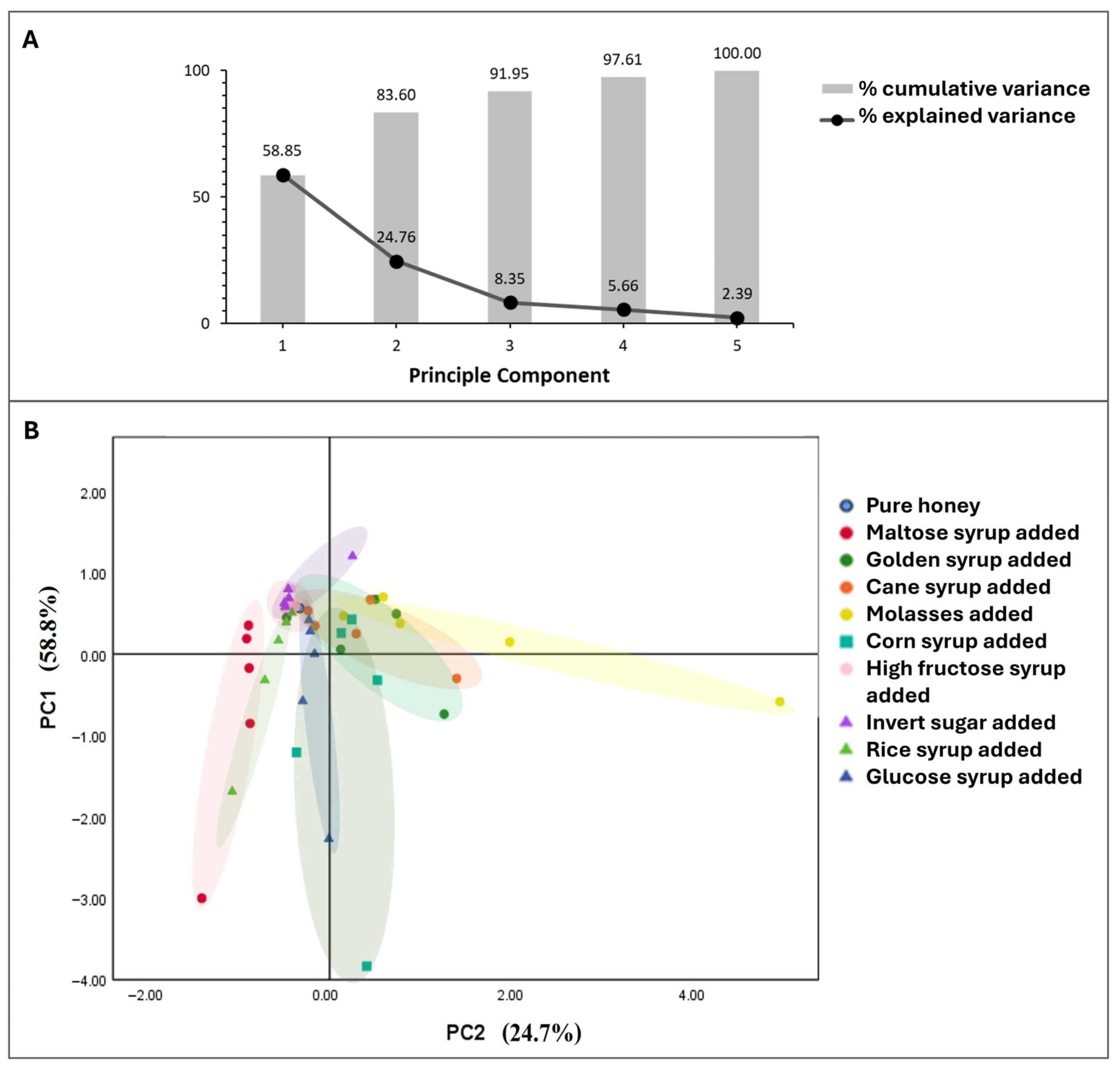
3.3.5. Chemometric Analysis of Deliberately Adulterated Honey
3.4. Authentication of Thai Honey
3.4.1. ẟ13C of Thai Honey
3.4.2. Pollen Composition of Thai Honey
3.4.3. Authentication Consideration
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| SCIRA | Stable carbon isotope ratio analysis |
| EA/LC-IRMS | Elemental analyzer/liquid chromatography–isotope ratio mass spectrometer |
| EA-IRMS | Elemental analyzer–isotope ratio mass spectrometer |
| CM-CRDS | Combustion module–cavity ring down spectroscopy |
| δ13CH | δ13C of bulk honey |
| δ13CP | δ13C of honey protein fraction |
| δ13CP-H | δ13C of protein fraction minus δ13C of bulk honey |
| HPLC | High-performance liquid chromatography |
References
- Powrel, P.J.; Sharma, S. Dynamics of Global Honey Trade: A Longitudinal Analysis from 1961 to 2022. Arch. Curr. Res. Int. 2024, 24, 92–103. [Google Scholar] [CrossRef]
- Terrab, A.; González, A.G.; Díez, M.J.; Heredia, F.J. Characterisation of Moroccan Unifloral Honeys Using Multivariate Analysis. Eur. Food Res. Technol. 2003, 218, 88–95. [Google Scholar] [CrossRef]
- Alcivar-Saldaña, J.J.; Rodriguez-Monroy, M.A.; Carrillo-Miranda, L.; Canales-Martinez, M.M. Botanical Origin and Biological Properties of Honey and Propolis from Cuautitlan, State of Mexico, Mexico. Antioxidants 2024, 13, 874. [Google Scholar] [CrossRef]
- Bunket, A.; Na Lampang, K.; Intanon, M.; Chaisowwong, W. Knowledge, Attitudes, and Practices of Beekeepers Regarding Good Agricultural Practice in Northern Thailand. Vet. Integr. Sci. 2026, 24, e2026021. [Google Scholar]
- Kafle, L.; Mabuza, T.Z. An Analysis of Longan Honey from Taiwan and Thailand Using Flow Cytometry and Physicochemical Analysis. Foods 2024, 13, 3772. [Google Scholar] [CrossRef] [PubMed]
- Disayathanoowat, T.; Judprasong, K.; Boonsirichai, K.; Kamdee, K.; Sinpoo, C.; Phokasem, P.; Takewwong, T.; Segsarnviriya, C.; Esor, J.; Fungklin, R.; et al. The Standard of Thai Honey; Chiang Mai University: Chiang Mai, Thailand, 2025; p. 8. [Google Scholar]
- Wongsiri, S.; Thapa, R.; Kongpitak, P. Longan: A Major Honey Plant in Thailand. Bee World 1998, 79, 23–28. [Google Scholar] [CrossRef]
- Narjes, M.E.; Lippert, C. Regional Differences in Farmers’ Preferences for a Native Bee Conservation Policy: The Case of Farming Communities in Northern and Eastern Thailand. PLoS ONE 2021, 16, e0251206. [Google Scholar] [CrossRef] [PubMed]
- Wanjai, C.; Sringarm, K.; Santasup, C.; Pak-Uthai, S.; Chantawannakul, P. Physicochemical and Microbiological Properties of Longan, Bitter Bush, Sunflower and Litchi Honeys Produced by Apis mellifera in Northern Thailand. J. Apic. Res. 2012, 51, 36–44. [Google Scholar] [CrossRef]
- Chaikham, P.; Prangthip, P. Alteration of Antioxidative Properties of Longan Flower-Honey after High Pressure, Ultra-Sonic and Thermal Processing. Food Biosci. 2015, 10, 1–7. [Google Scholar] [CrossRef]
- Tangtragoon, T.; Kawaree, R.; Posingtong, R. Pollen Analysis and Chemical Characterization of Longan Honey in Northern Thailand. J. Agric. Res. Ext. 2017, 34, 37–47. [Google Scholar]
- Pichai, P.; Budkrajang, P. Quality of Honey Produced in Chiang Mai, Lamphun and Chiang Rai Provinces. Bull. Dept. Med. Sci. 2022, 64, 181–196. [Google Scholar]
- Ministry of Public Health. Notification of Ministry of Public Health (No. 211) B.E. 2543 (2000) Re: Honey. R. Thai Gov. Gaz. 2001, 118, 96–98. [Google Scholar]
- Hatch, M.D.; Slack, C.R. Photosynthesis by Sugar-Cane Leaves. A New Carboxylation Reaction and the Pathway of Sugar Formation. Biochem. J. 1966, 101, 103–111. [Google Scholar] [CrossRef]
- O’Leary, M.H. Carbon Isotopes in Photosynthesis: Fractionation Techniques May Reveal New Aspects of Carbon Dynamics in Plants. BioScience 1988, 38, 328–336. [Google Scholar] [CrossRef]
- Sage, R.F. The Evolution of C4 Photosynthesis. New Phytol. 2004, 161, 341–370. [Google Scholar] [CrossRef]
- Farquhar, G.D.; Ehleringer, J.R.; Hubick, K.T. Carbon Isotope Discrimination and Photosynthesis. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1989, 40, 503–537. [Google Scholar] [CrossRef]
- Winkler, F.J.; Schmidt, H.L. Einsatzmöglichkeiten der 13C-Isotopen-Massenspektrometrie in der Lebensmitteluntersuchung. Z. Lebensm. Unters. Forsch. 1980, 171, 85–94. [Google Scholar] [CrossRef]
- AOAC International. Official Method 991.41. C4 Plant Sugars in Honey. In Official Methods of Analysis of AOAC International, 16th ed.; AOAC International: Arlington, VA, USA, 1995. [Google Scholar]
- AOAC International. Official Method 978.17. Corn and Cane Sugar Products in Honey. In Official Methods of Analysis of AOAC International, 20th ed.; AOAC International: Rockville, MD, USA, 2016. [Google Scholar]
- AOAC International. Official Method 998.12. C4 Plant Sugars in Honey. In Official Methods of Analysis of AOAC International, 20th ed.; AOAC International: Rockville, MD, USA, 2016. [Google Scholar]
- Vîjan, L.E.; Mazilu, I.C.; Enache, C.; Enache, S.; Topală, C.M. Botanical Origin Influence on Some Honey Physicochemical Characteristics and Antioxidant Properties. Foods 2023, 12, 2134. [Google Scholar] [CrossRef]
- von der Ohe, W.; Persano Oddo, L.; Piana, M.L.; Morlot, M.; Martin, P. Harmonized Methods of Melissopalynology. Apidologie 2004, 35, S18–S25. [Google Scholar] [CrossRef]
- Louveaux, J.; Maurizio, A.; Vorwohl, G. Methods of Melissopalynology. Bee World 1978, 59, 139–154. [Google Scholar] [CrossRef]
- Berman, E.; Gupta, M.; Gabrielli, C.; Garland, T.; McDonnell, J.J. High-Frequency Field Deployable Isotope Analyzer for Hydrological Applications. Water Resour. Res. 2009, 45, W10201. [Google Scholar] [CrossRef]
- Chesson, L.A.; Bowen, G.J.; Ehleringer, J.R. Analysis of the Hydrogen and Oxygen Stable Isotope Ratios of Beverage Waters without Prior Water Extraction Using Isotope Ratio Infrared Spectroscopy. Rapid Commun. Mass Spectrom. 2010, 24, 3133–3138. [Google Scholar] [CrossRef] [PubMed]
- AOAC International. Official Method 980.13. Fructose, Glucose, Lactose, Maltose and Sucrose in Milk Chocolate. In Official Methods of Analysis of AOAC International, 18th ed.; AOAC International: Gaithersburg, MD, USA, 2010. [Google Scholar]
- White, J.W., Jr.; Winters, K. Honey Protein as Internal Standard for Stable Carbon Isotope Ratio Detection of Adulteration of Honey. J. Assoc. Off. Anal. Chem. 1989, 72, 907–911. [Google Scholar] [CrossRef]
- Chen, H.; Karion, A.; Rella, C.W.; Winderlich, J.; Gerbig, C.; Filges, A.; Newberger, T.; Sweeney, C.; Tans, P.P. Accurate Measurements of Carbon Monoxide in Humid Air Using the Cavity Ring-Down Spectroscopy (CRDS) Technique. Atmos. Meas. Tech. 2013, 6, 1031–1040. [Google Scholar] [CrossRef]
- Kamdee, K.; Naksuriyawong, S.; Uapoonphol, N.; Fungklin, R.; Esor, J.; Permnamtip, V.; Meepho, S.; Judprasong, K. Evaluation of Honey Authenticity in Thailand by Analysis of Carbon Stable Isotope Ratio Using Elemental Analyser Coupled to Isotope Ratio Mass Spectrometry and Cavity Ring-Down Spectrometry. Int. J. Food Sci. Technol. 2023, 58, 2458–2464. [Google Scholar] [CrossRef]
- El Hawari, K.; Al Iskandarani, M.; Jaber, F.; Ezzeddine, R.; Ziller, L.; Perini, M.; Bontempo, L.; Pellegrini, M.; Camin, F. Evaluation of Honey Authenticity in Lebanon by Analysis of Carbon Stable Isotope Ratio Using Elemental Analyzer and Liquid Chromatography Coupled to Isotope Ratio Mass Spectrometry. J. Mass Spectrom. 2021, 56, e4730. [Google Scholar] [CrossRef] [PubMed]
- Mantha, M.; Urban, J.R.; Mark, W.A.; Chernyshev, A.; Kubachka, K.M. Direct Comparison of Cavity Ring Down Spectrometry and Isotope Ratio Mass Spectrometry for Detection of Sugar Adulteration in Honey Samples. J. AOAC Int. 2018, 101, 1857–1863. [Google Scholar] [CrossRef]
- Elflein, L.; Raezke, K.P. Improved Detection of Honey Adulteration by Measuring Differences between 13C/12C Stable Carbon Isotope Ratios of Protein and Sugar Compounds with a Combination of Elemental Analyzer-Isotope Ratio Mass Spectrometry and Liquid Chromatography-Isotope Ratio Mass Spectrometry (δ13C-EA/LC-IRMS). Apidologie 2008, 39, 574–587. [Google Scholar]
- Dua, A.; Bahman, S.; Kelly, S.; Dogra, S.; Sharma, K. Authentication of Indian Honey Based on Carbon Stable Isotope Ratio Analysis—Verification of Indian Regulatory Criteria. Foods 2025, 14, 1289. [Google Scholar] [CrossRef]
- Schievano, E.; Piana, L.; Tessari, M. Automatic NMR-Based Protocol for Assessment of Honey Authenticity. Food Chem. 2023, 420, 136094. [Google Scholar] [CrossRef]
- Meepho, S.; Kamdee, K.; Saengkorakot, C.; Thapprathum, P.; Judprasong, K. Comparison between Isotope Ratio Mass Spectrometry (IRMS) and Cavity Ring-Down Spectroscopy (CRDS) for Analysing the Carbon Isotope Ratio and Detection of Adulteration in Coconut Water. Int. J. Food Sci. Technol. 2021, 56, 6611–6617. [Google Scholar] [CrossRef]
- Ghosh, S.; Lee, D.G.; Jung, C. A Comparative Study on the Two Different Methods IRMS and CRDS for Estimation of δ13C (‰) of Honey Samples. J. Apic. 2018, 33, 99–105. [Google Scholar] [CrossRef]
- Wang, C.; Guo, L.; Li, Y.; Wang, Z. Systematic Comparison of C3 and C4 Plants Based on Metabolic Network Analysis. BMC Syst. Biol. 2012, 6, S9. [Google Scholar] [CrossRef]
- Bräutigam, A.; Gowik, U. Photorespiration Connects C3 and C4 Photosynthesis. J. Exp. Bot. 2016, 67, 2953–2962. [Google Scholar] [CrossRef]
- White, J.W., Jr.; Doner, L.W. Mass Spectrometric Detection of High-Fructose Corn Syrup in Honey by Use of 13C/12C Ratio: Collaborative Study. J. Assoc. Off. Anal. Chem. 1978, 61, 746–750. [Google Scholar]
- Rossmann, A. Determination of Stable Isotope Ratios in Food Analysis. Food Rev. Int. 2001, 17, 347–381. [Google Scholar] [CrossRef]
- Padovan, G.J.; De Jong, D.; Rodrigues, L.P.; Marchini, J.S. Detection of Adulteration of Commercial Honey Samples by the 13C/12C Isotopic Ratio. Food Chem. 2003, 82, 633–636. [Google Scholar] [CrossRef]
- Desrochers, J.; Brye, K.R.; Pollock, E.D. Long-Term Agricultural Practice Effects on Carbon and Nitrogen Isotopes of Soil Organic Matter Fractions. Agrosyst. Geosci. Environ. 2022, 5, e20232. [Google Scholar] [CrossRef]
- Ma, X.; Gong, L.; Yang, Y.; Ding, Z.; Li, X. Mineralization and Fixed Stable Carbon Isotopic Characteristics of Organic Carbon in Cotton Fields with Different Continuous Cropping Years. Agronomy 2023, 13, 804. [Google Scholar] [CrossRef]
- Liu, Y.; Hu, J.; Wang, G.; Yang, H.; Hong, L.; Xu, J.; Wang, H. Can Stable Carbon Isotope Fingerprints Be Competent for Geographic Traceability of Rice? Food Chem. 2024, 455, 139819. [Google Scholar] [CrossRef]
- Thomatou, A.-A.; Mazarakioti, E.C.; Zotos, A.; Kokkotos, E.; Kontogeorgos, A.; Patakas, A.; Ladavos, A. Stable Isotope Ratio Analysis for the Geographic Origin Discrimination of Greek Beans “Gigantes-Elefantes” (Phaseolus coccineus L.). Foods 2024, 13, 2107. [Google Scholar] [CrossRef]
- Güçlü, H.; Yücel, P.; Oktar, O.; Yazıcı, N.; Ocak, S.B. Carbon Isotope Ratios in Honey and Proteins: A Novel Method for Regional Differentiation. J. Food Compos. Anal. 2025, 144, 107676. [Google Scholar] [CrossRef]
- Camin, F.; Boner, M.; Bontempo, L.; Fauhl-Hassek, C.; Kelly, S.D.; Riedl, J.; Rossmann, A. Stable Isotope Techniques for Verifying the Declared Geographical Origin of Food in Legal Cases. Trends Food Sci. Technol. 2017, 61, 176–187. [Google Scholar] [CrossRef]
- Peterson, D.M.; Budde, A.D.; Henson, C.A.; Jones, B.L. Detecting Corn Syrup in Barley Malt Extracts. Cereal Chem. 2001, 78, 349–353. [Google Scholar] [CrossRef]
- Waworuntu, J.S. Sugar Content Composition of Various Types of Honey Produced by Apis Mellifera L.: A Review. J. Penelit. Pendidik. IPA 2024, 10, 98–106. [Google Scholar] [CrossRef]
- Moniruzzaman, M.; Khalil, M.I.; Sulaiman, S.A.; Gan, S.H. Physicochemical and Antioxidant Properties of Malaysian Honeys Produced by Apis cerana, Apis dorsata and Apis mellifera. BMC Complement. Altern. Med. 2013, 13, 43. [Google Scholar] [CrossRef]
- Mongi, R.J.; Ruhembe, C.C. Sugar Profile and Sensory Properties of Honey from Different Geographical Zones and Botanical Origins in Tanzania. Heliyon 2024, 10, e38094. [Google Scholar] [CrossRef] [PubMed]
- Zulkiflee, N.S.; Zoolfakar, A.S.; Rani, R.A.; Aryani, D.; Zolkapli, M. Detection and Classification of Honey Adulteration Combined with Multivariate Analysis. Int. J. Integr. Eng. 2022, 14, 262–272. [Google Scholar] [CrossRef]
- Codex Alimentarius Commission. Standard for Honey (CXS 12-1981); FAO/WHO: Rome, Italy, 2022.
- Chen, C.-T.; Chen, B.-Y.; Nai, Y.-S.; Chang, Y.-M.; Chen, K.-H.; Chen, Y.-W. Novel Inspection of Sugar Residue and Origin in Honey Based on the 13C/12C Isotopic Ratio and Protein Content. J. Food Drug Anal. 2019, 27, 175–183. [Google Scholar] [CrossRef] [PubMed]
- Won, S.-R.; Lee, D.-C.; Ko, S.H.; Kim, J.-W.; Rhee, H.-I. Honey Major Protein Characterization and Its Application to Adulteration Detection. Food Res. Int. 2008, 41, 952–956. [Google Scholar] [CrossRef]
- Narjes, M.E.; Lippert, C. Longan Fruit Farmers’ Demand for Policies Aimed at Conserving Native Pollinating Bees in Northern Thailand. Ecosyst. Serv. 2016, 18, 58–67. [Google Scholar] [CrossRef]
- Chuttong, B.; Chanbang, Y.; Sringarm, K.; Burgett, M. Physicochemical Profiles of Honey Produced from Monofloral Longan, Rubber, and Sunflower in Northern Thailand. Chiang Mai Univ. J. Nat. Sci. 2016, 15, 115–126. [Google Scholar]
- Suwannapong, G.; Benbow, M.E.; Nieh, J.C. Biology of Thai Honeybees: Natural History and Threats. Insect. Soc. 2011, 58, 495–506. [Google Scholar]
- Rattanawannee, A.; Chanchao, C.; Wongsiri, S. Melissopalynological Analysis and Floral Preferences of Honeybees in Thailand. Palynology 2017, 41, 339–353. [Google Scholar]
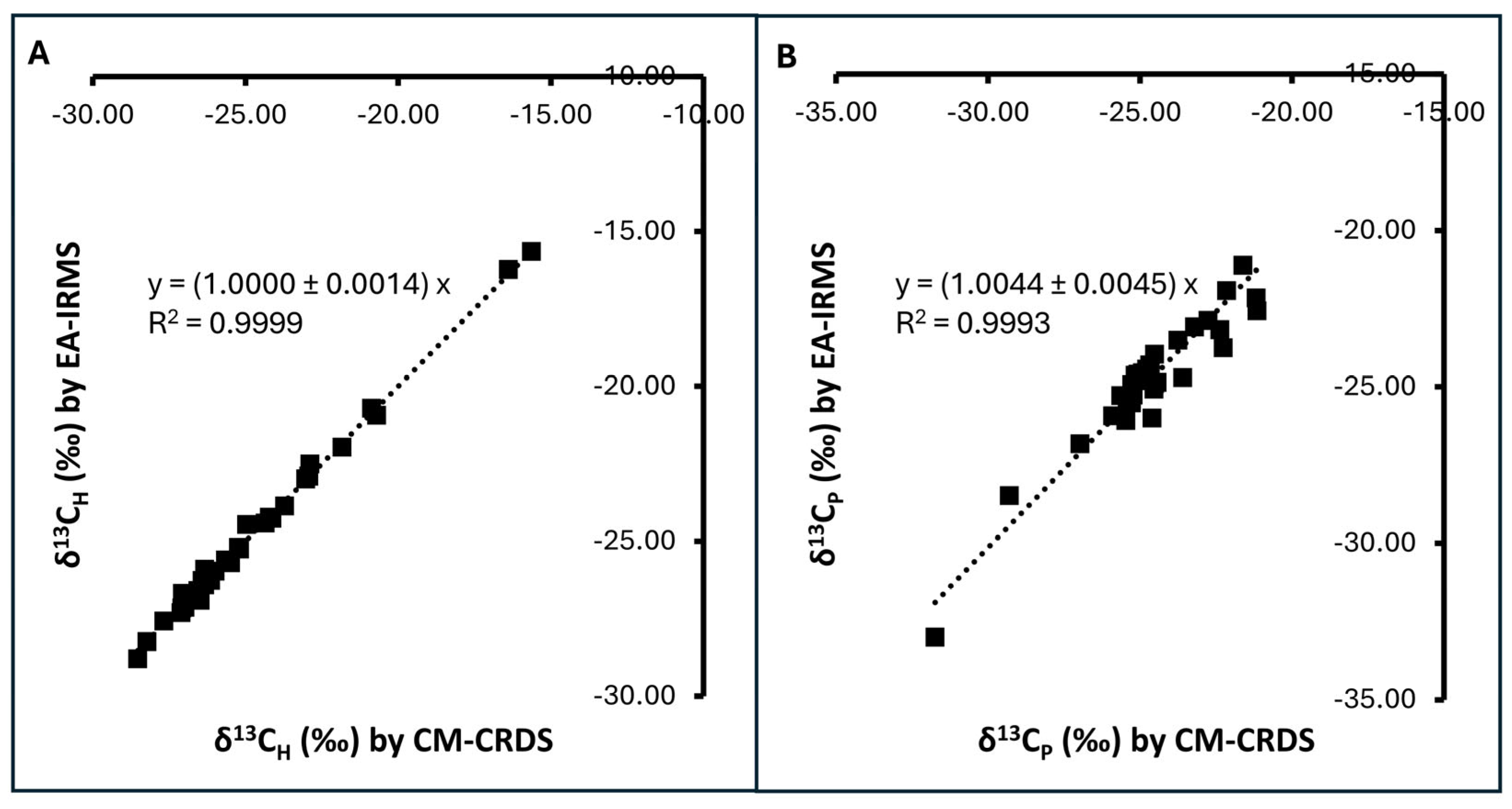
| Method | δ13CH 1 | δ13CP 1 | ∆δ13CP-H |
|---|---|---|---|
| EA/LC-IRMS | −26.43 a | −25.57 b | 0.86 |
| EA-IRMS | −26.51 a | −26.00 a | 0.51 |
| CM-CRDS | −26.26 a | −25.68 b | 0.58 |
| Syrup Type | Labeled Source | δ13C (‰) | Deduced Plant Source 1 |
|---|---|---|---|
| Cane syrup | Sugarcane | −13.04 ± 0.26 | C4 |
| Corn Syrup | Corn | −11.76 ± 0.09 | C4 |
| Golden syrup | Sugarcane | −12.78 ± 0.29 | C4 |
| Molasses | Sugarcane | −13.23 ± 0.10 | C4 |
| Maltose syrup | Rice and barley | −18.83 ± 0.21 | C4 and C3 |
| High-fructose syrup | Cassava | −26.92 ± 0.32 | C3 |
| Invert sugar | Sugar beet | −27.69 ± 0.29 | C3 |
| Rice syrup | Rice | −28.29 ± 0.20 | C3 |
| Glucose syrup | Cassava | −27.20 ± 0.21 | C3 |
| Honey Samples | Declared Origin | δ13CH 1,3 | δ13CP 1,3 | ∆δ13CP-H | δ13CH <−22.00‰ 2 | ∆δ13CP-H >−1.00‰ 2 |
|---|---|---|---|---|---|---|
| Longan A01 | Longan blossom | −26.42 ± 0.12 | −23.60 ± 0.06 | 2.82 | A | A |
| Longan B01 | Longan blossom | −22.89 ± 0.08 | −22.76 ± 0.03 | 0.13 | A | A |
| Longan C04 | Longan blossom | −24.36 ± 0.15 | −21.18 ± 0.13 | 3.18 | A | A |
| Longan CP01 | Longan blossom | −23.03 ± 0.28 | −24.53 ± 0.12 | −1.50 | A | R |
| Longan CP02 | Longan blossom | −25.19 ± 0.06 | −25.17 ± 0.00 | 0.03 | A | A |
| Longan CP03 | Longan blossom | −23.72 ± 0.03 | −24.66 ± 0.01 | −0.94 | A | A |
| Longan CP04 | Longan blossom | −16.40 ± 0.41 * | −21.61 ± 0.03 | −5.22 | R | R |
| Longan CP05 | Longan blossom | −24.96 ± 0.03 | −23.21 ± 0.21 | 1.75 | A | A |
| Longan CP06 | Longan blossom | −26.34 ± 0.11 | −25.23 ± 0.12 | 1.11 | A | A |
| Longan CP07 | Longan blossom | −20.87 ± 0.39 | −22.15 ± 0.20 | −1.28 | R | R |
| Longan CP08 | Longan blossom | −24.14 ± 0.26 | −23.76 ± 0.12 | 0.38 | A | A |
| Longan CP09 | Longan blossom | −25.49 ± 0.08 | −25.90 ± 0.14 | −0.41 | A | A |
| Longan CP10 | Longan blossom | −25.23 ± 0.07 | −25.02 ± 0.08 | 0.21 | A | A |
| Longan F01 | Longan blossom | −26.48 ± 0.22 | −25.28 ± 0.18 | 1.20 | A | A |
| Longan H01 | Longan blossom | −27.11 ± 0.15 | −24.60 ± 0.16 | 2.51 | A | A |
| Longan H03 | Longan blossom | −26.14 ± 0.14 | −24.54 ± 0.05 | 1.60 | A | A |
| Longan L01 | Longan blossom | −27.68 ± 0.05 | −25.47 ± 0.06 | 2.21 | A | A |
| Longan N01 | Longan blossom | −21.84 ± 0.04 | −22.37 ± 0.16 | −0.53 | R | A |
| Longan P01 | Longan blossom | −26.16 ± 0.01 | −25.42 ± 0.44 | 0.73 | A | A |
| Longan P02 | Longan blossom | −24.22 ± 0.03 | −25.28 ± 0.00 | −1.06 | A | R |
| Longan P03 | Longan blossom | −25.66 ± 0.01 | −24.69 ± 0.01 | 0.97 | A | A |
| Longan P04 | Longan blossom | −26.29 ± 0.02 | −24.92 ± 0.02 | 1.37 | A | A |
| Longan P05 | Longan blossom | −25.57 ± 0.02 | −24.78 ± 0.04 | 0.79 | A | A |
| Longan P06 | Longan blossom | −27.08 ± 0.08 | −25.05 ± 0.12 | 2.03 | A | A |
| Longan P07 | Longan blossom | −26.56 ± 0.01 | −25.07 ± 0.03 | 1.49 | A | A |
| Longan P09 | Longan blossom | −26.98 ± 0.07 | −25.13 ± 0.00 | 1.85 | A | A |
| Wildflower C06 | Wild flowers | −15.64 ± 0.11 * | −21.15 ± 0.17 | −5.51 | R | R |
| Wildflower H04 | Wild flowers | −22.93 ± 0.07 | −24.44 ± 0.01 | −1.51 | A | R |
| Wildflower P08 | Wild flowers | −26.00 ± 0.05 | −25.64 ± 0.05 | 0.36 | A | A |
| Lychee C05 | Lychee blossom | −20.70 ± 0.20 | −22.26 ± 0.05 | −1.56 | R | R |
| Lychee H02 | Lychee blossom | −26.33 ± 0.07 | −24.52 ± 0.05 | 1.81 | A | A |
| Sunflower H01 | Sunflower blossom | −27.07 ± 0.03 | −26.98 ± 0.03 | 0.09 | A | A |
| Sunflower K01 | Sunflower blossom | −28.53 ± 0.19 | −29.30 ± 0.07 | −0.77 | A | A |
| Coffee H01 | Coffee blossom | −28.23 ± 0.09 | −31.75 ± 0.01 * | −3.52 | A | R |
| Honey Samples | Declared Origin | Predominant Pollen (>45%) | Secondary Pollen (16–45%) | Minor Pollen (3–15.9%) | Monofloral Honey as Claimed 1 |
|---|---|---|---|---|---|
| Longan A01 | Longan blossom | Mimosa pudica (72%) | Bidens pilosa L. (13%) | N | |
| Longan B01 | Longan blossom | Mimosa pudica (46%) | Dimocarpus longan L. (41%) | N | |
| Longan C04 | Longan blossom | Dimocarpus longan L. (50%) | Mimosa pudica (18%) | Y | |
| Longan CP01 | Longan blossom | Dimocarpus longan L. (41%) Mimosa pudica (28%) | N | ||
| Longan CP02 | Longan blossom | Dimocarpus longan L. (46%) | Mimosa pudica (21%) | Y | |
| Longan CP03 | Longan blossom | Dimocarpus longan L. (50%) | Mimosa pudica (31%) | Y | |
| Longan CP04 | Longan blossom | Dimocarpus longan L. (48%) | Mimosa pudica (35%) | Y | |
| Longan CP05 | Longan blossom | Dimocarpus longan L. (44%) Mimosa pudica (36%) | N | ||
| Longan CP06 | Longan blossom | Dimocarpus longan L. (44%) Mimosa pudica (28%) | N | ||
| Longan CP07 | Longan blossom | Mimosa pudica (52%) | Dimocarpus longan L. (29%) | N | |
| Longan CP08 | Longan blossom | Mimosa pudica (38%) Mimosa pigra (28%) Dimocarpus longan L. (25%) | N | ||
| Longan CP09 | Longan blossom | Mimosa pigra (82%) | Mimosa pudica (15%) | N | |
| Longan CP10 | Longan blossom | Dimocarpus longan L. (66%) | Leucaena leucocephala L. (18%) | Y | |
| Longan F01 | Longan blossom | Mimosa pudica (52%) | Bidens pilosa L. (21%) | N | |
| Longan H01 | Longan blossom | Mimosa pudica (69%) | Dimocarpus longan L. (18%) | N | |
| Longan H03 | Longan blossom | Dimocarpus longan L. (41%) | Mimosa pudica (15%) | N | |
| Longan L01 | Longan blossom | Dimocarpus longan L. (52%) | Mimosa pudica (20%) | Y | |
| Longan N01 | Longan blossom | Mimosa pudica (60%) | Salicaceae (29%) | N | |
| Longan P01 | Longan blossom | Dimocarpus longan L. (45%) Mimosa pudica (34%) | N | ||
| Longan P02 | Longan blossom | Mimosa pudica (32%) Dimocarpus longan L. (29%) | N | ||
| Longan P03 | Longan blossom | Mimosa pudica (47%) | Dimocarpus longan L. (35%) | N | |
| Longan P04 | Longan blossom | Dimocarpus longan L. (40%) Mimosa pudica (37%) | N | ||
| Longan P05 | Longan blossom | Mimosa pudica (66%) | Dimocarpus longan L. (18%) | N | |
| Longan P06 | Longan blossom | Dimocarpus longan L. (54%) | Mimosa pudica (27%) | Y | |
| Longan P07 | Longan blossom | Dimocarpus longan L. (37%) Mimosa pudica (25%) | N | ||
| Longan P09 | Longan blossom | Dimocarpus longan L. (55%) | Mimosa pudica (24%) | Y | |
| Wildflower C06 | Wild flowers | Mimosa pudica (81%) | Bidens pilosa L. 7% | Y | |
| Wildflower H04 | Wild flowers | Mimosa pudica (61%) | Fabaceae 12% | Y | |
| Wildflower P08 | Wild flowers | Mimosa pudica (62%) | Dimocarpus longan L. (22%) | Y | |
| Lychee C05 | Lychee blossom | Mimosa pudica (31%) Litchi chinensis (25%) | N | ||
| Lychee H02 | Lychee blossom | Litchi chinensis (26%) Polygonaceae family (21%) | N | ||
| Sunflower H01 | Sunflower blossom | Helianthus annuus (37%) Salicaceae family (26%) | N | ||
| Sunflower K01 | Sunflower blossom | Helianthus annuus (88%) | Fabaceae 7% | Y | |
| Coffee H01 | Coffee blossom | Coffea (93%) | Poaceae (3%) | Y |
| Honey Samples | δ13C Criteria 1 | Monofloral Honey Criteria 2 | Final Consideration 3 |
|---|---|---|---|
| Longan A01 | A | N | F |
| Longan B01 | A | N | F |
| Longan C04 | R | Y | F |
| Longan CP01 | R | N | F |
| Longan CP02 | A | Y | P |
| Longan CP03 | A | Y | P |
| Longan CP04 | R | Y | F |
| Longan CP05 | A | N | F |
| Longan CP06 | A | N | F |
| Longan CP07 | R | N | F |
| Longan CP08 | A | N | F |
| Longan CP09 | A | N | F |
| Longan CP10 | A | Y | P |
| Longan F01 | A | N | F |
| Longan H01 | A | N | F |
| Longan H03 | A | N | F |
| Longan L01 | A | Y | P |
| Longan N01 | R | N | F |
| Longan P01 | A | N | F |
| Longan P02 | R | N | F |
| Longan P03 | A | N | F |
| Longan P04 | A | N | F |
| Longan P05 | A | N | F |
| Longan P06 | A | Y | P |
| Longan P07 | A | N | F |
| Longan P09 | A | Y | P |
| Wildflower C06 | R | Y | F |
| Wildflower H04 | R | Y | F |
| Wildflower P08 | A | Y | P |
| Lychee C05 | R | N | F |
| Lychee H02 | A | N | F |
| Sunflower H01 | A | N | F |
| Sunflower K01 | A | Y | P |
| Coffee H01 | R | Y | F |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Judprasong, K.; Sinpoo, C.; Naksuriyawong, S.; Kamdee, K.; Meepho, S.-a.; Phokasem, P.; Saengkorakot, C.; Fungklin, R.; Uapoonphol, N.; Disayathanoowat, T.; et al. A Synergistic Approach Combining Stable Carbon Isotope Ratio Analysis and Melissopalynology for the Authentication of Honey from Thailand. Foods 2025, 14, 3850. https://doi.org/10.3390/foods14223850
Judprasong K, Sinpoo C, Naksuriyawong S, Kamdee K, Meepho S-a, Phokasem P, Saengkorakot C, Fungklin R, Uapoonphol N, Disayathanoowat T, et al. A Synergistic Approach Combining Stable Carbon Isotope Ratio Analysis and Melissopalynology for the Authentication of Honey from Thailand. Foods. 2025; 14(22):3850. https://doi.org/10.3390/foods14223850
Chicago/Turabian StyleJudprasong, Kunchit, Chainarong Sinpoo, Sasiwimon Naksuriyawong, Kiattipong Kamdee, Sang-arun Meepho, Patcharin Phokasem, Chakrit Saengkorakot, Ratchai Fungklin, Nichtima Uapoonphol, Terd Disayathanoowat, and et al. 2025. "A Synergistic Approach Combining Stable Carbon Isotope Ratio Analysis and Melissopalynology for the Authentication of Honey from Thailand" Foods 14, no. 22: 3850. https://doi.org/10.3390/foods14223850
APA StyleJudprasong, K., Sinpoo, C., Naksuriyawong, S., Kamdee, K., Meepho, S.-a., Phokasem, P., Saengkorakot, C., Fungklin, R., Uapoonphol, N., Disayathanoowat, T., Esor, J., Thongphichai, W., & Boonsirichai, K. (2025). A Synergistic Approach Combining Stable Carbon Isotope Ratio Analysis and Melissopalynology for the Authentication of Honey from Thailand. Foods, 14(22), 3850. https://doi.org/10.3390/foods14223850








