Protective Effects of Cyanidin-3-O-Glucoside Against Neurotoxin Acrylamide Through Alleviating Mitochondrial Dysfunction
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
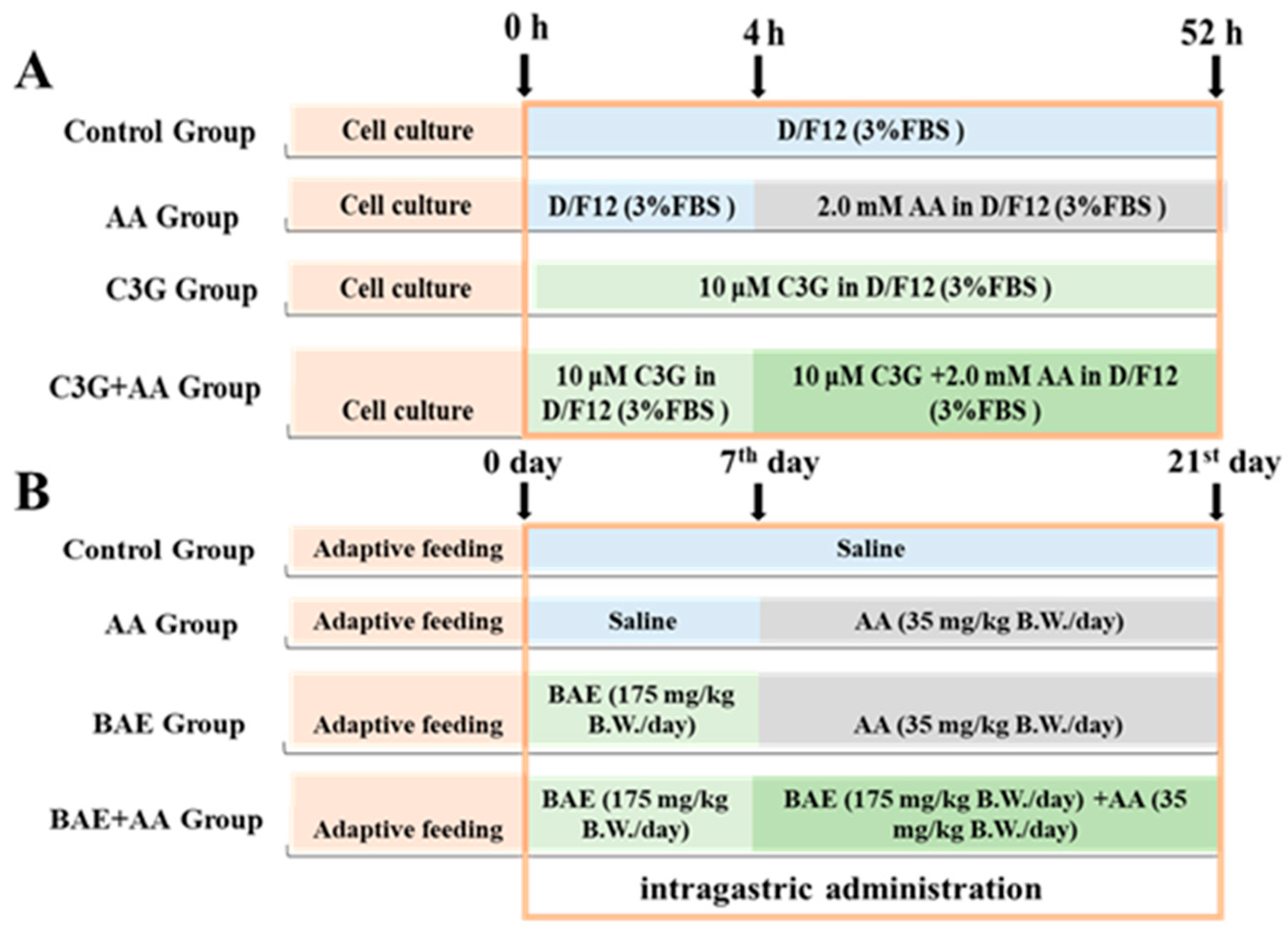
2.2. Cell and Animal Treatment
2.3. MTT Assay
2.4. Measurement of ROS Generation
2.5. Measurement of GSH Levels and MDA Content
2.6. Mitochondrial Membrane Potential Assay
2.7. Mitochondrial ROS Detection
2.8. AO/EB Double Staining
2.9. Extracellular Flux Analyses
2.10. Quantitative Real-Time PCR
2.11. Western Blot Analysis
2.12. Statistical Analysis
3. Results
3.1. C3G Alleviates AA-Induced Cytotoxicity and Oxidative Stress
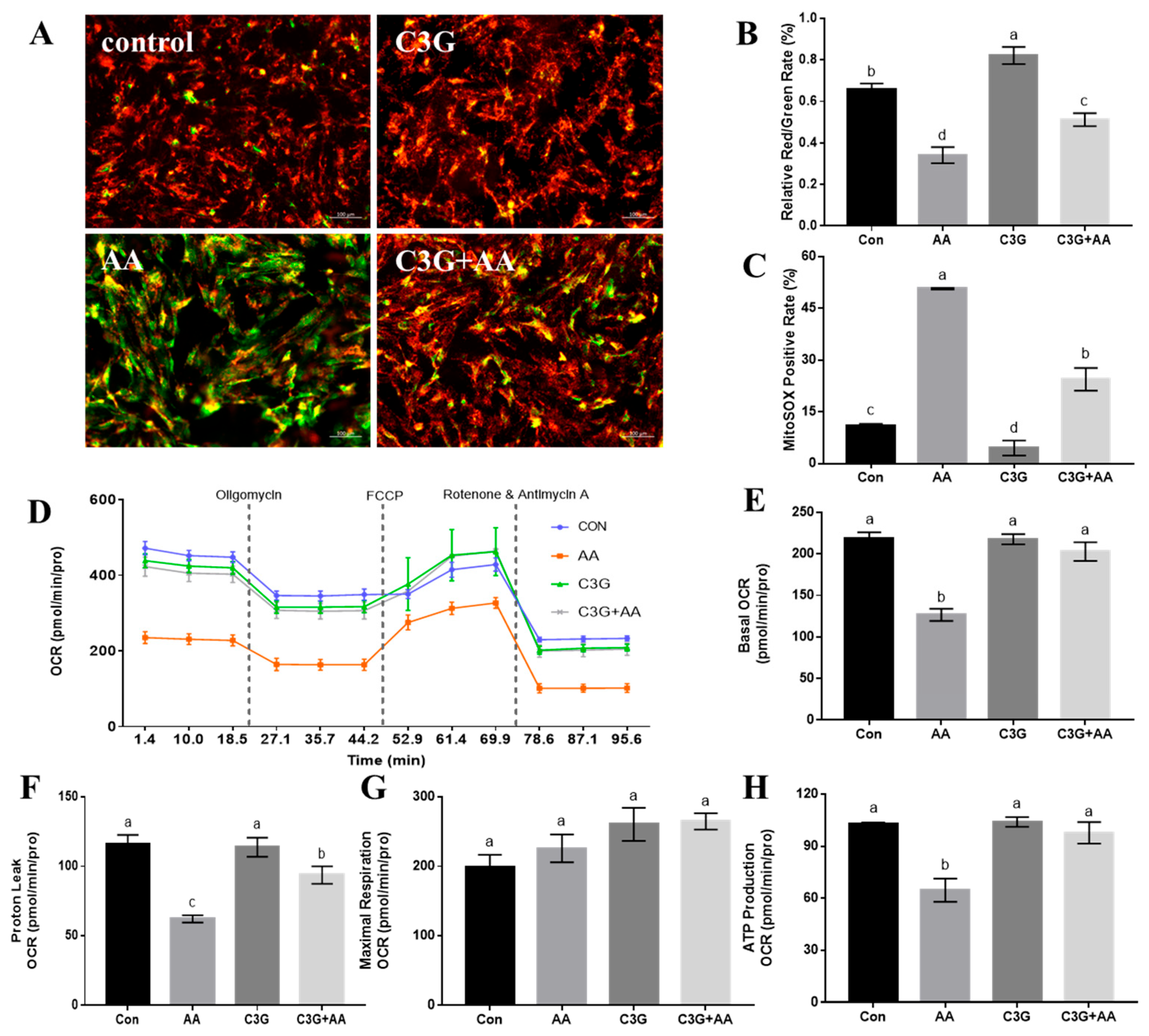
3.2. C3G Suppresses AA-Induced Damage to Mitochondrial Structure, Redox Balance, and Respiratory Function
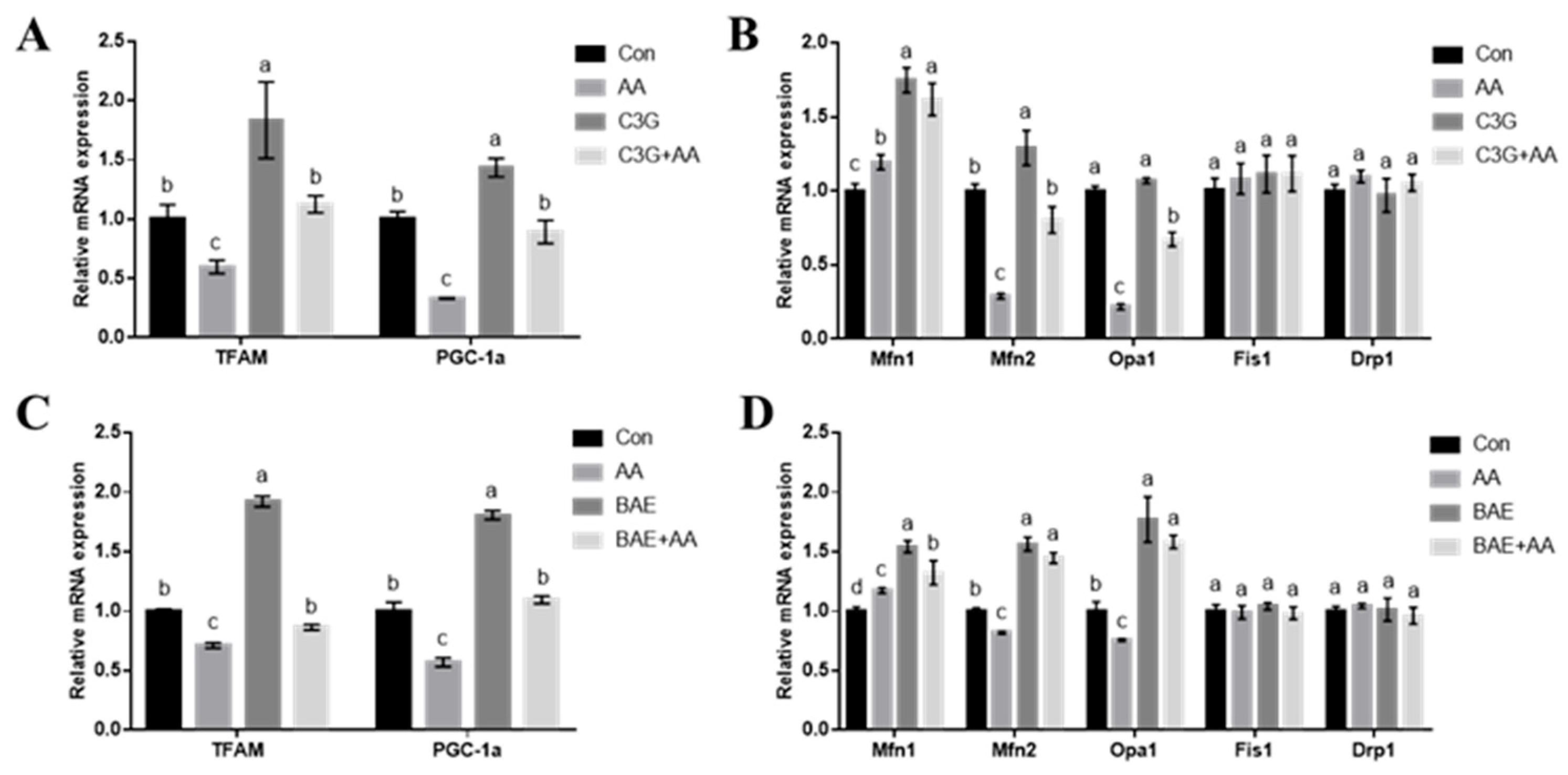
3.3. C3G Ameliorates AA-Caused Mitochondrial Function-Related Gene Expression Abnormalities
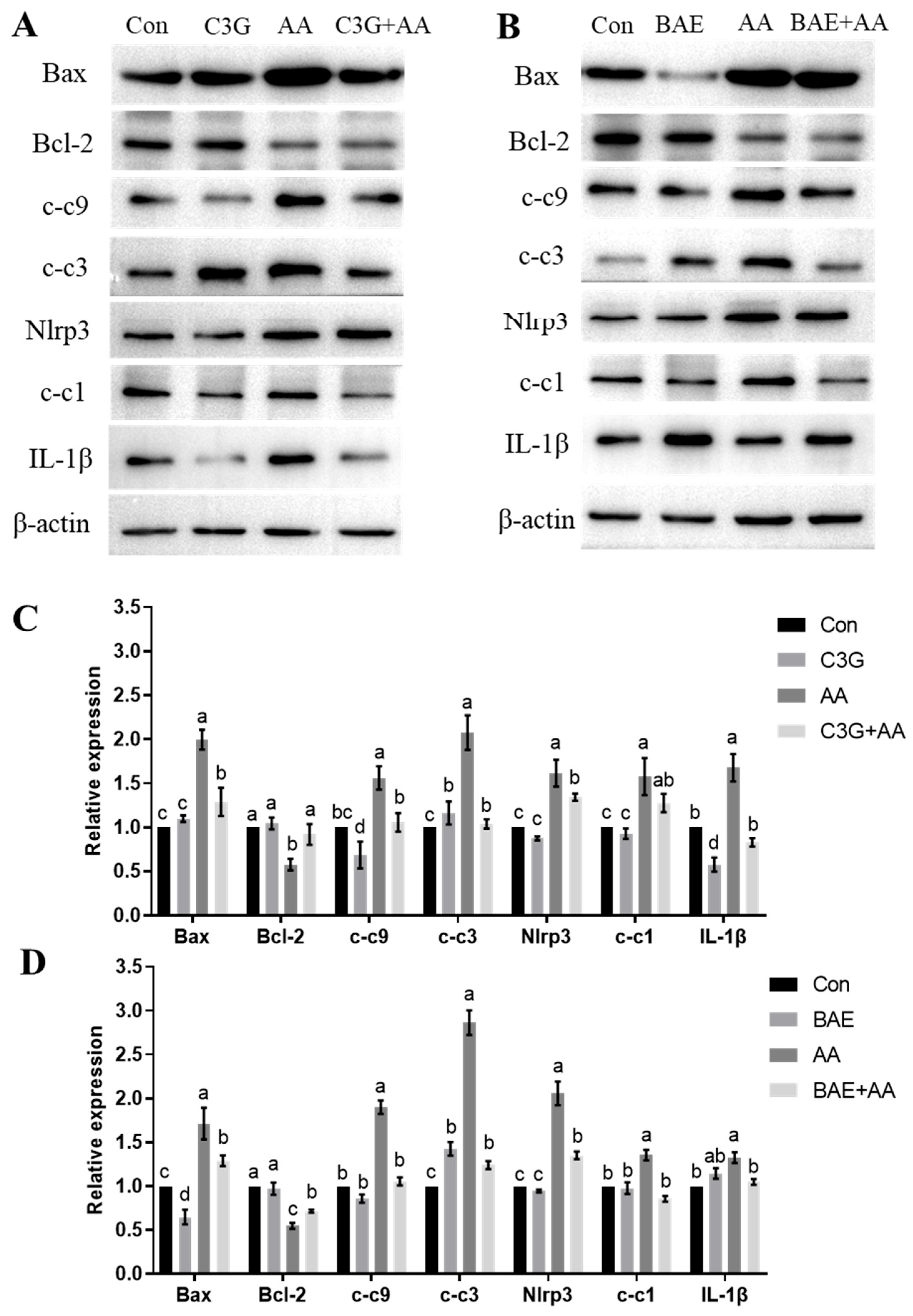
3.4. C3G Mitigates AA-Induced Apoptosis and Inflammatory Response
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AA | Acrylamide |
| C3G | Cyanidin-3-O-glucoside |
| ROS | Reactive oxygen species |
| PGC-1α | Peroxisome proliferator activator receptor gamma coactivator-1α |
| TFAM | Mitochondrial transcription factor A |
| CNS | Central nervous system |
| BAE | Blueberry anthocyanin extract |
| MTT | Thiazolyl blue tetrazolium bromide |
| DCFH-DA | 2′,7′-dichlorofluorescein diacetate |
| JC-1 | 5,5′,6,6′-tetrachloro-1,1′,3,3′-tetraethylbenzimidazolyl carbocyanine iodide |
| FBS | Fetal bovine serum |
| PBS | Phosphate-buffered saline |
| GSH | Glutathione |
| MDA | Malondialdehyde |
| MMP | Mitochondrial membrane potential |
| mtROS | Mitochondrial ROS |
| c-c9 | Cleaved caspase-9 |
| c-c3 | Cleaved caspase-3 |
| c-c1 | Cleaved caspase-1 |
Appendix A
Appendix A.1
| Gene | Forward (5′-3′) | Reverse (5′-3′) |
|---|---|---|
| Mfn1 | CAGCAGCAGAGAAGAGGGTTTAT | CAGCACACTGGGGGTAGGAT |
| Mfn2 | AGTGTCAAGACCGTGAACCA | ACACATCAGCATCCAGGCAA |
| Drp1 | GCTAGATGTGCCAGTTCCAGT | TGTGCCATGTCCTCGGATTC |
| Opa1 | GGCACTTCAAGGTCGTCTCA | CACTGCTCTTGGGTCCGATT |
| Fis1 | ACGCCTGCCGTTACTTCTTC | GCAACCCTGCAATCCTTCAC |
| TFAM | AAATGGCTGAAGTTGGGCGAAGTG | AGCTTCTTGTGCCCAATCCCAATG |
| PGC-1α | CCGAGAATTCATGGAGCAAT | GTGTGAGGAGGGTCATCGTT |
| GAPDH | AGTGCCAGCCTCGTCTCATA | GGTAACCAGGCGTCCGATAC |
Appendix A.2
| Name | Dilution | Manufacturer | Catalog |
|---|---|---|---|
| Anti-Bcl-2 antibody [E17] | 1:1000 | abacm | ab194583 |
| Anti-Bax antibody [E63] | 1:1000 | abacm | ab32503 |
| Anti-Cytochrome C antibody [EPR1327] | 1:1000 | abcam | ab133504 |
| Cleaved Caspase-3 (Asp175) (5A1E) Rabbit mAb | 1:1000 | Cell Signaling Technology | #9664 |
| Caspase-9 (C9) Mouse mAb | 1:1000 | Cell Signaling Technology | #9508 |
| Cleaved-Caspase 1 Rabbit pAb | 1:500 | Chengdu Zen-Bioscience | 341030 |
| IL-1 beta Rabbit pAb | 1:500 | Chengdu Zen-Bioscience | 511369 |
| NLRP3 Rabbit pAb | 1:500 | Chengdu Zen-Bioscience | 381207 |
| β-Actin Antibody | 1:1000 | Cell Signaling Technology | #4967 |
References
- Bin-Jumah, M.; Abdel-Fattah, A.M.; Saied, E.M.; El-Seedi, H.R.; Abdel-Daim, M.M. Acrylamide-induced peripheral neuropathy: Manifestations, mechanisms, and potential treatment modalities. Environ. Sci. Pollut. Res. Int. 2021, 28, 13031–13046. [Google Scholar] [CrossRef] [PubMed]
- Peivasteh-Roudsari, L.; Karami, M.; Barzegar-Bafrouei, R.; Samiee, S.; Karami, H.; Tajdar-Oranj, B.; Mahdavi, V.; Alizadeh, A.M.; Sadighara, P.; Oliveri Conti, G.; et al. Toxicity, metabolism, and mitigation strategies of acrylamide: A comprehensive review. Int. J. Environ. Health Res. 2024, 34, 1–29. [Google Scholar] [CrossRef]
- Bušová, M.; Bencko, V.; Veszelits Laktičová, K.; Holcátová, I.; Vargová, M. Risk of exposure to acrylamide. Cent. Eur. J. Public. Health 2020, 28, S43–S46. [Google Scholar] [CrossRef] [PubMed]
- Tareke, E.; Rydberg, P.; Karlsson, P.; Eriksson, S.; Törnqvist, M. Analysis of acrylamide, a carcinogen formed in heated foodstuffs. J. Agric. Food Chem. 2002, 50, 4998–5006. [Google Scholar] [CrossRef]
- Mottram, D.S.; Wedzicha, B.L.; Dodson, A.T. Acrylamide is formed in the Maillard reaction. Nature 2002, 419, 448–449. [Google Scholar] [CrossRef] [PubMed]
- Rasool, A.; Luo, X.; Zhang, Q.; Jia, C.; Zhao, S.; Liu, R.; Rong, J.; Zhou, G.; Wang, B.; Kuai, J.; et al. Acrylamide and Advanced Glycation End Products in Frying Food: Formation, Effects, and Harmfulness. Foods 2025, 14, 3313. [Google Scholar] [CrossRef]
- Rifai, L.; Saleh, F.A. A Review on Acrylamide in Food: Occurrence, Toxicity, and Mitigation Strategies. Int. J. Toxicol. 2020, 39, 93–102. [Google Scholar] [CrossRef]
- Koszucka, A.; Nowak, A.; Nowak, I.; Motyl, I. Acrylamide in human diet, its metabolism, toxicity, inactivation and the associated European Union legal regulations in food industry. Crit. Rev. Food Sci. Nutr. 2020, 60, 1677–1692. [Google Scholar] [CrossRef]
- Lindeman, B.; Johansson, Y.; Andreassen, M.; Husøy, T.; Dirven, H.; Hofer, T.; Knutsen, H.K.; Caspersen, I.H.; Vejrup, K.; Paulsen, R.E.; et al. Does the food processing contaminant acrylamide cause developmental neurotoxicity? A review and identification of knowledge gaps. Reprod. Toxicol. 2021, 101, 93–114. [Google Scholar] [CrossRef]
- Zhang, L.; Yang, L.; Luo, Y.; Dong, L.; Chen, F. Acrylamide-Induced Hepatotoxicity Through Oxidative Stress: Mechanisms and Interventions. Antioxid. Redox Signal. 2023, 38, 1122–1137. [Google Scholar] [CrossRef]
- Zhao, M.; Zhang, B.; Deng, L. The Mechanism of Acrylamide-Induced Neurotoxicity: Current Status and Future Perspectives. Front. Nutr. 2022, 9, 859189. [Google Scholar] [CrossRef]
- Teleanu, D.M.; Niculescu, A.G.; Lungu, I.I.; Radu, C.I.; Vladâcenco, O.; Roza, E.; Costăchescu, B.; Grumezescu, A.M.; Teleanu, R.I. An Overview of Oxidative Stress, Neuroinflammation, and Neurodegenerative Diseases. Int. J. Mol. Sci. 2022, 23, 5938. [Google Scholar] [CrossRef] [PubMed]
- Aroor, A.R.; Mandavia, C.; Ren, J.; Sowers, J.R.; Pulakat, L. Mitochondria and Oxidative Stress in the Cardiorenal Metabolic Syndrome. Cardiorenal Med. 2012, 2, 87–109. [Google Scholar] [CrossRef]
- Liu, Z.; Song, G.; Zou, C.; Liu, G.; Wu, W.; Yuan, T.; Liu, X. Acrylamide induces mitochondrial dysfunction and apoptosis in BV-2 microglial cells. Free Radic. Biol. Med. 2015, 84, 42–53. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.H.; Yang, C.H.; Wang, Y.S.; Lee, J.G.; Cheng, C.H.; Chou, C.C. Acrylamide-induced mitochondria collapse and apoptosis in human astrocytoma cells. Food Chem. Toxicol. 2013, 51, 446–452. [Google Scholar] [CrossRef]
- Yang, L.; Dong, L.; Zhang, L.; Bai, J.; Chen, F.; Luo, Y. Acrylamide Induces Abnormal mtDNA Expression by Causing Mitochondrial ROS Accumulation, Biogenesis, and Dynamics Disorders. J. Agric. Food Chem. 2021, 69, 7765–7776. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Cao, X.; Hu, X.; Li, S.; Wang, J. The anti-apoptotic, antioxidant and anti-inflammatory effects of curcumin on acrylamide-induced neurotoxicity in rats. BMC Pharmacol. Toxicol. 2020, 21, 62. [Google Scholar] [CrossRef]
- Mattioli, R.; Francioso, A.; Mosca, L.; Silva, P. Anthocyanins: A Comprehensive Review of Their Chemical Properties and Health Effects on Cardiovascular and Neurodegenerative Diseases. Molecules 2020, 25, 3809. [Google Scholar] [CrossRef]
- Victoria-Campos, C.I.; Ornelas-Paz, J.J.; Rios-Velasco, C.; Ruiz-Cruz, S.; Ornelas-Paz, J.; Del Toro-Sánchez, C.L.; Márquez-Ríos, E.; Calderón-Loera, R. Relevance of Anthocyanin Metabolites Generated During Digestion on Bioactivity Attributed to Intact Anthocyanins. Foods 2024, 13, 4066. [Google Scholar] [CrossRef]
- Sadowska-Bartosz, I.; Bartosz, G. Antioxidant Activity of Anthocyanins and Anthocyanidins: A Critical Review. Int. J. Mol. Sci. 2024, 25, 12001. [Google Scholar] [CrossRef]
- Talavera, S.; Felgines, C.; Texier, O.; Besson, C.; Gil-Izquierdo, A.; Lamaison, J.L. Remesy C(2005) Anthocyanin metabolism in rats their distribution to digestive area, kidney, and brain. J. Agric. Food Chem. 2005, 53, 3902–3908. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Chen, H.; Zhang, W.; Ding, Y.; Zhao, T.; Zhang, M.; Mao, G.; Feng, W.; Wu, X.; Yang, L. Bioaccessibility and biotransformation of anthocyanin monomers following in vitro simulated gastric-intestinal digestion and in vivo metabolism in rats. Food Funct. 2019, 10, 6052–6061. [Google Scholar] [CrossRef]
- Olivas-Aguirre, F.J.; Rodrigo-García, J.; Martínez-Ruiz, N.D.; Cárdenas-Robles, A.I.; Mendoza-Díaz, S.O.; Álvarez-Parrilla, E.; González-Aguilar, G.A.; de la Rosa, L.A.; Ramos-Jiménez, A.; Wall-Medrano, A. Cyanidin-3-O-glucoside: Physical-Chemistry, Foodomics and Health Effects. Molecules 2016, 21, 1264. [Google Scholar] [CrossRef]
- Oumeddour, D.Z.; Al-Dalali, S.; Zhao, L.; Zhao, L.; Wang, C. Recent advances on cyanidin-3-O-glucoside in preventing obesity-related metabolic disorders: A comprehensive review. Biochem. Biophys. Res. Commun. 2024, 729, 150344. [Google Scholar] [CrossRef]
- Molonia, M.S.; Occhiuto, C.; Muscarà, C.; Speciale, A.; Bashllari, R.; Villarroya, F.; Saija, A.; Cimino, F.; Cristani, M. Cyanidin-3-O-glucoside restores insulin signaling and reduces inflammation in hypertrophic adipocytes. Arch. Biochem. Biophys. 2020, 691, 108488. [Google Scholar] [CrossRef]
- Zhang, J.; Wu, J.; Liu, F.; Tong, L.; Chen, Z.; Chen, J.; He, H.; Xu, R.; Ma, Y.; Huang, C. Neuroprotective effects of anthocyanins and its major component cyanidin-3-O-glucoside (C3G) in the central nervous system: An outlined review. Eur. J. Pharmacol. 2019, 858, 172500. [Google Scholar] [CrossRef]
- Baek, H.; Sanjay Park, M.; Lee, H.J. Cyanidin-3-O-glucoside protects the brain and improves cognitive function in APPswe/PS1ΔE9 transgenic mice model. J. Neuroinflammation 2023, 20, 268. [Google Scholar] [CrossRef] [PubMed]
- Song, J.; Zhao, M.; Liu, X.; Zhu, Y.; Hu, X.; Chen, F. Protection of cyanidin-3-glucoside against oxidative stress induced by acrylamide in human MDA-MB-231 cells. Food Chem. Toxicol. 2013, 58, 306–310. [Google Scholar] [CrossRef] [PubMed]
- Verkhratsky, A.; Semyanov, A. Astrocytes in Ageing. Subcell. Biochem. 2023, 103, 253–277. [Google Scholar] [CrossRef]
- Santiago-Balmaseda, A.; Aguirre-Orozco, A.; Valenzuela-Arzeta, I.E.; Villegas-Rojas, M.M.; Pérez-Segura, I.; Jiménez-Barrios, N.; Hurtado-Robles, E.; Rodríguez-Hernández, L.D.; Rivera-German, E.R.; Guerra-Crespo, M.; et al. Neurodegenerative Diseases: Unraveling the Heterogeneity of Astrocytes. Cells 2024, 13, 921. [Google Scholar] [CrossRef]
- Fang, Z.; Luo, Y.; Ma, C.; Dong, L.; Chen, F. Blueberry Anthocyanins Extract Attenuates Acrylamide-Induced Oxidative Stress and Neuroinflammation in Rats. Oxid. Med. Cell Longev. 2022, 2022, 7340881. [Google Scholar] [CrossRef]
- Wang, P.; Ji, R.; Ji, J.; Chen, F. Changes of metabolites of acrylamide and glycidamide in acrylamide-exposed rats pretreated with blueberry anthocyanins extract. Food Chem. 2019, 274, 611–619. [Google Scholar] [CrossRef]
- Sadatomi, D.; Nakashioya, K.; Mamiya, S.; Honda, S.; Kameyama, Y.; Yamamura, Y.; Tanimura, S.; Takeda, K. Mitochondrial function is required for extracellular ATP-induced NLRP3 inflammasome activation. J. Biochem. 2017, 161, 503–512. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Dong, L.; Zhang, L.; Li, D.; Luo, Y.; Chen, F. Acrylamide-induced autophagy-lysosomal pathway dysfunction contributing to neurotoxicity through targeting transcription factor EB. Food Front. 2023, 4, 1324–1336. [Google Scholar] [CrossRef]
- Sun, J.; Li, M.; Zou, F.; Bai, S.; Jiang, X.; Tian, L.; Ou, S.; Jiao, R.; Bai, W. Protection of cyanidin-3-O-glucoside against acrylamide- and glycidamide-induced reproductive toxicity in leydig cells. Food Chem. Toxicol. 2018, 119, 268–274. [Google Scholar] [CrossRef]
- Zhao, M.; Zhang, B.; Deng, L.; Zhao, L. Acrylamide Induces Neurotoxicity in SH-SY5Y Cells via NLRP3-mediated Pyroptosis. Mol. Neurobiol. 2023, 60, 596–609. [Google Scholar] [CrossRef]
- Yan, D.; Yao, J.; Liu, Y.; Zhang, X.; Wang, Y.; Chen, X.; Liu, L.; Shi, N.; Yan, H. Tau hyperphosphorylation and P-CREB reduction are involved in acrylamide-induced spatial memory impairment: Suppression by curcumin. Brain Behav. Immun. 2018, 71, 66–80. [Google Scholar] [CrossRef]
- Dag, Y.; Yildirim, S.; Sengul, E.; Aykurt, F.; Gok, M.; Cinar, A. Therapeutic role of melatonin on acrylamide-induced neurotoxicity via reducing ER stress, inflammation, and apoptosis in a rat model. BMC Pharmacol. Toxicol. 2025, 26, 57. [Google Scholar] [CrossRef] [PubMed]
- Saleh, D.O.; Baraka, S.M.; Jaleel, G.A.A.; Hassan, A.; Ahmed-Farid, O.A. Eugenol alleviates acrylamide-induced rat testicular toxicity by modulating AMPK/p-AKT/mTOR signaling pathway and blood-testis barrier remodeling. Sci. Rep. 2024, 14, 1910. [Google Scholar] [CrossRef]
- Famurewa, A.C.; Elsawy, H.; Sedky, A. Thymoquinone Abrogates Acrylamide-Induced Cerebellar Toxicity via Modulation of Nuclear Factor Erythroid 2-Related Factor 2/Nuclear Factor Kappa B Signaling, Oxidative Neuroinflammation, and Neuroapoptosis in Rats. J. Med. Food 2024, 27, 1062–1069. [Google Scholar] [CrossRef] [PubMed]
- Yan, D.; Pan, X.; Yao, J.; Wang, D.; Wu, X.; Chen, X.; Shi, N.; Yan, H. MAPKs and NF-KB-Mediated Acrylamide-Induced Neuropathy in Rat Striatum and Human Neuroblastoma Cells SY5Y. J. Cell Biochem. 2019, 120, 3898–3910. [Google Scholar] [CrossRef]
- BoespÓug, E.L.; Eliassen, J.C.; Dudley, J.A.; Shidler, M.D.; Kalt, W.; Summer, S.S.; Stein, A.L.; Stover, A.N.; Krikorian, R. Enhanced neural activation with blueberry supplementation in mild cognitive impairment. Nutr. Neurosci. 2018, 21, 297–305. [Google Scholar] [CrossRef]
- Suresh, S.; Vellapandian, C. Cyanidin improves spatial memory and cognition in bisphenol A-induced rat model of Alzheimer’s-like neuropathology by restoring canonical Wnt signaling. Toxicol. Appl. Pharmacol. 2024, 487, 116953. [Google Scholar] [CrossRef]
- Zhang, J.; Shao, X.; Zhao, B.; Zhai, L.; Liu, N.; Gong, F.; Ma, X.; Pan, X.; Zhao, B.; Yuan, Z.; et al. Neurotoxicity of perfluorooctanoic acid and post-exposure recovery due to blueberry anthocyanins in the planarians Dugesia japonica. Environ. Pollut. 2020, 263, 114471. [Google Scholar] [CrossRef]
- Gilani, S.J.; Bin-Jumah, M.N.; Al-Abbasi, F.A.; Imam, S.S.; Alshehri, S.; Ghoneim, M.M.; Shahid Nadeem, M.; Afzal, M.; Alzarea, S.I.; Sayyed, N.; et al. Antiamnesic Potential of Malvidin on Aluminum Chloride Activated by the Free Radical Scavenging Property. ACS Omega 2022, 7, 24231–24240. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.S.; Kim, Y.R.; Song, I.G.; Ha, S.J.; Kim, Y.E.; Baek, N.I.; Hong, E.K. Cyanidin-3-glucoside isolated from mulberry fruit protects pancreatic β-cells against oxidative stress-induced apoptosis. Int. J. Mol. Med. 2015, 35, 405–412. [Google Scholar] [CrossRef] [PubMed]
- Ke, Z.; Liu, Y.; Wang, X.; Fan, Z.; Chen, G.; Xu, M.; Bower, K.A.; Frank, J.A.; Ou, X.; Shi, X.; et al. Cyanidin-3-glucoside ameliorates ethanol neurotoxicity in the developing brain. J. Neurosci. Res. 2011, 89, 1676–1684. [Google Scholar] [CrossRef]
- Zhang, Z.; Yuan, X.; Zhao, Z.; Liu, Y.; Zhou, Y.; Zhu, Z. Cyanidin-3-O-glucoside attenuates LPS-induced endometritis in mice via regulating PPARγ activation. Nat. Prod. Res. 2025, 39, 5136–5140. [Google Scholar] [CrossRef]
- Jia, Y.; Wu, C.; Kim, Y.S.; Yang, S.O.; Kim, Y.; Kim, J.S.; Jeong, M.Y.; Lee, J.H.; Kim, B.; Lee, S.; et al. A dietary anthocyanin cyanidin-3-O-glucoside binds to PPARs to regulate glucose metabolism and insulin sensitivity in mice. Commun. Biol. 2020, 3, 514. [Google Scholar] [CrossRef]
- Lyu, Q.; Deng, H.; Wang, S.; El-Seedi, H.; Cao, H.; Chen, L.; Teng, H. Dietary supplementation with casein/cyanidin-3-O-glucoside nanoparticles alters the gut microbiota in high-fat fed C57BL/6 mice. Food Chem. 2023, 412, 135494. [Google Scholar] [CrossRef]
- Huang, F.; Zhao, R.; Xia, M.; Shen, G.X. Impact of Cyanidin-3-Glucoside on Gut Microbiota and Relationship with Metabolism and Inflammation in High Fat-High Sucrose Diet-Induced Insulin Resistant Mice. Microorganisms 2020, 8, 1238. [Google Scholar] [CrossRef]
- Zhu, L.; Cao, F.; Hu, Z.; Zhou, Y.; Guo, T.; Yan, S.; Xie, Q.; Xia, X.; Yuan, H.; Li, G.; et al. Cyanidin-3-O-Glucoside Alleviates Alcoholic Liver Injury via Modulating Gut Microbiota and Metabolites in Mice. Nutrients 2024, 16, 694. [Google Scholar] [CrossRef]
- Klemmensen, M.M.; Borrowman, S.H.; Pearce, C.; Pyles, B.; Chandra, B. Mitochondrial dysfunction in neurodegenerative disorders. Neurotherapeutics 2024, 21, e00292. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Dong, L.; Yang, L.; Luo, Y.; Chen, F. MiR-27a-5p regulates acrylamide-induced mitochondrial dysfunction and intrinsic apoptosis via targeting Btf3 in rats. Food Chem. 2022, 368, 130816. [Google Scholar] [CrossRef]
- Liu, L.; Li, Y.; Chen, G.; Chen, Q. Crosstalk between mitochondrial biogenesis and mitophagy to maintain mitochondrial homeostasis. J. Biomed. Sci. 2023, 30, 86. [Google Scholar] [CrossRef]
- Scarpulla, R.C. Transcriptional paradigms in mammalian mitochondrial biogenesis and function. Physiol. Rev. 2008, 88, 611–638. [Google Scholar] [CrossRef]
- Fontecha-Barriuso, M.; Martin-Sanchez, D.; Martinez-Moreno, J.M.; Monsalve, M.; Ramos, A.M.; Sanchez-Niño, M.D.; Ruiz-Ortega, M.; Ortiz, A.; Sanz, A.B. The Role of PGC-1α and Mitochondrial Biogenesis in Kidney Diseases. Biomolecules 2020, 10, 347. [Google Scholar] [CrossRef] [PubMed]
- Chan, D.C. Mitochondrial fusion and fission in mammals. Annu. Rev. Cell Dev. Biol. 2006, 22, 79–99. [Google Scholar] [CrossRef]
- Chen, H.; Chomyn, A.; Chan, D.C. Disruption of fusion results in mitochondrial heterogeneity and dysfunction. J Biol. Chem. 2005, 280, 26185–26192. [Google Scholar] [CrossRef]
- Griparic, L.; van der Wel, N.N.; Orozco, I.J.; Peters, P.J.; van der Bliek, A.M. Loss of the intermembrane space protein Mgm1/OPA1 induces swelling and localized constrictions along the lengths of mitochondria. J. Biol. Chem. 2004, 279, 18792–18798. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.J.; Jeong, S.Y.; Karbowski, M.; Smith, C.L.; Youle, R.J. Roles of the mammalian mitochondrial fission and fusion mediators Fis1, Drp1, and Opa1 in apoptosis. Mol. Biol. Cell. 2004, 15, 5001–5011. [Google Scholar] [CrossRef] [PubMed]
- Qiang, Y.; Song, M.; Wang, S.; Liu, Z.; Shan, S.; Sun, Y.; Ni, W.; Chao, S.; Liu, Z.; Zhao, X.; et al. High-fat diet exacerbated motor dysfunction via necroptosis and neuroinflammation in acrylamide-induced neurotoxicity in mice. Ecotoxicol. Environ. Saf. 2024, 269, 115777. [Google Scholar] [CrossRef]
- Grel, H.; Woznica, D.; Ratajczak, K.; Kalwarczyk, E.; Anchimowicz, J.; Switlik, W.; Olejnik, P.; Zielonka, P.; Stobiecka, M.; Jakiela, S. Mitochondrial Dynamics in Neurodegenerative Diseases: Unraveling the Role of Fusion and Fission Processes. Int. J. Mol. Sci. 2023, 24, 13033. [Google Scholar] [CrossRef]
- Hou, Y.; Wang, Q.; Han, B.; Chen, Y.; Qiao, X.; Wang, L. CD36 promotes NLRP3 inflammasome activation via the mtROS pathway in renal tubular epithelial cells of diabetic kidneys. Cell Death Dis. 2021, 12, 523. [Google Scholar] [CrossRef]
- Zhao, M.; Deng, L.; Lu, X.; Fan, L.; Zhu, Y.; Zhao, L. The involvement of oxidative stress, neuronal lesions, neurotransmission impairment, and neuroinflammation in acrylamide-induced neurotoxicity in C57/BL6 mice. Environ. Sci. Pollut. Res. Int. 2022, 29, 41151–41167. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, L.; Zhang, L.; Dong, L.; Ma, Y.; Zhao, L.; Xu, R.; Chen, F.; Luo, Y. Protective Effects of Cyanidin-3-O-Glucoside Against Neurotoxin Acrylamide Through Alleviating Mitochondrial Dysfunction. Foods 2025, 14, 3826. https://doi.org/10.3390/foods14223826
Yang L, Zhang L, Dong L, Ma Y, Zhao L, Xu R, Chen F, Luo Y. Protective Effects of Cyanidin-3-O-Glucoside Against Neurotoxin Acrylamide Through Alleviating Mitochondrial Dysfunction. Foods. 2025; 14(22):3826. https://doi.org/10.3390/foods14223826
Chicago/Turabian StyleYang, Liuqing, Lujia Zhang, Li Dong, Yanli Ma, Lei Zhao, Ruoyang Xu, Fang Chen, and Yinghua Luo. 2025. "Protective Effects of Cyanidin-3-O-Glucoside Against Neurotoxin Acrylamide Through Alleviating Mitochondrial Dysfunction" Foods 14, no. 22: 3826. https://doi.org/10.3390/foods14223826
APA StyleYang, L., Zhang, L., Dong, L., Ma, Y., Zhao, L., Xu, R., Chen, F., & Luo, Y. (2025). Protective Effects of Cyanidin-3-O-Glucoside Against Neurotoxin Acrylamide Through Alleviating Mitochondrial Dysfunction. Foods, 14(22), 3826. https://doi.org/10.3390/foods14223826







