Purification and Characterization of Anti-Inflammatory Peptide Fractions from Enzymatic Hydrolysate of Abalone Viscera
Abstract
1. Introduction
2. Method
2.1. Collection and Preparation of Abalone Viscera
2.2. Enzymatic Hydrolysis
2.3. Compositional Analyses
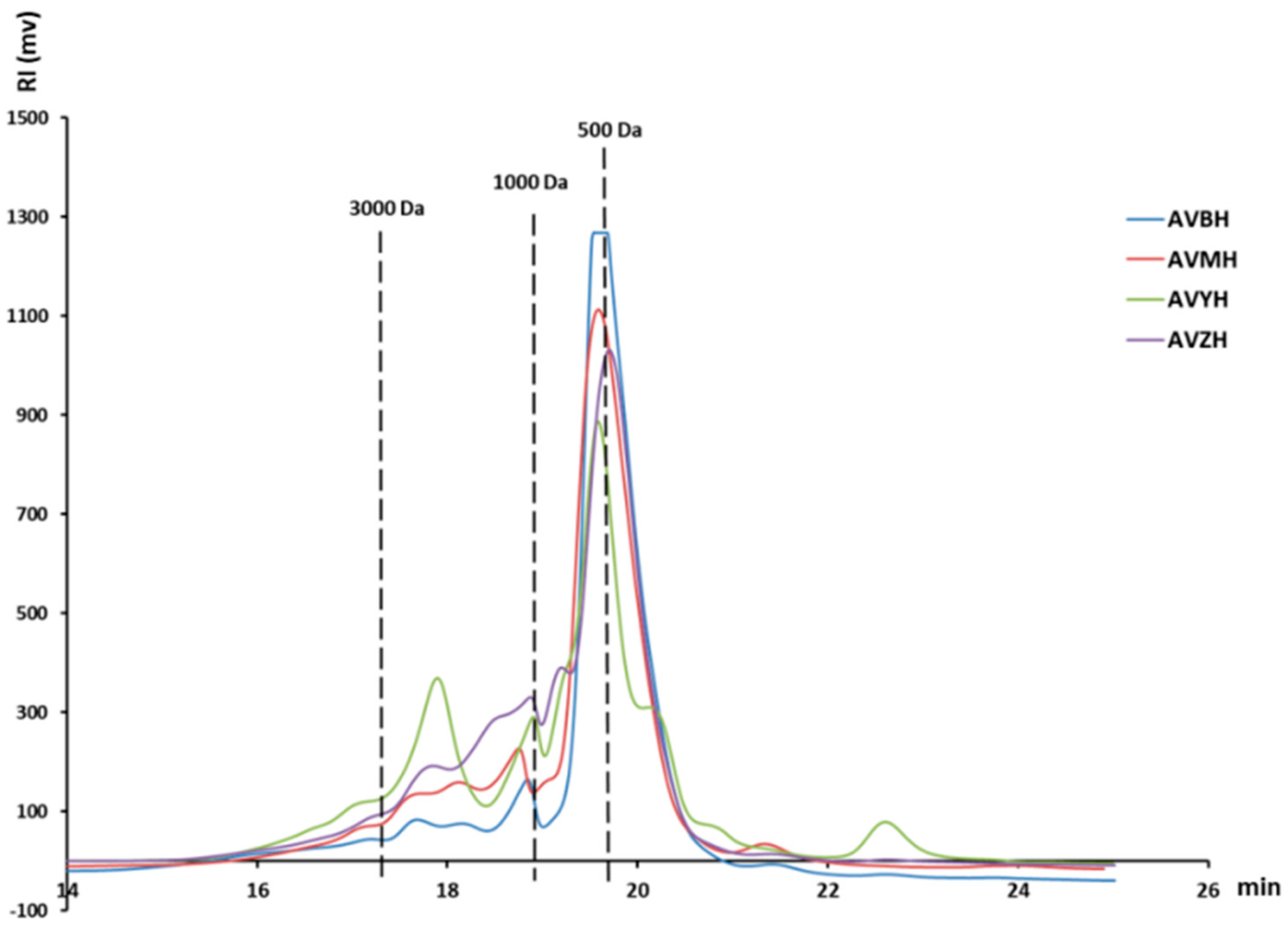
2.4. Molecular-Weight Distribution
2.5. Antioxidant and Anti-Inflammatory Assays
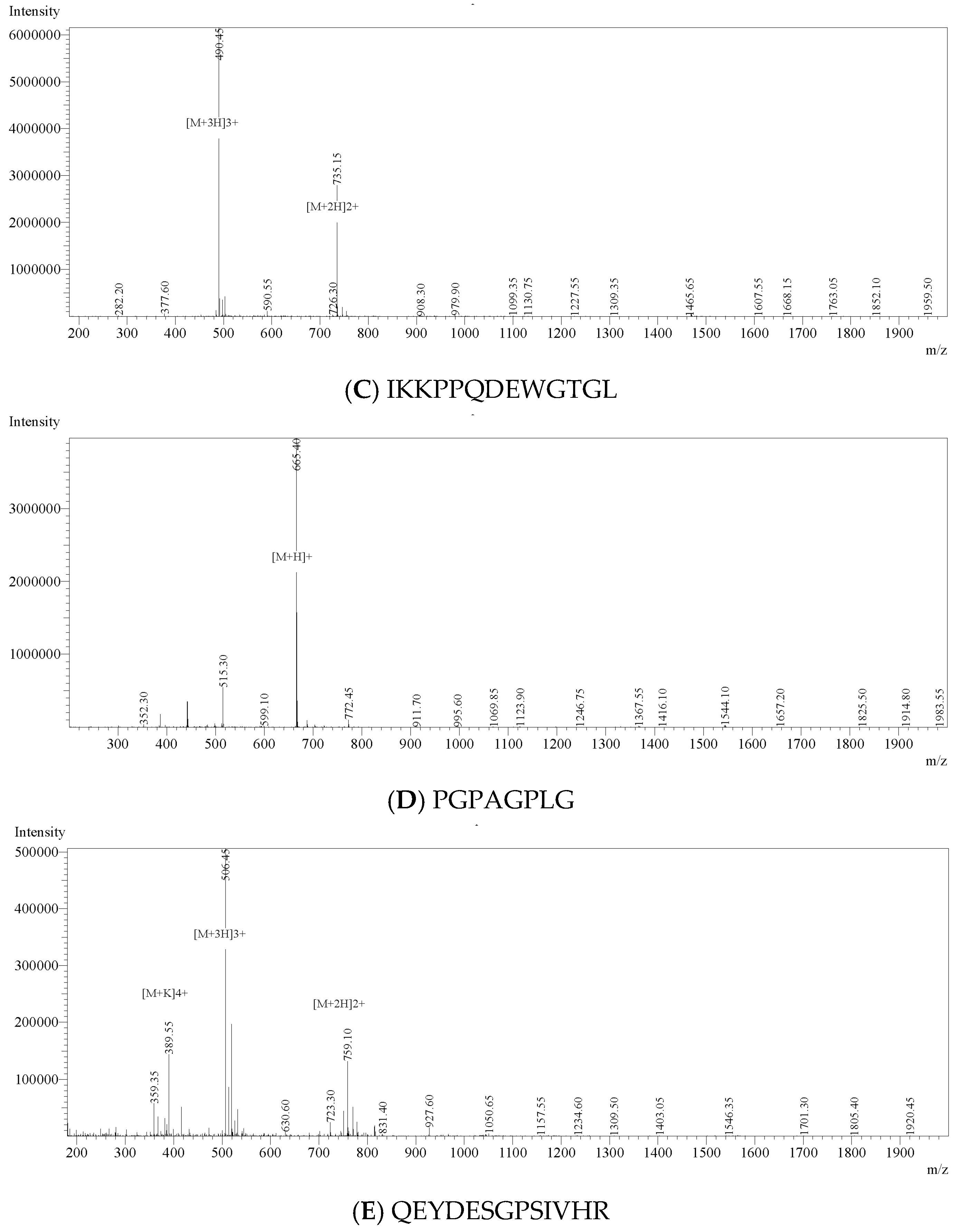
2.6. LC-MS/MS Profiling and Peptide Identification
2.7. Bioinformatic Analysis and Peptide Selection
2.8. Solid-Phase Synthesis of Anti-Inflammatory Peptides
2.9. Cell Culture and Treatment
2.10. Western Blotting
2.11. Quantitative Real-Time PCR (qPCR)
2.12. Statistical Analysis
3. Results
3.1. Compositional Characterisation of Abalone Visceral Hydrolysates
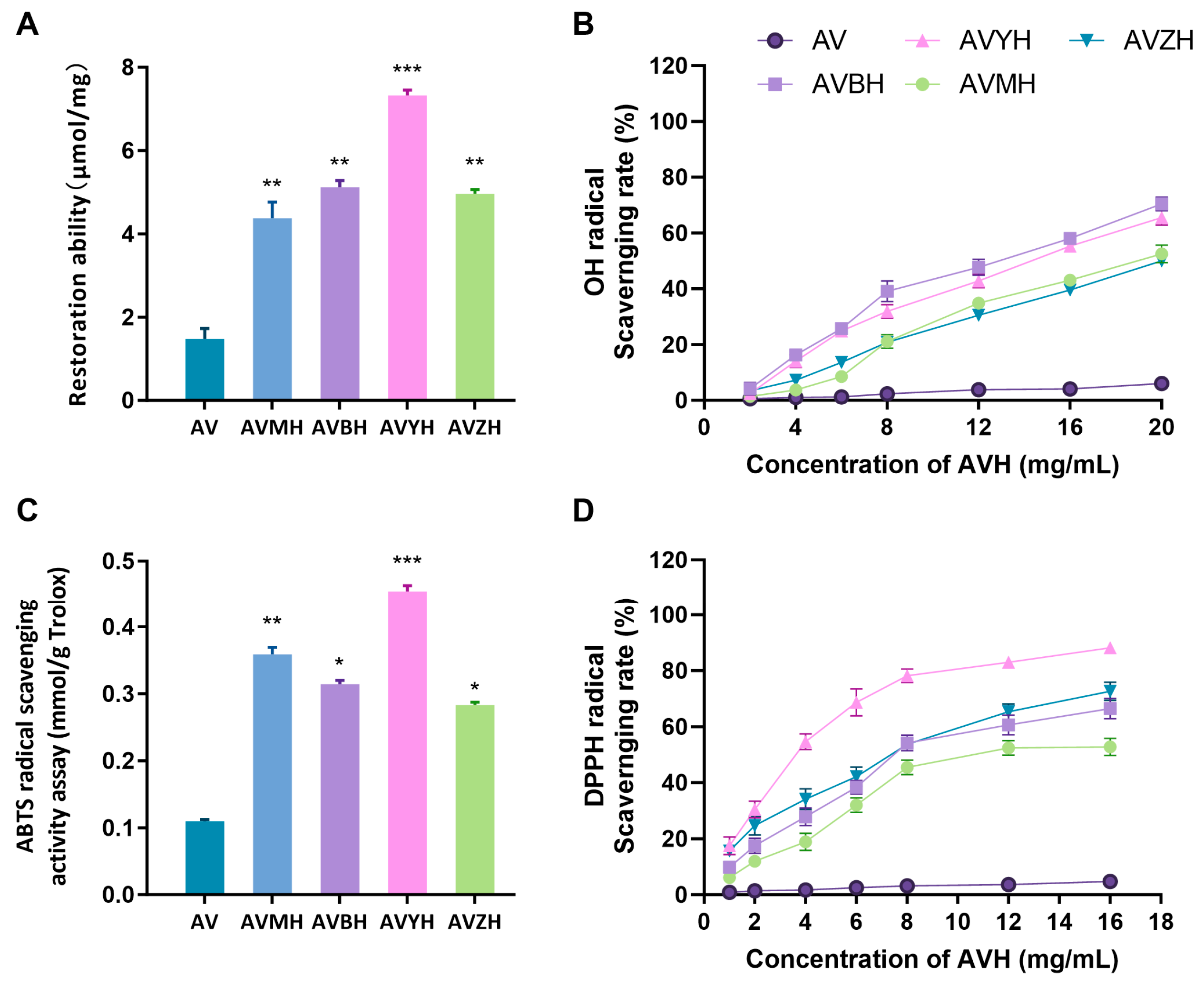
3.2. Antioxidant Capacity of the Enzymatic Hydrolysates
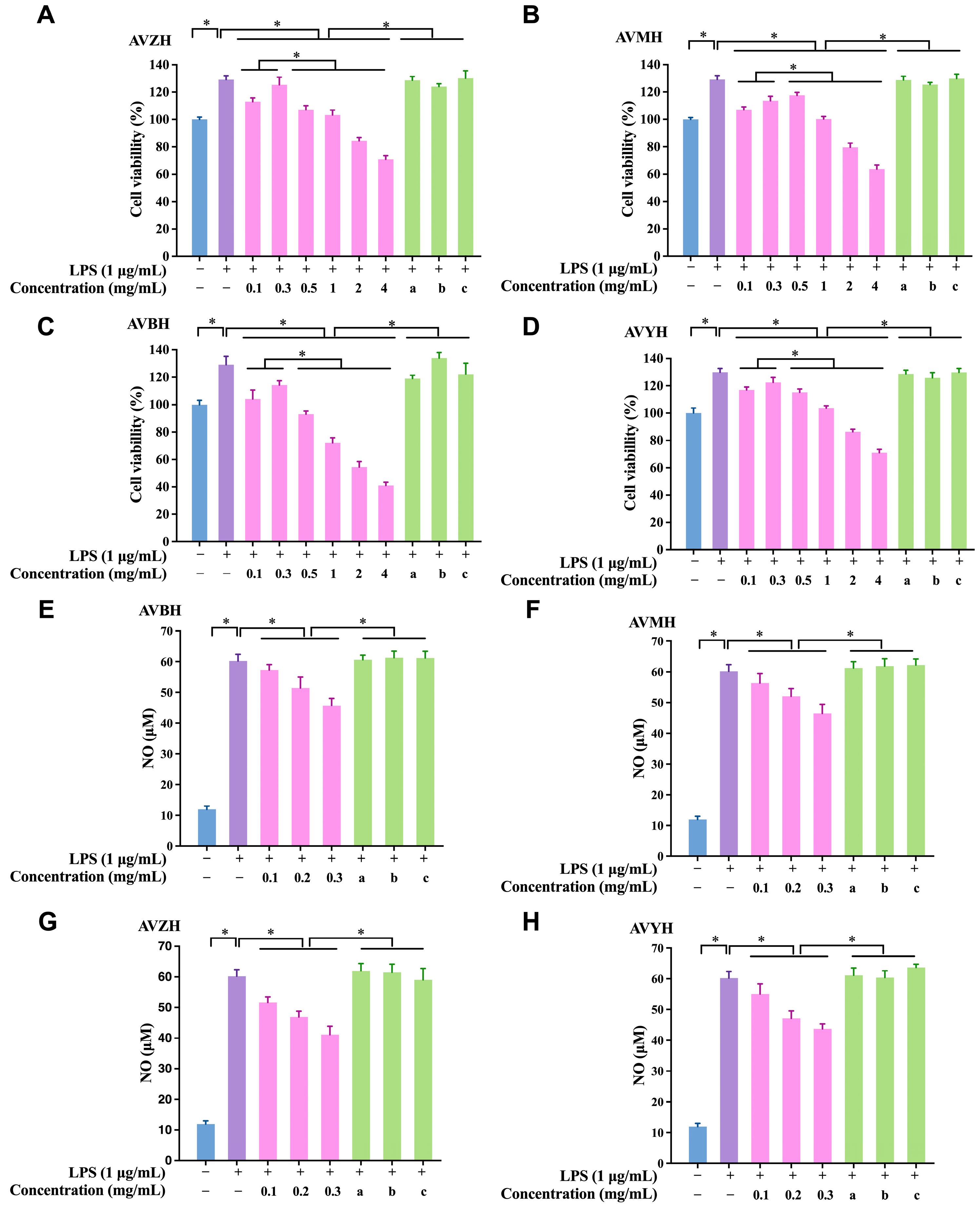
3.3. Effects of Hydrolysates on RAW264.7 Macrophage Viability and NO Production
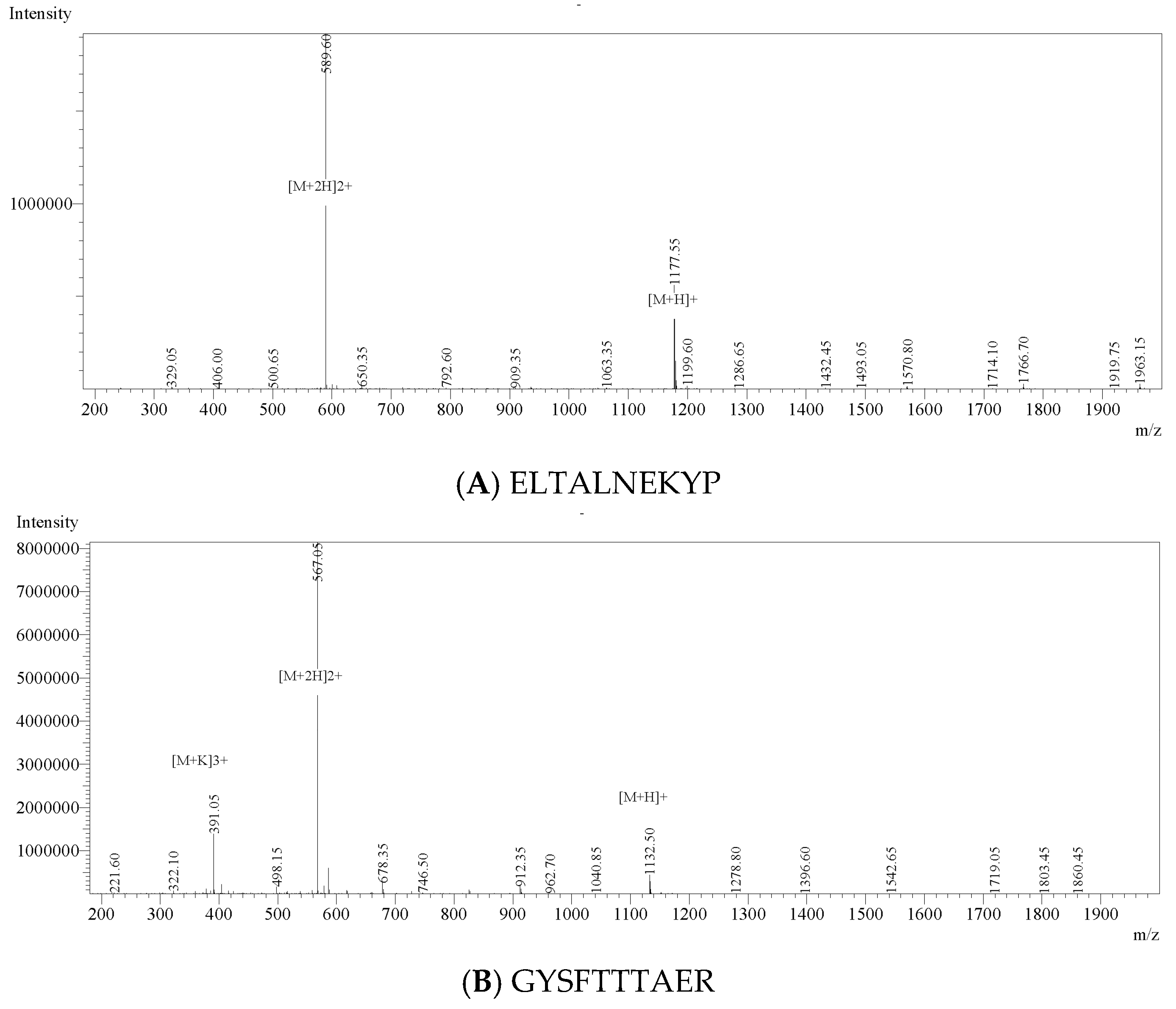
3.4. Identification, In Silico Screening and Chemical Validation of Anti-Inflammatory Peptides from AVZH
3.5. Impact of Synthetic Anti-Inflammatory Peptides on Cell Viability and NO Secretion
3.6. Effects of Anti-Inflammatory Peptides on Cytokine Secretion and Transcriptional Expression in RAW264.7 Cells
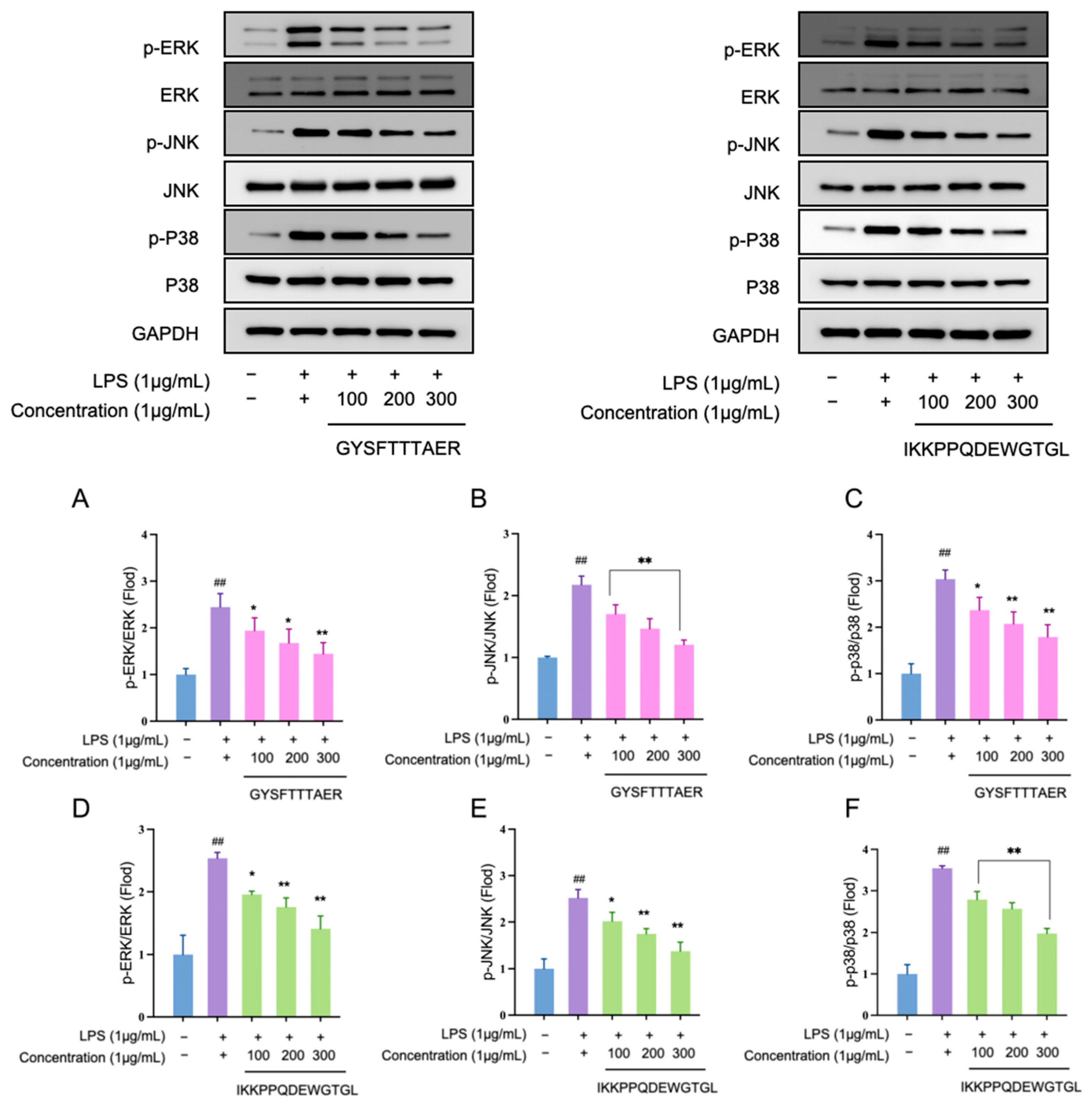
3.7. Anti-Inflammatory Peptides Suppress MAPK Signaling Cascades in RAW264.7 Cells
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| Abbreviation | Full Form |
| ACE | Angiotensin-I-converting Enzyme |
| ANOVA | Analysis of Variance |
| AV | Abalone Viscera |
| AVBH | Bromelain Hydrolysate of Abalone Viscera |
| AVMH | Papain Hydrolysate of Abalone Viscera |
| AVYH | Trypsin Hydrolysate of Abalone Viscera |
| AVZH | Neutral-protease Hydrolysate of Abalone Viscera |
| DPPH | 2,2-Diphenyl-1-picrylhydrazyl |
| ERK | Extracellular Signal-Regulated Kinase |
| ESI-MS | Electrospray Ionization Mass Spectrometry |
| FBS | Fetal Bovine Serum |
| GAPDH | Glyceraldehyde-3-Phosphate Dehydrogenase |
| HP-GPC | High-Performance Gel Permeation Chromatography |
| IL-1β | Interleukin-1 Beta |
| IL-6 | Interleukin-6 |
| JNK | C-Jun N-terminal Kinase |
| LC-MS/MS | Liquid Chromatography with Tandem Mass Spectrometry |
| LPS | Lipopolysaccharide |
| MAPK | Mitogen-activated Protein Kinase |
| NO | Nitric Oxide |
| p-ERK | Phosphorylated Extracellular Signal-Regulated Kinase |
| p-JNK | Phosphorylated C-Jun N-terminal Kinase |
| p-p38 | Phosphorylated p38 |
| p38 | p38 Mitogen-Activated Protein Kinase |
| RP-HPLC | Reversed-Phase High-Performance Liquid Chromatography |
| TNF-α | Tumour Necrosis Factor-alpha |
References
- Zhang, H.; Dhalla, N.S. The Role of Pro-Inflammatory Cytokines in the Pathogenesis of Cardiovascular Disease. Int. J. Mol. Sci. 2024, 25, 1082. [Google Scholar] [CrossRef]
- Thiruchenthooran, V.; Sánchez-López, E.; Gliszczyńska, A. Perspectives of the Application of Non-Steroidal Anti-Inflammatory Drugs in Cancer Therapy: Attempts to Overcome Their Unfavorable Side Effects. Cancers 2023, 15, 475. [Google Scholar] [CrossRef]
- Chen, P.; Ye, T.; Li, C.; Praveen, P.; Hu, Z.; Li, W.; Shang, C. Embracing the era of antimicrobial peptides with marine organisms. Nat. Prod. Rep. 2024, 41, 331–346. [Google Scholar] [CrossRef]
- Cheung, R.C.; Ng, T.B.; Wong, J.H. Marine Peptides: Bioactivities and Applications. Mar. Drugs 2015, 13, 4006–4043. [Google Scholar] [CrossRef]
- Bauer, J.; Segovia-Rendón, J.; Lorda, J.; Abadía-Cardoso, A.; Malpica-Cruz, L.; Alvarado-Graef, P.; Searcy-Bernal, R.; Vázquez-Vera, L.; Beas-Luna, R. Short-term effects of community-based marine reserves on green abalone, as revealed by population studies. Sci. Rep. 2024, 14, 955. [Google Scholar] [CrossRef]
- Tian, M.; Li, C.; Liu, L.; Xiang, F.; Li, W.; Li, C.; Liu, B.; Fang, T. Multistage Countercurrent Extraction of Abalone Viscera Oil and Its Hypolipidemic Action on High-Fat Diet-Induced Hyperlipidemia Mice. Nutrients 2025, 17, 3062. [Google Scholar] [CrossRef]
- Li, P.; Zhang, M.; Xie, D.; Zhang, X.; Zhang, S.; Gao, F.; Wang, Y.; Hsiao, C.D.; Li, X.; Liu, K. Characterization and bioactivities of phospholipids from squid viscera and gonads using ultra-performance liquid chromatography-Q-exactive orbitrap/mass spectrometry-based lipidomics and zebrafish models. Food Funct. 2021, 12, 7986–7996. [Google Scholar] [CrossRef] [PubMed]
- Rivera-Perez, C.; Ponce Gonzalez, X.P.; Hernandez-Savedra, N.Y. Antimicrobial and anticarcinogenic activity of bioactive peptides derived from abalone viscera (Haliotis fulgens and Haliotis corrugata). Sci. Rep. 2023, 13, 15185. [Google Scholar] [CrossRef]
- Liu, B.; Liu, L.; Li, C.; Guo, T.; Li, C.; Tian, M.; Fang, T. The Alleviating Effect of Abalone Viscera Collagen Peptide in DSS-Induced Colitis Mice: Effect on Inflammatory Cytokines, Oxidative Stress, and Gut Microbiota. Nutrients 2025, 17, 1926. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Chen, B.; Wang, S.; Xiao, Z.; Guo, H.; Wang, Z.; Qi, X.; Dong, S.; Zhao, Z. Maillard reaction products from Tilapia (Oreochromis mossambicus) scale collagen peptides conjugated with Galactooligosaccharides of different purities: Characterization, antioxidant activity, and impact on the growth of probiotics. Food Chem. X 2025, 29, 102733. [Google Scholar] [CrossRef] [PubMed]
- Xiang, Z.; Xue, Q.; Gao, P.; Yu, H.; Wu, M.; Zhao, Z.; Li, Y.; Wang, S.; Zhang, J.; Dai, L. Antioxidant peptides from edible aquatic animals: Preparation method, mechanism of action, and structure-activity relationships. Food Chem. 2023, 404, 134701. [Google Scholar] [CrossRef]
- Wang, Y.; Song, Y.; Chang, Y.; Liu, Y.; Chen, G.; Xue, C. Dynamic changes of peptidome and release of polysaccharide in sea cucumber (Apostichopus japonicus) hydrolysates depending on enzymatic hydrolysis approaches. Food Sci. Hum. Wellness 2022, 11, 1331–1341. [Google Scholar] [CrossRef]
- Minkiewicz, P.; Iwaniak, A.; Darewicz, M. BIOPEP-UWM Database of Bioactive Peptides: Current Opportunities. Int. J. Mol. Sci. 2019, 20, 5978. [Google Scholar] [CrossRef]
- Li, Q.; Zhang, C.; Chen, H.; Xue, J.; Guo, X.; Liang, M.; Chen, M. BioPepDB: An integrated data platform for food-derived bioactive peptides. Int. J. Food Sci. Nutr. 2018, 69, 963–968. [Google Scholar] [CrossRef] [PubMed]
- Zamyatnin, A.A.; Borchikov, A.S.; Vladimirov, M.G.; Voronina, O.L. The EROP-Moscow oligopeptide database. Nucleic Acids Res. 2006, 34, D261–D266. [Google Scholar] [CrossRef] [PubMed]
- Mooney, C.; Haslam, N.J.; Pollastri, G.; Shields, D.C. Towards the improved discovery and design of functional peptides: Common features of diverse classes permit generalized prediction of bioactivity. PLoS ONE 2012, 7, e45012. [Google Scholar] [CrossRef] [PubMed]
- Manavalan, B.; Shin, T.H.; Kim, M.O.; Lee, G. AIPpred: Sequence-Based Prediction of Anti-inflammatory Peptides Using Random Forest. Front. Pharmacol. 2018, 9, 276. [Google Scholar] [CrossRef]
- Khatun, M.S.; Hasan, M.M.; Kurata, H. PreAIP: Computational Prediction of Anti-inflammatory Peptides by Integrating Multiple Complementary Features. Front. Genet. 2019, 10, 129. [Google Scholar] [CrossRef]
- Shin, M.K.; Hwang, I.W.; Kim, Y.; Kim, S.T.; Jang, W.; Lee, S.; Bang, W.Y.; Bae, C.H.; Sung, J.S. Antibacterial and Anti-Inflammatory Effects of Novel Peptide Toxin from the Spider Pardosa astrigera. Antibiotics 2020, 9, 422. [Google Scholar] [CrossRef]
- Jia, J.; Liu, Q.; Liu, H.; Yang, C.; Zhao, Q.; Xu, Y.; Wu, W. Structure characterization and antioxidant activity of abalone visceral peptides-selenium in vitro. Food Chem. 2024, 433, 137398. [Google Scholar] [CrossRef]
- Suleria, H.A.; Masci, P.P.; Gobe, G.C.; Osborne, S.A. Therapeutic potential of abalone and status of bioactive molecules: A comprehensive review. Crit. Rev. Food Sci. Nutr. 2017, 57, 1742–1748. [Google Scholar] [CrossRef]
- Zhou, D.Y.; Zhu, B.; Qiao, L.; Wu, H.; Li, D.M.; Yang, J.F.; Murata, Y. In vitro antioxidant activity of enzymatic hydrolysates prepared from abalone (Haliotis discus hannai Ino) viscera. Food Bioprod. Process. 2012, 90, 148–154. [Google Scholar] [CrossRef]
- Hu, X.; Yang, Y.; Chang, C.; Li, J.; Su, Y.; Gu, L. The targeted development of collagen-active peptides based on composite enzyme hydrolysis: A study on the structure-activity relationship. Food Funct. 2024, 15, 401–410. [Google Scholar] [CrossRef] [PubMed]
- Gharehbeglou, P.; Sarabandi, K.; Akbarbaglu, Z. Insights into enzymatic hydrolysis: Exploring effects on antioxidant and functional properties of bioactive peptides from Chlorella proteins. J. Agric. Food Res. 2024, 16, 101129. [Google Scholar] [CrossRef]
- Rios-Herrera, G.D.; Salazar-Leyva, J.A.; Hernández, C.; Jiménez-Gutiérrez, L.R.; Sandoval-Gallardo, J.M.; Osuna-Ruiz, I.; Martínez-Montaño, E.; Pacheco-Aguilar, R.; Lugo-Sánchez, M.E.; Ramirez-Perez, J.S. Production of Protein Hydrolysates Using Marine Catfish Bagre panamensis Muscle or Casein as Substrates: Effect of Enzymatic Source and Degree of Hydrolysis on Antioxidant and Biochemical Properties. Appl. Biochem. Biotechnol. 2021, 193, 3214–3231. [Google Scholar] [CrossRef]
- Sánchez-Velázquez, O.A.; Cuevas-Rodríguez, E.O.; Mondor, M.; Ribéreau, S.; Arcand, Y.; Mackie, A.; Hernández-Álvarez, A.J. Impact of in vitro gastrointestinal digestion on peptide profile and bioactivity of cooked and non-cooked oat protein concentrates. Curr. Res. Food Sci. 2021, 4, 93–104. [Google Scholar] [CrossRef]
- Cheng, J.A.; Wang, D.; Yu, G.; Chen, S.; Ma, Z.; Wei, Y.; Zhao, X.; Li, C.; Wang, Y.; Zhang, Y.; et al. Bioactive Peptides Derived from Tuna: Screening, Extraction, Bioactivity, and Mechanism of Action. Mar. Drugs 2025, 23, 293. [Google Scholar] [CrossRef]
- Leiva-Portilla, D.; Martínez, R.; Bernal, C. Valorization of shrimp (Heterocarpus reedi) processing waste via enzymatic hydrolysis: Protein extractions, hydrolysates and antioxidant peptide fractions. Biocatal. Agric. Biotechnol. 2023, 48, 102625. [Google Scholar] [CrossRef]
- Chen, J.; Bai, W.; Cai, D.; Yu, Z.; Xu, B. Characterization and identification of novel anti-inflammatory peptides from Baijiao sea bass (Lateolabrax maculatus). LWT 2021, 147, 111521. [Google Scholar] [CrossRef]
- Shirazi, A.N.; El-Sayed, N.S.; Tiwari, R.K.; Tavakoli, K.; Parang, K. Cyclic Peptide Containing Hydrophobic and Positively Charged Residues as a Drug Delivery System for Curcumin. Curr. Drug Deliv. 2016, 13, 409–417. [Google Scholar] [CrossRef]
- Desantis, F.; Miotto, M.; Di Rienzo, L.; Milanetti, E.; Ruocco, G. Spatial organization of hydrophobic and charged residues affects protein thermal stability and binding affinity. Sci. Rep. 2022, 12, 12087. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.; Sharma, A.K.; Shastri, V.; Madhu, M.K.; Sharma, V.K. Prediction of anti-inflammatory proteins/peptides: An in silico approach. J. Transl. Med. 2017, 15, 7. [Google Scholar] [CrossRef] [PubMed]
- Gao, R.; Shu, W.; Shen, Y.; Sun, Q.; Bai, F.; Wang, J.; Li, D.; Li, Y.; Jin, W.; Yuan, L. Sturgeon protein-derived peptides exert anti-inflammatory effects in LPS-stimulated RAW264.7 macrophages via the MAPK pathway. J. Funct. Foods 2020, 72, 104044. [Google Scholar] [CrossRef]
- Zhang, X.; Yang, J.; Bian, Z.; Shi, D.; Cao, Z. Long noncoding RNA DANCR promotes nasopharyngeal carcinoma progression by interacting with STAT3, enhancing IL-6/JAK1/STAT3 signaling. Biomed. Pharmacother. 2019, 113, 108713. [Google Scholar] [CrossRef]
- Guha, S.; Majumder, K. Structural-features of food-derived bioactive peptides with anti-inflammatory activity: A brief review. J. Food Biochem. 2019, 43, e12531. [Google Scholar] [CrossRef]
- Chi, C.F.; Wang, B. Marine Bioactive Peptides-Structure, Function and Application. Mar. Drugs 2023, 21, 275. [Google Scholar] [CrossRef]


| Primer Name | Primer Sequence |
|---|---|
| GAPDH (mouse) F | ACCCTTAAGAGGGATGCTGC |
| GAPDH (mouse) R | CCCAATACGGCCAAATCCGT |
| TNF-α (mouse) F | TAGCCCACGTCGTAGCAAAC |
| TNF-α (mouse) R | ACAAGGTACAACCCATCGGC |
| IL-1β (mouse) F | TGCCACCTTTTGACAGTGATG |
| IL-1β (mouse) R | TGATGTGCTGCTGCGAGATT |
| IL-6 (mouse) F | CTCATTCTGCTCTGGAGCCC |
| IL-6 (mouse) R | CAACTGGATGGAAGTCTCTTGC |
| Sample | Components % | |||
|---|---|---|---|---|
| Total Sugars | Total Protein | Moisture Content | Others | |
| AVME | 21.82 ± 1.46 | 49.41 ± 1.12 | 9.14 ± 1.61 | 19.63 ± 2.55 |
| AVBE | 20.33 ± 3.09 | 48.43 ± 1.57 | 7.78 ± 0.29 | 23.46 ± 2.86 |
| AVZE | 19.13 ± 1.82 | 50.98 ± 2.86 | 8.7 ± 1.14 | 21.19 ± 2.73 |
| AVYE | 19.84 ± 1.51 | 50.69 ± 1.18 | 11.56 ± 0.13 | 17.91 ± 0.52 |
| Abalone Viscera powder | 18.54 ± 2.96 | 45.09 ± 2.48 | 7.99 ± 0.98 | 26.38 ± 0.49 |
| Accession | Sequence | Length | Mass/Da | Intensity | Theoretical Iso-Electric Point | Net Charge at pH 7 | Estimated Solubility |
|---|---|---|---|---|---|---|---|
| AAQ92368.1 | QEYDESGPSIVHR | 13 | 1515.70 | 44,101,000 | 4.65 | −1.9 | G |
| AAQ92368.1 | GYSFTTTAER | 10 | 1131.52 | 23,298,000 | 6 | 0 | G |
| AYD59980.1 | PTIIFEPGIDTHVLD | 15 | 1665.86 | 20,254,000 | 4.02 | −2.9 | P |
| QOD61783.1 | ELTALNEKYP | 10 | 1176.60 | 20,189,000 | 4.53 | −1 | G |
| AAQ92368.1 | SYELPDGQVITIGNER | 16 | 1789.88 | 18,912,000 | 4.14 | −2 | G |
| ADK60915.1 | IKKPPQDEWGTGL | 13 | 1467.77 | 12,707,000 | 6.07 | 0 | G |
| AAQ92368.1 | QEYDESGPSIVHR | 13 | 1515.70 | 12,408,000 | 4.65 | −1.9 | G |
| APB93432.1 | KHTLPDLPYDY | 11 | 1360.67 | 11,330,000 | 5.21 | −0.9 | G |
| ADK60915.1 | LKRVGPGLGEYQ | 12 | 1315.72 | 10,870,000 | 8.59 | 1 | G |
| AAQ92368.1 | DLYANTVLSGGTTMYPGIADR | 21 | 2230.06 | 8,836,800 | 4.21 | −1 | P |
| AYD59980.1 | NHGSQIGIPY | 10 | 1084.53 | 8,414,300 | 6.74 | 0.1 | P |
| ADK60915.1 | VGPGLGEYQFDHETLS | 16 | 1747.81 | 3,786,000 | 4.13 | −2.9 | G |
| AYL88760.1 | PGPAGPLG | 8 | 664.35 | 3,689,900 | 5.96 | 0 | P |
| AYD59980.1 | PTIIFEPGIDTHV | 13 | 1437.75 | 2,781,800 | 4.35 | −1.9 | P |
| QOD61838.1 | ITNNQVQVITQAP | 13 | 1428.70 | 2,387,900 | 5.52 | 0 | P |
| AAQ92368.1 | DLTDYLMK | 8 | 997.48 | 1,485,200 | 4.21 | −1 | G |
| UJF23575.1 | QAGGLMNLFTNN | 12 | 1295.58 | 1,485,200 | 5.52 | 0 | P |
| GDY27137.1 | NNGKVIVEVGQP | 12 | 1254.65 | 1,485,200 | 6 | 0 | G |
| Peptide Sequence | Peak # | Ret. Time (min) | Area % |
|---|---|---|---|
| QEYDESGPSIVHR | 2 | 10.295 | 95.076 |
| GYSFTTTAER | 2 | 12.295 | 98.192 |
| ELTALNEKYP | 4 | 11.231 | 98.439 |
| IKKPPQDEWGTGL | 3 | 9.770 | 95.282 |
| PGPAGPLG | 1 | 9.436 | 99.119 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, N.; Yang, Z.; Wu, C.; Chen, Y.; Chan, Z.; Zeng, R. Purification and Characterization of Anti-Inflammatory Peptide Fractions from Enzymatic Hydrolysate of Abalone Viscera. Foods 2025, 14, 3811. https://doi.org/10.3390/foods14223811
Wu N, Yang Z, Wu C, Chen Y, Chan Z, Zeng R. Purification and Characterization of Anti-Inflammatory Peptide Fractions from Enzymatic Hydrolysate of Abalone Viscera. Foods. 2025; 14(22):3811. https://doi.org/10.3390/foods14223811
Chicago/Turabian StyleWu, Nan, Ziyi Yang, Chaocheng Wu, Yuan Chen, Zhuhua Chan, and Runying Zeng. 2025. "Purification and Characterization of Anti-Inflammatory Peptide Fractions from Enzymatic Hydrolysate of Abalone Viscera" Foods 14, no. 22: 3811. https://doi.org/10.3390/foods14223811
APA StyleWu, N., Yang, Z., Wu, C., Chen, Y., Chan, Z., & Zeng, R. (2025). Purification and Characterization of Anti-Inflammatory Peptide Fractions from Enzymatic Hydrolysate of Abalone Viscera. Foods, 14(22), 3811. https://doi.org/10.3390/foods14223811






