Impact of Hydrogel-to-Oleogel Ratio and Presence of Carob Fruit Extracts on Formulated Bigels: Rheological, Thermal, Physicochemical and Microstructural Properties
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Bigels (BGs)
2.3. Rheological Characterization
2.3.1. Oscillatory Measurements
2.3.2. Steady-State Measurements
2.4. Thermal Behavior
2.5. Color Measurement, Physical Stability and Total Loss
2.6. Microscopy
2.7. Statistical Analysis
3. Results and Discussion
3.1. Effects of HG:OG Ratio and I-CFE and P-CFE Extracts on Rheological Properties of Bigels
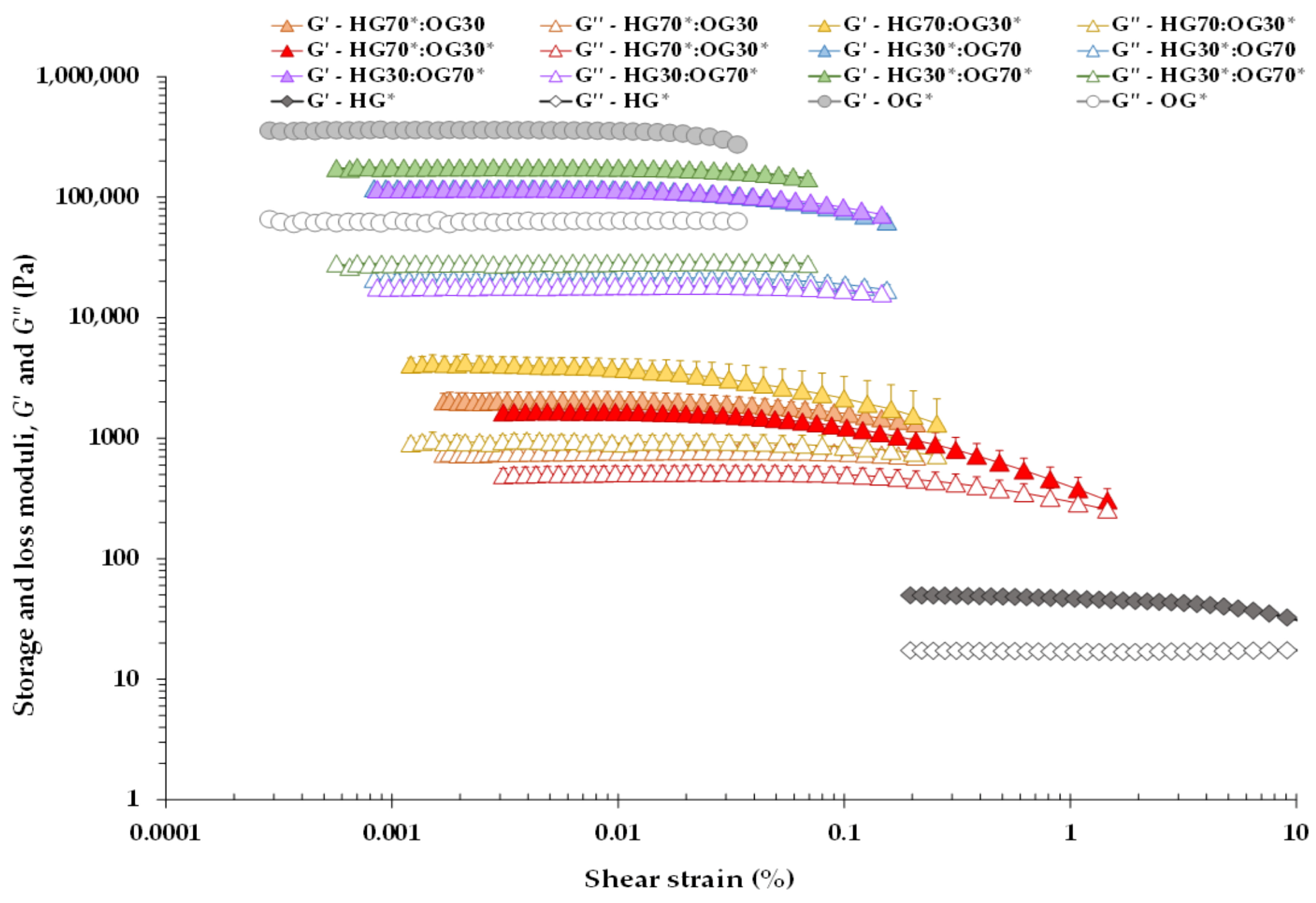
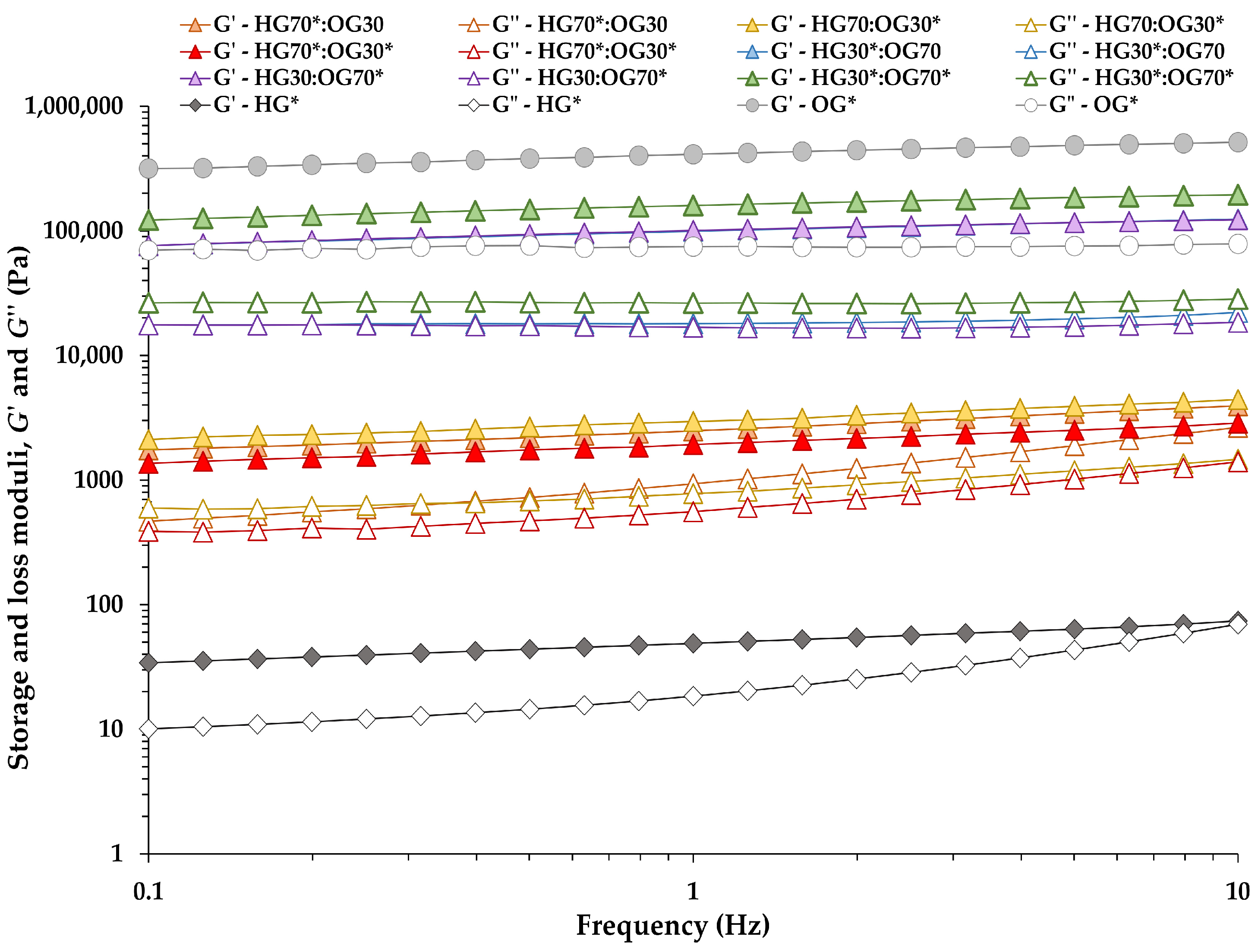
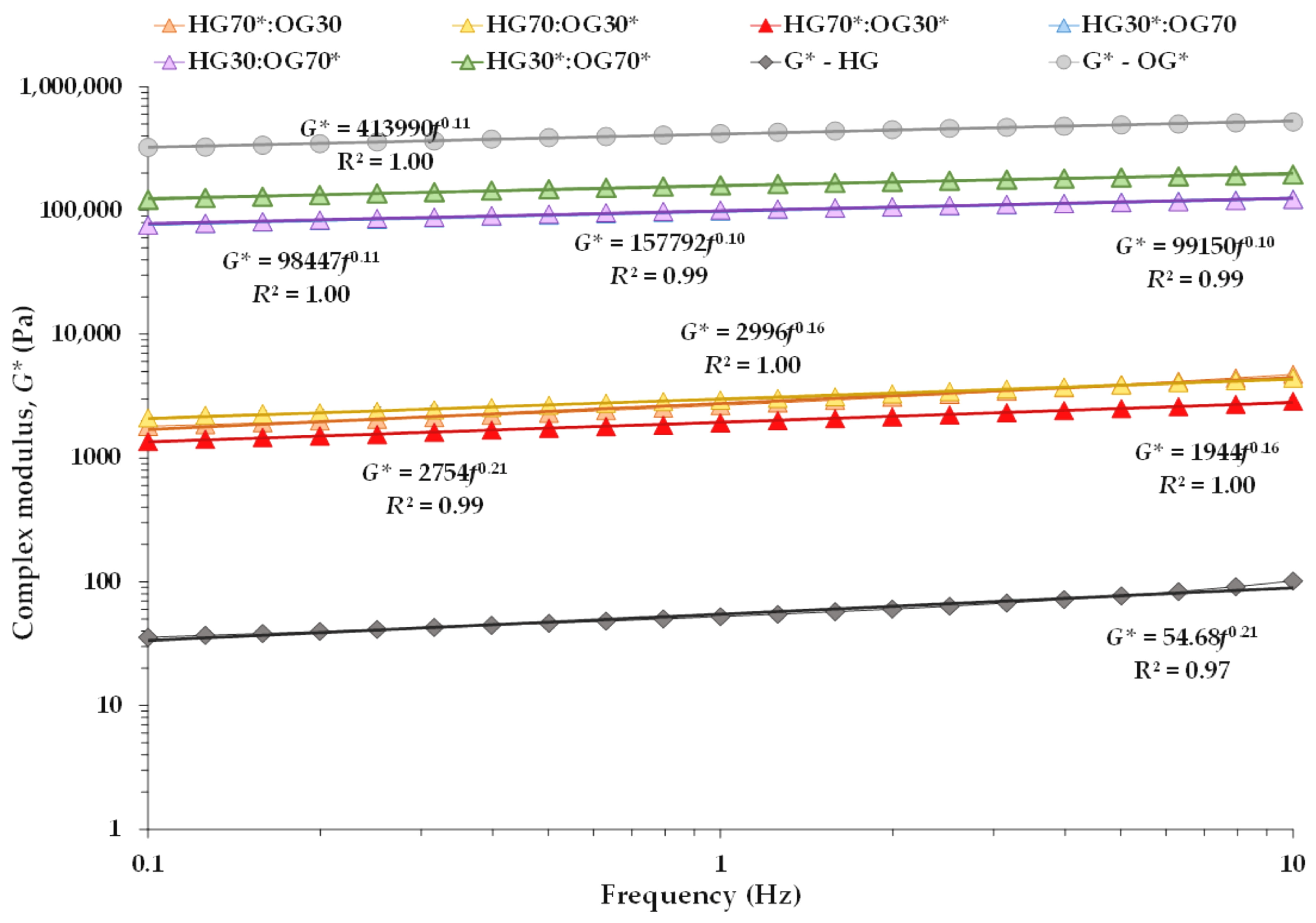
3.1.1. Oscillatory Measurements
3.1.2. Steady Shear Viscosities
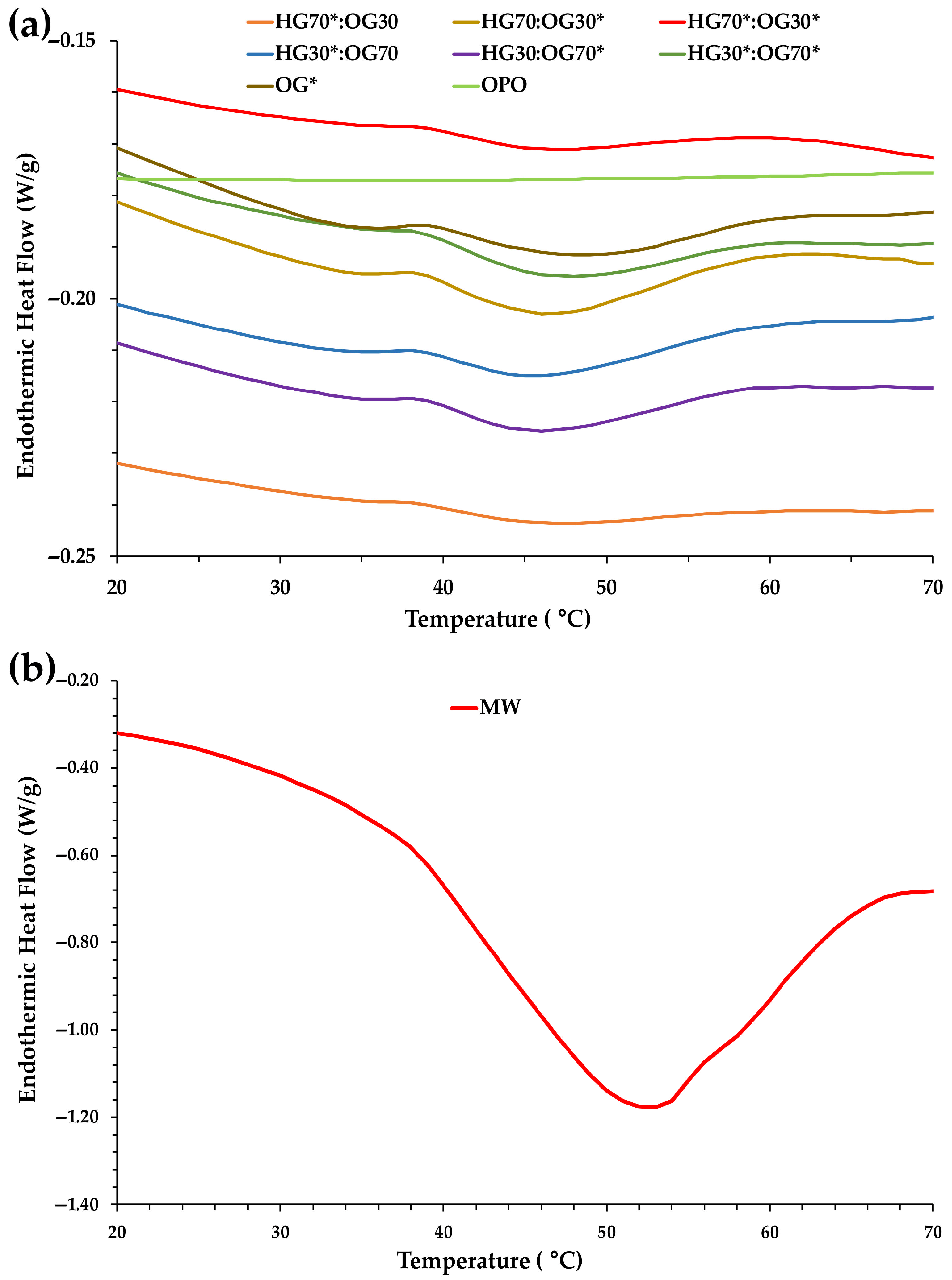
3.2. Effects of HG:OG Ratio and I-CFE and P-CFE Extracts on Thermal Properties of Bigels
3.3. Effects of HG:OG Ratio and I-CFE and P-CFE Extracts on Physicochemical Parameters of Bigels
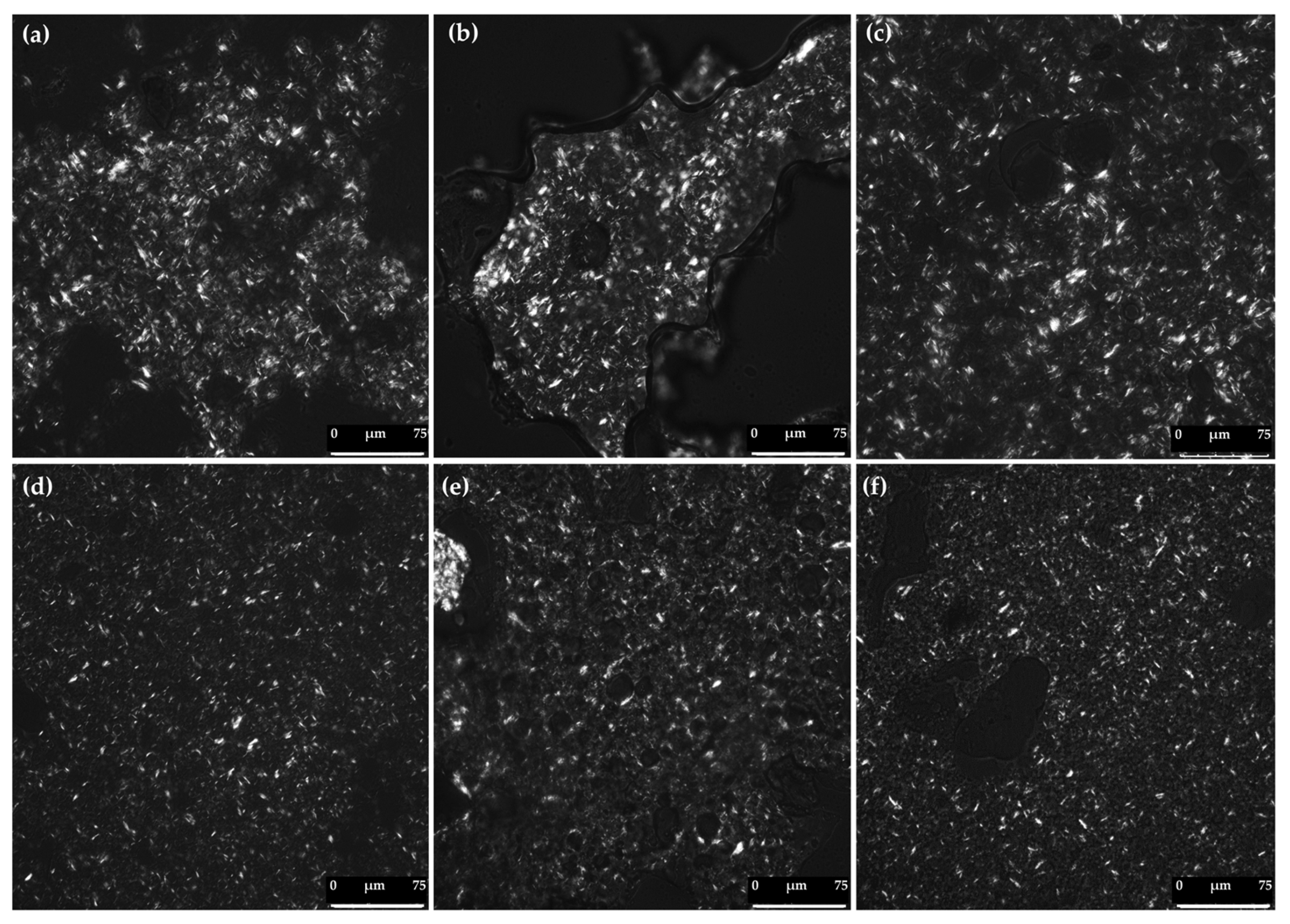
3.4. Polarized Light Microscopy of Bigels
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zampouni, K.; Dimakopoulou-Papazoglou, D.; Katsanidis, E. Food-Grade Bigel Systems: Formulation, Characterization, and Applications for Novel Food Product Development. Gels 2024, 10, 712. [Google Scholar] [CrossRef]
- Shakeel, A.; Lupi, F.R.; Gabriele, D.; Baldino, N.; De Cindio, B. Bigels: A Unique Class of Materials for Drug Delivery Applications. Soft Mater. 2018, 16, 77–93. [Google Scholar] [CrossRef]
- Martín-Illana, A.; Notario-Pérez, F.; Cazorla-Luna, R.; Ruiz-Caro, R.; Bonferoni, M.C.; Tamayo, A.; Veiga, M.D. Bigels as Drug Delivery Systems: From their Components to their Applications. Drug Discov. Today 2022, 27, 1008–1026. [Google Scholar] [CrossRef]
- Xie, D.; Hu, H.; Huang, Q.; Lu, X. Influence of Oleogel/Hydrogel Ratios and Emulsifiers on Structural and Digestion Properties of Food-Grade 3D Printed Bigels as Carriers for Quercetin and Catechin. Food Hydrocoll. 2023, 144, 108948. [Google Scholar] [CrossRef]
- Zheng, H.; Mao, L.; Cui, M.; Liu, J.; Gao, Y. Development of Food-Grade Bigels Based on κ-Carrageenan Hydrogel and Monoglyceride Oleogels as Carriers for β-Carotene: Roles of Oleogel Fraction. Food Hydrocoll. 2020, 105, 105855. [Google Scholar] [CrossRef]
- Zhu, Q.; Gao, J.; Han, L.; Han, K.; Wei, W.; Wu, T.; Li, J.; Zhang, M. Development and Characterization of Novel Bigels Based on Monoglyceride-Beeswax Oleogel and High Acyl Gellan Gum Hydrogel for Lycopene Delivery. Food Chem. 2021, 365, 130419. [Google Scholar] [CrossRef]
- Kaimal, A.M.; Singhal, R.S. A Bigel Based Formulation Protects Lutein Better in the Gastric Environment with Controlled Release and Antioxidant Profile than other Gel Based Systems. Food Chem. 2023, 423, 136304. [Google Scholar] [CrossRef]
- Loza-Rodríguez, N.; Millán-Sánchez, A.; López, O. Characteristics of a Lipid Hydrogel and Bigel as Matrices for Ascorbic Acid Stabilization. Gels 2023, 9, 649. [Google Scholar] [CrossRef]
- Saffold, A.C.; Acevedo, N.C. The Effect of Mono-Diglycerides on the Mechanical Properties, Microstructure, and Physical Stability of an Edible Rice Bran Wax–Gelatin Biphasic Gel System. J. Am. Oil Chem. Soc. 2022, 99, 1033–1043. [Google Scholar] [CrossRef]
- Ghiasi, F.; Golmakani, M.-T. Fabrication and Characterization of a Novel Biphasic System Based on Starch and Ethylcellulose as an Alternative Fat Replacer in a Model Food System. Innov. Food Sci. Emerg. Technol. 2022, 78, 103028. [Google Scholar] [CrossRef]
- Zampouni, K.; Filippou, A.; Papadimitriou, K.; Katsanidis, E. Evaluation of Bigel Systems as Potential Substitutes to Partially Replace Pork Backfat in Semi-Dry Sausages. Meat Sci. 2024, 208, 109392. [Google Scholar] [CrossRef]
- Baltuonytė, G.; Eisinaitė, V.; Kazernavičiūtė, R.; Vinauskienė, R.; Jasutienė, I.; Leskauskaitė, D. Novel Formulation of Bigel-Based Vegetable Oil Spreads Enriched with Lingonberry Pomace. Foods 2022, 11, 2213. [Google Scholar] [CrossRef]
- Álvarez, M.D.; Saiz, A.; Herranz, B.; Cofrades, S. Olive Pomace Oil Structuring for the Development of Healthy Puff Pastry Laminating Fats: The Effect of Chilling Storage on the Quality of Baked Products. Foods 2024, 13, 603. [Google Scholar] [CrossRef]
- Ikram, A.; Khalid, W.; Wajeeha Zafar, K.U.; Ali, A.; Afzal, M.F.; Aziz, A.; Faiz ul Rasool, I.; Al-Farga, A.; Aqlan, F.; Koraqi, H. Nutritional, biochemical, and clinical applications of carob: A review. Food Sci. Nutr. 2023, 11, 3641–3654. [Google Scholar] [CrossRef]
- Macho-González, A.; López-Oliva, M.E.; Garcimartín, A.; Sánchez-Muniz, F.J.; Benedí, J. Carob Fruit Extract-Enriched Meat Improves Pancreatic Beta-Cell Dysfunction, Hepatic Insulin Signaling and Lipogenesis in Late-Stage Type 2 Diabetes Mellitus Model. J. Nutr. Biochem. 2020, 84, 108461. [Google Scholar] [CrossRef]
- Azab, A. D-Pinitol—Active Natural Product from Carob with Notable Insulin Regulation. Nutrients 2022, 14, 1453. [Google Scholar] [CrossRef]
- Gabriele, D.; de Cindio, B.; D’Antona, P. A Weak Gel Model for Foods. Rheol. Acta 2001, 40, 120–127. [Google Scholar] [CrossRef]
- Zhou, M.; Li, B.; Wu, A.; Hu, Z.; Zhou, L.; Hu, H.; Fu, D. Preparation of a Two-Phase Gel System Based on Gelatin Hydrogel and Beeswax/Rice Bran Wax Oleogel and Eutectic Phase Behavior of Beeswax and Rice Bran Wax in Soybean Oil. LWT-Food Sci. Technol. 2025, 215, 117306. [Google Scholar] [CrossRef]
- Eisinaitė, V.; Jasutienė, I.; Vinauskienė, R.; Leskauskaitė, D. Development of Bigel Based Dysphagia-Oriented Products, Structured with Collagen and Carnauba Wax: Characterisation and Rheological Behaviour. Int. J. Food. Sci. Technol. 2023, 58, 145–153. [Google Scholar] [CrossRef]
- National Dysphagia Diet Task Force. Dysphagia diet task, and AD association. In National Dysphagia Diet: Standardization for Optimal Care; American Dietetic Association: Chicago, IL, USA, 2002. [Google Scholar]
- Álvarez, M.D.; Cofrades, S.; Espert, M.; Sanz, T.; Salvador, A. Development of Chocolates with Improved Lipid Profile by Replacing Cocoa Butter with an Oleogel. Gels 2021, 7, 220. [Google Scholar] [CrossRef]
- Mancini, F.; Montanari, L.; Peressini, D.; Fantozzi, P. Influence of Alginate Concentration and Molecular Weight on Functional Properties of Mayonnaise. LWT-Food Sci. Technol. 2002, 35, 517–525. [Google Scholar] [CrossRef]
- Solo-de-Zaldívar, B.; Tovar, C.A.; Borderías, A.J.; Herranz, B. Pasteurization and Chilled Storage of Restructured Fish Muscle Products Based on Glucomannan Gelation. Food Hydrocoll. 2015, 43, 418–426. [Google Scholar] [CrossRef]
- Richterová, V.; Gjevik, A.; Vaculík, O.; Vejrosta, J.; Pekař, M. Impact of Collagen on the Rheological and Transport Properties of Agarose Hydrogels. Gels 2025, 11, 396. [Google Scholar] [CrossRef]
- Lupi, F.R.; De Santo, M.P.; Ciuchi, F.; Baldino, N.; Gabriele, D. A Rheological Modelling and Microscopic Analysis of Bigels. Rheol. Acta 2017, 56, 753–763. [Google Scholar] [CrossRef]
- Kavya, M.; Priyanka, V.; Jacob, A.R.; Nisha, P. Investigating the Influence of Hydrogel and Oleogel Ratios on Physico Chemical Characteristics, Microstructure, Rheology, and Texture of a Food Grade Bigel. Biomacromolecules 2025, 26, 2800–2810. [Google Scholar] [CrossRef]
- Quilaqueo, M.; Iturra, N.; Contardo, I.; Millao, S.; Morales, E.; Rubilar, M. Food-Grade Bigels with Potential to Replace Saturated and Trans Fats in Cookies. Gels 2022, 8, 445. [Google Scholar] [CrossRef] [PubMed]
- Russo, N.; Avallone, P.R.; Grizzuti, N.; Pasquino, R. Stable O/W Emulsions by combining Pluronic L64 and Sodium Alginate. Colloids Surf. A: Physicochem. Eng. Asp. 2024, 701, 134776. [Google Scholar] [CrossRef]
- Li, A.; Gong, T.; Houa, Y.; Yang, X.; Guo, Y. Alginate-Stabilized Thixotropic Emulsion Gels and their Applications in Fabrication of Low-Fat Mayonnaise Alternatives. Int. J. Biol. Macromol. 2020, 146, 821–831. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Q.; Chen, K.; Cai, Z.; Li, Y.; Zhang, H. Phase Inversion Regulable Bigels Co-Stabilized by Chlorella Pyrenoidosa Protein and Beeswax: In-Vitro Digestion and Food 3D Printing. Int. J. Biol. Macromol. 2024, 277, 134540. [Google Scholar] [CrossRef]
- Dassanayake, L.S.K.; Kodali, D.R.; Ueno, S.; Sato, K. Physical Properties of Organogels Made of Rice Bran Wax and Vegetable Oils. In Edible Oleogels: Structure and Health Implications; Marangoni, A., Garti, N., Eds.; AOCS Press: Urbana, IL, USA, 2011; Chapter 7; pp. 149–172. [Google Scholar]
- Martins, A.J.; Guimarães, A.; Fuciños, P.; Sousa, P.; Venâncio, A.; Pastrana, L.M.; Cerqueira, M.A. Food-Grade Bigels: Evaluation of Hydrogel: Oleogel Ratio and Gelator Concentration on their Physicochemical Properties. Food Hydrocoll. 2023, 143, 108893. [Google Scholar] [CrossRef]
- Loza-Rodríguez, N.; Millán-Sánchez, A.; López, O. A Biocompatible Lipid-Based Bigel for Topical Applications. Eur. J. Pharm. Biopharm. 2023, 190, 24–34. [Google Scholar] [CrossRef]
- Oyom, W.; Strange, J.; Nowlin, K.; Tukur, P.; Ferdaus, M.J.; Faraji, H.; Tahergorabi, R. Development and Characterization of Bigel Systems as Carriers for Thyme Essential Oil Utilizing Hydrogel from Chicken Processing By-Products for Food Applications. Int. J. Biol. Macromol. 2025, 292, 139222. [Google Scholar] [CrossRef]


| Bigel (BG) | HG:OG Ratio | HG | OG |
|---|---|---|---|
| HG70*:OG30 | 70:30 | With I-CFE | Without P-CFE |
| HG70:OG30* | 70:30 | Without I-CFE | With P-CFE |
| HG70*:OG30* | 70:30 | With I-CFE | With P-CFE |
| HG30*:OG70 | 30:70 | With I-CFE | Without P-CFE |
| HG30:OG70* | 30:70 | Without I-CFE | With P-CFE |
| HG30*:OG70* | 30:70 | With I-CFE | With P-CFE |
| Control individual phases | |||
| HG* | 100:0 | With I-CFE | - |
| OG* | 0:100 | - | With P-CFE |
| Individual HG Phase | Individual OG Phase | ||||||
|---|---|---|---|---|---|---|---|
| Bigel (BG) | I-CFE | SA | Water | MW | Soy Lecithin | OPO | P-CFE |
| HG70*:OG30 | 68.60 | 1.40 | 0.00 | 3.75 | 0.075 | 26.18 | 0.00 |
| HG70:OG30* | 0.00 | 1.40 | 68.60 | 3.75 | 0.075 | 25.58 | 0.60 |
| HG70*:OG30* | 68.60 | 1.40 | 0.00 | 3.75 | 0.075 | 25.58 | 0.60 |
| HG30*:OG70 | 29.40 | 0.60 | 0.00 | 8.75 | 0.175 | 61.08 | 0.00 |
| HG30:OG70* | 0.00 | 0.60 | 29.40 | 8.75 | 0.175 | 59.68 | 1.40 |
| HG30*:OG70* | 29.40 | 0.60 | 0.00 | 8.75 | 0.175 | 59.68 | 1.40 |
| System | σmax (Pa) | γmax (%) | G*max (kPa) | tan δ (-) |
|---|---|---|---|---|
| HG70*:OG30 | 2.28 ± 0.00 C | 0.10 ± 0.01 C | 1.91 ± 0.21 A | 0.44 ± 0.00 C |
| HG70:OG30* | 0.50 ± 0.00 A | 0.01 ± 0.00 A | 3.72 ± 0.83 B | 0.27 ± 0.03 A |
| HG70*:OG30* | 0.82 ± 0.00 B | 0.06 ± 0.01 B | 1.50 ± 0.30 A | 0.37 ± 0.03 B |
| HG30*:OG70 | 31.64 ± 0.01 A | 0.03 ± 0.00 B | 107.34 ± 7.67 A | 0.20 ± 0.00 B |
| HG30:OG70* | 35.50 ± 0.00 B | 0.03 ± 0.01 B | 105.42 ± 16.39 A | 0.18 ± 0.01 A |
| HG30*:OG70* | 31.63 ± 0.00 A | 0.02 ± 0.00 A | 173.85 ± 11.25 B | 0.17 ± 0.00 A |
| HG* | 1.29 ± 0.00 | 2.80 ± 0.32 | 0.05 ± 0.01 | 0.39 ± 0.03 |
| OG* | 100.00 ± 0.00 | 0.03 ± 0.01 | 319.15 ± 83.15 | 0.20 ± 0.01 |
| System | G′ (kPa) | G″ (kPa) | tan δ (-) | A (kPa s1/z) | z (-) | R2 (Equation (1)) |
|---|---|---|---|---|---|---|
| HG70*:OG30 | 2.48 ± 0.12 B | 0.93 ± 0.04 C | 0.38 ± 0.00 C | 2.75 ± 0.12 B | 4.76 ± 0.06 A | 0.99 |
| HG70:OG30* | 2.95 ± 0.11 C | 0.78 ± 0.01 B | 0.27 ± 0.01 A | 3.11 ± 0.11 C | 6.17 ± 0.52 B | 0.99 |
| HG70*:OG30* | 1.93 ± 0.05 A | 0.56 ± 0.01 A | 0.29 ± 0.00 B | 2.05 ± 0.02 A | 5.80 ± 0.16 B | 0.99 |
| HG30*:OG70 | 99.25 ± 4.00 A | 17.99 ± 0.41 B | 0.18 ± 0.00 B | 100.23 ± 4.04 A | 9.74 ± 0.15 A | 1.00 |
| HG30:OG70* | 100.69 ± 1.92 A | 16.82 ± 0.12 A | 0.17 ± 0.00 A | 100.72 ± 1.88 A | 10.10 ± 0.14 B | 0.99 |
| HG30*:OG70* | 159.87 ± 3.10 B | 26.33 ± 0.28 C | 0.17 ± 0.00 A | 160.08 ± 2.72 B | 10.14 ± 0.13 B | 0.99 |
| HG* | 0.05 ± 0.00 | 0.02 ± 0.00 | 0.38 ± 0.01 | 0.05 ± 0.00 | 4.70 ± 0.17 | 0.97 |
| OG* | 411.03 ± 8.00 | 74.81 ± 2.02 | 0.18 ± 0.00 | 413.93±7.50 | 9.39±0.10 | 1.00 |
| System | η0.1 (Pa s) | η10 (Pa s) | η50 (Pa s) | η100 (Pa s) | K (Pa sn) | n (-) | R2 (Equation (2)) |
|---|---|---|---|---|---|---|---|
| HG70*:OG30 | 48.97 ± 3.17 B | 3.20 ± 0.16 B | 2.09 ± 0.11 C | 1.80 ± 0.10 C | 13.36 ± 0.90 B | 0.44 ± 0.01 A | 0.98 |
| HG70:OG30* | 7.88 ± 0.87 A | 2.94 ± 0.03 B | 1.71 ± 0.03 B | 1.28 ± 0.03 B | 4.95 ± 0.32 A | 0.74 ± 0.02 C | 0.99 |
| HG70*:OG30* | 10.97 ± 0.91 A | 1.72 ± 0.12 A | 1.13 ± 0.07 A | 1.01 ± 0.06 A | 4.85 ± 0.42 A | 0.58 ± 0.01 B | 0.98 |
| HG30*:OG70 | 147.43 ± 7.61 B | 2.86 ± 0.16 B | 1.39 ± 0.07 A,B | 1.13 ± 0.04 A | 22.27 ± 0.94 B | 0.20 ± 0.01 A | 1.00 |
| HG30:OG70* | 117.53 ± 1.96 A | 2.49 ± 0.11 A | 1.28 ± 0.07 A | 1.08 ± 0.07 A | 18.77 ± 0.65 A | 0.22 ± 0.01 A | 0.99 |
| HG30*:OG70* | 122.40 ± 3.36 A | 2.84 ± 0.17 A,B | 1.53 ± 0.05 B | 1.31 ± 0.01 B | 20.24 ± 0.78 A | 0.24 ± 0.02 B | 0.99 |
| HG* | 6.07 ± 0.27 | 1.17 ± 0.08 | 0.96 ± 0.08 | 0.90 ± 0.07 | 2.86 ± 0.08 | 0.63 ± 0.03 | 0.97 |
| OG* | 129.35 ± 3.65 | 2.20 ± 0.24 | 1.08 ± 0.04 | 0.93 ± 0.12 | 18.66 ± 1.55 | 0.18 ± 0.01 | 1.00 |
| Sample | Onset Temperature (TO, °C) | Peak Temperature (TP, °C) | Enthalpy (ΔHM, J/g) |
|---|---|---|---|
| HG70*:OG30 | 40.66 ± 0.61 A | 47.31 ± 1.21 A | 0.33 ± 0.05 A |
| HG70:OG30* | 40.85 ± 0.24 A | 49.08 ± 0.83 A | 1.31 ± 0.01 B |
| HG70*:OG30* | 41.01 ± 0.11 A | 47.51 ± 0.35 A | 0.41 ± 0.02 A |
| HG30*:OG70 | 42.39 ± 2.07 A | 48.68 ± 0.86 A | 0.99 ± 0.05 A |
| HG30:OG70* | 40.84 ± 0.05 A | 48.33 ± 0.16 A | 0.98 ± 0.03 A |
| HG30*:OG70* | 40.57 ± 0.16 A | 48.46 ± 0.18 A | 1.15 ± 0.02 B |
| OG* | 41.24 ± 0.00 | 50.88 ± 0.32 | 1.02 ± 0.01 |
| MW | 33.34 ± 2.96 | 51.42 ± 0.06 | 70.99 ± 1.41 |
| OPO | - | - | - |
| Sample | L* | a* | b* | Total Loss (%) |
|---|---|---|---|---|
| HG70*:OG30 | 26.91 ± 0.68 A | 4.61 ± 0.93 A | −2.15 ± 0.37 B | 2.06 ± 0.37 B |
| HG70:OG30* | 44.96 ± 0.30 C | 5.04 ± 0.13 A | 10.61 ± 0.14 C | 0.00 ± 0.00 A |
| HG70*:OG30* | 34.06 ± 0.28 B | 5.25 ± 0.14 B | −10.66 ± 0.15 A | 4.82 ± 0.36 C |
| HG30*:OG70 | 29.59 ± 0.81 A | 5.20 ± 0.46 B | 4.19 ± 0.43 B | 4.16 ±0.87 B |
| HG30:OG70* | 55.39 ± 0.35 C | 4.61 ± 0.16 A | 13.58 ± 0.50 C | 1.64 ± 0.38 A |
| HG30*:OG70* | 36.56 ± 0.28 B | 7.71 ± 0.13 C | −6.71 ± 0.11 A | 8.25 ± 0.54 C |
| I-CFE | 26.31 ± 0.40 | 5.11 ± 0.28 | −4.92 ± 0.11 | - |
| P-CFE | 63.13 ± 0.52 | 8.41 ± 0.17 | 16.01 ± 0.15 | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Álvarez, M.D.; Saiz, A.; Cofrades, S. Impact of Hydrogel-to-Oleogel Ratio and Presence of Carob Fruit Extracts on Formulated Bigels: Rheological, Thermal, Physicochemical and Microstructural Properties. Foods 2025, 14, 3753. https://doi.org/10.3390/foods14213753
Álvarez MD, Saiz A, Cofrades S. Impact of Hydrogel-to-Oleogel Ratio and Presence of Carob Fruit Extracts on Formulated Bigels: Rheological, Thermal, Physicochemical and Microstructural Properties. Foods. 2025; 14(21):3753. https://doi.org/10.3390/foods14213753
Chicago/Turabian StyleÁlvarez, María Dolores, Arancha Saiz, and Susana Cofrades. 2025. "Impact of Hydrogel-to-Oleogel Ratio and Presence of Carob Fruit Extracts on Formulated Bigels: Rheological, Thermal, Physicochemical and Microstructural Properties" Foods 14, no. 21: 3753. https://doi.org/10.3390/foods14213753
APA StyleÁlvarez, M. D., Saiz, A., & Cofrades, S. (2025). Impact of Hydrogel-to-Oleogel Ratio and Presence of Carob Fruit Extracts on Formulated Bigels: Rheological, Thermal, Physicochemical and Microstructural Properties. Foods, 14(21), 3753. https://doi.org/10.3390/foods14213753







