Functional Potential of Sweet Cherry Cultivars Grown in New Zealand: Effects of Processing on Nutritional and Bioactive Properties
Highlights
- Potassium was the most abundant mineral in cherries, followed by phosphorus, calcium, and magnesium.
- Some cultivars of fresh cherries had higher potassium content than others.
- Some processed cultivars provide higher phenolic metabolites, such as neochlorogenic acid.
- New Zealand cherries offer dietary value and health benefits owing to their nutrients and bioactives.
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Harvest, Processing, and Preparation
2.2. Chemicals
2.3. Proximate Composition
2.4. Amino Acid Profile
2.5. Sugars
2.6. Vitamins
2.7. Minerals
2.8. Total Phenolic Content
2.9. Phenolic Metabolites
2.9.1. Phenolic Compounds (Non Anthocyanins)
2.9.2. Anthocyanins
2.10. Statistical Analysis
3. Results and Discussion
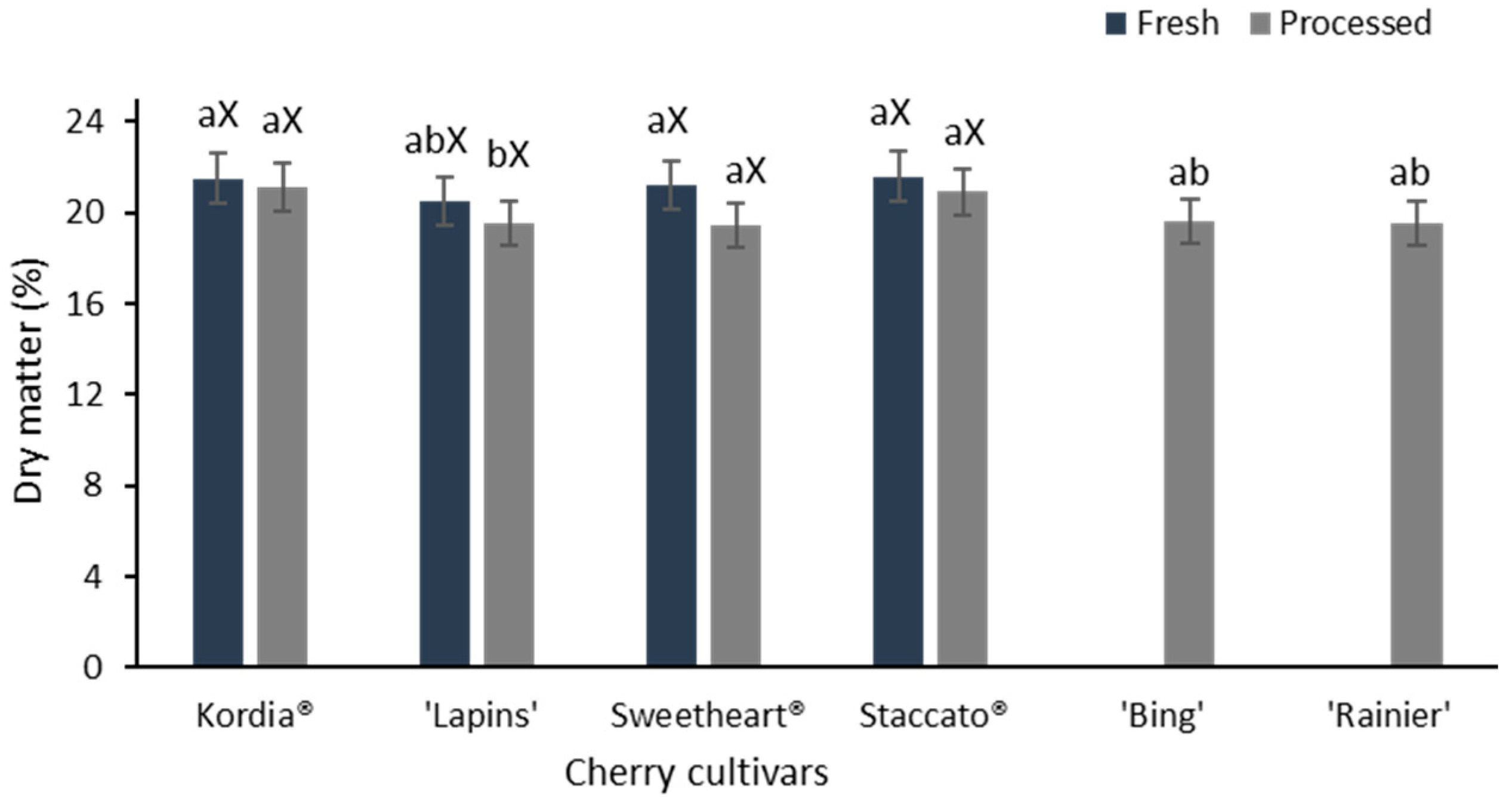
3.1. Dry Matter Content
3.2. Chemical Composition
3.2.1. Vitamins
3.2.2. Minerals
3.3. Sugar Profiles
3.4. Amino Acid Profile
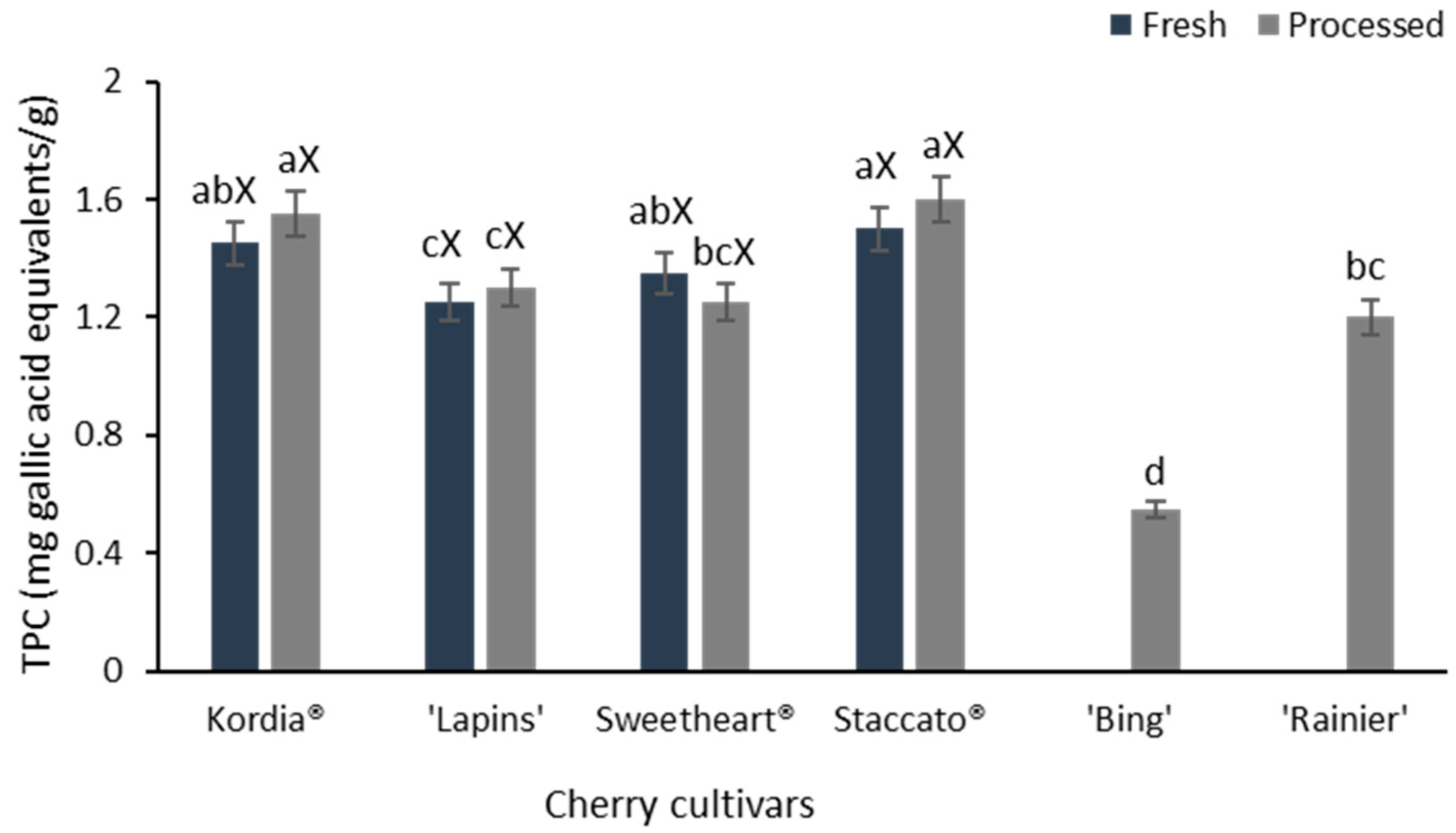
3.5. Total Phenolic Content (TPC)
3.6. Phenolic Composition
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Blando, F.; Oomah, B.D. Sweet and sour cherries: Origin, distribution, nutritional composition and health benefits. Trends Food Sci. Technol. 2019, 86, 517–529. [Google Scholar] [CrossRef]
- Pacifico, S.; Galasso, S.; Piccolella, S.; Kremer, D.; Stabentheiner, E.; Jukić, M.; Žarković, N.; Tuberoso, C.I.G. Chemical composition, nutritional value and antioxidant properties of autochthonous Prunus avium cultivars from Campania Region. Food Res. Int. 2014, 64, 188–199. [Google Scholar] [CrossRef]
- Ballistreri, G.; Continella, A.; Gentile, A.; Amenta, M.; Fabroni, S.; Rapisarda, P. Fruit quality and bioactive compounds relevant to human health of sweet cherry (Prunus avium L.) cultivars grown in Italy. Food Chem. 2013, 140, 630–638. [Google Scholar] [CrossRef]
- Crisosto, C.H.; Crisosto, G.M.; Metheney, P. Consumer acceptance of ‘Brooks’ and ‘Bing’ cherries is mainly dependent on fruit SSC and visual skin color. Postharvest Biol. Technol. 2003, 28, 159–167. [Google Scholar] [CrossRef]
- Esti, M.; Cinquanta, L.; Sinesio, F.; Moneta, E.; Di Matteo, M. Physicochemical and sensory fruit characteristics of two sweet cherry cultivars after cool storage. Food Chem. 2002, 76, 399–405. [Google Scholar] [CrossRef]
- Prvulovic, D.; Malenčić, Đ.; Popović, M.; Ljubojević, M.; Ognjanov, V. Phenolic compounds in sweet cherry (Prunus avium L.) petioles and their antioxidant properties. Res. J. Agric. Sci. 2011, 43, 198–202. [Google Scholar]
- Cooper, A.J.; Sharp, S.J.; Lentjes, M.A.H.; Luben, R.N.; Khaw, K.T.; Wareham, N.J.; Forouhi, N.G. Fruit and vegetable intake and type 2 diabetes: EPIC-InterAct prospective study and meta-analysis. Eur. J. Clin. Nutr. 2012, 66, 1082–1092. [Google Scholar] [CrossRef]
- Grosso, G.; Stepaniak, U.; Micek, A.; Kozela, M.; Stefler, D.; Bobak, M.; Pająk, A. Dietary polyphenol intake and risk of type 2 diabetes in the Polish arm of the HAPIEE study. Br. J. Nutr. 2017, 118, 60–68. [Google Scholar] [CrossRef]
- McCune, L.M.; Kubota, C.; Stendell-Hollis, N.R.; Thomson, C.A. Cherries and health: A review. Crit. Rev. Food Sci. Nutr. 2011, 51, 1–12. [Google Scholar] [CrossRef]
- Kelley, D.S.; Adkins, Y.; Laugero, K.D. A review of the health benefits of cherries. Nutrients 2018, 10, 368. [Google Scholar] [CrossRef]
- Chiva-Blanch, G.; Badimon, L. Effects of polyphenol intake on metabolic syndrome: Current evidences from human trials. Oxid. Med. Cell. Longev. 2017, 2017, 5812401. [Google Scholar] [CrossRef]
- Prior, R.L. Fruits and vegetables in the prevention of cellular oxidative damage. Am. J. Clin. Nutr. 2003, 78, 570S–578S. [Google Scholar] [CrossRef]
- Szajdek, A.; Borowska, E.J. Bioactive compounds and health-promoting properties of berry fruits: A review. Plant Foods Hum. Nutr. 2008, 63, 147–156. [Google Scholar] [CrossRef]
- FNew Zealand Horticulture Export Authority. Summerfruit (Cherries, Apricots, Peaches, Nectarines, Plums). Available online: https://hea.co.nz/2012-05-11-03-05-28/summerfruit-trade (accessed on 12 February 2025).
- Pissard, A.; Baeten, V.; Dardenne, P.; Fernández Pierna, J.A.; Baeten, V.; Pierna, J.A.F. Determination of total phenolic compound content and antioxidant activity in cherry species and cultivars. J. Berry Res. 2016, 6, 81–91. [Google Scholar] [CrossRef]
- Kim, D.-O.; Heo, H.J.; Kim, Y.J.; Yang, H.S.; Lee, C.Y. Sweet and sour cherry phenolics and their protective effects on neuronal cells. J. Agric. Food Chem. 2005, 53, 9921–9927. [Google Scholar] [CrossRef] [PubMed]
- AOAC International. Official Methods of Analysis of AOAC International, 17th ed.; AOAC International: Arlington, VA, USA, 2000. [Google Scholar]
- Lee, S.K.; Kader, A.A. Preharvest and postharvest factors influencing vitamin C content of horticultural crops. Postharvest Biol. Technol. 2000, 20, 207–220. [Google Scholar] [CrossRef]
- McClure, S. Simultaneous determination of total vitamins B1, B2, B3, and B6 in infant formula and related nutritionals by enzymatic digestion and LC-MS/MS—A multi-laboratory testing study final action: AOAC method 2015.14. J. AOAC Int. 2020, 103, 1060–1072. [Google Scholar] [CrossRef]
- Ismail, F.; Talpur, F.N.; Memon, A. Determination of water-soluble vitamins in fruits and vegetables marketed in Sindh, Pakistan. Pak. J. Nutr. 2013, 12, 197–202. [Google Scholar] [CrossRef]
- Sami, R.; Li, Y.; Qi, B.; Wang, S.; Zhang, Q.; Han, F.; Ma, Y.; Jing, J.; Jiang, L. HPLC analysis of water-soluble vitamins (B2, B3, B6, B12, and C) and fat-soluble vitamins (E, K, D, A, and β-carotene) of okra (Abelmoschus esculentus). J. Chem. 2014, 2014, 831357. [Google Scholar] [CrossRef]
- Tabart, J.; Kevers, C.; Pincemail, J.; Defraigne, J.O.; Dommes, J. Antioxidant and anti-inflammatory activities of Ribes nigrum extracts. Food Chem. 2012, 131, 1116–1122. [Google Scholar] [CrossRef]
- Özkaya, O.; Yildiz, K.; Cambazoglu, B. Influence of fast cold chain and modified atmosphere packaging storage on postharvest quality of early season-harvested sweet cherries. J. Food Process. Preserv. 2015, 39, 2119–2128. [Google Scholar] [CrossRef]
- Chockchaisawasdee, S.; Golding, J.B.; Vuong, Q.V.; Papoutsis, K.; Stathopoulos, C.E. Sweet cherry: Composition, postharvest preservation, processing and trends for its future use. Trends Food Sci. Technol. 2016, 55, 72–83. [Google Scholar] [CrossRef]
- Bastos, C.; Barros, L.; Dueñas, M.; Calhelha, R.C.; Queiroz, M.J.R.P.; Santos-Buelga, C.; Ferreira, I.C.F.R. Chemical characterisation and bioactive properties of Prunus avium L.: The widely studied fruits and the unexplored stems. Food Chem. 2015, 173, 1045–1053. [Google Scholar] [CrossRef] [PubMed]
- Gardiner, M.A.; Beyer, R.; Melton, L.D. Sugar and anthocyanidin content of two processing-grade sweet cherry cultivars and cherry products. N. Z. J. Crop Hortic. Sci. 1993, 21, 213–218. [Google Scholar] [CrossRef]
- Bohn, T.; Bouayed, J. Apples: An apple a day, still keeping the doctor away? In Nutritional Composition and Antioxidant Properties of Fruits and Vegetables; Elsevier: London, UK, 2020; pp. 595–612. [Google Scholar]
- The New Zealand Institute for Plant & Food Research Limited and the New Zealand Ministry of Health. The Concise New Zealand Food Composition Tables, 14th Edition 2021; New Zealand Food Composition Database: Palmerston North, New Zealand, 2022; Available online: https://www.foodcomposition.co.nz/downloads/concise-14-edition.pdf (accessed on 12 February 2025).
- Serradilla, M.J.; Martín, A.; Hernández, A.; López-Corrales, M.; Córdoba, M.G.; Sánchez, M.A.; Casado, M.L. Composition of the cherry (Prunus avium L. and Prunus cerasus L.; Rosaceae). In Nutritional Composition of Fruit Cultivars; Academic Press: London, UK, 2016; pp. 127–147. [Google Scholar]
- Ferretti, G.; Bacchetti, T.; Belleggia, A.; Neri, D. Cherry antioxidants: From farm to table. Molecules 2010, 15, 6993–7005. [Google Scholar] [CrossRef]
- Jänes, H.; Arus, L.; Kikas, A.; Libek, A. Some biological properties and fruit quality parameters of new sweet cherry cultivars and perspective selections. Agron. Res. 2010, 8, 583–588. [Google Scholar]
- Leong, S.Y.; Oey, I. Effects of processing on anthocyanins, carotenoids and vitamin C in summer fruits and vegetables. Food Chem. 2012, 133, 1577–1587. [Google Scholar] [CrossRef]
- Fonseca, L.R.S.; Oliveira, A.P.; Santos, S.A.O.; Silvestre, A.J.D.; Caleja, C.; Barros, L.; Ferreira, I.C.F.R. Sweet cherries as anti-cancer agents: From bioactive compounds to function. Molecules 2021, 26, 2941. [Google Scholar] [CrossRef]
- Dismore, M.L.; Haytowitz, D.B.; Holden, J.M.; Gebhardt, S.E.; Peterson, J.W. Vitamin K content of nuts and fruits in the US diet. J. Am. Diet. Assoc. 2003, 103, 1650–1652. [Google Scholar] [CrossRef]
- Food Standards Australia New Zealand. Annual Report 2023-24; Food Standards Australia New Zealand: Canberra, Australia, 2024. Available online: https://www.foodstandards.gov.au/sites/default/files/2024-10/FSANZ%20-%20Annual%20Report%202023%E2%80%9324.pdf (accessed on 12 February 2025).
- Girard, B.; Kopp, T. Physicochemical characteristics of selected sweet cherry cultivars. J. Agric. Food Chem. 1998, 46, 471–476. [Google Scholar] [CrossRef]
- Papp, N.; Szilvássy, B.; Abrankó, L.; Szabó, T.; Pfeiffer, P.; Szabó, Z.; Hegedűs, A. Main quality attributes and antioxidants in Hungarian sour cherries: Identification of genotypes with enhanced functional properties. Int. J. Food Sci. Technol. 2010, 45, 395–402. [Google Scholar] [CrossRef]
- Gündoğdu, M.; Bilge, U. Determination of organics, phenolics, sugars and vitamin C contents of some cherry cultivars (Prunus avium). Int. J. Agric. Biol. 2012, 14, 719–724. [Google Scholar]
- Sîrbu, S.; Niculaua, M.; Chiriţă, O. Physico-chemical and antioxidant properties of new sweet cherry cultivars from Iaşi, Romania. Agron. Res. 2012, 10, 341–350. [Google Scholar]
- Hayaloglu, A.A.; Demir, N. Physicochemical characteristics, antioxidant activity, organic acid and sugar contents of 12 sweet cherry (Prunus avium L.) cultivars grown in Turkey. J. Food Sci. 2015, 80, C564–C570. [Google Scholar] [CrossRef]
- Vosnjak, M.; Mrzlic, D.; Usenik, V. Summer pruning of sweet cherry: A way to control sugar content in different organs. J. Sci. Food Agric. 2022, 102, 1216–1224. [Google Scholar] [CrossRef]
- Hrotkó, K. Progress in cherry rootstock research. In V International Cherry Symposium; Acta Horticulturae: Leuven, Belgium, 2005; Volume 795, pp. 123–130. [Google Scholar]
- Balducci, F.; Blasi, P.; Negro, D.; Vagnoni, E.; Proietti, P.; Bartolini, S.; Mencarelli, F. The rootstock effects on vigor, production and fruit quality in sweet cherry (Prunus avium L.). J. Berry Res. 2019, 9, 249–265. [Google Scholar] [CrossRef]
- Yilmaz, U.N.; Akin, M.; Akca, Y.; Ercisli, S.; Balik, H.I. Effects of rootstock and training system on tree canopy, fruit quality and phytochemicals of ‘0900 Ziraat’ and ‘Regina’ sweet cherry cultivars. Braz. Arch. Biol. Technol. 2023, 66, e23220688. [Google Scholar] [CrossRef]
- Serrano, M.; Guillén, F.; Martínez-Romero, D.; Castillo, S.; Valero, D. Chemical constituents and antioxidant activity of sweet cherry at different ripening stages. J. Agric. Food Chem. 2005, 53, 2741–2745. [Google Scholar] [CrossRef] [PubMed]
- Huang, R.; Xu, C. An overview of the perception and mitigation of astringency associated with phenolic compounds. Compr. Rev. Food Sci. Food Saf. 2021, 20, 1036–1074. [Google Scholar] [CrossRef]
- Malundo, T.M.; Baldwin, E.A.; Moshonas, M.G.; Baker, R.A.; Shewfelt, R.L. Sugars and acids influence flavor properties of mango (Mangifera indica). J. Am. Soc. Hortic. Sci. 2001, 126, 115–121. [Google Scholar] [CrossRef]
- Monirujjaman, M.; Ferdouse, A. Metabolic and physiological roles of branched-chain amino acids. Adv. Mol. Biol. 2014, 2014, 364976. [Google Scholar] [CrossRef]
- Usenik, V.; Fabčič, J.; Štampar, F. Sugars, organic acids, phenolic composition and antioxidant activity of sweet cherry (Prunus avium L.). Food Chem. 2008, 107, 185–192. [Google Scholar] [CrossRef]
- Haminiuk, C.W.I.; Maciel, G.M.; Plata-Oviedo, M.S.V.; Peralta, R.M. Phenolic compounds in fruits—An overview. Int. J. Food Sci. Technol. 2012, 47, 2023–2044. [Google Scholar] [CrossRef]
- Soong, Y.-Y.; Barlow, P.J. Antioxidant activity and phenolic content of selected fruit seeds. Food Chem. 2004, 88, 411–417. [Google Scholar] [CrossRef]
- Wada, L.; Ou, B. Antioxidant activity and phenolic content of Oregon caneberries. J. Agric. Food Chem. 2002, 50, 3495–3500. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves, B.; Landbo, A.-K.; Let, M.; Silva, A.P.; Nunes, C.; Rosenfeld, H.J. Effect of ripeness and postharvest storage on the phenolic profiles of cherries (Prunus avium L.). J. Agric. Food Chem. 2004, 52, 523–530. [Google Scholar] [CrossRef]
- Valero, D.; Serrano, M. Postharvest Biology and Technology for Preserving Fruit Quality; CRC Press: Boca Raton, FL, USA, 2010. [Google Scholar]
- Kiprovski, B.; Malenčić, D.; Popović, M.; Prvulović, D.; Milić, S.; Ljubojević, M. Postharvest changes in primary and secondary metabolites of sweet cherry cultivars induced by Monilinia laxa. Postharvest Biol. Technol. 2018, 144, 46–54. [Google Scholar] [CrossRef]
- Food Standards Australia New Zealand. Annual Report 2017–2018; Food Standards Australia New Zealand: Canberra, Australia, 2018. Available online: https://www.foodstandards.gov.au/sites/default/files/publications/annualreport201718/Documents/Food%20Standards%20Annual%20Report%202017-18.pdf (accessed on 12 February 2025).
| Nutrient | Kordia® | ‘Lapins’ | Sweetheart® | Staccato® | ‘Bing’ | ‘Rainier’ | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| Fresh | Processed | Fresh | Processed | Fresh | Processed | Fresh | Processed | Processed | Processed | |
| Ash (g/100 g) | 0.5 ± 0.01 aX | 0.3 ± 0.02 cY | 0.3 ± 0.02 cX | 0.3 ± 0.02 cY | 0.3 ± 0.02 cX | 0.3 ± 0.02 cX | 0.4 ± 0.02 bX | 0.4 ± 0.02 bX | 0.2 ± 0.01 d | 0.3 ± 0.02 c |
| Fat (g/100 g) | 0.6 ± 0.01 cY | 0.8 ± 0.04 aX | 0.5 ± 0.01 dX | 0.6 ± 0.03 cX | 0.6 ± 0.03 cX | 0.6 ± 0.03 cX | 0.6 ± 0.03 cX | 0.5 ± 0.03 dX | 0.5 ± 0.03 d | 0.7 ± 0.04 b |
| Protein (g/100 g) | 1.0 ± 0.02 aX | 0.8 ± 0.04 cX | 0.7 ± 0.01 dX | 0.8 ± 0.05 bX | 1.1 ± 0.06 aX | 0.8 ± 0.04 cX | 0.8 ± 0.04 cX | 0.8 ± 0.04 bX | 0.9 ± 0.05 b | 0.9 ± 0.05 b |
| Total available Carb (g/100 g) | 17 ± 0.34 bX | 16.7 ± 0.84 bX | 16.8 ± 0.34 cX | 15.2 ± 0.76 cX | 17.7 ± 0.89 bX | 17.5 ± 0.88 bX | 18.6 ± 0.93 aX | 17.3 ± 0.87 aX | 16.3 ± 0.82 c | 15.1 ± 0.76 d |
| TDF (g/100 g) | 1.2 ± 0.02 cX | 1.3 ± 0.06 aX | 0.8 ± 0.01 eX | 1.1 ± 0.06 bX | 1.1 ± 0.05 dX | 1.1 ± 0.06 bX | 1.0 ± 0.06 dX | 1.1 ± 0.06 bX | 1.1 ± 0.06 b | 1.2 ± 0.06 a |
| SDF (g/100 g) | 0.4 ± 0.01 aX | 0.6 ± 0.03 aX | 0.2 ± 0.0 cX | 0.5 ± 0.03 aX | 0.5 ± 0.03 bX | 0.5 ± 0.03 aX | 0.3± 0.02 aX | 0.5 ± 0.03 aX | 0.5 ± 0.03 a | 0.6 ± 0.03 a |
| IDF (g/100 g) | 0.8 ± 0.02 aX | 0.7 ± 0.04 bX | 0.6 ± 0.01 cX | 0.6 ± 0.03 cX | 0.6 ± 0.03 cX | 0.6 ± 0.03 cX | 0.7 ± 0.04 bX | 0.6 ± 0.03 cX | 0.6 ± 0.03 c | 0.6 ± 0.03 c |
| Vit C (mg/100 g) | 4.42 ± 0.22 bX | 4.64 ± 0.23 bX | 4.13 ± 0.21 bX | 4.09 ± 0.2 bX | 4.92 ± 0.25 bX | 5.32 ± 0.27 aX | 5.7 ± 0.29 aX | 5.5 ± 0.28 aX | 3.13 ± 0.16 c | 3.83 ± 0.19 c |
| Vit B1 (mg/100 g) | 0.03 ± 00 aX | 0.02 ± 00 bX | 0.02 ± 00 bX | 0.02 ± 00 bX | 0.03 ± 00 aX | 0.02 ± 00 bX | 0.03 ± 00 aX | 0.02 ± 0 bX | 0.02 ± 00 b | 0.03 ± 00 a |
| Vit B2 (mg/100 g) | 0.04 ± 00 aX | 0.04 ± 00 aX | 0.03 ± 00 bX | 0.03 ± 00 bX | 0.04 ± 00 aX | 0.04 ± 00 aX | 0.04 ± 00 aX | 0.03 ±0 bX | 0.03 ± 00 b | 0.03 ± 00 b |
| Vit B3 (mg/100 g) | 0.05 ± 00 d | <0.01 | 0.06 ±00 cX | 0.05 ± 00 dX | 0.07 ± 00 b | <0.01 | 0.06 ± 00 c | <0.01 | 0.15 ± 0.01 a | 0.03 ± 00 e |
| Vit B6 (mg/100 g) | 0.03 ± 00 eX | 0.03 ± 0 eX | 0.07 ± 00 cX | 0.07 ± 00 cX | 0.09 ± 00 aX | 0.07 ± 00 cX | 0.08 ± 00 bX | 0.08 ± 0 aX | 0.07 ± 00 c | 0.06 ± 00 d |
| Vit E, ATE (mg/100 g) | 0.2 ± 0.01 aX | 0.2 ± 0.01 aX | 0.1 ± 0.01 bX | 0.1 ± 0.01 bX | 0.1 ± 0.01 bX | 0.2 ± 0.01 aX | 0.2 ± 0.01 aX | 0.2 ± 0.01 aX | 0.1 ± 0.01 b | 0.1 ± 0.01 b |
| Vit K (mg/100 g) | <0.003 | 0.00391 ± 0.0002 a | <0.003 | <0.003 | <0.003 | <0.003 | <0.003 | <0.003 | <0.003 | <0.003 |
| Vit A, RE (mg/100 g) | <0.0025 | <0.0025 | 0.0038 ± 0.00019 b | <0.0025 | 0.0055 ± 0.00028 a | <0.0025 | <0.0025 | <0.0025 | ND | <0.0025 |
| Calcium (mg/100 g) | 11.6 ± 5.8 iX | 12.2 ± 6.2 hX | 11.3 ± 5.65 jY | 13.1 ± 6.5 cX | 12.5 ± 6.25 gX | 13.3 ± 6.5 dX | 14.8 ± 7.4 bX | 14.2 ± 7.1 aX | 12.6 ± 6.3 f | 12.8 ± 6.4 e |
| Magnesium (mg/100 g) | 11.2 ± 5.6 cX | 9.9 ± 4.9 gY | 9.5 ± 4.75 hY | 10.3 ± 5.2 dX | 12.0 ± 6.0 aX | 10.7 ± 5.4 dY | 10.5 ± 5.25 fY | 11.0 ± 5.5 bX | 10.6 ± 5.3 e | 11.2 ± 5.6 c |
| Potassium (mg/100 g) | 220.0 ± 110 aX | 192.0 ± 96 cY | 184.0 ± 92 fY | 188.0 ± 94 eX | 220.0 ± 110 aX | 190.0 ± 95 dY | 220.0 ± 110 aX | 220.0 ± 110 aX | 177.0 ± 88.5 g | 200.0 ± 100 b |
| Phosphorus (mg/100 g) | 24.0 ± 12 bX | 21.0 ± 10.5 eY | 19.8 ± 9.9 fX | 18.6 ± 9.3 fX | 25.0 ± 12.5 aX | 21.0 ± 10.5 dY | 21.0 ± 10.5 eY | 22.0 ± 11 cX | 18.3 ± 9.15 g | 21.0 ± 10.5 e |
| Iron (mg/100 g) | 0.26 ± 0.13 bX | 0.19 ± 0.01 fY | 0.18 ± 0.09 hY | 0.20 ± 0.10 eX | 0.28 ± 0.14 aX | 0.21 ± 0.11 dY | 0.18 ± 0.09 hY | 0.20 ± 0.10 eX | 0.23 ± 0.12 c | 0.19 ± 0.10 g |
| Copper (mg/100 g) | 0.13 ± 0.06 cX | 0.104 ± 0.05 eY | 0.195 ± 0.01 aX | 0.083 ± 0.04 eY | 0.137 ± 0.07 bX | 0.083 ± 0.04 eY | 0.083 ± 0.04 fY | 0.105 ± 0.05 dX | 0.082 ± 0.04 g | 0.081 ± 0.04 h |
| Manganese (mg/100 g) | 0.066 ± 0.03 eX | 0.06 ± 0.03 gY | 0.051 ± 0.03 hY | 0.058 ± 0.03 gX | 0.065 ± 0.03 fY | 0.104 ± 0.05 bX | 0.072 ± 0.04 dY | 0.092 ± 0.05 cX | 0.119 ± 0.06 a | 0.062 ± 0.03 g |
| Zinc (mg/100 g) | 0.07 ± 0.04 cX | 0.06 ± 0.03 cX | 0.13 ± 0.07 aX | 0.09 ± 0.05 bY | 0.13 ± 0.07 aX | 0.13 ± 0.07 aX | 0.09 ± 0.05 bY | 0.09 ± 0.05 bY | 0.09 ± 0.05 b | 0.08 ± 0.04 c |
| Sugar (g/100 g) | Kordia® | ‘Lapins’ | Sweetheart® | Staccato® | ‘Bing’ | ‘Rainier’ | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| Fresh | Processed | Fresh | Processed | Fresh | Processed | Fresh | Processed | Processed | Processed | |
| Glucose | 7.3 ± 0.15 bX | 7.3 ± 0.15 bX | 7.1 ± 0.14 bX | 6.6 ± 0.13 cX | 7.9 ± 0.16 aX | 7.5 ± 0.15 bX | 8.0 ± 0.16 aX | 7.4 ± 0.15 bY | 6.8 ± 0.14 c | 6.6 ± 0.13 c |
| Fructose | 6.5 ± 0.13 aX | 6.5 ± 0.13 aX | 6.2 ±0.12 aX | 5.7 ± 0.11 bX | 6.6 ± 0.13 aX | 6.0 ± 0.12 bY | 6.6 ± 0.13 aX | 6.2 ± 0.12 aX | 6.1 ± 0.12 b | 5.8 ± 0.12 c |
| Maltose | <0.1 | <0.1 | <0.1 | <0.1 | <0.1 | <0.1 | <0.1 | <0.1 | <0.1 | <0.1 |
| Sucrose | <0.1 | <0.1 | <0.1 | <0.1 | <0.1 | <0.1 | <0.1 | <0.1 | <0.1 | <0.1 |
| Galactose | <0.1 | <0.1 | <0.1 | <0.1 | <0.1 | <0.1 | <0.1 | <0.1 | <0.1 | <0.1 |
| Total sugar | 13.8 ± 0.28 aX | 13.8 ± 0.28 aX | 13.2 ± 0.26 bX | 12.2 ± 0.24 bX | 14.5 ± 0.29 aX | 13.1 ± 0.26 bY | 14.5 ± 0.29 aX | 13.6 ± 0.27 aX | 12.9 ± 0.26 c | 12.4 ± 0.25 c |
| Amino Acid (mg/g) | Kordia® | ‘Lapins’ | Sweetheart® | Staccato® | Bing’ | ‘Rainier’ | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| Fresh | Processed | Fresh | Processed | Fresh | Processed | Fresh | Processed | Processed | Processed | |
| Aspartic Acid | 3.75 ± 0.19 aX | 2.19 ± 0.11 cY | 1.52 ± 0.08 eY | 2.11 ± 0.11 cX | 3.61 ± 0.18 aX | 1.74 ± 0.09 dY | 1.59 ± 0.08 bY | 2.0 ± 0.1 cX | 3.01 ± 0.15 b | 2.79 ± 0.14 b |
| Threonine | 0.24 ± 0.01 aX | 0.22 ± 0.01 aY | 0.18 ± 0.01 cY | 0.21 ± 0.01 aX | 0.24 ± 0.01 aX | 0.22 ± 0.01 aX | 0.23 ± 0.01 aX | 0.21 ± 0.01 aX | 0.19 ± 0.01 b | 0.2 ± 0.01 b |
| Serine | 0.28 ± 0.01 aX | 0.25 ± 0.01 aX | 0.2 ± 0.01 cY | 0.25 ± 0.01 aX | 0.26 ± 0.01 aX | 0.25 ± 0.01 aX | 0.28 ± 0.01 aX | 0.24 ± 0.01 aX | 0.22 ± 0.01 b | 0.21 ± 0.01 b |
| Glutamic Acid | 0.65 ± 0.03 aX | 0.56 ± 0.03 bY | 0.44 ± 0.02 dY | 0.52 ± 0.03 bX | 0.62 ± 0.03 aX | 0.54 ± 0.03 bY | 0.53 ± 0.03 bX | 0.51 ± 0.03 bX | 0.59 ± 0.03 a | 0.52 ± 0.03 c |
| Proline | 0.49 ± 0.02 bX | 0.40 ± 0.02 dY | 0.33 ± 0.02 eX | 0.33 ± 0.02 eX | 0.57 ± 0.03 aX | 0.39 ± 0.02 dY | 0.45 ± 0.02 cX | 0.40 ± 0.02 cX | 0.44 ± 0.02 c | 0.35 ± 0.02 e |
| Glycine | 0.26 ± 0.01 aX | 0.25 ± 0.01 aX | 0.23 ± 0.01 bY | 0.24 ± 0.01 aX | 0.25 ± 0.01 aX | 0.25 ± 0.01 aX | 0.26 ± 0.01 aX | 0.24 ± 0.01 aX | 0.22 ± 0.01 b | 0.21 ± 0.01 b |
| Alanine | 0.24 ± 0.01 aX | 0.24 ± 0.01 aX | 0.2 ± 0.01 bY | 0.23 ± 0.01 aX | 0.23 ± 0.01 bY | 0.24 ± 0.01 aX | 0.24 ± 0.01 aX | 0.23 ± 0.01 aX | 0.21 ± 0.01 b | 0.21 ± 0.01 b |
| Valine | 0.23 ± 0.01 aX | 0.23 ± 0.01 aX | 0.2 ± 0.01 bY | 0.22 ± 0.01 aX | 0.23 ± 0.01 aX | 0.23 ± 0.01 aX | 0.23 ± 0.01 aX | 0.22 ± 0.01 aX | 0.2 ± 0.01 b | 0.2 ± 0.01 b |
| Isoleucine | 0.33 ± 0.02 aX | 0.3 ± 0.01 aX | 0.23 ± 0.01 cY | 0.29 ± 0.01 aX | 0.3 ± 0.02 aX | 0.26 ± 0.01 bY | 0.31 ± 0.02 aX | 0.25 ± 0.01 bY | 0.25 ± 0.01 b | 0.26 ± 0.01 b |
| Leucine | 0.30 ± 0.02 aX | 0.31 ± 0.02 aX | 0.27 ± 0.01 aX | 0.28 ± 0.01 aX | 0.29 ± 0.01 aX | 0.30 ± 0.02 aX | 0.30 ± 0.02 aX | 0.29 ± 0.01 aX | 0.26 ± 0.01 b | 0.26 ± 0.01 b |
| Tyrosine | 0.19 ± 0.01 aX | 0.18 ± 0.01 aX | 0.16 ± 0.01 aY | 0.18 ± 0.01 aX | 0.19 ± 0.01 aX | 0.18 ± 0.01 aX | 0.19 ± 0.01 aX | 0.18 ± 0.01 aX | 0.16 ± 0.01 Y | 0.16 ± 0.01 Y |
| Phenylalanine | 0.21 ± 0.01 aX | 0.21 ± 0.01 aX | 0.18 ± 0.01 bY | 0.21 ± 0.01 aX | 0.21 ± 0.01 aX | 0.21 ± 0.01 aX | 0.22 ± 0.01 aX | 0.21 ± 0.01 aX | 0.18 ± 0.01 bY | 0.18 ± 0.01 bY |
| Histidine | 0.13 ± 0.01 aX | 0.12 ± 0.01 aX | 0.09 ± 0.01 bY | 0.10 ± 0.01 aX | 0.13 ± 0.01 aX | 0.11 ± 0.01 aX | 0.11 ± 0.01 aX | 0.11 ± 0.01 aX | 0.10 ± 0.01 a | 0.10 ± 0.01 a |
| Lysine | 0.30 ± 0.02 aX | 0.31 ± 0.02 aX | 0.27 ± 0.01 bY | 0.28 ± 0.02 aX | 0.31 ± 0.02 aX | 0.31 ± 0.02 aX | 0.30 ± 0.02 aX | 0.30 ± 0.02 aX | 0.26 ± 0.01 b | 0.26 ± 0.01 b |
| Arginine | 0.21 ± 0.01 aX | 0.20 ± 0.01 aX | 0.17 ± 0.01 bX | 0.19 ± 0.01 aX | 0.20 ± 0.01 aX | 0.19 ± 0.01 aX | 0.20 ± 0.01 aX | 0.18 ± 0.01 aX | 0.18 ± 0.01 a | 0.17 ± 0.01 a |
| Cysteine | 0.08 ± 0.01 bY | 0.07 ± 0.01 bY | 0.07 ± 0.01 bY | 0.07 ± 0.01 bY | 0.11 ± 0.01 aX | 0.10 ± 0.01 aX | 0.10 ± 0.01 aX | 0.09 ± 0.01 aX | 0.05 ± 0.01 c | 0.06 ± 0.01 c |
| Methionine | 0.12 ± 0.01 cX | 0.11 ± 0.01 cX | 0.11 ± 0.01 cX | 0.13 ± 0.01 aX | 0.13 ± 0.01 bX | 0.18 ± 0.01 aX | 0.15 ± 0.01 aX | 0.15 ± 0.01 aX | 0.12 ± 0.01 c | 0.12 ± 0.01 c |
| Tryptophan | 0.06 ± 0.01 bY | 0.04 ± 0.01 bY | 0.04 ± 0.01 bY | 0.05 ± 0.01 bY | 0.09 ± 0.01 aX | 0.05 ± 0.01 bY | 0.05 ± 0.01 bY | 0.09 ± 0.01 aX | 0.05 ± 0.01 b | 0.05 ± 0.01 b |
| Phenolic Metabolite (mg/100 g FW) | Kordia® | ‘Lapins’ | Staccato® | Sweetheart® | ‘Bing’ | ‘Rainier’ | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| Fresh | Processed | Fresh | Processed | Fresh | Processed | Fresh | Processed | Processed | Processed | |
| Hydroxycinnamic acid | ||||||||||
| 3,5-Dicaffeoylquinic acid | 1.85 | 1.83 | 1.99 | 2.29 | 2.28 | 2.43 | 1.61 | 2.02 | 2.70 | 1.16 |
| 3-p-Coumaroyl quinic acid | 21.41 | 34.21 | 3.70 | 5.35 | 26.27 | 22.57 | 15.93 | 19.00 | 6.68 | 1.95 |
| Chlorogenic acid | 2.27 | 3.68 | 2.14 | 3.21 | 3.15 | 3.00 | 1.86 | 3.02 | 3.52 | 1.79 |
| Ferulic acid | nd | nd | 0.09 | 0.11 | nd | nd | nd | nd | 0.08 | 0.08 |
| Neochlorogenic acid | 20.47 | 34.51 | 28.05 | 40.96 | 29.50 | 27.72 | 17.87 | 25.10 | 44.26 | 22.78 |
| trans-4-p-Coumaroyl quinic acid | nd | 0.63 | 0.08 | 0.12 | 0.99 | 0.96 | 0.47 | 0.85 | 0.12 | 0.05 |
| trans-5-p-Coumaroyl quinic acid | 0.13 | 0.16 | 0.08 | 0.14 | 0.11 | 0.13 | nd | 0.08 | 0.14 | 0.07 |
| Flavanol | ||||||||||
| Catechin | 1.32 | 1.76 | 0.60 | 0.65 | 0.84 | 0.83 | 0.57 | 0.81 | 0.83 | 0.34 |
| Epicatechin | 2.97 | 4.14 | 2.51 | 3.74 | 7.89 | 7.25 | 3.76 | 4.89 | 3.94 | 0.95 |
| Flavonol | ||||||||||
| Kaempferol 3-rutinoside | 0.45 | 0.80 | 0.20 | 0.45 | 0.61 | 0.55 | 0.29 | 0.57 | 0.47 | 0.64 |
| Quercetin 3-galactoside | nd | nd | nd | nd | nd | nd | nd | 0.01 | nd | nd |
| Quercetin 3-glucoside | 0.55 | 0.73 | 0.16 | 0.22 | 0.39 | 0.25 | 0.30 | 0.17 | 0.26 | 0.06 |
| Quercetin 3-rhamnoside | 0.03 | 0.06 | 0.01 | 0.03 | 0.04 | 0.03 | 0.02 | 0.02 | 0.03 | 0.02 |
| Quercetin 3-rutinoside | 3.45 | 4.34 | 1.99 | 2.38 | 3.24 | 2.68 | 2.66 | 2.93 | 2.46 | 1.03 |
| Flavanone | ||||||||||
| Sakuranetin | 0.03 | 0.03 | 0.00 | 0.01 | 0.00 | 0.00 | 0.01 | 0.01 | 0.00 | 0.01 |
| Sakuranin | 0.32 | 0.41 | 0.08 | 0.12 | 0.19 | 0.11 | 0.09 | 0.09 | 0.10 | 0.08 |
| Procyanidin | ||||||||||
| Procyanidin B1 | 0.94 | 1.28 | 0.46 | 0.48 | 1.04 | 1.00 | 0.60 | 0.91 | 0.59 | 0.14 |
| Procyanidin B2 | 1.14 | 1.70 | 1.03 | 1.56 | 4.16 | 3.34 | 1.76 | 2.80 | 1.50 | 0.37 |
| Procyanidin B5 | 0.21 | 0.29 | 0.16 | 0.24 | 0.57 | 0.44 | 0.22 | 0.31 | 0.22 | 0.07 |
| Procyanidin B7 | 0.32 | 0.43 | 0.16 | 0.21 | 0.44 | 0.36 | 0.22 | 0.24 | 0.21 | 0.06 |
| Anthocyanin | ||||||||||
| Cyanidin 3-glucoside | 6.73 | 8.81 | 3.14 | 2.93 | 8.77 | 6.19 | 7.39 | 3.85 | 3.97 | nd |
| Cyanidin 3-rutinoside | 48.44 | 58.77 | 45.90 | 40.16 | 68.30 | 66.33 | 65.98 | 80.42 | 46.55 | 1.26 |
| Peonidin 3-rutinoside | 3.09 | 3.28 | 4.28 | 4.04 | 6.28 | 6.48 | 8.83 | 10.13 | 4.29 | nd |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rashidinejad, A.; Ahmmed, F.; Lister, C.; Stoklosinski, H. Functional Potential of Sweet Cherry Cultivars Grown in New Zealand: Effects of Processing on Nutritional and Bioactive Properties. Foods 2025, 14, 3749. https://doi.org/10.3390/foods14213749
Rashidinejad A, Ahmmed F, Lister C, Stoklosinski H. Functional Potential of Sweet Cherry Cultivars Grown in New Zealand: Effects of Processing on Nutritional and Bioactive Properties. Foods. 2025; 14(21):3749. https://doi.org/10.3390/foods14213749
Chicago/Turabian StyleRashidinejad, Ali, Fatema Ahmmed, Carolyn Lister, and Halina Stoklosinski. 2025. "Functional Potential of Sweet Cherry Cultivars Grown in New Zealand: Effects of Processing on Nutritional and Bioactive Properties" Foods 14, no. 21: 3749. https://doi.org/10.3390/foods14213749
APA StyleRashidinejad, A., Ahmmed, F., Lister, C., & Stoklosinski, H. (2025). Functional Potential of Sweet Cherry Cultivars Grown in New Zealand: Effects of Processing on Nutritional and Bioactive Properties. Foods, 14(21), 3749. https://doi.org/10.3390/foods14213749





