Glycyrrhiza glabra L. Extracts Prevent LPS-Induced Inflammation in RAW264.7 Cells by Targeting Pro-Inflammatory Cytokines, Mediators and the JAK/STAT Signaling Pathway
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Plant Material Recovery, Extraction Procedure and Hydrolysis Process
2.3. HPLC Analyses
2.4. Cell Culture
2.5. Cell Viability (SRB) Assay
2.6. Cytokine Measurements
2.7. Nitric Oxide (NO) Production Inhibition
2.8. Western Blot Analysis
2.9. Statistical Analyses
3. Results and Discussion
3.1. HPLC Analyses
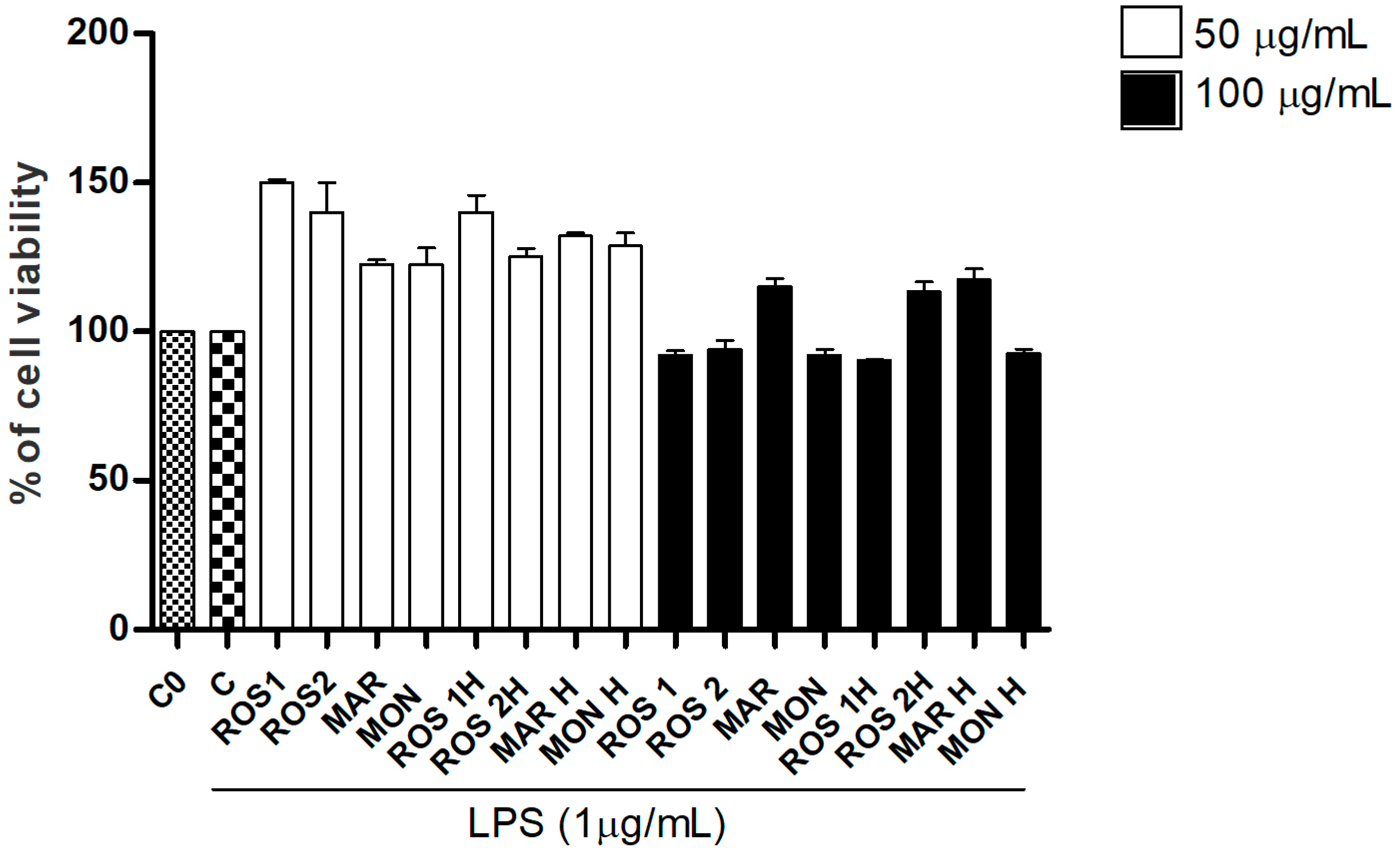
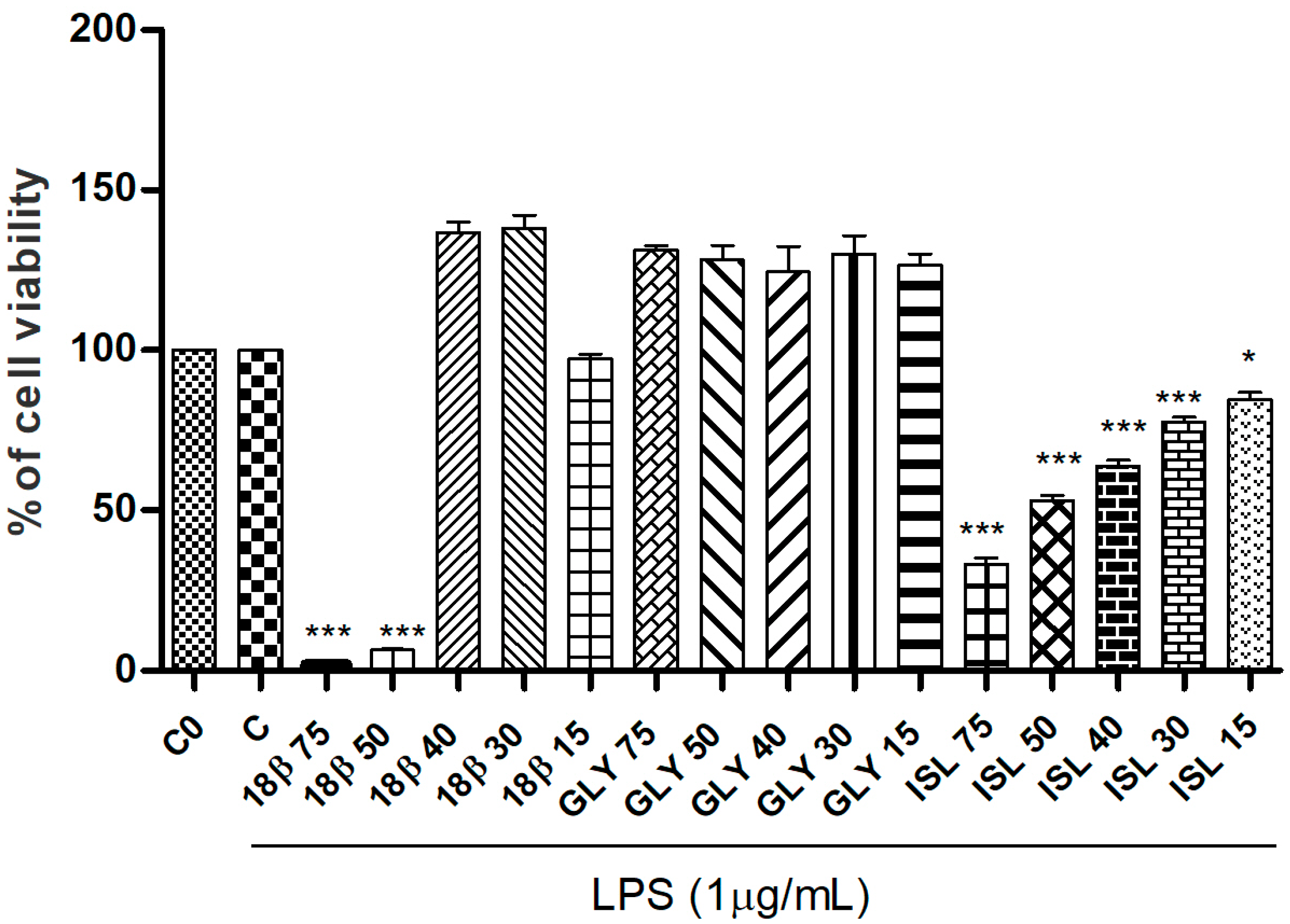
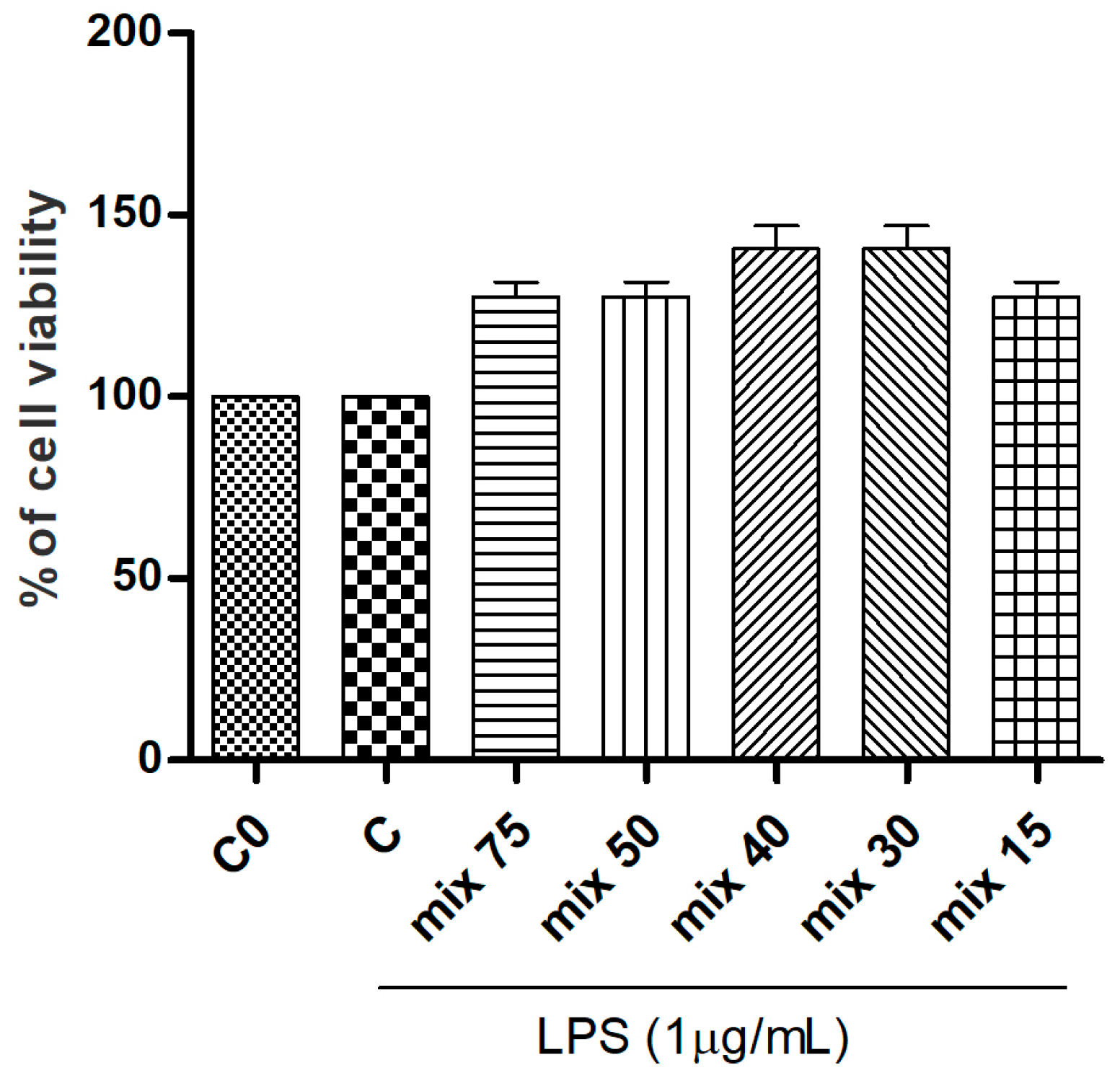
3.2. Effects of G. glabra L. Extracts and Standards in RAW264.7 Cell Viability
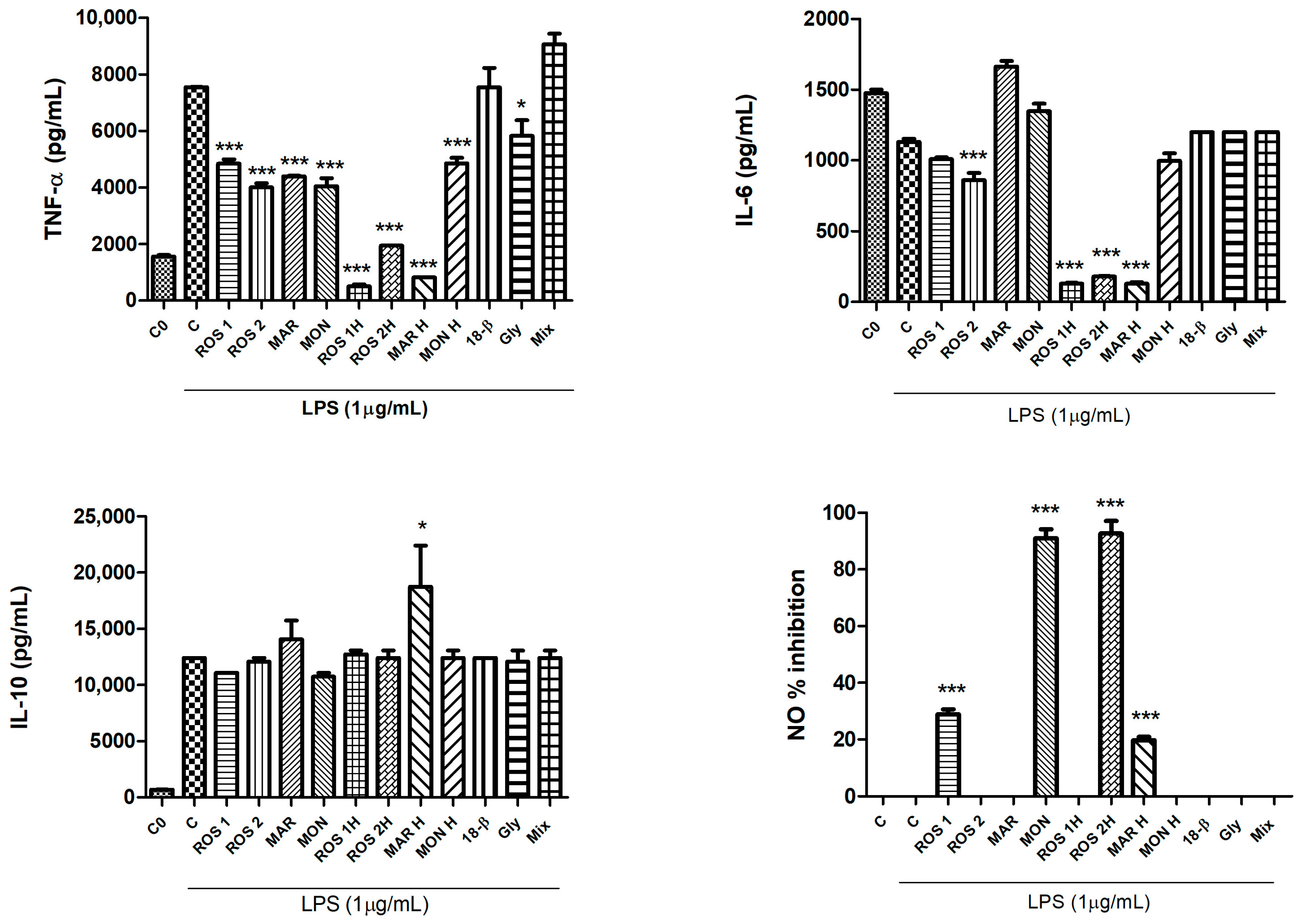
3.3. Effects of G. glabra L. Extracts and Standards on LPS-Induced Production of Pro-Inflammatory Cytokines (TNF-α, IL-6), the NO Mediator and the Induction of the Anti-Inflammatory Cytokine (IL-10)
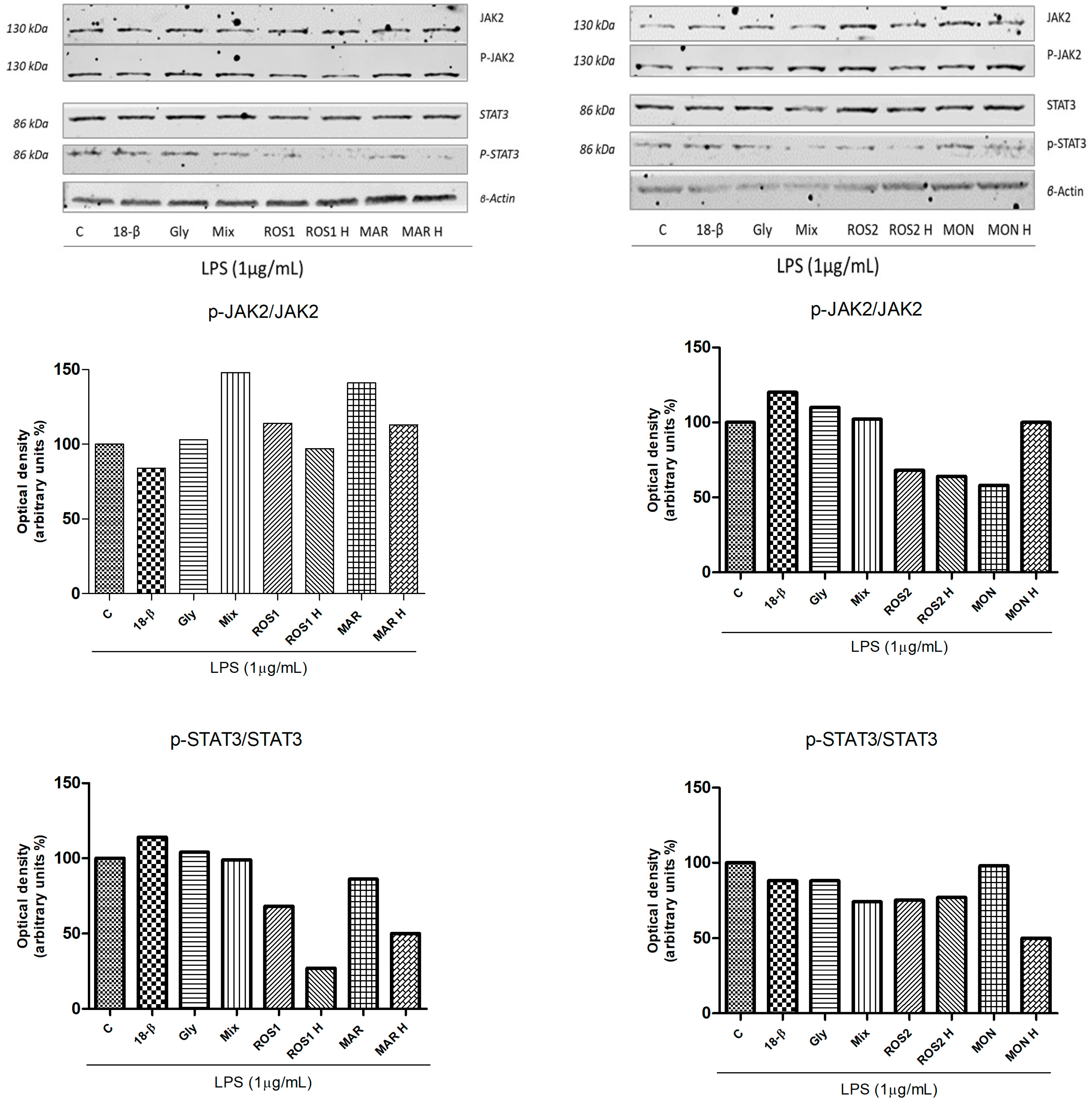
3.4. Effects of G. glabra L. Extracts and Standards on LPS-Induced Activation of the JAK/STAT Signaling Pathway
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| EMA | European Medicine Agency |
| NSAIDs | Non-steroidal anti-inflammatory drugs |
| TNF-α | Tumor Necrosis Factor-α |
| IL-6 | Interleukin-6 |
| NO | Nitric Oxide |
| IL-10 | Interleukin-10 |
| LPS | Lipopolysaccharide |
| JAK2 | Janus Kinase |
| STAT-3 | Signal Transducer and Activator of Transcription 3 |
| 11β-HSD2 | 11 beta-hydroxysteroid dehydrogenase |
| FDA | Food and Drug Administration |
| GRAS | Generally Recognized As Safe |
| ADI | Admitted Daily Intake |
| HPLC | High Performance Liquid Chromatography |
| DMEM | Dulbecco’s Modified Eagle’s Medium |
| FBS | Fetal Bovin Serum |
| PBS | Phosphate Buffered Saline |
| BSA | Bovine Serum Albumin |
| TCA | Trichloroacetic Acid |
| SRB | Sulphorhodamine B |
| ISL | Isoliquiritigenin |
| Gly | Glycyrrhizin |
| 18-β | 18-β glycyrrhetinic acid |
| PGE-2 | Prostaglandin E2 |
| TXB-2 | Thromboxane B2 |
| LTB-4 | Leukotriene B4 |
| COX-2 | Cyclooxygenase-2 |
| iNOS | Inducible Nitrix Oxide Synthase |
| ROS | Reactive Oxygen Species |
References
- Narayanankutty, A.; Famurewa, A.C.; Oprea, E. Natural bioactive compounds and human health. Molecules 2024, 29, 3372. [Google Scholar] [CrossRef]
- Walia, A.; Gupta, A.K.; Sharma, V. Role of bioactive compounds in human health. Acta Sci. Med. Sci. 2019, 3, 25–33. [Google Scholar]
- Samtiya, M.; Aluko, R.E.; Dhewa, T.; Moreno-Rojas, J.M. Potential health benefits of plant food-derived bioactive components: An overview. Foods 2021, 10, 839. [Google Scholar] [CrossRef]
- Najmi, A.; Javed, S.A.; Al Bratty, M.; Alhazmi, H.A. Modern approaches in the discovery and development of plant-based natural products and their analogues as potential therapeutic agents. Molecules 2022, 27, 349. [Google Scholar] [CrossRef]
- Hossain, M.S.; Wazed, M.A.; Asha, S.; Amin, M.R.; Shimul, I.M. Dietary phytochemicals in health and disease: Mechanisms, clinical evidence, and applications—A comprehensive review. Food Sci. Nutr. 2025, 13, e70101. [Google Scholar] [CrossRef]
- Sharma, V.; Katiyar, A.; Agrawal, R.C. Glycyrrhiza glabra: Chemistry and Pharmacological Activity. In Sweeteners; Springer: Cham, Switzerland, 2018; pp. 87–100. [Google Scholar]
- Hasan, M.K.; Ara, I.; Mondal, M.S.A.; Kabir, Y. Phytochemistry, pharmacological activity, and potential health benefits of Glycyrrhiza glabra. Heliyon 2021, 7, e07240. [Google Scholar] [CrossRef] [PubMed]
- El-Saber Batiha, G.; Magdy Beshbishy, A.; El-Mleeh, A.; M. Abdel-Daim, M.; Prasad Devkota, H. Traditional uses, bioactive chemical constituents, and pharmacological and toxicological activities of Glycyrrhiza glabra L. (Fabaceae). Biomolecules 2020, 10, 352. [Google Scholar] [CrossRef]
- Sidhu, P.; Shankargouda, S.; Rath, A.; Ramamurthy, P.H.; Fernandes, B.; Singh, A.K. Therapeutic benefits of liquorice in dentistry. J. Ayurveda Integr. Med. 2020, 11, 82–88. [Google Scholar] [CrossRef]
- European Medicines Agency. Draft European Union Herbal Monograph on Glycyrrhiza glabra L.; Gycyrrhiza inflata Bat.; Glycyrrhiza uralensis Fisch., Radix—Revision 1. Available online: https://www.ema.europa.eu/en/medicines/herbal/liquiritiae-radix (accessed on 12 October 2025).
- Isbrucker, R.A.; Burdock, G.A. Risk and safety assessment on the consumption of Licorice root (Glycyrrhiza sp.), its extract and powder as a food ingredient, with emphasis on the pharmacology and toxicology of glycyrrhizin. Regul. Toxicol. Pharmacol. 2006, 46, 167–192. [Google Scholar] [CrossRef]
- Richard, S.A. Exploring the pivotal immunomodulatory and anti-inflammatory potentials of glycyrrhizic and glycyrrhetinic acids. Mediat. Inflamm. 2021, 1, 6699560. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Shang, E.; Zhao, J.; Fan, X.; Duan, J.; Qian, D.; Tao, W.; Tang, Y. Data mining and frequency analysis for licorice as a “Two-Face” herb in Chinese Formulae based on Chinese Formulae Database. Phytomedicine 2014, 21, 1281–1286. [Google Scholar] [CrossRef]
- Peng, F.; Du, Q.; Peng, C.; Wang, N.; Tang, H.; Xie, X.; Shen, J.; Chen, J. A review: The pharmacology of isoliquiritigenin. Phytother. Res. 2015, 29, 969–977. [Google Scholar] [CrossRef]
- Crusz, S.M.; Balkwill, F.R. Inflammation and cancer: Advances and new agents. Nat. Rev. Clin. Oncol. 2015, 12, 584–596. [Google Scholar] [CrossRef]
- Gurpinar, E.; Grizzle, W.E.; Piazza, G.A. COX-independent mechanisms of cancer chemoprevention by anti-inflammatory drugs. Front. Oncol. 2013, 3, 181. [Google Scholar] [CrossRef]
- Groner, B.; von Manstein, V. Jak Stat signaling and cancer: Opportunities, benefits and side effects of targeted inhibition. Mol. Cell. Endocrinol. 2017, 451, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Jang, S.; Lee, A.Y.; Lee, A.R.; Choi, G.; Kim, H.K. Optimization of ultrasound-assisted extraction of glycyrrhizic acid from licorice using response surface methodology. Integr. Med. Res. 2017, 6, 388–394. [Google Scholar] [CrossRef]
- Zhang, Y.; Cao, J.; Wang, Y.; Xiao, S. Simultaneous determination of glycyrrhizin and 15 flavonoids in licorice and blood by high performance liquid chromatography with ultraviolet detector. Int. Sch. Res. Not. 2013, 2013, 786151. [Google Scholar] [CrossRef]
- Conforti, F.; Perri, M.R.; Guerrini, A.; Sacchetti, G.; Statti, G. Lavandula austroapennina and Lavandula angustifolia essential oils and bioactive components: In vitro anti-denaturation effect of lavender from the Pollino massif (Southern Italy). Plant Biosyst. 2023, 157, 339–345. [Google Scholar] [CrossRef]
- Mazzei, R.; Piacentini, E.; Nardi, M.; Poerio, T.; Bazzarelli, F.; Procopio, A.; Di Gioia, M.L.; Rizza, P.; Ceraldi, L.; Morelli, C.; et al. Production of plant-derived oleuropein aglycone by a combined membrane process and evaluation of its breast anticancer properties. Front. Bioeng. Biotechnol. 2020, 8, 908. [Google Scholar] [CrossRef]
- Perri, M.R.; Pellegrino, M.; Marrelli, M.; Aquaro, S.; Cavaliere, F.; Grande, F.; Occhiuzzi, M.A.; Lupia, C.; Toma, C.C.; Conforti, F.; et al. Identification of Pinosylvin in Pinus nigra subsp. laricio: A Naturally Occurring Stilbenoid Suppressing LPS-Induced Expression of Pro-Inflammatory Cytokines and Mediators and Inhibiting the JAK/STAT Signaling Pathway. Pharmaceuticals 2023, 16, 718. [Google Scholar] [CrossRef] [PubMed]
- Musolino, V.; Marrelli, M.; Perri, M.R.; Palermo, M.; Gliozzi, M.; Mollace, V.; Conforti, F. Centranthus ruber (L.) DC. and Tropaeolum majus L.: Phytochemical Profile, In Vitro Anti-Denaturation Effects and Lipase Inhibitory Activity of Two Ornamental Plants Traditionally Used as Herbal Remedies. Molecules 2023, 28, 32. [Google Scholar] [CrossRef]
- Perri, M.R.; Pellegrino, M.; Aquaro, S.; Cavaliere, F.; Lupia, C.; Uzunov, D.; Marrelli, M.; Conforti, F.; Statti, G. Cachrys spp. from Southern Italy: Phytochemical Characterization and JAK/STAT Signaling Pathway Inhibition. Plants 2022, 11, 2913. [Google Scholar] [CrossRef]
- Esmaeili, H.; Karami, A.; Hadian, J.; Saharkhiz, M.J.; Ebrahimi, S.N. Variation in the phytochemical contents and antioxidant activity of Glycyrrhiza glabra populations collected in Iran. Ind. Crops Prod. 2019, 137, 248–259. [Google Scholar] [CrossRef]
- Eghlima, G.; Tafreshi, Y.M.; Aghamir, F.; Ahadi, H.; Hatami, M. Regional environmental impacts on growth traits and phytochemical profiles of Glycyrrhiza glabra L. for enhanced medicinal and industrial use. BMC Plant Biol. 2025, 25, 116. [Google Scholar] [CrossRef] [PubMed]
- Graebin, C.S. The Pharmacological Activities of Glycyrrhizinic Acid (“Glycyrrhizin”) and Glycyrrhetinic Acid. In Sweeteners; Springer: Cham, Switzerland, 2018; pp. 245–261. [Google Scholar]
- Li, C.; Eom, T.; Jeong, Y. Glycyrrhiza glabra (L.) extract inhibits LPS-induced inflammation in RAW macrophages. J. Nutr. Sci. Vitaminol. 2015, 61, 375–381. [Google Scholar] [CrossRef]
- Thiyagarajan, P.; Chandrasekaran, C.V.; Deepak, H.B.; Agarwal, A. Modulation of lipopolysaccharide-induced pro-inflammatory mediators by an extract of Glycyrrhiza glabra and its phytoconstituents. Inflammopharmacology 2011, 19, 235–241. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.K.; Oh, S.M.; Kwon, H.S.; Oh, Y.S.; Lim, S.S.; Shin, H.K. Anti-inflammatory effect of roasted licorice extracts on lipopolysaccharide-induced inflammatory responses in murine macrophages. Biochem. Biophys. Res. Commun. 2006, 345, 1215–1223. [Google Scholar] [CrossRef]
- Bisht, D.; Rashid, M.; Arya, R.K.K.; Kumar, D.; Chaudhary, S.K.; Rana, V.S.; Sethiya, N.K. Revisiting liquorice (Glycyrrhiza glabra L.) as anti-inflammatory, antivirals and immunomodulators: Potential pharmacological applications with mechanistic insight. Phytomed. Plus 2022, 2, 100206. [Google Scholar] [CrossRef]
- Wang, H.L.; Li, Y.X.; Niu, Y.T.; Zheng, J.; Wu, J.; Shi, G.J.; Ma, L.; Niu, Y.; Sun, T.; Yu, J.Q. Observing anti-inflammatory and anti-nociceptive activities of glycyrrhizin through regulating COX-2 and pro-inflammatory cytokines expressions in mice. Inflammation 2015, 38, 2269–2278. [Google Scholar] [CrossRef]
- Bodet, C.; La, V.D.; Gafner, S.; Bergeron, C.; Grenier, D. A licorice extract reduces lipopolysaccharide-induced proinflammatory cytokine secretion by macrophages and whole blood. J. Periodontol. 2008, 79, 1752–1761. [Google Scholar] [CrossRef]
- Wang, C.Y.; Kao, T.C.; Lo, W.H.; Yen, G.C. Glycyrrhizic acid and 18β-glycyrrhetinic acid modulate lipopolysaccharide-induced inflammatory response by suppression of NF-κB through PI3K p110δ and p110γ inhibitions. J. Agric. Food Chem. 2011, 59, 7726–7733. [Google Scholar] [CrossRef]
- Zhou, J.X.; Wink, M. Evidence for anti-inflammatory activity of isoliquiritigenin, 18β glycyrrhetinic acid, ursolic acid, and the traditional Chinese medicine plants Glycyrrhiza glabra and Eriobotrya japonica, at the molecular level. Medicines 2019, 6, 55. [Google Scholar] [CrossRef] [PubMed]
- Han, M.H.; Lee, M.H.; Hong, S.H.; Choi, Y.H.; Moon, J.S.; Song, M.K.; Kim, M.J.; Shin, S.J.; Hwang, H.J. Comparison of anti-inflammatory activities among ethanol extracts of Sophora flavescens, Glycyrrhiza uralensis and Dictamnus dasycarpus, and their mixtures in RAW 246.7 murine macrophages. J. Life Sci. 2014, 24, 329–335. [Google Scholar] [CrossRef]
- Yoon, T.S.; Cheon, M.S.; Kim, S.J.; Lee, A.; Moon, B.C.; Chun, J.M.; Choo, B.K.; Kim, H.K. Evaluation of solvent extraction on the anti-inflammatory efficacy of Glycyrrhiza uralensis. Korean J. Med. Crop Sci. 2010, 18, 28–33. [Google Scholar]
- Wu, T.Y.; Khor, T.O.; Saw, C.L.L.; Loh, S.C.; Chen, A.I.; Lim, S.S.; Park, J.H.Y.; Kong, A.N.T. Anti-inflammatory/Anti-oxidative stress activities and differential regulation of Nrf2-mediated genes by non-polar fractions of tea Chrysanthemum zawadskii and licorice Glycyrrhiza uralensis. AAPS J. 2011, 13, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Nunes, C.D.R.; Barreto Arantes, M.; de Menezes, F.P.S.; da Leandro, C.L.; de Souza, P.M.; de Pereira, M.L.; Vieira, I.J.C.; de Barros, O.D. Plants as sources of anti-inflammatory agents. Molecules 2020, 25, 3726. [Google Scholar] [CrossRef] [PubMed]
- Montoro, P.; Maldini, M.; Russo, M.; Postorino, S.; Piacente, S.; Pizza, C. Metabolic profiling of roots of liquorice (Glycyrrhiza glabra) from different geographical areas by ESI/MS/MS and determination of major metabolites by LC-ESI/MS and LC-ESI/MS/MS. J. Pharm. Biomed. Anal. 2011, 54, 535–544. [Google Scholar] [CrossRef]
- Semenescu, I.; Avram, S.; Similie, D.; Minda, D.; Diaconeasa, Z.; Muntean, D.; Lazar, A.E.; Gurgus, D.; Danciu, C. Phytochemical, Antioxidant, Antimicrobial and Safety Profile of Glycyrrhiza glabra L. Extract Obtained from Romania. Plants 2024, 13, 3265. [Google Scholar] [CrossRef]
| G. glabra Sample Origin | Material | Raw/Hydrolyzed Extracts Codes | Coordinates |
|---|---|---|---|
| Montalto Uffugo (CS), Italy | Collected | MON/MON H | 39°24′ N; 16°09′ E |
| Rossano (CS), Italy | Commercially available | ROS1/ROS1 H | - |
| Rossano (CS), Italy | Commercially available | ROS2/ROS2 H | - |
| Morocco | Commercially available | MAR/MAR H | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Perri, M.R.; Pellegrino, M.; Toma, C.-C.; Prezioso, P.; Tagliaferri, V.; Marrelli, M.; Conforti, F.; Statti, G. Glycyrrhiza glabra L. Extracts Prevent LPS-Induced Inflammation in RAW264.7 Cells by Targeting Pro-Inflammatory Cytokines, Mediators and the JAK/STAT Signaling Pathway. Foods 2025, 14, 3746. https://doi.org/10.3390/foods14213746
Perri MR, Pellegrino M, Toma C-C, Prezioso P, Tagliaferri V, Marrelli M, Conforti F, Statti G. Glycyrrhiza glabra L. Extracts Prevent LPS-Induced Inflammation in RAW264.7 Cells by Targeting Pro-Inflammatory Cytokines, Mediators and the JAK/STAT Signaling Pathway. Foods. 2025; 14(21):3746. https://doi.org/10.3390/foods14213746
Chicago/Turabian StylePerri, Maria Rosaria, Michele Pellegrino, Claudia-Crina Toma, Pierfrancesco Prezioso, Vincenzo Tagliaferri, Mariangela Marrelli, Filomena Conforti, and Giancarlo Statti. 2025. "Glycyrrhiza glabra L. Extracts Prevent LPS-Induced Inflammation in RAW264.7 Cells by Targeting Pro-Inflammatory Cytokines, Mediators and the JAK/STAT Signaling Pathway" Foods 14, no. 21: 3746. https://doi.org/10.3390/foods14213746
APA StylePerri, M. R., Pellegrino, M., Toma, C.-C., Prezioso, P., Tagliaferri, V., Marrelli, M., Conforti, F., & Statti, G. (2025). Glycyrrhiza glabra L. Extracts Prevent LPS-Induced Inflammation in RAW264.7 Cells by Targeting Pro-Inflammatory Cytokines, Mediators and the JAK/STAT Signaling Pathway. Foods, 14(21), 3746. https://doi.org/10.3390/foods14213746








