Optimising Green Pressurised Liquid Extraction and Sustainability Assessment of Carotenoid-Rich Extracts from Daucus carota L. Pomace
Abstract
1. Introduction
2. Materials and Methods
2.1. Standards and Materials
2.2. Samples
2.3. Optimisation of PLE Conditions by an Experimental Design
2.4. Qualitative and Quantitative Analysis of Carotenoids and Polar Fraction of Extract by HPLC-UV/Vis
2.5. Statistical Analysis
3. Results and Discussion
3.1. Evaluation of Chemical Composition of Carrot By-Product
3.2. Optimisation of Carotenoid Extraction by PLE
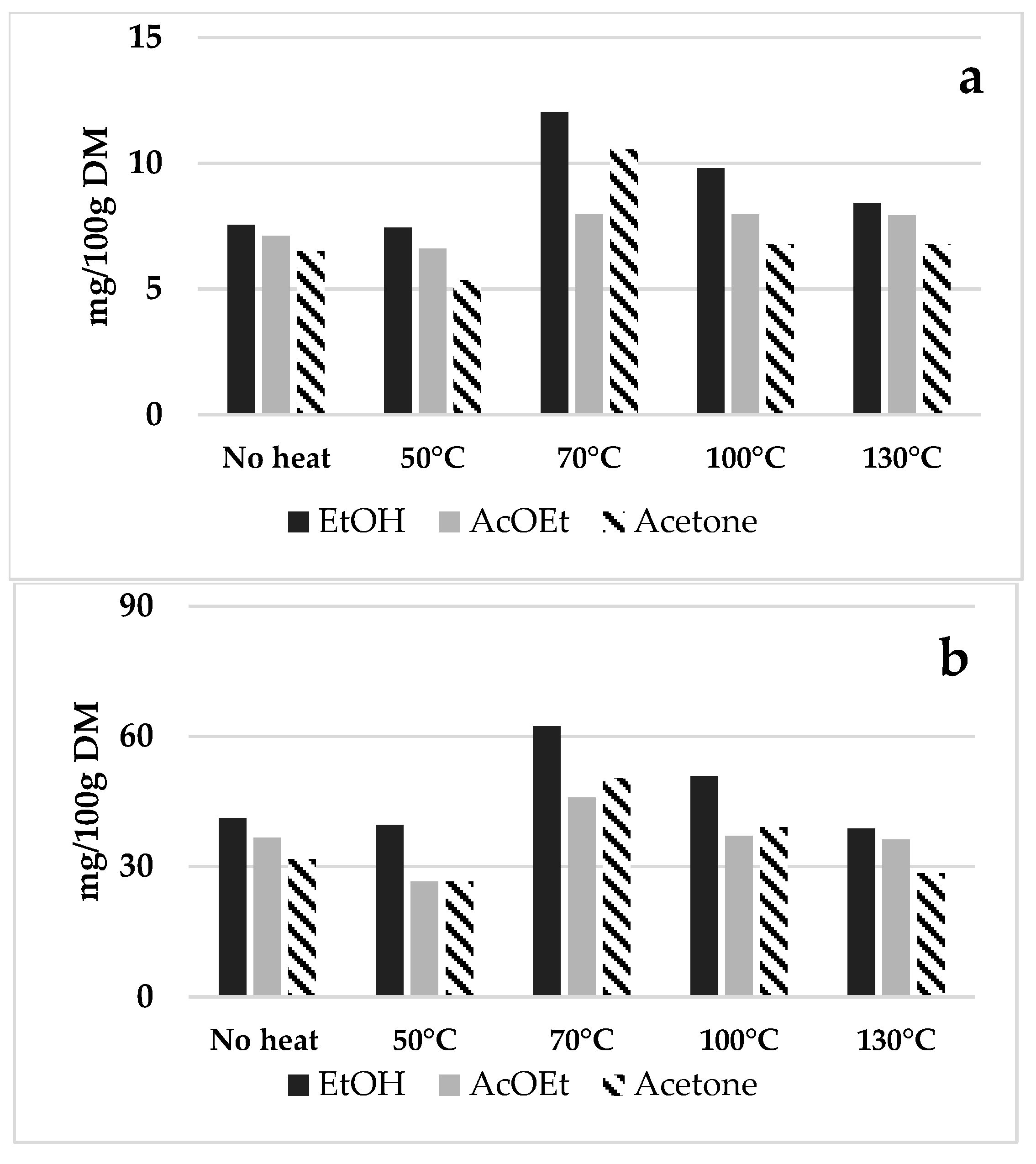
3.2.1. Preliminary Selection of Solvent Composition and Temperature
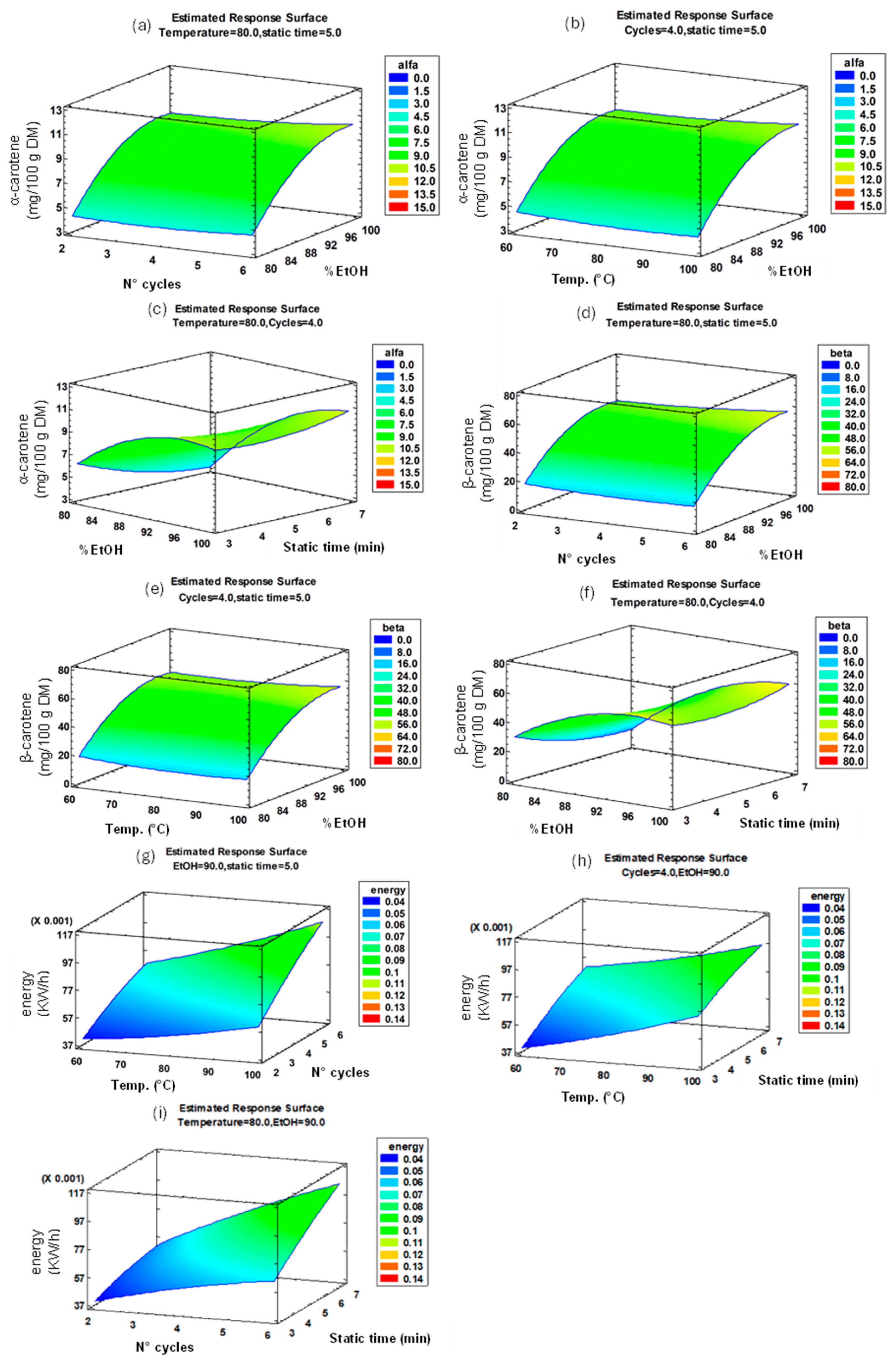
3.2.2. Response Surface Design of PLE Process
3.3. Quantitative Analysis of PLE Extract
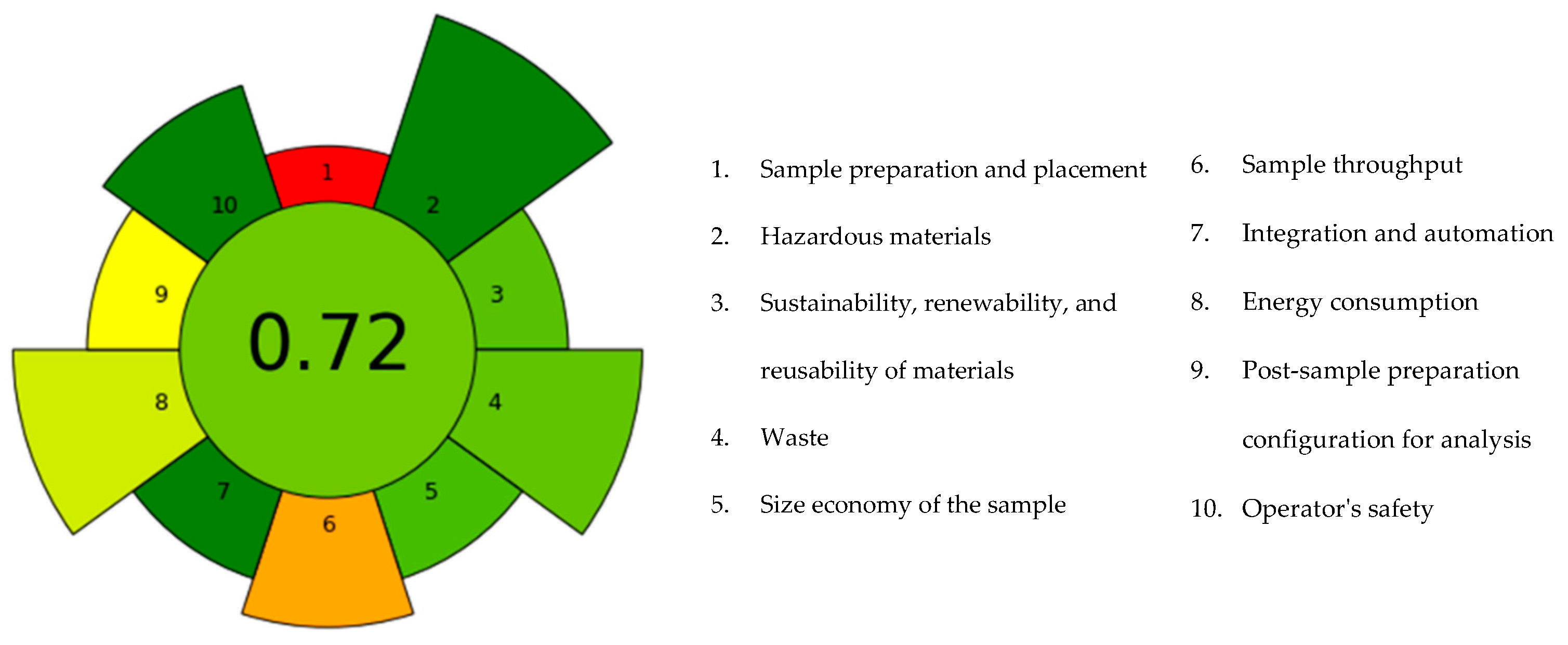
3.4. Greenness of PLE Optimised Process
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| MeCN | Acetonitrile |
| ANOVA | Analysis of variance |
| CCD | Central composite experimental design |
| EtOH | Ethanol |
| AcOEt | Ethyl acetate |
| HCOOH | Formic acid |
| GSP | Green sample preparation |
| Hex | Hexane |
| HRMS | High-resolution mass spectrometry |
| IPA | Isopropyl alcohol |
| MeOH | Methanol |
| PLE | Pressurised liquid extraction |
| GRAS | Recognised as safe |
| UPLC | Ultra-pressure liquid chromatography |
| H2O | Water |
| TEA | Techno-economic assessment |
References
- FAO. The State of Food and Agriculture 2019. Moving Forward on Food Loss and Waste Reduction; Food and Agriculture Organization of the United Nations: Rome, Italy, 2019. [Google Scholar]
- Santos, D.; Lopes da Silva, J.A.; Pintado, M. Fruit and vegetable by-products’ flours as ingredients: A review on production process, health benefits and technological functionalities. LWT 2022, 154, 112707. [Google Scholar] [CrossRef]
- Kultys, E.; Kurek, M.A. Green Extraction of Carotenoids from Fruit and Vegetable Byproducts: A Review. Molecules 2022, 27, 518. [Google Scholar] [CrossRef]
- Tiwari, S.; Upadhyay, N.; Singh, A.K.; Meena, G.S.; Arora, S. Organic solvent-free extraction of carotenoids from carrot bio-waste and its physico-chemical properties. J. Food Sci. Technol. 2019, 56, 4678–4687. [Google Scholar] [CrossRef]
- Sharma, H.K. Carrots production, processing, and nutritional quality. In Handbook of Vegetables and Vegetable Processing; Wiley: Hoboken, NJ, USA, 2018; Volume 2, pp. 589–608. [Google Scholar] [CrossRef]
- Singh, B.K.; Koley, T.K.; Maurya, A.; Singh, P.M.; Singh, B. Phytochemical and antioxidative potential of orange, red, yellow, rainbow and black coloured tropical carrots (Daucus carota subsp. sativus Schubl. & Martens). Physiol. Mol. Biol. Plants 2018, 24, 899–907. [Google Scholar] [CrossRef] [PubMed]
- Hager, T.J.; Howard, L.R. Processing Effects on Carrot Phytonutrients. Hortscience 2006, 41, 74–79. [Google Scholar] [CrossRef]
- Sharma, K.D.; Karki, S.; Thakur, N.S.; Attri, S. Chemical composition, functional properties and processing of carrot—A review. J. Food Sci. Technol. 2012, 49, 22–32. [Google Scholar] [CrossRef]
- Kalt, W. Effects of production and processing factors on major fruit and vegetable antioxidants. J. Food Sci. 2005, 70, R11–R19. [Google Scholar] [CrossRef]
- Saini, R.K.; Keum, Y.S. Carotenoid extraction methods: A review of recent developments. Food Chem. 2018, 240, 90–103. [Google Scholar] [CrossRef] [PubMed]
- Ilyas, M.; Rana, F.A.; Riaz, M. Apocarotenoids. In Carotenoids: Structure and Function in the Human Body; Springer: Berlin/Heidelberg, Germany, 2021; pp. 125–146. [Google Scholar] [CrossRef]
- Rivera-Madrid, R.; Carballo-Uicab, V.M.; Cárdenas-Conejo, Y.; Aguilar-Espinosa, M.; Siva, R. Overview of carotenoids and beneficial effects on human health. In Carotenoids: Properties, Processing and Applications; Academic Press: Cambridge, MA, USA, 2020; pp. 1–40. [Google Scholar] [CrossRef]
- Müller, L.; Caris-Veyrat, C.; Lowe, G.; Böhm, V. Lycopene and Its Antioxidant Role in the Prevention of Cardiovascular Diseases—A Critical Review. Crit. Rev. Food Sci. Nutr. 2016, 56, 1868–1879. [Google Scholar] [CrossRef]
- Eggersdorfer, M.; Wyss, A. Carotenoids in human nutrition and health. Arch. Biochem. Biophys. 2018, 652, 18–26. [Google Scholar] [CrossRef]
- Simkin, A.J. Carotenoids and Apocarotenoids in Planta: Their Role in Plant Development, Contribution to the Flavour and Aroma of Fruits and Flowers, and Their Nutraceutical Benefits. Plants 2021, 10, 2321. [Google Scholar] [CrossRef] [PubMed]
- Kaur, P.; Subramanian, J.; Singh, A. Green extraction of bioactive components from carrot industry waste and evaluation of spent residue as an energy source. Sci. Rep. 2022, 12, 16607. [Google Scholar] [CrossRef]
- Yara-Varón, E.; Fabiano-Tixier, A.S.; Balcells, M.; Canela-Garayoa, R.; Bily, A.; Chemat, F. Is it possible to substitute hexane with green solvents for extraction of carotenoids? A theoretical versus experimental solubility study. RSC Adv. 2016, 6, 27750–27759. [Google Scholar] [CrossRef]
- Cha, K.H.; Lee, H.J.; Koo, S.Y.; Song, D.G.; Lee, D.U.; Pan, C.H. Optimization of pressurized liquid extraction of carotenoids and chlorophylls from Chlorella vulgaris. J. Agric. Food Chem. 2010, 58, 793–797. [Google Scholar] [CrossRef]
- Mustafa, A.; Trevino, L.M.; Turner, C. Pressurized hot ethanol extraction of carotenoids from carrot by-products. Molecules 2012, 17, 1809–1818. [Google Scholar] [CrossRef]
- Mantiniotou, M.; Athanasiadis, V.; Kalompatsios, D.; Lalas, S.I. Optimization of carotenoids and other antioxidant compounds extraction from carrot peels using response surface methodology. Biomass 2024, 5, 3. [Google Scholar] [CrossRef]
- Amran, M.A.; Palaniveloo, K.; Fauzi, R.; Satar, N.M.; Mohidin, T.B.M.; Mohan, G.; Razak, S.A.; Arunasalam, M.; Nagappan, T.; Seelan, J.S.S. Value-Added Metabolites from Agricultural Waste and Application of Green Extraction Techniques. Sustainability 2021, 13, 11432. [Google Scholar] [CrossRef]
- Belwal, T.; Ezzat, S.M.; Rastrelli, L.; Bhatt, I.D.; Daglia, M.; Baldi, A.; Devkota, H.P.; Orhan, I.E.; Patra, J.K.; Das, G.; et al. A critical analysis of extraction techniques used for botanicals: Trends, priorities, industrial uses and optimization strategies. TrAC Trends Anal. Chem. 2018, 100, 82–102. [Google Scholar] [CrossRef]
- Figueroa, J.G.; Borrás-Linares, I.; Lozano-Sánchez, J.; Quirantes-Piné, R.; Segura-Carretero, A. Optimization of drying process and pressurized liquid extraction for recovery of bioactive compounds from avocado peel by-product. Electrophoresis 2018, 39, 1908–1916. [Google Scholar] [CrossRef] [PubMed]
- Mustafa, A.; Turner, C. Pressurized liquid extraction as a green approach in food and herbal plants extraction: A review. Anal. Chim. Acta 2011, 703, 8–18. [Google Scholar] [CrossRef] [PubMed]
- Pagano, I.; Campone, L.; Celano, R.; Piccinelli, A.L.; Rastrelli, L. Green non-conventional techniques for the extraction of polyphenols from agricultural food by-products: A review. J. Chromatogr. A 2021, 1651, 462295. [Google Scholar] [CrossRef]
- Schmiedeskamp, A.; Schreiner, M.; Baldermann, S. Impact of cultivar selection and thermal processing by air drying, air frying, and deep frying on the carotenoid content and stability and antioxidant capacity in carrots. J. Agric. Food Chem. 2022, 70, 1629–1639. [Google Scholar] [CrossRef]
- Pagano, I.; Sánchez-Camargo, A.d.P.; Mendiola, J.A.; Campone, L.; Cifuentes, A.; Rastrelli, L.; Ibañez, E. Selective extraction of high-value phenolic compounds from distillation wastewater of basil (Ocimum basilicum L.) by pressurized liquid extraction. Electrophoresis 2018, 39, 1884–1891. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, J.; Shivhare, U.S.; Sandhu, K.S. Thermal degradation kinetics of carotenoids and visual color of papaya puree. J. Food Sci. 2002, 67, 2692–2695. [Google Scholar] [CrossRef]
- Carneiro, C.; Alhaji, A.; da Silva, C.; de Sousa, R.D.C.S.d.; Monteiro, S.; Coimbra, J. Potential Challenges of the Extraction of Carotenoids and Fatty Acids from Pequi (Caryocar brasiliense) Oil. Foods 2023, 12, 1907. [Google Scholar] [CrossRef] [PubMed]
- Umair, M.; Jabbar, S.; Nasiru, M.M.; Lu, Z.; Zhang, J.; Abid, M.; Murtaza, M.A.; Kieliszek, M.; Zhao, L. Ultrasound-assisted extraction of carotenoids from carrot pomace and their optimization through response surface methodology. Molecules 2021, 26, 6763. [Google Scholar] [CrossRef]
- Mordi, R.C.; Ademosun, O.T.; Ajanaku, C.O.; Olanrewaju, I.O.; Walton, J.C. Free radical mediated oxidative degradation of carotenes and xanthophylls. Molecules 2020, 25, 1038. [Google Scholar] [CrossRef]
- Borsarelli, C.D.; Mercadante, A.Z. 12 Thermal and photochemical degradation of carotenoids. In Carotenoids: Physical, Chemical, and Biological Functions and Properties; CRC Press: Boca Raton, FL, USA, 2009; pp. 229–250. [Google Scholar]
- Dias, M.G.; Camões, M.F.G.; Oliveira, L. Carotenoid stability in fruits, vegetables and working standards–Effect of storage temperature and time. Food Chem. 2014, 156, 37–41. [Google Scholar] [CrossRef]
- Tang, Y.C.; Chen, B.H. Pigment change of freeze-dried carotenoid powder during storage. Food Chem. 2000, 69, 11–17. [Google Scholar] [CrossRef]
- Arshad, M.T.; Maqsood, S.; Ikram, A.; Khan, A.A.; Raza, A.; Ahmad, A.; Gnedeka, K.T. Encapsulation Techniques of Carotenoids and Their Multifunctional Applications in Food and Health: An Overview. Food Sci. Nutr. 2025, 13, e70310. [Google Scholar] [CrossRef]
- Wojnowski, W.; Tobiszewski, M.; Pena-Pereira, F.; Psillakis, E. AGREEprep—Analytical greenness metric for sample preparation. TrAC Trends Anal. Chem. 2022, 149, 116553. [Google Scholar] [CrossRef]
- Psillakis, E.; Chisvert, A.; Cagliero, C.; Ozkan, S.A.; Segundo, M.; Mester, Z. Greenness of official sample preparation standard methods. Chem. Int. 2024, 46, 26–30. [Google Scholar] [CrossRef]
- Rodriguez, J.M.F.; Corazza, M.L.; Kruger, R.L.; Khalil, N.M.; de Campos, D.; da Silva, V.R. Economic evaluation of pressurized liquid extraction system scale-up: A potential new business opportunity for yerba mate (Ilex paraguariensis St.-Hil.) waste reuse. Sep. Sci. Technol. 2024, 59, 1712–1722. [Google Scholar] [CrossRef]
- Viganó, J.; Zabot, G.L.; Martínez, J. Supercritical fluid and pressurized liquid extractions of phytonutrients from passion fruit by-products: Economic evaluation of sequential multi-stage and single-stage processes. J. Supercrit. Fluids 2017, 122, 88–98. [Google Scholar] [CrossRef]

| Run | Temperature | Cycles | EtOH | Static Time | α-Carotene | β-Carotene | Energy Consumption |
|---|---|---|---|---|---|---|---|
| °C | n° | % | min | mg/100 g DM | mg/100 g DM | kW/h | |
| 1 | 80 | 4 | 80 | 7 | 3.06 | 11.3 | 0.078 |
| 2 | 80 | 4 | 90 | 5 | 8.34 | 41.6 | 0.072 |
| 3 | 80 | 6 | 100 | 5 | 11.08 | 61.8 | 0.084 |
| 4 | 80 | 6 | 80 | 5 | 4.18 | 11.8 | 0.085 |
| 5 | 80 | 6 | 90 | 3 | 9.41 | 52.6 | 0.063 |
| 6 | 80 | 4 | 100 | 3 | 10.67 | 60.1 | 0.051 |
| 7 | 100 | 4 | 90 | 3 | 10.30 | 57.0 | 0.074 |
| 8 | 60 | 2 | 90 | 5 | 8.51 | 46.6 | 0.037 |
| 9 | 60 | 4 | 90 | 7 | 9.19 | 51.2 | 0.073 |
| 10 | 60 | 6 | 90 | 5 | 9.65 | 53.6 | 0.069 |
| 11 | 80 | 2 | 80 | 5 | 3.5 | 11.5 | 0.048 |
| 12 | 100 | 4 | 80 | 5 | 3.07 | 11.4 | 0.086 |
| 13 | 80 | 4 | 100 | 7 | 9.20 | 50.5 | 0.079 |
| 14 | 100 | 2 | 90 | 5 | 9.84 | 54.2 | 0.055 |
| 15 | 80 | 2 | 90 | 7 | 9.38 | 52.3 | 0.056 |
| 16 | 100 | 4 | 90 | 7 | 9.60 | 53.8 | 0.098 |
| 17 | 80 | 2 | 100 | 5 | 9.80 | 53.2 | 0.049 |
| 18 | 60 | 4 | 100 | 5 | 10.17 | 55.0 | 0.058 |
| 19 | 80 | 4 | 90 | 5 | 9.63 | 52.4 | 0.070 |
| 20 | 80 | 2 | 90 | 3 | 8.64 | 47.7 | 0.040 |
| 21 | 80 | 6 | 90 | 7 | 9.07 | 50.4 | 0.106 |
| 22 | 100 | 4 | 100 | 5 | 9.42 | 51.5 | 0.087 |
| 23 | 100 | 6 | 90 | 5 | 9.29 | 50.5 | 0.109 |
| 24 | 60 | 4 | 90 | 3 | 8.42 | 45.4 | 0.041 |
| 25 | 80 | 4 | 80 | 3 | 8.29 | 44.3 | 0.052 |
| 26 | 80 | 4 | 90 | 5 | 7.92 | 43.6 | 0.072 |
| 27 | 60 | 4 | 80 | 5 | 4.68 | 21.2 | 0.057 |
| Predicted Optimised A | Predicted Optimised B | Optimised B | |
|---|---|---|---|
| α-Carotene (mg/100 g DM) | 11.20 | 11.49 | 11.28 ± 0.71 |
| β-Carotene (mg/100 g DM) | 63.74 | 65.59 | 67.42 ± 2.33 |
| Energy consumption (kW/h) | 0.070 | 0.052 | 0.054 ± 0.02 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Favaretto, L.; Pagliari, S.; Cannavacciuolo, C.; Campone, L.; Labra, M. Optimising Green Pressurised Liquid Extraction and Sustainability Assessment of Carotenoid-Rich Extracts from Daucus carota L. Pomace. Foods 2025, 14, 3740. https://doi.org/10.3390/foods14213740
Favaretto L, Pagliari S, Cannavacciuolo C, Campone L, Labra M. Optimising Green Pressurised Liquid Extraction and Sustainability Assessment of Carotenoid-Rich Extracts from Daucus carota L. Pomace. Foods. 2025; 14(21):3740. https://doi.org/10.3390/foods14213740
Chicago/Turabian StyleFavaretto, Lidia, Stefania Pagliari, Ciro Cannavacciuolo, Luca Campone, and Massimo Labra. 2025. "Optimising Green Pressurised Liquid Extraction and Sustainability Assessment of Carotenoid-Rich Extracts from Daucus carota L. Pomace" Foods 14, no. 21: 3740. https://doi.org/10.3390/foods14213740
APA StyleFavaretto, L., Pagliari, S., Cannavacciuolo, C., Campone, L., & Labra, M. (2025). Optimising Green Pressurised Liquid Extraction and Sustainability Assessment of Carotenoid-Rich Extracts from Daucus carota L. Pomace. Foods, 14(21), 3740. https://doi.org/10.3390/foods14213740







