Risk Assessment of Dietary Exposure to Fluoride from Follow-On Milk Consumption
Abstract
1. Introduction
2. Material and Methods
2.1. Samples
- Starter or Type 1 formulas: Used from birth to the fourth or sixth month of the infant’s life. Their composition aims to mimic that of breast milk and must fully cover the infant’s nutritional needs.
- Follow-on or Type 2 formulas: Used from the fourth or sixth month of the infant’s life. This type of milk does not provide enough energy to cover the metabolic needs of the newborn, so complementary feeding must be given. About 500 mL per day is recommended, providing about half of the daily dietary intake.
- Growing-up or Type 3 formulas: These are dairy products offered as a transition between follow-on milk (Type 2, for babies 6 to 12 months) and cow’s milk. Their composition is adapted to the nutritional requirements of children at this stage of growth, which differ from those of younger infants.
- Hypoallergenic FH (protein hydrolysate) formulas: These formulas are included among the special types. They contain predigested proteins that do not require enzymatic hydrolysis by the pancreas and are more easily absorbed in the proximal small intestine. They are produced from milk formulas treated with heat, enzymatic hydrolysis, and ultrafiltration to remove higher-molecular-weight peptides, thereby reducing antigenicity. Some infant formulas are also based on synthetic amino acids with no antigenic load. Their osmolarity is usually much higher than that of conventional formulas, and they are primarily indicated for cases in which absorption is compromised, digestive diseases with fat malabsorption, or allergy to cow’s milk proteins.
- Lactose-free milk: Also included among special formulas. In these, lactose is replaced by other carbohydrates such as dextrin maltose, glucose polymers, sucrose or glucose. Their main indication is for infants with congenital or acquired lactase deficiency or with galactosemia.
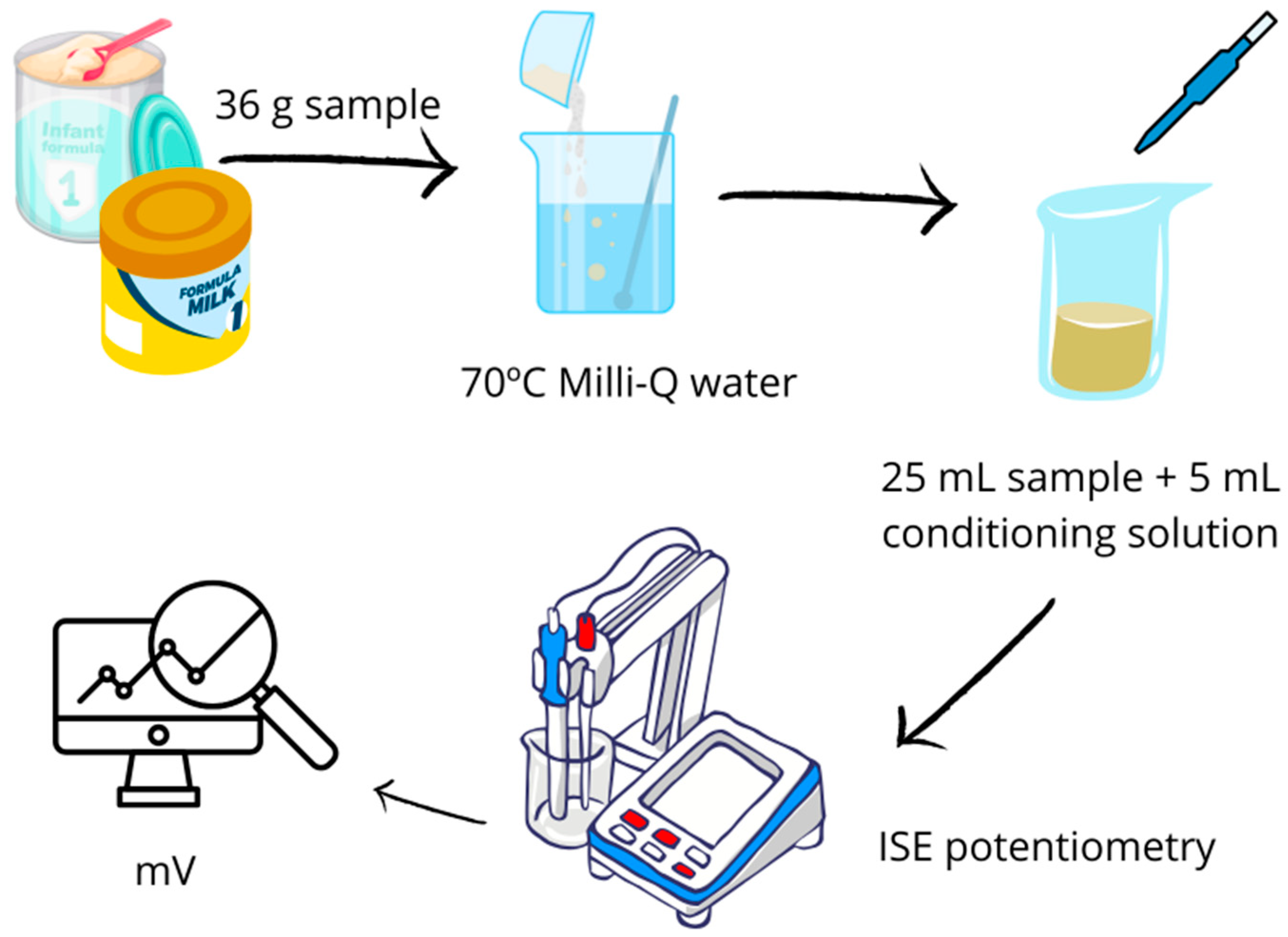
2.2. Sample Treatment and Fluoride Determination
2.3. Quality and Validation of the Method
2.4. Dietary Intake Assessment
2.5. Statistical Analysis
3. Results and Discussion
3.1. Fluoride Concentration in the Samples Tested
3.2. Comparison of Determined Fluoride Content with Labeling
3.3. Comparison with Other Authors
| Reference | Conc. Fluoride (mg/L) | Age of the Population (Months) |
|---|---|---|
| [59] | 0.53–1.33 | 6 |
| [65] | 0.05–0.28 | <24 |
| [66] | 0.031–0.532 | 1 to 12 |
| [67] | 0.01–0.75 | Unknown |
| [62] | 0.002–0.282 | 0–24 |
| [63] | 0–0.4 | 6–12 |
| [68] | 0.009–0.197 | 0–12 |
| [64] | 0.003–0.035 (liquid formulas) | 0–12 |
| 0.010–1.2 (solid formular) | ||
| [69] | 0.01–0.92 (milk-based formulas) | 0–12 |
| 0.13–1.11 (soy-based formulas) | ||
| [5] | 0.031–0.07 | 0–12 |
| Present study, 2025 | 0.004–8.09 (poder) | 0–12 |
| 0.03–9.02 (ready for consumption) |
3.4. Dietary Intake Assessment and Risk Characterization
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gómez Fernández-Vegue, M. Recommendations of the Spanish Association of Pediatrics on Complementary Feeding. Madrid, Spain. 2018. Available online: https://www.fen.org.es/storage/app/media/publicaciones%20nueva%20web%202020/recomendaciones_aep_sobre_alimentacio_n_complementaria_nov2018_v3_final.pdf (accessed on 5 April 2025).
- Nagel, E.M.; Howland, M.A.; Pando, C.; Stang, J.; Mason, S.M.; Fields, D.A.; Demerath, E.W. Maternal Psychological Distress and Lactation and Breastfeeding Outcomes: A Narrative Review. Clin. Ther. 2022, 44, 215–227. [Google Scholar] [CrossRef]
- Iwuagwu, C.; Chen, M.J.; Hoyt-Austin, A.E.; Kair, L.; Fix, M.; Schwarz, E.B. Awareness of the Maternal Health Benefits of Lactation Among U.S. Pregnant Individuals. Women’s Health Issues 2024, 34, 283–290. [Google Scholar] [CrossRef]
- Rollins, N.C.; Bhandari, N.; Hajeebhoy, N.; Horton, S.; Lutter, C.K.; Martines, J.C.; Piwoz, E.G.; Ritcher, L.M.; Victora, C.G. Why invest, and what it will take to improve breastfeeding practices? Lancet 2016, 387, 491–504. [Google Scholar] [CrossRef]
- Satti, A.S.; Muppa, R.; Kotha, R.S.; Koya, S.; Kantipudi, M.J.N.; Siva Harika, D.C. A comparative evaluation of the fluoride content in commercially available infant formulae in India: An in vitro study. J. Indian Soc. Pedod. Prev. Dent. 2023, 41, 328–334. [Google Scholar] [CrossRef]
- Iguacel, I.; Monje, L.; Cabero, M.J.; Moreno Aznar, L.A.; Samper, M.P.; Rodríguez-Palmero, M.; Rivero, M.; Rodríguez, G. Feeding patterns and growth trajectories in breast-fed and formula-fed infants during the introduction of complementary food. Hosp. Nutr. 2019, 36, 777–785. [Google Scholar] [CrossRef]
- Pinho-Gomes, A.C.; Morelli, G.; Jones, A.; Woodward, M. Association of lactation with maternal risk of type 2 diabetes: A systematic review and meta-analysis of observational studies. Diabetes Obes. Metab. 2021, 23, 1902–1916. [Google Scholar] [CrossRef]
- Tschiderer, L.; Willeit, P.; Peters, S.A.E. The cardiovascular benefits of breastfeeding to mothers. Expert Rev. Cardiovasc. Ther. 2022, 20, 589–592. [Google Scholar] [CrossRef]
- Picaud, J. Review highlights the importance of donor human milk being available for very low birth weight infants. Acta Paediatr. 2022, 111, 1127–1133. [Google Scholar] [CrossRef]
- Bustos, R.; Copaja, D.; Bancalari, A. Congenital candidiasis in a very low birth weight newborn. Chil. J. Pediatr. 2003, 74, 193–196. [Google Scholar] [CrossRef]
- Prieto Regueiro, B.; Gómez Santos, G.; Diéguez Perez, M. Prolonged Artificial Breastfeeding Associated with Oral Habits, Malocclusions and Sociodemographic Characteristics in Spanish Preschoolers: Observational Study. Span. J. Hum. Nutr. Diet. 2022, 26 (Suppl. S2), 1284. [Google Scholar] [CrossRef]
- Martín-Ramos, S.; Domínguez-Aurrecoechea, B.; García Vera, C.; Lorente García Mauriño, A.M.; Sánchez Almeida, E.; Solís-Sánchez, G. Breastfeeding in Spain and the factors related to its establishment and maintenance: LAyDI Study (PAPenRed). Prim. Care 2024, 56, 102772. [Google Scholar] [CrossRef]
- Giovannini, M.; Verduci, E.; Gregori, D.; Ballali, S.; Soldi, S.; Ghisleni, D.; Riva, E.; PLAGOS Trial Study Group. Prebiotic Effect of an Infant Formula Supplemented with Galacto-Oligosaccharides: Randomized Multicenter Trial. J. Am. Coll. Nutr. 2014, 33, 385–393. [Google Scholar] [CrossRef]
- Rodríguez Fernández, A. Benefits of Artificial Breastfeeding. Systematic Review. Bachelor’s Thesis, University of La Coruña, Galicia, Spain, 2017. Available online: https://ruc.udc.es/dspace/bitstream/handle/2183/19401/RodriguezFernandez_Ana_TFG_2017.pdf (accessed on 15 April 2025).
- Parks, E.P.; Shaikhkhalil, A.; Sainath, N.N.; Mitchell, J.A.; Brownell, J.N.; Stallings, V.A. Feeding healthy infants, children, and adolescents. In Nelson Textbook of Pediatrics, 21st ed.; Kliegman, R.M., St. Geme, J.W., Blum, N.J., Shah, S.S., Tasker, R.C., Wilson, K.M., Eds.; Elsevier: Philadelphia, PA, USA, 2020. [Google Scholar]
- Seery, A. Normal infant feeding. In Conn’s Current Therapy; Kellerman, R.D., Rakel, D.P., Heidelbaugh, J.J., Lee, E.M., Eds.; Elsevier: Philadelphia, PA, USA, 2023. [Google Scholar]
- AAP (American Academy of Pediatrics). Amount and Schedule of Baby Formula Feedings; AAP (American Academy of Pediatrics): Itasca, IL, USA, 2022; Updated 16 May 2022; Available online: www.healthychildren.org/English/ages-stages/baby/formula-feeding/Pages/amount-and-schedule-of-formula-feedings.aspx (accessed on 5 April 2025).
- Hossein Mahvi, A.; Ghanbarian, M.; Ghanbarian, M.; Khosravi, A.; Ghanbarian, M. Determination of fluoride concentration in powdered milk in Iran 2010. Br. J. Nutr. 2012, 107, 1077–1079. [Google Scholar] [CrossRef]
- Jiménez-Reyes, M.; Mireles-Martínez, M.M.; González-Amaro, A.M.; Nava-Juárez, R.; Maltos-Miranda, C. Fluoride exposure doses from the consumption of different types of milk in residents of an area with endemic hydrofluorosis in Mexico. Ann. Pediatr. 2019, 89, 371–378. Available online: https://analesdepediatria.org/es-dosis-exposicion-fluoruros-por-el-articulo-S1695403318303710 (accessed on 6 April 2025).
- Till, C.; Green, R.; Flora, D.; Hornung, R.; Martinez-Mier, E.A.; Blazer, M.; Farmus, L.; Ayotte, P.; Muckle, G.; Lanphear, B. Fluoride exposure from infant formula and child IQ in a canadian birth cohort. Environ. Int. 2020, 134, 105315. Available online: https://www.sciencedirect.com/science/article/pii/S0160412019326145?via%3Dihub (accessed on 17 April 2025). [CrossRef]
- Maruszewska, A.; Żwierełło, W.; Skórka-Majewicz, M.; Baranowska-Bosiacka, I.; Wszołek, A.; Janda, K.; Kulis, D.; Kapczuk, P.; Chlubek, D.; Gutowska, I. Modified Baby Milk-Bioelements Composition and Toxic Elements Contamination. Molecules 2021, 26, 4184. [Google Scholar] [CrossRef]
- ATSDR (Agency for Toxic Substances and Disease Registry). Toxicological Profile for Fluorides, Hydrogen Fluoride, and Fluorine; Department of Health and Human Services, Public Health Service: Atlanta, GA, USA, 2003.
- Rodríguez, I.; Jaudenes, J.R.; Hardisson, A.; Paz, S.; Rubio, C.; Gutiérrez, A.J.; Burgos, A.; Revert, C. Correction to: Potentiometric Determination of Fluoride Concentration in Beers. Biol. Trace Elem. Res. 2018, 181, 184. [Google Scholar] [CrossRef]
- Jaudenes, J.R.; Gutiérrez, Á.J.; Paz, S.; Rubio, C.; Hardisson, A. Fluoride Risk Assessment from Consumption of Different Foods Commercialized in a European Region. Appl. Sci. 2020, 10, 6582. [Google Scholar] [CrossRef]
- EFSA (European Food Safety Authority). EFSA NDA Panel on Dietetic Products, Nutrition and Allergies. Scientific opinion on Dietary Reference Values for fluoride. EFSA J. 2013, 11, 3332. [Google Scholar] [CrossRef]
- DenBesten, P.; Li, W. Chronic fluoride toxicity: Dental fluorosis. Monogr. Oral Sci. 2011, 22, 81–96. [Google Scholar] [CrossRef]
- Maita Vilca, L. Behavior of Fluoride Concentration in Toothpastes Prescribed for Children. Ph.D. Thesis, Alas Peruanas University, Arequipá, Peru, 2017. [Google Scholar]
- Cobos, C.; Stefany, M. Relationship Between the Use of Fluoride Pastes and the Prevalence of Fluorosis in Pediatric Patients. Bachellor’s Thesis, University of Guayaquil, Guayaquil, Ecuador, 2022. Available online: http://repositorio.ucsg.edu.ec/handle/3317/12249 (accessed on 4 April 2025).
- Susheela, A.K.; Toteja, G.S. Prevention & control of fluorosis & linked disorders: Developments in the 21st century—Reaching out to patients in the community & hospital settings for recovery. Indian J. Med. Res. 2018, 148, 539–547. [Google Scholar]
- Nilchian, F.; Asgary, I.; Mastan, F. The effect of dental fluorosis on the quality of life of female high school and precollege students of high fluoride-concentrated area. J. Int. Soc. Prev. Community Dent. 2018, 8, 314–319. Available online: https://www.jispcd.org/article.asp?issn=2231-0762;year=2018;volume=8;issue=4;spage=314;epage=319;aulast=Nilchian (accessed on 10 April 2025). [CrossRef]
- Revelo-Mejía, I.A.; Hardisson, A.; Rubio, C.; Gutiérrez, Á.J.; Paz, S. Dental fluorosis: The risk of misdiagnosis—A review. Biol. Trace Elem. Res. 2020, 199, 1762–1770. Available online: https://link.springer.com/article/10.1007/s12011-020-02296-4 (accessed on 15 April 2025). [CrossRef]
- Khan, S.A.; Singh, R.K.; Navit, S.; Chadha, D.; Johri, N.; Navit, P.; Sharma, A.; Bahuguna, R. Relationship Between Dental Fluorosis and Intelligence Quotient of School Going Children in and Around Lucknow District: A Cross-Sectional Study. J. Clin. Diagn. Res. JCDR 2015, 9, ZC10–ZC15. [Google Scholar] [CrossRef]
- Razdan, P.; Patthi, B.; Kumar, J.K.; Agnihotri, N.; Chaudhari, P.; Prasad, M.; Razdan, P. Effect of fluoride concentration in drinking water on intelligence quotient of 12–14-year-old children in mathura district: A cross-sectional study. J. Int. Soc. Prev. Community Dent. 2017, 7, 252–258. [Google Scholar] [CrossRef]
- Grandjean, P. Developmental fluoride neurotoxicity: An updated review. Environ. Health 2019, 18, 110. Available online: https://link.springer.com/article/10.1186/s12940-019-0551-x#citeas (accessed on 6 April 2025). [CrossRef]
- Waugh, D.T. Fluoride exposure induces inhibition of sodium/iodide symporter (NIS) contributing to impaired iodine absorption and iodine deficiency: Molecular mechanisms of inhibition and implications for public health. Int. J. Environ. Res. Public Health 2019, 16, 1086. Available online: https://www.mdpi.com/1660-4601/16/6/1086 (accessed on 17 April 2025). [CrossRef]
- EFSA Scientific Committee; Bennekou, S.H.; Allende, A.; Bearth, A.; Casacuberta, J.; Castle, L.; Coja, T.; Crépet, A.; Hoogen-boom, R.; Knutsen, H.; et al. Updated consumer risk assessment of fluoride in food and drinking water including the contribution from other sources of oral exposure. EFSA J. 2025, 23, e9478. [Google Scholar] [CrossRef]
- Ullah, R.; Zafar, M.S.; Shahani, N. Potential fluoride toxicity from oral medicaments: A review. Iran. J. Basic Med. Sci. 2017, 20, 841–848. [Google Scholar] [CrossRef]
- Waugh, D.T.; Godfrey, M.; Limeback, H.; Potter, W. Black Tea Source, Production, and Consumption: Assessment of Health Risks of Fluoride Intake in New Zealand. J. Environ. Public Health 2017, 2017, 5120504. [Google Scholar] [CrossRef]
- Guth, S.; Hüser, S.; Roth, A.; Degen, G.; Diel, P.; Edlund, K.; Eisenbrand, G.; Engel, K.H.; Epe, B.; Grune, T.; et al. Toxicity of fluoride: Critical evaluation of evidence for human developmental neurotoxicity in epidemiological studies, animal experiments and in vitro analyses. Arch. Toxicol. 2020, 94, 1375–1415. [Google Scholar] [CrossRef]
- Prabhakar, A.; Abdulkhayarkutty, K.; Cheruvallil, S.V.; Sudhkaran, P. Effect of endemic fluorosis on cognitive function of school children in alappuzha district, kerala: Across sectional study. Ann. Indian Acad. Neurol. 2021, 24, 715–720. Available online: https://www.annalsofian.org/text.asp?2021/24/715/300179 (accessed on 11 April 2025). [CrossRef]
- Ren, C.; Zhang, P.; Yao, X.; Li, H.; Chen, R.; Zhang, C.; Geng, D. The cognitive impairment and risk factors of the older people living in high fluorosis areas: DKK1 need attention. BMC Public Health 2021, 21, 2237. Available online: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8656079/ (accessed on 10 April 2025). [CrossRef]
- Fishta, A.; Thakur, R.; Sharma, K.C.; Thakur, N.; Patial, B. Effects of Fluoride Toxicity on Female Reproductive System of Mammals: A Meta-Analysis. Biol. Trace Elem. Res. 2025, 203, 646–669. [Google Scholar] [CrossRef]
- Abanto Alvarez, J.; Rezende, K.M.; Salazar Marocho, S.M.; Alves, B.T.F.; Celiberti, P.; Ciamponi, A.L. Dental fluorosis: Exposure, prevention and management. J. Clin. Exp. Dent. 2009, 14, 103–107. Available online: http://www.medicinaoral.com/pubmed/medoralv14_i2_pE103.pdf (accessed on 6 April 2025).
- Tamer, M.N.; Kale Köroğlu, B.; Arslan, Ç.; Akdoğan, M.; Köroğlu, M.; Çam, H.; Yildiz, M. Osteosclerosis due to endemic fluorosis. Sci. Total Environ. 2007, 373, 43–48. [Google Scholar] [CrossRef]
- Bhagavatula, P.; Levy, S.M.; Broffitt, B.; Weber-Gasparoni, K.; Warren, J.J. Timing of fluoride intake and dental fluorosis on late-erupting permanent teeth. Community Dent. Oral Epidemiol. 2016, 44, 32–45. [Google Scholar] [CrossRef]
- Sellami, M.; Riahi, H.; Maatallah, K.; Ferjani, H.; Bouaziz, M.C.; Ladeb, M.F. Skeletal fluorosis: Don’t miss the diagnosis. Skelet. Radiol. 2019, 49, 345–357. Available online: https://link.springer.com/article/10.1007/s00256-019-03302-0 (accessed on 15 April 2025). [CrossRef]
- IOM (Institute of Medicine). Standing Committee on the Scientific Evaluation of Dietary Reference Intakes. In Dietary Reference Intakes for Calcium, Phosphorus, Magnesium, Vitamin D, and Fluoride; National Academies Press: Washington, DC, USA, 1997. [Google Scholar]
- Alejandro-Vega, S.; Hardisson, A.; Rubio, C.; Gutiérrez, Á.J.; Jaudenes-Marrero, J.R.; Paz-Montelongo, S. Soft Drinks as a Dietary Source of Fluoride Exposure. Biol. Trace Elem. Res. 2024, 202, 3816–3828. [Google Scholar] [CrossRef]
- Erney, R.M.; Black, C.K. Fluoride Determination in Milk, Soy, and Water-Based Products Using Ion-Selective Electrode and Direct Measurement Technique: Single-Laboratory Validation, First Action 2022.05. J. AOAC Int. 2024, 107, 103–111. [Google Scholar] [CrossRef]
- Alejandro-Vega, S.; Ruiz-Benitez-de-Lugo, S.; Hevia-Loredo, D.; Paz-Montelongo, S.; Hardisson, A.; Rubio-Armendariz, C.; Gutiérrez-Fernández, Á.J.; Jáudenes-Marrero, J.R. Fluoride Risk Assessment of Different Brands of Coffee Commercialized in the Canary Islands. Appl. Sci. 2024, 14, 7400. [Google Scholar] [CrossRef]
- IOM. Institute of Medicine (US) Committee on Dietary Risk Assessment in the WIC Program. In Dietary Risk Assessment in the WIC Program; National Academies Press: Washington, DC, USA, 2002. Available online: https://www.ncbi.nlm.nih.gov/books/NBK220560/ (accessed on 5 April 2025).
- Paz Montelongo, S.; Rubio Armendáriz, C.; Gutiérrez Fernández, Á.; Revert Gironés, C.; González Weller, D.; Caballero Mesa, J.M.; de la Torre, A.H. Evaluación del Riesgo Toxicológico de los Alimentos: Manual de Problemas; Universidad de La Laguna: San Cristóbal de La Laguna, Spain, 2018. [Google Scholar]
- Nachar, N. The Mann-Whitney U: A test for assessing whether two independent samples come from the same distribution. Tutor. Quant. Methods Psychol. 2008, 4, 13–20. [Google Scholar] [CrossRef]
- Stephens, M.A. Anderson-Darling Test—Wiley StatsRef Statistics Reference Online; Wiley & Sons: Hoboken, NJ, USA, 2017; pp. 1–6. [Google Scholar]
- Razali, N.M.; Wah, Y.B. Power comparisons of Shapiro–Wilk, Kolmogorov–Smirnov, Lilliefors and Anderson–Darling tests. J. Stat. Model. Anal. 2011, 2, 21–33. [Google Scholar]
- Shapiro, S.S. Goodness-of-Fit Tests for Normality; John Wiley & Sons: New York, NY, USA, 2017. [Google Scholar]
- Jamaludin, M.Z.; Hussin, N.H.; Harun, M.H.; Said, N.S. A review of normality tests for statistical analysis. J. Phys. Conf. Ser. 2019, 1366, 012012. [Google Scholar]
- Sheskin, D.J. Handbook of Parametric and Nonparametric Statistical Procedures; Chapman and Hall/CRC: Boca Raton, FL, USA, 2020. [Google Scholar]
- Nishijima, M.T.; Koga, H.; Maki, Y.; Takaesu, Y. A comparison of daily fluoride intakes from food samples in Japan and Brazil. Bull. Tokyo Dent. Coll. 1993, 34, 43–50. [Google Scholar]
- EU (European Union). REGULATION (EU) No 609/2013 of the European Parliament and of the Council of 12 June 2013 on food intended for infants and young children, food for special medical purposes, and total diet replacement for weight control and repealing Council Directive 92/52/EEC, Commission Directives 96/8/EC, 1999/21/EC, 2006/125/EC and 2006/141/EC, Directive 2009/39/EC of the European Parliament and of the Council and Commission Regulations (EC) No 41/2009 and (EC) No 953/2009. Off. J. Eur. Union 2013, 181, 35–36. [Google Scholar]
- EU (European Union). Commission Delegated Regulation (EU) 2016/127 of 25 September 2015 supplementing Regulation (EU) No 609/2013 of the European Parliament and of the Council as regards the specific compositional and information requirements for infant formula and follow-on formula and as regards requirements on information relating to infant and young child feeding. Off. J. Eur. Communities 2016, 59, 1–29. [Google Scholar]
- Bussell, R.M.; Nichol, R.; Toumba, K.J. Fluoride levels in UK infant milks. Eur. Arch. Paediatr. Dent. 2016, 17, 177–185. [Google Scholar] [CrossRef]
- Agha, Y.; Kowash, M.; Hussein, I.; Al Salami, A.; Al-Halabi, M. Fluoride concentration of commercially available infant formulae in the United Arab Emirates. Eur. Arch. Paediatr. Dent. 2020, 21, 657–666. [Google Scholar] [CrossRef]
- Chandio, N.; John, J.R.; Floyd, S.; Gibson, E.; Wong, D.K.Y.; Levy, S.M.; Heilman, J.R.; Arora, A. Fluoride Content of Ready-to-Eat Infant Foods and Drinks in Australia. Int. J. Environ. Res. Public Health 2022, 19, 14087. [Google Scholar] [CrossRef]
- Van Winkle, S.; Levy, S.M.; Kiritsy, M.C.; Heilman, J.R.; Wefel, J.S.; Marshall, T. Water and formula fluoride concentrations: Significance for infants fed formula. Pediatr. Dent. 1995, 17, 305–310. [Google Scholar]
- Silva, M.; Reynolds, E.C. Fluoride content of infant formulae in Australia. Aust. Dent. J. 1996, 41, 37–42. [Google Scholar] [CrossRef]
- Buzalaf, M.A.; Granjeiro, J.M.; Damante, C.A.; de Ornelas, F. Fluoride content of infant formulas prepared with deionized, bottled mineral and fluoridated drinking water. ASDC J. Dent. Child. 2001, 68, 37–41. [Google Scholar]
- Mohd Desa, S.; Muhamad, N.A.; Mohd Nor, N.A.; Abdul Razak, F.; Abdul Manan, N.S.; Ab-Murat, N.; Marhazlina, J. A laboratory study of fluoride concentration in infant formulas marketed in Malaysia and estimation of daily intake. Int. Food Res. J. 2020, 27, 893–902. Available online: http://www.ifrj.upm.edu.my/27%20(05)%202020/DONE%20-%2013%20-%20IFRJ20023.R2.pdf (accessed on 10 April 2025).
- Velez-León, E.; Pacheco-Quito, E.-M.; Díaz-Dosque, M.; Tobar-Almache, D. Worldwide Variations in Fluoride Content in Beverages for Infants. Children 2023, 10, 1896. [Google Scholar] [CrossRef]

| Sample | Brand | Type | Sample | Brand | Type |
|---|---|---|---|---|---|
| 1 | I | Type 1 | 22 | I | Type 3 |
| 2 | M | 23 | |||
| 3 | L | 24 | |||
| 4 | F | 25 | B | ||
| 5 | E | 40 | L | ||
| 6 | F | 43 | N | ||
| 7 | L | 44 | |||
| 8 | C | 45 | |||
| 9 | I | 46 | |||
| 10 | 27 | B | Hydrolyzed | ||
| 11 | 28 | K | |||
| 12 | D | 29 | I | ||
| 21 | H | 30 | |||
| 32 | A | 31 | B | ||
| 33 | K | 35 | I | ||
| 34 | G | 36 | M | ||
| 38 | B | 37 | I | ||
| 42 | J | ||||
| 13 | I | Type 2 | |||
| 14 | |||||
| 15 | L | ||||
| 16 | F | ||||
| 17 | D | ||||
| 18 | B | ||||
| 19 | I | ||||
| 20 | I | ||||
| 26 | E | ||||
| 39 | D | ||||
| 41 | A |
| Fluoride Conc. (mg/L) ± SD | Min.–Max. Value | |
|---|---|---|
| Type | ||
| Type 1 | 0.75 ± 0.35 | 0.27–1.54 |
| Type 2 | 0.53 ± 0.22 | 0.16–0.99 |
| Type 3 | 0.47 ± 0.58 | 0.03–1.75 |
| Hydrolyzed | 3.38 ± 2.78 | 0.77–7.32 |
| Presentation format | ||
| Powder | 1.02 ± 1.35 | 0.004–8.09 |
| Ready for consumption | 1.54 ± 2.36 | 0.03–9.02 |
| Sample | Fluoride Conc. (mg/100 mL) | Declared Value (mg/100 mL) | Difference (%) |
|---|---|---|---|
| 1 | 0.094 | 0.01 | 840.00 |
| 2 | 0.046 | 0.006 | 666.67 |
| 3 | 0.078 | 0.01 | 680.00 |
| 4 | 0.107 | 0.00069 | 15,407.25 |
| 6 | 0.055 | 0.00066 | 8233.33 |
| 7 | 0.048 | 0.01 | 380.00 |
| 8 | 0.074 | 0.0065 | 1038.46 |
| 9 | 0.037 | 0.01 | 270.00 |
| 10 | 0.057 | 0.01 | 470.00 |
| 11 | 0.078 | 0.000079 | 98,634.18 |
| 13 | 0.059 | 0.008 | 637.50 |
| 14 | 0.044 | 0.01 | 340.00 |
| 15 | 0.068 | 0.01 | 580.00 |
| 16 | 0.069 | 0.00069 | 9900.00 |
| 18 | 0.045 | 0.000371 | 12,029.38 |
| 20 | 0.04 | 0.01 | 300.00 |
| 25 | 0.038 | 0.00069 | 5407.25 |
| 28 | 0.476 | 0.06 | 693.33 |
| 29 | 0.333 | 0.21 | 58.57 |
| 30 | 0.124 | 1 | −87.60 |
| 32 | 0.075 | 0.006 | 1150.00 |
| 33 | 0.039 | 0.06 | −35.00 |
| 34 | 0.127 | 0.001 | 12,600.00 |
| 38 | 0.154 | 0.000358 | 42,916.76 |
| 41 | 0.016 | 0.006 | 166.67 |
| 42 | 0.107 | 0.01 | 970.00 |
| Product Type | 1 Serving | % UL | 3 Servings | % UL | 7 Servings | % UL | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Infants | Children | Infants | Children | Infants | Children | |||||||
| EDI (mg/Day) | 0–6 Months | 7–12 Months | 1–3 Years | EDI (mg/Day) | 0–6 Months | 7–12 Months | 1–3 Years | EDI (mg/Day) | 0–6 Months | 7–12 Months | 1–3 Years | |
| Type 1 | 0.02 | 3.43 | 2.67 | 1.85 | 0.07 | 10.4 | 8.11 | 5.62 | 0.17 | 24.3 | 18.9 | 13.1 |
| 0.07 | 9.71 | 7.56 | 5.23 | 0.20 | 29.0 | 22.6 | 15.6 | 0.47 | 67.6 | 52.6 | 36.4 | |
| 0.14 | 19.9 | 15.4 | 10.7 | 0.42 | 59.4 | 46.2 | 32.0 | 0.97 | 139 | 108 | 74.6 | |
| Type 2 | 0.01 | 2.00 | 1.56 | 1.08 | 0.04 | 6.14 | 4.78 | 3.31 | 0.10 | 14.4 | 11.2 | 7.77 |
| 0.05 | 7.00 | 5.44 | 3.77 | 0.15 | 20.9 | 16.2 | 11.2 | 0.34 | 48.6 | 37.8 | 26.2 | |
| 0.09 | 12.7 | 9.89 | 6.85 | 0.27 | 38.1 | 29.7 | 20.5 | 0.62 | 89.1 | 69.3 | 48.0 | |
| Type 3 | 0.00 | 0.43 | 0.33 | 0.23 | 0.01 | 1.14 | 0.89 | 0.62 | 0.02 | 2.71 | 2.11 | 1.46 |
| 0.04 | 6.14 | 4.78 | 3.31 | 0.13 | 18.6 | 14.4 | 10.0 | 0.30 | 43.1 | 33.6 | 23.2 | |
| 0.16 | 22.6 | 17.6 | 12.2 | 0.47 | 67.6 | 52.6 | 36.4 | 1.12 | 158.2 | 123.2 | 85.4 | |
| Hydrolyzed | 0.07 | 9.86 | 7.67 | 5.31 | 0.21 | 29.7 | 23.1 | 16.0 | 0.49 | 69.3 | 53.9 | 37.3 |
| 0.30 | 43.4 | 33.8 | 23.4 | 0.91 | 130 | 101 | 70.2 | 2.1 | 303.8 | 236.6 | 163.8 | |
| 0.66 | 94.1 | 73.2 | 50.7 | 1.98 | 282.3 | 219.6 | 152.1 | 4.62 | 658.7 | 512.4 | 354.9 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cerdán-Pérez, S.; Paz-Montelongo, S.; Alejandro-Vega, S.; Rubio, C.; Gutiérrez, Á.J.; Hardisson, A.; de la Cruz Morales, C.; Revelo-Mejía, I.A.; Darias-Rosales, J.; Pérez-Rodríguez, N.; et al. Risk Assessment of Dietary Exposure to Fluoride from Follow-On Milk Consumption. Foods 2025, 14, 3728. https://doi.org/10.3390/foods14213728
Cerdán-Pérez S, Paz-Montelongo S, Alejandro-Vega S, Rubio C, Gutiérrez ÁJ, Hardisson A, de la Cruz Morales C, Revelo-Mejía IA, Darias-Rosales J, Pérez-Rodríguez N, et al. Risk Assessment of Dietary Exposure to Fluoride from Follow-On Milk Consumption. Foods. 2025; 14(21):3728. https://doi.org/10.3390/foods14213728
Chicago/Turabian StyleCerdán-Pérez, Santiago, Soraya Paz-Montelongo, Samuel Alejandro-Vega, Carmen Rubio, Ángel J. Gutiérrez, Arturo Hardisson, Chaxiraxi de la Cruz Morales, Inés A. Revelo-Mejía, Javier Darias-Rosales, Natalia Pérez-Rodríguez, and et al. 2025. "Risk Assessment of Dietary Exposure to Fluoride from Follow-On Milk Consumption" Foods 14, no. 21: 3728. https://doi.org/10.3390/foods14213728
APA StyleCerdán-Pérez, S., Paz-Montelongo, S., Alejandro-Vega, S., Rubio, C., Gutiérrez, Á. J., Hardisson, A., de la Cruz Morales, C., Revelo-Mejía, I. A., Darias-Rosales, J., Pérez-Rodríguez, N., & Revert, C. (2025). Risk Assessment of Dietary Exposure to Fluoride from Follow-On Milk Consumption. Foods, 14(21), 3728. https://doi.org/10.3390/foods14213728










