Biopreservation of Hericium erinaceus By-Products via Lactic Acid Fermentation: Effects on Functional and Technological Properties
Abstract
1. Introduction
2. Materials and Methods
2.1. Mushroom Pre-Treatment and Preparation
2.2. Strains Preparation, Inoculation and Microbiology Analysis
2.3. Physicochemical and Functional Analyses
2.3.1. pH, TA, TSS Determination
2.3.2. Color Characterization
2.3.3. Rheology Measurements
2.3.4. Determination of Total Phenolic Content and Antioxidant Capacity
2.3.5. Determination of Biogenic Amines
2.4. Statistical Analysis
3. Results
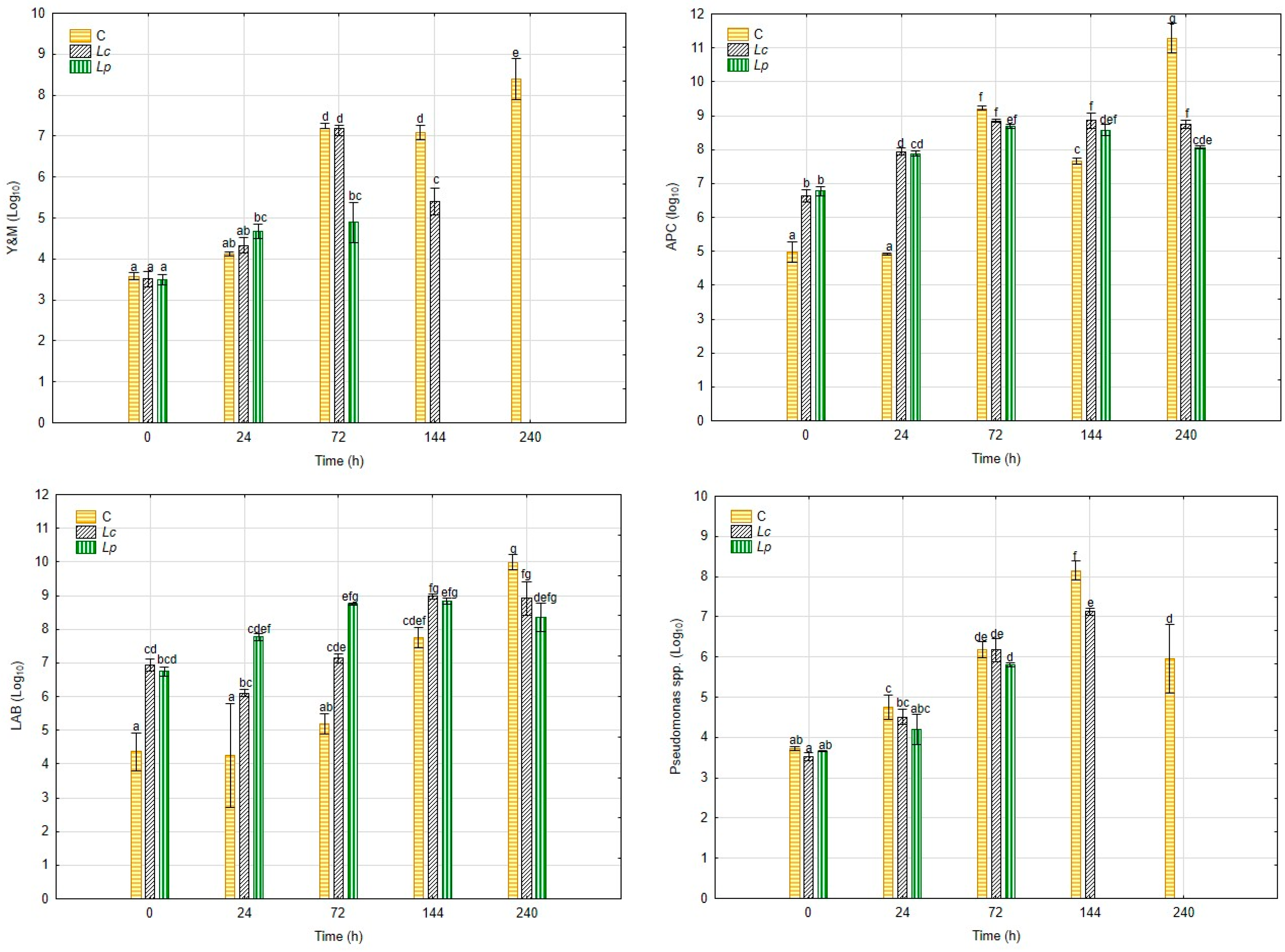
3.1. Dynamic of Microbiological Loads
3.2. Physicochemical and Functional Properties
3.2.1. pH, TA, and TSS of the Fermented Products
3.2.2. Color Evaluation
3.2.3. Rheology Properties
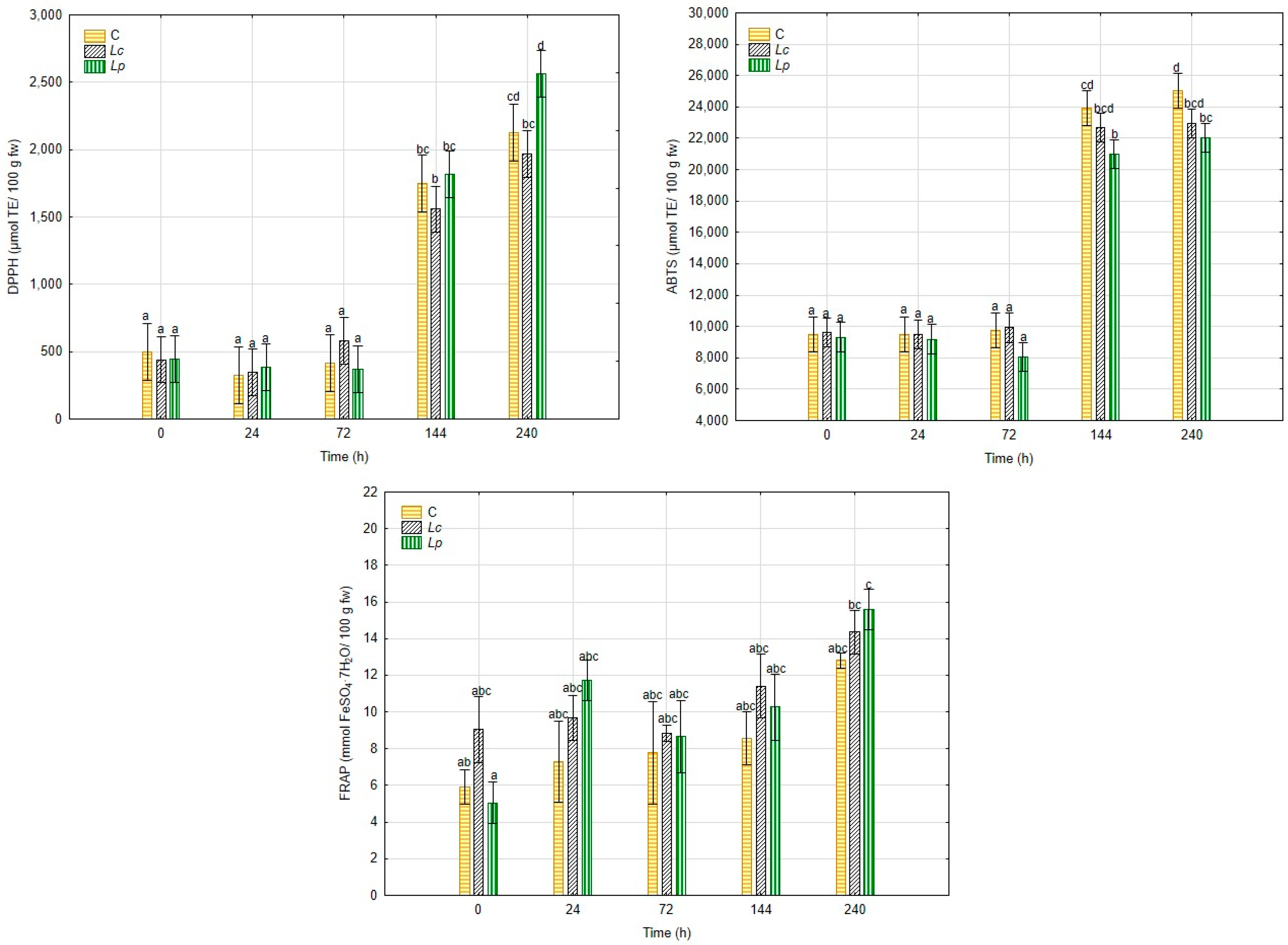
3.2.4. Determination of Total Phenolic Content and Antioxidant Capacity
3.2.5. Determination of Biogenic Amines
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sionek, B.; Szydłowska, A.; Kołożyn-Krajewska, D. The Role of Microorganisms and Their Antibacterial Compounds in Food Biopreservation. Appl. Sci. 2024, 14, 5557. [Google Scholar] [CrossRef]
- Marco, M.L.; Heeney, D.; Binda, S.; Cifelli, C.J.; Cotter, P.D.; Foligné, B.; Gänzle, M.; Kort, R.; Pasin, G.; Pihlanto, A.; et al. Health Benefits of Fermented Foods: Microbiota and Beyond. Curr. Opin. Biotechnol. 2017, 44, 94–102. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.Y.; Bong, Y.J.; Jeong, J.K.; Lee, S.; Kim, B.Y.; Park, K.Y. Heterofermentative Lactic Acid Bacteria Dominate in Korean Commercial Kimchi. Food Sci. Biotechnol. 2016, 25, 541–545. [Google Scholar] [CrossRef]
- Ahansaz, N.; Tarrah, A.; Pakroo, S.; Corich, V.; Giacomini, A. Lactic Acid Bacteria in Dairy Foods: Prime Sources of Antimicrobial Compounds. Fermentation 2023, 9, 964. [Google Scholar] [CrossRef]
- Bartkiene, E.; Zokaityte, E.; Starkute, V.; Mockus, E.; Klupsaite, D.; Lukseviciute, J.; Bogomolova, A.; Streimikyte, A.; Ozogul, F. Biopreservation of Wild Edible Mushrooms (Boletus Edulis, Cantharellus, and Rozites Caperata) with Lactic Acid Bacteria Possessing Antimicrobial Properties. Foods 2022, 11, 1800. [Google Scholar] [CrossRef]
- Kingcha, Y.; Pumpuang, L.; Adunphatcharaphon, S.; Chantarasakha, K.; Santiyanont, P.; Klomtun, M.; Janyaphisan, T.; Kongtong, K.; Phonsatta, N.; Panya, A.; et al. Potential Use of Lactiplantibacillus Plantarum BCC 4352 as a Functional Starter Culture for Fermenting Thai Pork Sausage (Nham). Fermentation 2024, 10, 145. [Google Scholar] [CrossRef]
- Silva, C.C.G.; Silva, S.P.M.; Ribeiro, S.C. Application of Bacteriocins and Protective Cultures in Dairy Food Preservation. Front. Microbiol. 2018, 9, 594. [Google Scholar] [CrossRef]
- Zapaśnik, A.; Sokołowska, B.; Bryła, M. Role of Lactic Acid Bacteria in Food Preservation and Safety. Foods 2022, 11, 1283. [Google Scholar] [CrossRef]
- Rossi, F.; Amadoro, C.; Gasperi, M.; Colavita, G. Lactobacilli Infection Case Reports in the Last Three Years and Safety Implications. Nutrients 2022, 14, 1178. [Google Scholar] [CrossRef]
- Mathur, H.; Beresford, T.P.; Cotter, P.D. Health Benefits of Lactic Acid Bacteria (Lab) Fermentates. Nutrients 2020, 12, 1679. [Google Scholar] [CrossRef] [PubMed]
- Gunawardena, S.; Nadeeshani, H.; Amarasinghe, V.; Liyanage, R. Bioactive Properties and Therapeutic Aspects of Fermented Vegetables: A Review. Food Prod. Process. Nutr. 2024, 6, 31. [Google Scholar] [CrossRef]
- Kalinowska, M.; Gołebiewska, E.; Zawadzka, M.; Choińska, R.; Koronkiewicz, K.; Piasecka-Jóźwiak, K.; Bujak, M. Sustainable Extraction of Bioactive Compound from Apple Pomace through Lactic Acid Bacteria (LAB) Fermentation. Sci. Rep. 2023, 13, 19310. [Google Scholar] [CrossRef] [PubMed]
- Leonard, W.; Zhang, P.; Ying, D.; Adhikari, B.; Fang, Z. Fermentation Transforms the Phenolic Profiles and Bioactivities of Plant-Based Foods. Biotechnol. Adv. 2021, 49, 107763. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.; Wu, T.; Chu, X.; Tang, S.; Cao, W.; Liang, F.; Fang, Y.; Pan, S.; Xu, X. Fermented Blueberry Pomace with Antioxidant Properties Improves Fecal Microbiota Community Structure and Short Chain Fatty Acids Production in an in Vitro Mode. LWT 2020, 125, 109260. [Google Scholar] [CrossRef]
- Sarıtaş, S.; Portocarrero, A.C.M.; Miranda López, J.M.; Lombardo, M.; Koch, W.; Raposo, A.; El-Seedi, H.R.; de Brito Alves, J.L.; Esatbeyoglu, T.; Karav, S.; et al. The Impact of Fermentation on the Antioxidant Activity of Food Products. Molecules 2024, 29, 3941. [Google Scholar] [CrossRef]
- Contato, A.G.; Conte-Junior, C.A. Lion’s Mane Mushroom (Hericium Erinaceus): A Neuroprotective Fungus with Antioxidant, Anti-Inflammatory, and Antimicrobial Potential—A Narrative Review. Nutrients 2025, 17, 1307. [Google Scholar] [CrossRef] [PubMed]
- Silva, M.; Vida, M.; Ramos, A.C.; Lidon, F.J.; Reboredo, F.H.; Gonçalves, E.M. Storage Temperature Effect on Quality and Shelf-Life of Hericium Erinaceus Mushroom. Horticulturae 2025, 11, 158. [Google Scholar] [CrossRef]
- Chen, Z.; Gao, H.; Wu, W.; Chen, H.; Fang, X.; Han, Y.; Mu, H. Effects of Fermentation with Different Microbial Species on the Umami Taste of Shiitake Mushroom (Lentinus edodes). LWT 2021, 141, 110889. [Google Scholar] [CrossRef]
- Xu, Y.; Tian, Y.; Ma, R.; Liu, Q.; Zhang, J. Effect of Plasma Activated Water on the Postharvest Quality of Button Mushrooms, Agaricus bisporus. Food Chem. 2016, 197, 436–444. [Google Scholar] [CrossRef]
- Tirado-Kulieva, V.; Quijano-Jara, C.; Avila-George, H.; Castro, W. Predicting the Evolution of PH and Total Soluble Solids during Coffee Fermentation Using Near-Infrared Spectroscopy Coupled with Chemometrics. Curr. Res. Food Sci. 2024, 9, 100788. [Google Scholar] [CrossRef]
- Maleke, M.; Doorsamy, W.; Abrahams, A.M.; Adefisoye, M.A.; Masenya, K.; Adebo, O.A. Influence of Fermentation Conditions (Temperature and Time) on the Physicochemical Properties and Bacteria Microbiota of Amasi. Fermentation 2022, 8, 57. [Google Scholar] [CrossRef]
- He, Y.; Hu, M.; He, W.; Li, Y.; Liu, S.; Hu, X.; Nie, S.; Yin, J.; Xie, M. Volatile Compound Dynamics during Blueberry Fermentation by Lactic Acid Bacteria and Its Potential Associations with Bacterial Metabolism. Food Biosci. 2024, 59, 103639. [Google Scholar] [CrossRef]
- Bartkiene, E.; Zarovaite, P.; Starkute, V.; Mockus, E.; Zokaityte, E.; Zokaityte, G.; Rocha, J.M.; Ruibys, R.; Klupsaite, D. Changes in Lacto-Fermented Agaricus Bisporus (White and Brown Varieties) Mushroom Characteristics, Including Biogenic Amine and Volatile Compound Formation. Foods 2023, 12, 2441. [Google Scholar] [CrossRef]
- Saha Turna, N.; Chung, R.; McIntyre, L. A Review of Biogenic Amines in Fermented Foods: Occurrence and Health Effects. Heliyon 2024, 10, e24501. [Google Scholar] [CrossRef]
- Gao, X.; Li, C.; He, R.; Zhang, Y.; Wang, B.; Zhang, Z.-H.; Ho, C.-T. Research Advances on Biogenic Amines in Traditional Fermented Foods: Emphasis on Formation Mechanism, Detection and Control Methods. Food Chem. 2023, 405, 134911. [Google Scholar] [CrossRef]
- Maglione, M.; Kochlamazashvili, G.; Eisenberg, T.; Rácz, B.; Michael, E.; Toppe, D.; Stumpf, A.; Wirth, A.; Zeug, A.; Müller, F.E.; et al. Spermidine Protects from Age-Related Synaptic Alterations at Hippocampal Mossy Fiber-CA3 Synapses. Sci. Rep. 2019, 9, 19616. [Google Scholar] [CrossRef]
- Madeo, F.; Hofer, S.J.; Pendl, T.; Bauer, M.A.; Eisenberg, T.; Carmona-Gutierrez, D.; Kroemer, G. Nutritional Aspects of Spermidine. Annu. Rev. Nutr. 2020, 40, 135–159. [Google Scholar] [CrossRef] [PubMed]
- Eisenberg, T.; Abdellatif, M.; Schroeder, S.; Primessnig, U.; Stekovic, S.; Pendl, T.; Harger, A.; Schipke, J.; Zimmermann, A.; Schmidt, A.; et al. Cardioprotection and Lifespan Extension by the Natural Polyamine Spermidine. Nat. Med. 2016, 22, 1428–1438. [Google Scholar] [CrossRef] [PubMed]
- ISO 4833-1:2013; Microbiology of the Food Chain—Horizontal Method for the Enumeration of Microorganisms—Part 1: Colony Count at 30 Degrees by the Pour Plate Technique. International Organization for Standardization Publications: Geneva, Switzerland, 2013.
- ISO 15214:1998; Microbiology of Food and Animal Feeding Stuffs—Horizontal Method for the Emumeration of Mesophilic Lactic Acid Bacteria—Colony-Count Technique at 30 °C. International Organization for Standardization Publications: Geneva, Switzerland, 1998.
- ISO 13720:2010; Meat and Meat Products—Enumeration of Presumptive Pseudomonas Spp. International Organization for Standardization Publications: Geneva, Switzerland, 2010.
- ISO 21527-1:2008; Microbiology of Food and Animal Feeding Stuffs—Horizontal Method for the Enumeration of Yeasts and Moulds—Part 1: Colony Count Technique in Products with Water Activity Greater than 0.95. International Organization for Standardization Publications: Geneva, Switzerland, 2008.
- Dias, J.; Coelho, P.; Alvarenga, N.B.; Duarte, R.V.; Saraiva, J.A. Evaluation of the Impact of High Pressure on the Storage of Filled Traditional Chocolates. Innov. Food Sci. Emerg. Technol. 2018, 45, 36–41. [Google Scholar] [CrossRef]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventós, R.M. [14] Analysis of Total Phenols and Other Oxidation Substrates and Antioxidants by Means of Folin-Ciocalteu Reagent. Methods Enzymol. 1999, 299, 152–178. [Google Scholar]
- Yu, M.; Gouvinhas, I.; Rocha, J.; Barros, A.I.R.N.A. Phytochemical and Antioxidant Analysis of Medicinal and Food Plants towards Bioactive Food and Pharmaceutical Resources. Sci. Rep. 2021, 11, 10041. [Google Scholar] [CrossRef]
- Pereira, N.; Ramos, A.C.; Alves, M.; Alves, V.D.; Roseiro, C.; Vida, M.; Moldão, M.; Abreu, M. Gluten-Free Sweet Potato Flour: Effect of Drying Method and Variety on the Quality and Bioactivity. Molecules 2024, 29, 5771. [Google Scholar] [CrossRef] [PubMed]
- Roseiro, C.; Santos, C.; Sol, M.; Silva, L.; Fernandes, I. Prevalence of Biogenic Amines during Ripening of a Traditional Dry Fermented Pork Sausage and Its Relation to the Amount of Sodium Chloride Added. Meat Sci. 2006, 74, 557–563. [Google Scholar] [CrossRef] [PubMed]
- StatSoft, Inc. Statistica (Data Analysis Software System), version 8.0; StatSoft, Inc.: Tulsa, OK, USA, 2007.
- Jurášková, D.; Ribeiro, S.C.; Silva, C.C.G. Exopolysaccharides Produced by Lactic Acid Bacteria: From Biosynthesis to Health-Promoting Properties. Foods 2022, 11, 156. [Google Scholar] [CrossRef] [PubMed]
- Mahdhi, A.; Leban, N.; Chakroun, I.; Chaouch, M.A.; Hafsa, J.; Fdhila, K.; Mahdouani, K.; Majdoub, H. Extracellular Polysaccharide Derived from Potential Probiotic Strain with Antioxidant and Antibacterial Activities as a Prebiotic Agent to Control Pathogenic Bacterial Biofilm Formation. Microb. Pathog. 2017, 109, 214–220. [Google Scholar] [CrossRef]
- Zheng, H.-G.; Chen, J.-C.; Ahmad, I. Preservation of King Oyster Mushroom by the Use of Different Fermentation Processes. J. Food Process Preserv. 2018, 42, e13396. [Google Scholar] [CrossRef]
- Saraiva, M.; Correia, C.B.; Cunha, I.C.; Maia, C.; Bonito, C.C.; Furtado, R.; Calhau, M.A. Interpretação de Resultados de Ensaios Microbiológicos Em Alimentos Prontos Para Consumo e Em Superfícies Do Ambiente de Preparação e Distribuição Alimentar Valores-Guia; Instituto Nacional de Saúde Doutor Ricardo Jorge: Lisbon, Portugal, 2019. [Google Scholar]
- Abdul Hakim, B.N.; Xuan, N.J.; Oslan, S.N.H. A Comprehensive Review of Bioactive Compounds from Lactic Acid Bacteria: Potential Functions as Functional Food in Dietetics and the Food Industry. Foods 2023, 12, 2850. [Google Scholar] [CrossRef]
- Kambire, O.; Yao, K.M.; Detto, K.; Kamate, M. Microbiological and Physicochemical Variations during Spontaneous Fermentation of Plantain Must. Int. J. Food Sci. 2023, 2023, 8611252. [Google Scholar] [CrossRef] [PubMed]
- Prakash, I.; Shankar, S.R.; Sneha, H.P.; Kumar, P.; Om, H.; Basavaraj, K.; Murthy, P.S. Metabolomics and Volatile Fingerprint of Yeast Fermented Robusta Coffee: A Value Added Coffee. LWT 2022, 154, 112717. [Google Scholar] [CrossRef]
- Li, H.; Guo, A.; Wang, H. Mechanisms of Oxidative Browning of Wine. Food Chem. 2008, 108, 1–13. [Google Scholar] [CrossRef]
- de Oliveira, C.M.S.; Grisi, C.V.B.; de Silva, G.S.; Lopes Neto, J.H.P.; de Medeiros, L.L.; dos Santos, K.M.O.; Cardarelli, H.R. Use of Lactiplantibacillus Plantarum CNPC 003 for the Manufacture of Functional Skimmed Fresh Cheese. Int. Dairy J. 2023, 141, 105628. [Google Scholar] [CrossRef]
- Wang, J.; Wu, T.; Fang, X.; Yang, Z. Manufacture of Low-Fat Cheddar Cheese by Exopolysaccharide-Producing Lactobacillus Plantarum JLK0142 and Its Functional Properties. J. Dairy Sci. 2019, 102, 3825–3838. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Sun, Y.; Gao, T.; Wu, Y.; Sun, H.; Zhu, Q.; Liu, C.; Zhou, C.; Han, Y.; Tao, Y. Fermentation and Storage Characteristics of “Fuji” Apple Juice Using Lactobacillus Acidophilus, Lactobacillus Casei and Lactobacillus Plantarum: Microbial Growth, Metabolism of Bioactives and in Vitro Bioactivities. Front. Nutr. 2022, 9, 833906. [Google Scholar] [CrossRef] [PubMed]
- Filannino, P.; Di Cagno, R.; Gobbetti, M. Metabolic and Functional Paths of Lactic Acid Bacteria in Plant Foods: Get out of the Labyrinth. Curr. Opin. Biotechnol. 2018, 49, 64–72. [Google Scholar] [CrossRef]
- Ruiz-Capillas, C.; Herrero, A. Impact of Biogenic Amines on Food Quality and Safety. Foods 2019, 8, 62. [Google Scholar] [CrossRef]



| Time (h) | Treatment | TSS (%) | TA (g LA/100 g) |
|---|---|---|---|
| 0 | Control | 7.4 ± 0.3 a | 0.2 ± 0.0 a |
| Lp. plantarum | 7.6 ± 0.0 a | 0.2 ± 0.0 a | |
| Ls. rhamnosus | 7.8 ± 0.1 a | 0.2 ± 0.1 a | |
| 24 | Control | 7.8 ± 0.0 a | 0.2 ± 0.0 a |
| Lp. plantarum | 8.9 ± 0.2 a | 0.2 ± 0.0 a | |
| Ls. rhamnosus | 8.1 ± 0.2 a | 0.2 ± 0.0 a | |
| 72 | Control | 7.9 ± 0.0 a | 0.3 ± 0.0 ab |
| Lp. plantarum | 8.4 ± 0.4 a | 0.7 ± 0.0 bc | |
| Ls. rhamnosus | 8.1 ± 0.0 a | 0.3 ± 0.0 ab | |
| 144 | Control | 7.8 ± 0.2 a | 0.8 ± 0.0 cd |
| Lp. plantarum | 8.8 ± 0.6 a | 1.0 ± 0.0 cde | |
| Ls. rhamnosus | 8.6 ± 0.5 a | 0.9 ± 0.0 cd | |
| 240 | Control | 8.4 ± 0.0 a | 1.4 ± 0.2 cde |
| Lp. plantarum | 8.4 ± 0.1 a | 1.6 ± 0.4 de | |
| Ls. rhamnosus | 8.4 ± 0.3 a | 1.7 ± 0.2 e |
| Time (h) | Samples | L | a* | b* | ∆E |
|---|---|---|---|---|---|
| 0 | Control | 67.7 ± 4.5 bc | 1.2 ± 0.3 ab | 29.0 ± 3.9 ab | - |
| Lp. plantarum | 72.5 ± 1.4 ef | 1.5 ± 0.5 abc | 29.8 ± 1.4 b | - | |
| Ls. rhamnosus | 71.7 ± 2.2 def | 1.5 ± 0.4 ab | 27.8 ± 2.6 ab | - | |
| 24 | Control | 71.8 ± 1.0 def | 1.7 ± 0.2 abcd | 29.6 ± 3.2 ab | 5.0 ± 1.9 abc |
| Lp. plantarum | 73.3 ± 1.3 f | 2.2 ± 0.6 cde | 33.3 ± 2.0 c | 4.1 ± 1.6 ac | |
| Ls. rhamnosus | 72.4 ± 1.1 ef | 2.8 ± 0.7 e | 34.5 ± 1.5 c | 7.0 ± 1.4 bd | |
| 72 | Control | 70.5 ± 1.3 cdef | 3.0 ± 0.5 e | 33.2 ± 2.8 c | 6.1 ± 1.3 abd |
| Lp. plantarum | 70.1 ± 0.8 ced | 2.4 ± 0.8 de | 34.1 ± 3.0 c | 8.3 ± 2.0 abc | |
| Ls. rhamnosus | 69.7 ± 1.6 cd | 4.3 ± 0.3 f | 35.2 ± 2.0 c | 5.2 ± 2.8 d | |
| 144 | Control | 69.3 ± 1.6 bcd | 1.1 ± 0.5 ab | 28.6 ± 0.9 ab | 1.7 ± 0.5 c |
| Lp. plantarum | 67.5 ± 1.2 bc | 1.1 ± 0.4 ab | 28.2 ± 0.5 ab | 5.0 ± 0.9 abc | |
| Ls. rhamnosus | 66.6 ± 1.1 b | 0.9 ± 0.6 bcd | 28.9 ± 1.1 ab | 5.3 ± 1.2 ab | |
| 240 | Control | 61.7 ± 1.5 a | 1.1 ± 0.3 a | 27.5 ± 1.4 ab | 6.4 ± 1.6 abd |
| Lp. plantarum | 61.6 ± 2.1 a | 1.5 ± 0.5 abc | 26.7 ± 1.1 a | 11.4 ± 2.2 e | |
| Ls. rhamnosus | 60.8 ± 1.6 a | 1.7 ± 0.3 abcd | 27.7 ± 1.5 ab | 11.0 ± 1.5 e |
| Sample | Tryptamine | Phenylethylamine | Putrescine | Cadaverine | Histamine | Tyramine | Spermidine | Spermine |
|---|---|---|---|---|---|---|---|---|
| Ci | 13.6 ± 2.3 a | 5.3 ± 3.1 a | 4.8 ± 2.0 a | 15.1 ± 0.9 a | 6.4 ± 0.5 a | 27.2 ± 0.8 b | 25.3 ± 0.1 a | 12.3 ± 0.4 a |
| Cf | 29.7 ± 4.7 b | 4.0 ± 0.0 a | 2.3 ± 0.3 a | 70.3 ± 15.2 b | 6.6 ± 1.0 a | 14.8 ± 4.9 ab | 32.7 ± 15.9 a | 11.0 ± 1.1 a |
| Lci | 10.4 ± 0.8 a | 0.7 ± 0.6 a | 2.4 ± 0.1 a | 7.2 ± 0.8 a | 5.5 ± 0.0 a | 9.5 ± 0.5 a | 29.0 ± 7.5 a | 11.6 ± 1.0 a |
| Lcf | 9.7 ± 0.6 a | 4.1 ± 0.1 a | 3.6 ± 0.4 a | 10.5 ± 0.1 a | 5.8 ± 0.7 a | 9.6 ± 2.3 a | 19.7 ± 10.0 a | 15.6 ± 3.1 a |
| Lpi | 7.4 ± 0.4 a | 1.4 ± 0.4 a | 3.0 ± 0.0 a | 14 ± 2.0 a | 6.8 ± 1.1 a | 12.6 ± 0.2 a | 45.2 ± 1.1 a | 10.8 ± 0.7 a |
| Lpf | 15.7 ± 0.3 a | 3.8 ± 1.1 a | 5.1 ± 1.2 a | 13.0 ± 0.3 a | 6.3 ± 0.2 a | 12.3 ± 0.1 a | 45.5 ± 11.2 a | 11.6 ± 1.1 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Silva, M.; Vida, M.; Ramos, A.C.; Roseiro, L.C.; Alvarenga, N.; Gomes, S.; Lidon, F.C.; Reboredo, F.H.; Gonçalves, E.M. Biopreservation of Hericium erinaceus By-Products via Lactic Acid Fermentation: Effects on Functional and Technological Properties. Foods 2025, 14, 3721. https://doi.org/10.3390/foods14213721
Silva M, Vida M, Ramos AC, Roseiro LC, Alvarenga N, Gomes S, Lidon FC, Reboredo FH, Gonçalves EM. Biopreservation of Hericium erinaceus By-Products via Lactic Acid Fermentation: Effects on Functional and Technological Properties. Foods. 2025; 14(21):3721. https://doi.org/10.3390/foods14213721
Chicago/Turabian StyleSilva, Mafalda, Manuela Vida, Ana Cristina Ramos, Luísa Cristina Roseiro, Nuno Alvarenga, Sandra Gomes, Fernando C. Lidon, Fernando H. Reboredo, and Elsa M. Gonçalves. 2025. "Biopreservation of Hericium erinaceus By-Products via Lactic Acid Fermentation: Effects on Functional and Technological Properties" Foods 14, no. 21: 3721. https://doi.org/10.3390/foods14213721
APA StyleSilva, M., Vida, M., Ramos, A. C., Roseiro, L. C., Alvarenga, N., Gomes, S., Lidon, F. C., Reboredo, F. H., & Gonçalves, E. M. (2025). Biopreservation of Hericium erinaceus By-Products via Lactic Acid Fermentation: Effects on Functional and Technological Properties. Foods, 14(21), 3721. https://doi.org/10.3390/foods14213721










