Aquafaba Hydrolysates as Functional Ingredients in Muffin Cakes: Effects on Physicochemical Properties, Quality Attributes, and Antioxidant Activity
Abstract
1. Introduction
2. Material and Methods
2.1. Material
2.2. Enzymatic Hydrolysis
2.3. Degree of Hydrolysis (DH)
2.4. SDS-PAGE (Sodium Dodecyl Sulfate–Polyacrylamide Gel) Electrophoresis Analysis
2.5. Muffin Preparation
2.6. The Flow Behavior of Muffin Batter
2.7. Some Physicochemical and Quality Properties of Muffins
2.8. Texture Profile Analysis
2.9. Oxidative Stability
2.10. Thermal Properties (Differential Scanning Calorimetry Analysis)
2.11. Preparation of Muffin Extracts for Bioactivity Assessments
2.12. Antioxidant Capacity Assays
2.12.1. DPPH Assay
2.12.2. ABTS Assay
2.13. Statistical Analysis
3. Result and Discussion
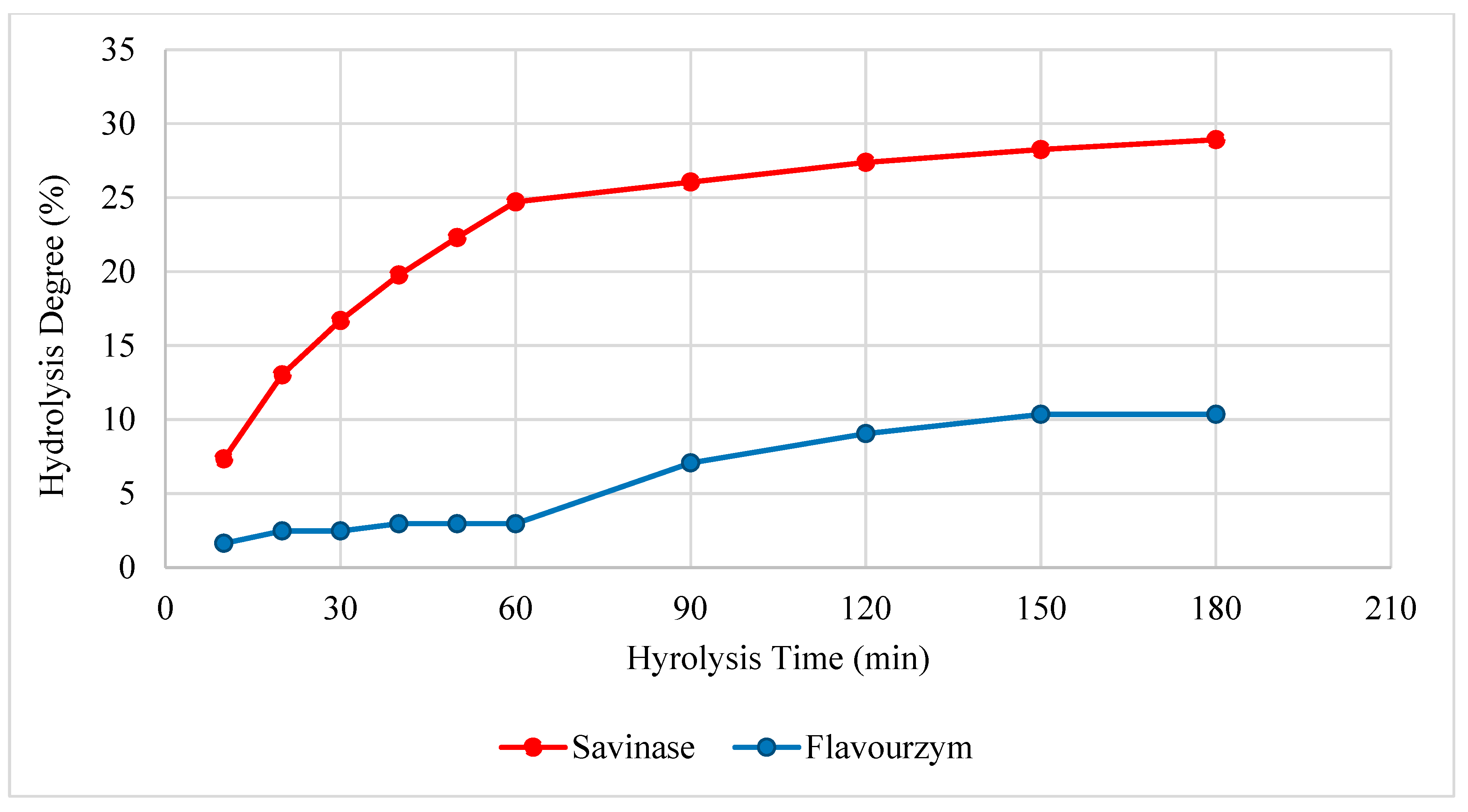
3.1. Degree of Hydrolysis (DH%)
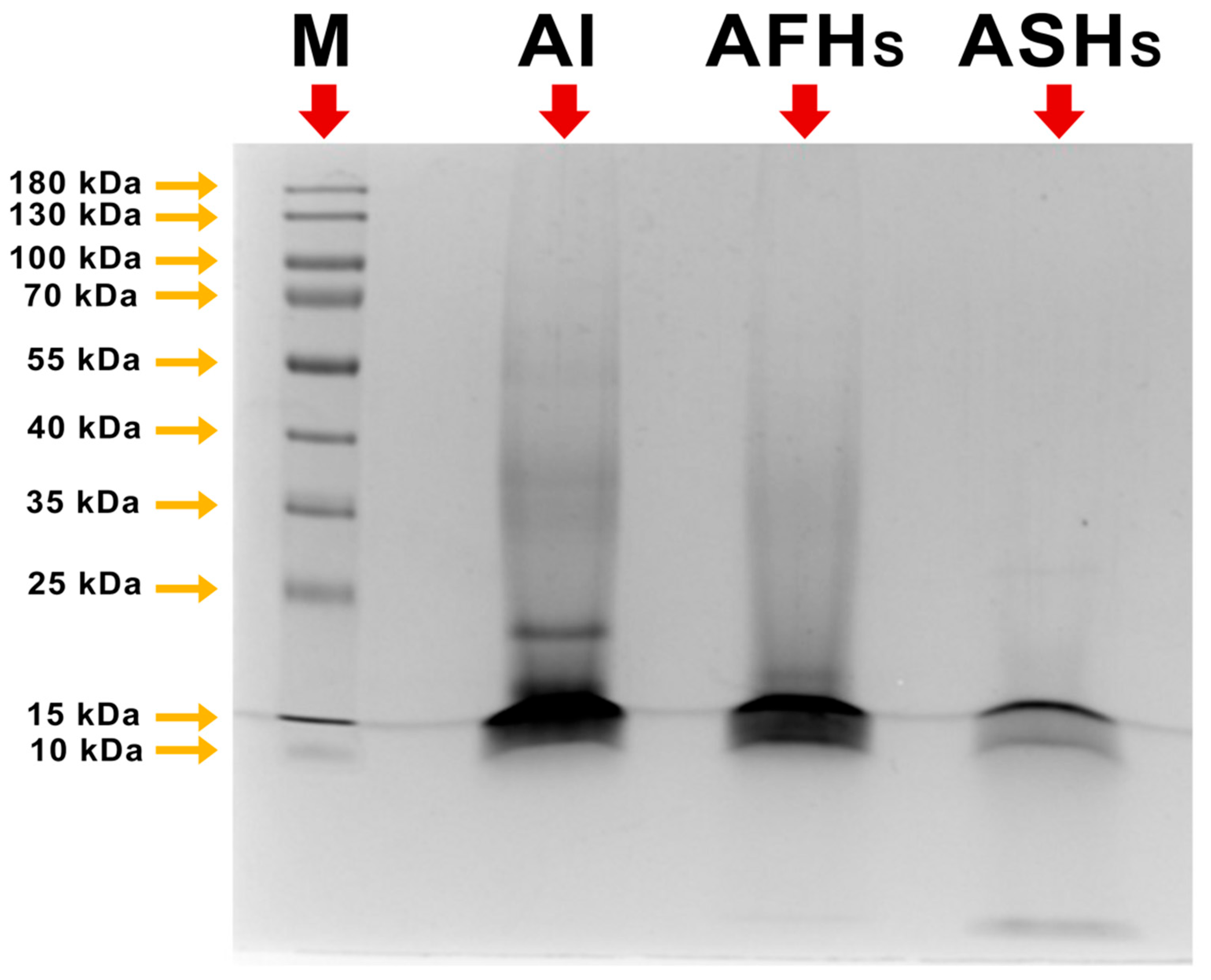
3.2. Molecular Weight Profile (SDS-PAGE Analysis)
3.3. Physicochemical and Color Properties of Muffin Cake Samples
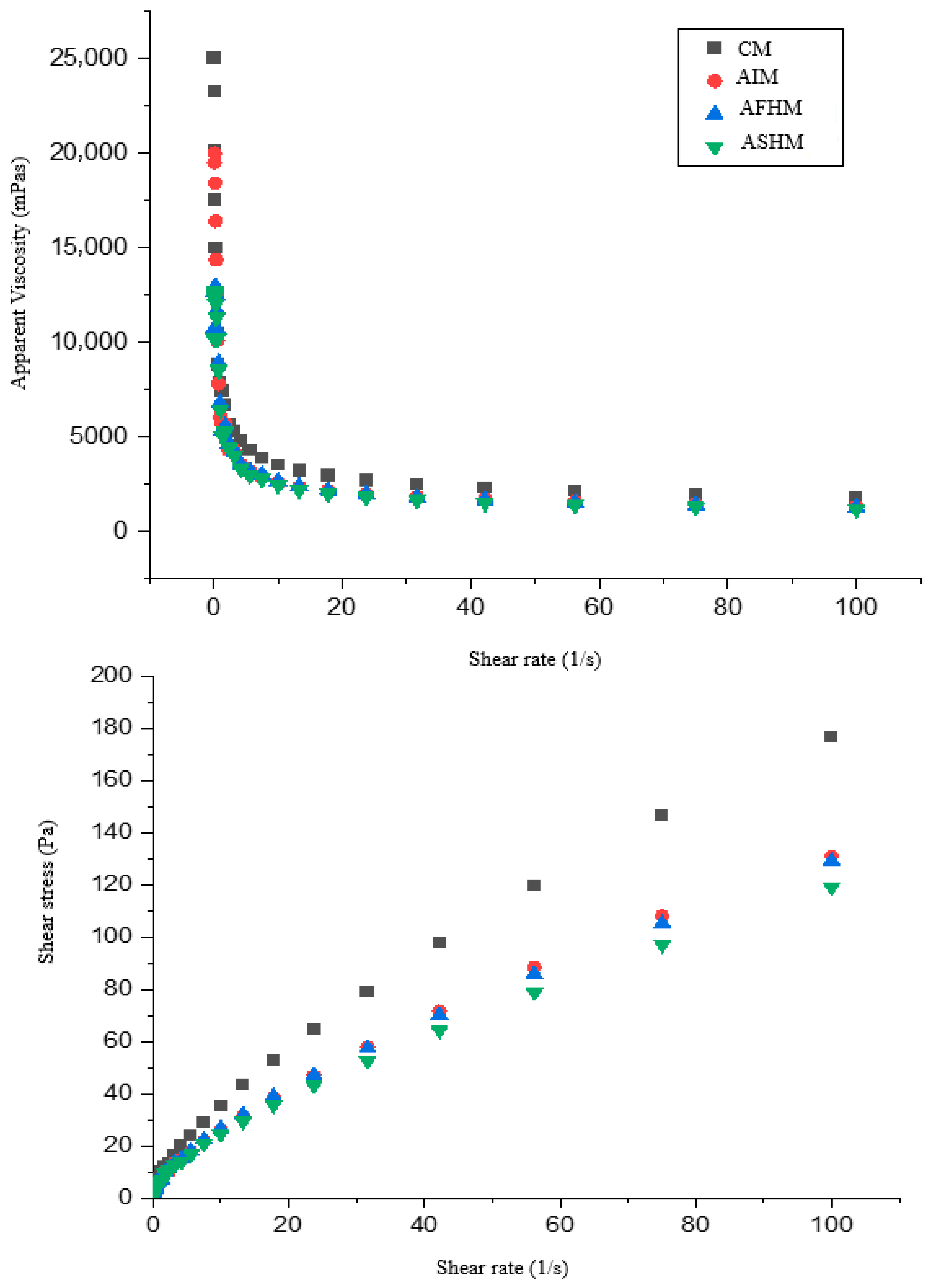
3.4. Rheological Properties of Muffin Batter
3.5. Texture Profile Analysis
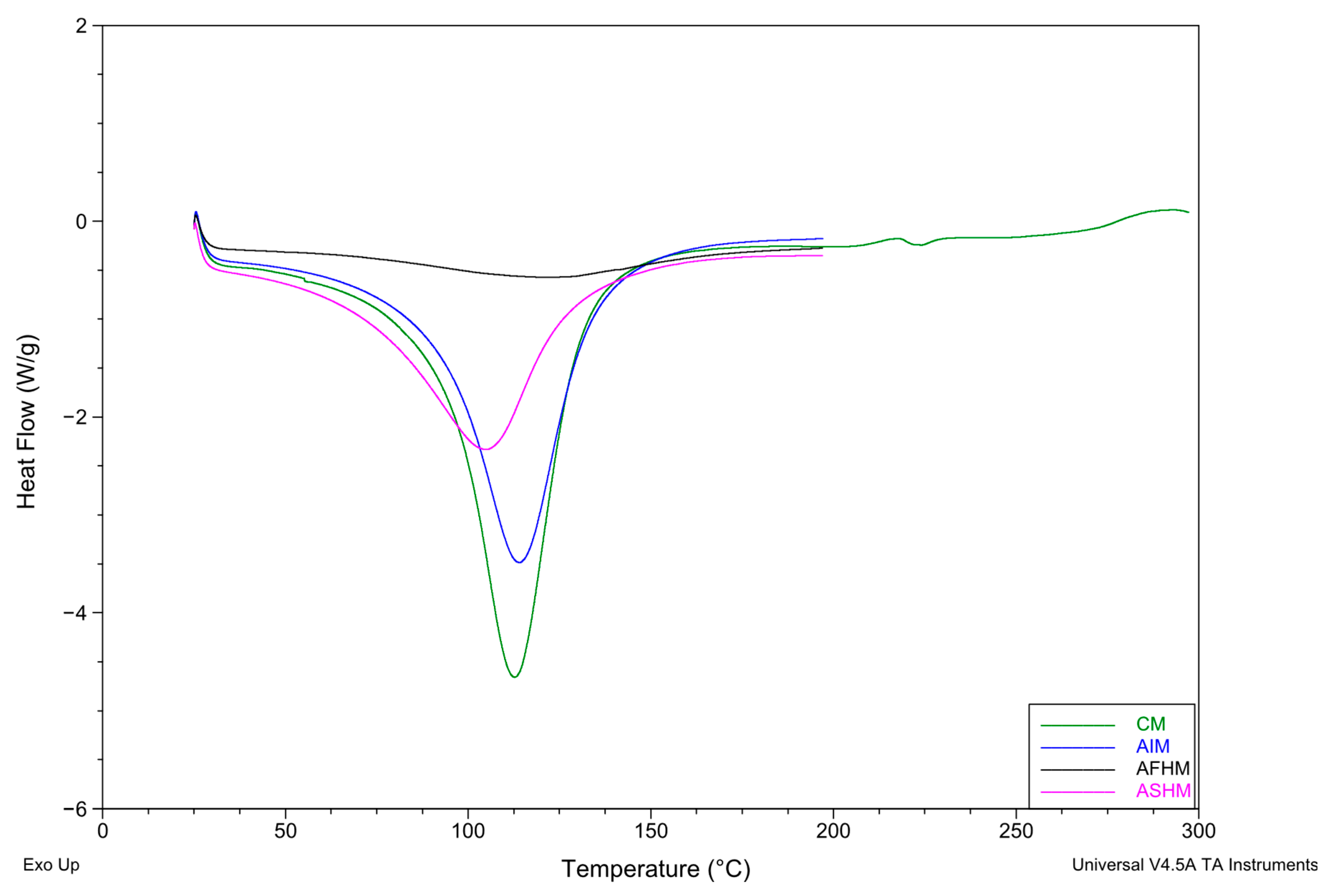
3.6. Thermal Properties (Differential Scanning Calorimetry Analysis)
3.7. Oxidative Stability
3.8. Antioxidant Capacity
4. Conclusions
Supplementary Materials
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tachie, C.; Nwachukwu, I.D.; Aryee, A.N.A. Trends and Innovations in the Formulation of Plant-Based Foods. Food Prod. Process. Nutr. 2023, 5, 16. [Google Scholar] [CrossRef]
- Fu, Q.; Zhao, J.; Rong, S.; Han, Y.; Liu, F.; Chu, Q.; Wang, S.; Chen, S. Research Advances in Plant Protein-Based Products: Protein Sources, Processing Technology, and Food Applications. J. Agric. Food Chem. 2023, 71, 15429–15444. [Google Scholar] [CrossRef] [PubMed]
- Thakur, S.; Pandey, A.K.; Verma, K.; Shrivastava, A.; Singh, N. Plant-Based Protein as an Alternative to Animal Proteins: A Review of Sources, Extraction Methods and Applications. Int. J. Food Sci. Technol. 2024, 59, 488–497. [Google Scholar] [CrossRef]
- Yazici, G.N.; Taspinar, T.; Ozer, M.S. Aquafaba: A Multifunctional Ingredient in Food Production. Biol. Life Sci. Forum 2022, 18, 24. [Google Scholar] [CrossRef]
- Stasiak, J.; Stasiak, D.M.; Libera, J. The Potential of Aquafaba as a Structure-Shaping Additive in Plant-Derived Food Technology. Appl. Sci. 2023, 13, 4122. [Google Scholar] [CrossRef]
- Bekiroglu, H.; Karimidastjerd, A.; Ozmen, D.; Toker, O.S.; Inan, M.; Sagdic, O.; Dertli, E. Improvement of Some Techno-Functional Properties of Aquafaba by Pre-Fermentation with Lactobacillus Plantarum MA2. Food Biosci. 2023, 54, 102807. [Google Scholar] [CrossRef]
- Gasparre, N.; Rosell, C.M.; Boukid, F. Enzymatic Hydrolysis of Plant Proteins: Tailoring Characteristics, Enhancing Functionality, and Expanding Applications in the Food Industry. Food Bioprocess Technol. 2025, 18, 3272–3287. [Google Scholar] [CrossRef]
- Wouters, A.G.B.; Rombouts, I.; Fierens, E.; Brijs, K.; Delcour, J.A. Relevance of the Functional Properties of Enzymatic Plant Protein Hydrolysates in Food Systems. Compr. Rev. Food Sci. Food Saf. 2016, 15, 786–800. [Google Scholar] [CrossRef]
- Nawaz, M.A.; Buckow, R.; Jegasothy, H.; Stockmann, R. Enzymatic Hydrolysis Improves the Stability of UHT Treated Faba Bean Protein Emulsions. Food Bioprod. Process. 2022, 132, 200–210. [Google Scholar] [CrossRef]
- Ghinea, C.; Ungureanu-Comăniță, E.D.; Țâbuleac, R.M.; Oprea, P.S.; Coșbuc, E.D.; Gavrilescu, M. Cost-Benefit Analysis of Enzymatic Hydrolysis Alternatives for Food Waste Management. Foods 2025, 14, 488. [Google Scholar] [CrossRef]
- Vogelsang-O’Dwyer, M.; Sahin, A.W.; Arendt, E.K.; Zannini, E. Enzymatic Hydrolysis of Pulse Proteins as a Tool to Improve Techno-Functional Properties. Foods 2022, 11, 1307. [Google Scholar] [CrossRef] [PubMed]
- Amiri, M.; Hassani, B.; Babapour, H.; Nikmanesh, A.; Hosseini, S.E.; Asadi, G.; Abedinia, A. Optimization of Enzyme Hydrolysis to Improve Functional and Structural Properties of Microalgae Protein Extract. J. Food Sci. 2025, 90, e70129. [Google Scholar] [CrossRef] [PubMed]
- Dent, T.; Campanella, O.; Maleky, F. Enzymatic Hydrolysis of Soy and Chickpea Protein with Alcalase and Flavourzyme and Formation of Hydrogen Bond Mediated Insoluble Aggregates. Curr. Res. Food Sci. 2023, 6, 100487. [Google Scholar] [CrossRef] [PubMed]
- Fierens, E.; Brijs, K.; Delcour, J.A. Emulsifying and Foaming Properties of Okara Protein Hydrolysates. Cereal Chem. 2016, 93, 71–76. [Google Scholar] [CrossRef]
- Güneş, Z.S.; Şişman, S.; Özarda, Ö.; Gülseren, İ. Bioactive, Textural and Sensory Attributes of Soft Confections Enriched with Plant Protein Hydrolysates. J. Food Meas. Charact. 2024, 18, 5534–5540. [Google Scholar] [CrossRef]
- Sung, W.C.; Tan, C.X.; Lai, P.H.; Wang, S.T.; Chiou, T.Y.; Lee, W.J. Enhancing the Functional and Emulsifying Properties of Potato Protein via Enzymatic Hydrolysis with Papain and Bromelain for Gluten-Free Cake Emulsifiers. Foods 2025, 14, 978. [Google Scholar] [CrossRef]
- Cermeño, M.; Dermiki, M.; Kleekayai, T.; Cope, L.; McManus, R.; Ryan, C.; Felix, M.; Flynn, C.; FitzGerald, R.J. Effect of Enzymatically Hydrolysed Brewers’ Spent Grain Supplementation on the Rheological, Textural and Sensory Properties of Muffins. Future Foods 2021, 4, 100085. [Google Scholar] [CrossRef]
- Ma, C.M.; Li, X.H.; Wang, X.P.; Song, C.L.; Zhao, X.H. Impact of the Enzyme-Hydrolyzed Pumpkin (Cucurbita moschata Duch.) Pulp on the Chemical and Textural Features of Cake Batter and Chiffon Cake. ACS Food Sci. Technol. 2025, 5, 2787–2794. [Google Scholar] [CrossRef]
- Ghanbarinia, S.; Ariaii, P.; Safari, R.; Najafian, L. The Effect of Hydrolyzed Sesame Meal Protein on the Quality and Shelf Life of Hamburgers during Refrigerated Storage. Anim. Sci. J. 2022, 93, 13729. [Google Scholar] [CrossRef]
- Bekiroglu, H.; Karaman, S.; Bozkurt, F.; Sagdic, O. Characterization of Some Physicochemical, Textural, and Antioxidant Properties of Muffins Fortified with Hydrolyzed Whey Protein. Food Sci. Nutr. 2024, 12, 8105–8117. [Google Scholar] [CrossRef]
- Mohammadi, M.; Salami, M.; Yarmand, M.; Emam-Djomeh, Z.; McClements, D.J. Production and Characterization of Functional Bakery Goods Enriched with Bioactive Peptides Obtained from Enzymatic Hydrolysis of Lentil Protein. J. Food Meas. Charact. 2022, 16, 3402–3409. [Google Scholar] [CrossRef]
- Akbin, A.C.; Turabi Yolacaner, E.; Sumnu, G. Effects of Legume-Based Aquafaba on Batter Rheology and Quality Characteristics of Microwave-Infrared Baked Cakes. Phys. Fluids 2025, 37, 2025. [Google Scholar] [CrossRef]
- Yazici, G.N.; Taspinar, T.; Binokay, H.; Agcam, E.; Agirman, B.; Ozer, M.S. Assessment of Physicochemical Properties and Staling Characteristics of Eggless Gluten-Free Cakes with Aquafaba. J. Food Meas. Charact. 2025, 19, 2557–2573. [Google Scholar] [CrossRef]
- Adler-Nissen, J. Methods in Food Protein Hydrolysis. In Enzymic Hydrolysis of Food Proteins; Elsevier Applied Science Publishers: London, UK, 1986. [Google Scholar]
- Laemmli, U.K. Cleavage of Structural Proteins during the Assembly of the Head of Bacteriophage T4. Nature 1970, 227, 680–685. [Google Scholar] [CrossRef] [PubMed]
- American Association of Cereal Chemists. AACC Approved Methods of the AACC, 8th ed.; American Association of Cereal Chemists: Saint Paul, MN, USA, 1990. [Google Scholar]
- Quiles, A.; Llorca, E.; Schmidt, C.; Reißner, A.M.; Struck, S.; Rohm, H.; Hernando, I. Use of Berry Pomace to Replace Flour, Fat or Sugar in Cakes. Int. J. Food Sci. Technol. 2018, 53, 1579–1587. [Google Scholar] [CrossRef]
- George, L. Official Methods of Analysis of AOAC International; AOAC Internatıonal: Rockville, MD, USA, 2006. [Google Scholar] [CrossRef]
- Ammar, I.; Gharsallah, H.; Ben Brahim, A.; Attia, H.; Ayadi, M.A.; Hadrich, B.; Felfoul, I. Optimization of Gluten-free Sponge Cake Fortified with Whey Protein Concentrate Using Mixture Design Methodology. Food Chem. 2021, 343, 128457. [Google Scholar] [CrossRef]
- Kemski, M.M.; Cottonaro, A.; Vittadini, E.; Vodovotz, Y. Development of Gluten-Free Muffins Made from Breadfruit and Unripe Plantain Flours. Int. J. Food Sci. Technol. 2022, 57, 2980–2991. [Google Scholar] [CrossRef]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a Free Radical Method to Evaluate Antioxidant Activity. LWT-Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Evans, C.R. Antioxidant Actvity Applying an Improved ABTS Radical Cation Decolorization Assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Bozkurt, F.; Bekiroglu, H.; Dogan, K.; Karasu, S.; Sagdic, O. Technological and Bioactive Properties of Wheat Glutenin Hydrolysates Prepared with Various Commercial Proteases. LWT 2021, 149, 111787. [Google Scholar] [CrossRef]
- Garcia-Mora, P.; Peñas, E.; Frias, J.; Martínez-Villaluenga, C. Savinase, the Most Suitable Enzyme for Releasing Peptides from Lentil (Lens culinaris Var. Castellana) Protein Concentrates with Multifunctional Properties. J. Agric. Food Chem. 2014, 62, 4166–4174. [Google Scholar] [CrossRef] [PubMed]
- Cui, Q.; Sun, Y.; Zhou, Z.; Cheng, J.; Guo, M. Effects of Enzymatic Hydrolysis on Physicochemical Properties and Solubility and Bitterness of Milk Protein Hydrolysates. Foods 2021, 10, 2462. [Google Scholar] [CrossRef] [PubMed]
- Sbroggio, M.F.; Montilha, M.S.; de Figueiredo, V.R.G.; Georgetti, S.R.; Kurozawa, L.E. Influence of the Degree of Hydrolysis and Type of Enzyme on Antioxidant Activity of Okara Protein Hydrolysates. Food Sci. Technol. 2016, 36, 375–381. [Google Scholar] [CrossRef]
- Xu, Y.; Galanopoulos, M.; Sismour, E.; Ren, S.; Mersha, Z.; Lynch, P.; Almutaimi, A. Effect of Enzymatic Hydrolysis Using Endo- and Exo-Proteases on Secondary Structure, Functional, and Antioxidant Properties of Chickpea Protein Hydrolysates. J. Food Meas. Charact. 2020, 14, 343–352. [Google Scholar] [CrossRef]
- Vogelsang-O’Dwyer, M.; Sahin, A.W.; Bot, F.; O’Mahony, J.A.; Bez, J.; Arendt, E.K.; Zannini, E. Enzymatic Hydrolysis of Lentil Protein Concentrate for Modification of Physicochemical and Techno-Functional Properties. Eur. Food Res. Technol. 2023, 249, 573–586. [Google Scholar] [CrossRef]
- Choden, N.; Odelli, D.; Casanova, F.; Petersen, H.O.; Ajalloueian, F.; Feyissa, A.H. Effect of the Extraction Process Parameters on Aquafaba Composition and Foaming Properties. Appl. Food Res. 2023, 3, 100354. [Google Scholar] [CrossRef]
- Imbart, S.; Régnault, S.; Bernard, C. Effects of Germination and Fermentation on the Emulsifying Properties of Cowpea (Vigna unguiculata L. Walp.) Proteins. J. Food Meas. Charact. 2016, 10, 119–126. [Google Scholar] [CrossRef]
- Di Francesco, A.; De Santis, M.A.; Lanzoni, A.; Pittalà, M.G.G.; Saletti, R.; Flagella, Z.; Cunsolo, V. Mass Spectrometry Characterization of the SDS-PAGE Protein Profile of Legumins and Vicilins from Chickpea Seed. Foods 2024, 13, 887. [Google Scholar] [CrossRef]
- Pascual-Bustamante, S.; Raya-Perez, J.C.; Aguirre-Mancilla, C.L.; Ramírez-Pimental, J.G. Chemical and Protein Characterization of Two Varieties of Chickpea (Cicer Arietinum): Costa 2004 and El Patrón. Plants 2024, 13, 2125. [Google Scholar] [CrossRef]
- Hunsakul, K.; Laokuldilok, T.; Sakdatorn, V.; Klangpetch, W.; Brennan, C.S.; Utama-ang, N. Optimization of Enzymatic Hydrolysis by Alcalase and Flavourzyme to Enhance the Antioxidant Properties of Jasmine Rice Bran Protein Hydrolysate. Sci. Rep. 2022, 12, 12582. [Google Scholar] [CrossRef]
- Kutlu, G.; Yılmaz, S.; Karabulut, A.E. Development of a New Vegan Muffin Formulation: Assessing Its Quality and Sensory Characteristics. Eur. Food Sci. Eng. 2024, 5, 26–34. [Google Scholar] [CrossRef]
- Mojtahedi, E.; Yilmaz, H. Evaluation of Techno-Functional Properties of Fava Bean Aquafaba Powder in Vegan Muffins: Effects of Locust Bean Gum and Foam-Mat Drying. Food Chem. X 2025, 26, 102316. [Google Scholar] [CrossRef] [PubMed]
- Haslubis, M.I.; Arifin, N. Physical Properties, Nutritional Composition and Sensory Acceptance of Eggless Pumpkin Muffin Prepared Using Plant-Based Ingredients. J. Adv. Res. Des. 2024, 118, 56–71. [Google Scholar] [CrossRef]
- Shaabani, S.; Yarmand, M.S.; Kiani, H.; Emam-Djomeh, Z. The Effect of Chickpea Protein Isolate in Combination with Transglutaminase and Xanthan on the Physical and Rheological Characteristics of Gluten Free Muffins and Batter Based on Millet Flour. LWT 2018, 90, 362–372. [Google Scholar] [CrossRef]
- Damian, J.J.; Huo, S.; Serventi, L. Phytochemical Content and Emulsifying Ability of Pulses Cooking Water. Eur. Food Res. Technol. 2018, 244, 1647–1655. [Google Scholar] [CrossRef]
- Sivaraj, D.; Dalbhagat, C.G.; Venugopal, A.P.; Thivya, P.; Nimbkar, S.; Gowda, N.A.N.; Mishra, S.; Kambhampati, V. Aquafaba as a Sustainable and Plant-Based Egg Alternative: Recent Advances in Extraction, Nutritional Insights, and Functional Characterization. Food Bioprocess Technol. 2025, 18, 8927–8953. [Google Scholar] [CrossRef]
- Koriyama, T.; Iijima, K.; Hosoya, T. Optimizing Chickpea Cooking Water (Aquafaba): Enhancing Superior Foaming and Emulsifying Properties Through Concentration Protocols. Gastronomy 2025, 3, 3. [Google Scholar] [CrossRef]
- Prieto-Vázquez del Mercado, P.; Mojica, L.; Morales-Hernández, N. Protein Ingredients in Bread: Technological, Textural and Health Implications. Foods 2022, 11, 2399. [Google Scholar] [CrossRef]
- Erem, E.; Icyer, N.C.; Tatlisu, N.B.; Kilicli, M.; Kaderoglu, G.H.; Toker, Ö.S. A New Trend among Plant-Based Food Ingredients in Food Processing Technology: Aquafaba. Crit. Rev. Food Sci. Nutr. 2021, 63, 4467–4484. [Google Scholar] [CrossRef]
- Mustafa, R.; He, Y.; Shim, Y.Y.; Reaney, M.J.T. Aquafaba, Wastewater from Chickpea Canning, Functions as an Egg Replacer in Sponge Cake. Int. J. Food Sci. Technol. 2018, 53, 2247–2255. [Google Scholar] [CrossRef]
- Qasem, A.A.A.; Alamri, M.S.; Mohamed, A.A.; Hussain, S.; Mahmood, K.; Ibraheem, M.A. Effect of Okra Gum on Pasting and Rheological Properties of Cake-Batter. J. Food Meas. Charact. 2017, 11, 827–834. [Google Scholar] [CrossRef]
- Brown, J.; Hu, R.; Xiao, R.; Liu, R.; Li, Y.; Getty, K. Enzyme-Modified Soy Protein Hydrolysates in Muffins. Cereal Technol. Getreidetechnol. 2023, 77, 68–83. [Google Scholar]
- Sung, M.-J.; Park, Y.-S.; Chang, H.-G. Quality Characteristics of Sponge Cake Supplemented with Soy Fiber Flour. Food Sci. Biotechnol. 2006, 15, 860–865. [Google Scholar]
- Guiné, R.P.F. Textural Properties of Bakery Products: A Review of Instrumental and Sensory Evaluation Studies. Appl. Sci. 2022, 12, 8628. [Google Scholar] [CrossRef]
- Ozón, B.; Cotabarren, J.; Geier, F.R.; Kise, M.P.; García-Pardo, J.; Parisi, M.G.; Obregón, W.D. Development of Fortified Breads Enriched with Plant-Based Bioactive Peptides Derived from the Chia (Salvia hispanica L.) Expeller. Foods 2023, 12, 3382. [Google Scholar] [CrossRef] [PubMed]
- Karimi, A.; Ahmadi Gavlighi, H.; Amini Sarteshnizi, R.; Udenigwe, C.C. Effect of Maize Germ Protein Hydrolysate Addition on Digestion, İn Vitro Antioxidant Activity and Quality Characteristics of Bread. J. Cereal Sci. 2021, 97, 2025. [Google Scholar] [CrossRef]
- Alemán-Huerta, M.E.; Castillo-Cázares, B.A.; Márquez-Reyes, J.M.; Báez-González, J.G.; Quintero-Zapata, I.; Gandarilla-Pacheco, F.L.; de Luna-Santillana, E.d.J.; Treviño-Garza, M.Z. Muffin-Type Bakery Product Based on Mexican Mesquite (Prosopis spp.) Flour: Texture Profile, Acceptability, and Physicochemical Properties. Foods 2023, 12, 3587. [Google Scholar] [CrossRef] [PubMed]
- Oprea, O.B.; Sannan, S.; Tolstorebrov, I.; Claussen, I.C.; Gaceu, L. Effects of Fish Protein Hydrolysate on the Nutritional, Rheological, Sensorial, and Textural Characteristics of Bread. Foods 2024, 13, 698. [Google Scholar] [CrossRef]
- Grossi Bovi Karatay, G.; Rebellato, A.P.; Joy Steel, C.; Dupas Hubinger, M. Chickpea Aquafaba-Based Emulsions as a Fat Replacer in Pound Cake: Impact on Cake Properties and Sensory Analysis. Foods 2022, 11, 2484. [Google Scholar] [CrossRef]
- Asaithambi, N.; Singha, P.; Singh, S.K. Recent Application of Protein Hydrolysates in Food Texture Modification. Crit. Rev. Food Sci. Nutr. 2023, 63, 10412–10443. [Google Scholar] [CrossRef]
- Ryan, M.; McEvoy, E.; Duignan, S.; Crowley, C.; Fenelon, M.; O’Callaghan, D.M.; FitzGerald, R.J. Thermal Stability of Soy Protein Isolate and Hydrolysate Ingredients. Food Chem. 2008, 108, 503–510. [Google Scholar] [CrossRef] [PubMed]
- Yarnpakdee, S.; Benjakul, S.; Kristinsson, H.G.; Kishimura, H. Antioxidant and Sensory Properties of Protein Hydrolysate Derived from Nile Tilapia (Oreochromis Niloticus) by One- and Two-Step Hydrolysis. J. Food Sci. Technol. 2015, 52, 3336–3349. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Feng, L.; Wu, Y.; Han, Y.; Yao, X.; Li, Q.; Liu, M.; Cao, Y. Structural Characteristics, Functional Properties and Nutritional Value of Walnut Protein by Limited Enzymatic Hydrolysis. LWT 2024, 197, 115923. [Google Scholar] [CrossRef]
- Hamed, F.; Elgaoud, I.; Eljoudi, S.; Deracinois, B.; Flahaut, C.; Nedjar, N.; Barkia, A. Diplodus Protein Hydrolysates: Antioxidant and Antibacterial Properties and Identification of Biopeptides. Waste Biomass Valorization 2024, 15, 4309–4323. [Google Scholar] [CrossRef]
- Shuai, X.; Gao, L.; Geng, Q.; Li, T.; He, X.; Chen, J.; Liu, C.; Dai, T. Effects of Moderate Enzymatic Hydrolysis on Structure and Functional Properties of Pea Protein. Foods 2022, 11, 2368. [Google Scholar] [CrossRef]
- Wang, J.; Wang, G.; Chen, N.; An, F.; Zhang, R.; Zhang, Y.; Rahman, M.U.; Zhang, Y. Characterization of Structural, Functional and Antioxidant Properties and Amino Acid Composition of Pepsin-Derived Glutelin-1 Hydrolysate from Walnut Processing by-Products. RSC Adv. 2021, 11, 19158–19168. [Google Scholar] [CrossRef]
- Bazsefidpar, N.; Ghandehari Yazdi, A.P.; Karimi, A.; Yahyavi, M.; Amini, M.; Ahmadi Gavlighi, H.; Simal-Gandara, J. Brewers Spent Grain Protein Hydrolysate as a Functional Ingredient for Muffins: Antioxidant, Antidiabetic, and Sensory Evaluation. Food Chem. 2024, 435, 137565. [Google Scholar] [CrossRef]
- Peng, X.; Kong, B.; Xia, X.; Liu, Q. Reducing and Radical-Scavenging Activities of Whey Protein Hydrolysates Prepared with Alcalase. Int. Dairy J. 2010, 20, 360–365. [Google Scholar] [CrossRef]
- Kong, B.; Xiong, Y.L. Antioxidant Activity of Zein Hydrolysates in a Liposome System and the Possible Mode of Action. J. Agric. Food Chem. 2006, 54, 6059–6068. [Google Scholar] [CrossRef]
- Li, Y.; Jiang, B.; Zhang, T.; Mu, W.; Liu, J. Antioxidant and Free Radical-Scavenging Activities of Chickpea Protein Hydrolysate (CPH). Food Chem. 2008, 106, 444–450. [Google Scholar] [CrossRef]
- Munteanu, I.G.; Apetrei, C. Analytical Methods Used in Determining Antioxidant Activity: A Review. Int. J. Mol. Sci. 2021, 22, 3380. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Ma, M.; Yu, Z.; Du, S. kui Preparation and Identification of Antioxidant Peptides from Cottonseed Proteins. Food Chem. 2021, 352, 129399. [Google Scholar] [CrossRef] [PubMed]
- Mareček, V.; Mikyška, A.; Hampel, D.; Čejka, P.; Neuwirthová, J.; Malachová, A.; Cerkal, R. ABTS and DPPH Methods as a Tool for Studying Antioxidant Capacity of Spring Barley and Malt. J. Cereal Sci. 2017, 73, 40–45. [Google Scholar] [CrossRef]
- Korhonen, H.; Pihlanto, A. Bioactive Peptides: Production and Functionality. Int. Dairy J. 2006, 16, 945–960. [Google Scholar] [CrossRef]
- You, L.; Zhao, M.; Cui, C.; Zhao, H.; Yang, B. Effect of Degree of Hydrolysis on the Antioxidant Activity of Loach (Misgurnus Anguillicaudatus) Protein Hydrolysates. Innov. Food Sci. Emerg. Technol. 2009, 10, 235–240. [Google Scholar] [CrossRef]
- Jamdar, S.N.; Rajalakshmi, V.; Pednekar, M.D.; Juan, F.; Yardi, V.; Sharma, A. Influence of Degree of Hydrolysis on Functional Properties, Antioxidant Activity and ACE Inhibitory Activity of Peanut Protein Hydrolysate. Food Chem. 2010, 121, 178–184. [Google Scholar] [CrossRef]
- Zhao, D.; Liu, X. Purification, Identification and Evaluation of Antioxidant Peptides from Pea Protein Hydrolysates. Molecules 2023, 28, 2952. [Google Scholar] [CrossRef]
- Bekiroglu, H.; Bozkurt, F.; Karadag, A.; Ahhmed, A.M.; Sagdic, O. The Effects of Different Protease Treatments on the Techno-Functional, Structural, and Bioactive Properties of Bovine Casein. Prep. Biochem. Biotechnol. 2022, 52, 1097–1108. [Google Scholar] [CrossRef]
- Senadheera, T.R.L.; Dave, D.; Shahidi, F. Antioxidant Potential and Physicochemical Properties of Protein Hydrolysates from Body Parts of North Atlantic Sea Cucumber (Cucumaria frondosa). Food Prod. Process. Nutr. 2021, 3, 3. [Google Scholar] [CrossRef]
- Włodarczyk, K.; Zienkiewicz, A.; Szydłowska-Czerniak, A. Radical Scavenging Activity and Physicochemical Properties of Aquafaba-Based Mayonnaises and Their Functional Ingredients. Foods 2022, 11, 1129. [Google Scholar] [CrossRef]
| Sample | Moisture (%) | Protein (%) | Oil (%) | AW (%) | Ash (%) | Specific Volume (mL/g) | Crust Color Properties | Crumb Color Properties | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| L* | a* | b* | ΔE* | L* | a* | b* | ΔE* | |||||||
| CM | 21.88 ± 0.17 a | 9.9 ± 0.19 b | 12.83 ± 0.66 a | 0.77 ± 0.00 a | 1.56 ± 0.11 d | 1.04 ± 0.09 b | 69.12 ± 0.07 a | 4.14 ± 0.21 d | 34.25 ± 0.28 b | - | 74.23 ± 0.26 a | −0.65 ± 0.02 d | 24.32 ± 0.15 c | - |
| AIM | 21.57 ± 0.61 ab | 12.22 ± 0.02 ba | 12.11 ± 0.06 a | 0.76 ± 0.00 a | 1.70 ± 0.16 c | 1.89 ± 0.04 a | 63.96 ± 0.33 b | 8.08 ± 0.34 c | 36.13 ± 0.25 a | 3.39 ± 0.06 c | 73.24 ± 0.19 b | −0.53 ± 0.01 c | 26.92 ± 0.28 a | 2.30 ± 0.07 a |
| AFHM | 20.99 ± 0.06 b | 12.29 b ± 0.09 a | 12.76 ± 0.02 a | 0.74 ± 0.01 a | 1.90 ± 0.02 a | 1.96 ± 0.04 a | 58.80 ± 0.65 c | 12.50 ± 0.27 b | 33.85 ± 0.12 b | 4.42 ± 0.02 a | 72.66 ± 0.15 c | −0.49 ± 0.02 b | 25.97 ± 0.06 b | 2.18 ± 0.02 a |
| ASHM | 21.06 ± 0.01 b | 12.60 ± 0.19 a | 12.88 ± 0.15 a | 0.74 ± 0.01 a | 1.84 ± 0.28 b | 2.23 ± 0.17 a | 57.06 ± 0.06 d | 13.54 ± 0.09 a | 29.99 ± 0.45 a | 4.11 ± 0.03 b | 72.18 ± 0.14 c | −0.43 ± 0.01 a | 25.83 ± 0.32 b | 1.86 ± 0.01 b |
| Sample | K (Pa·sn) | n | R2 |
|---|---|---|---|
| CM | 9.031 ± 0.04 a | 0.616 ± 0.01 a | 0.994 |
| AIM | 7.555 ± 0.03 b | 0.579 ± 0.00 b | 0.989 |
| AFHM | 6.725 ± 0.06 c | 0.625 ± 0.00 a | 0.990 |
| ASHM | 6.390 ± 0.13 d | 0.615 ± 0.01 a | 0.989 |
| Sample | Hardness (g) | Springiness | Cohesiveness | Gumminess (g) | Chewiness (g) | Resilience |
| CM | 4043 ± 81 a | 0.88 ± 0.01 b | 0.58 ± 0.02 b | 2323 ± 92 a | 2100 ± 72 a | 0.26 ± 0.01 b |
| AIM | 3511 ± 93 b | 0.90 ± 0.01 a | 0.66 ± 0.02 a | 2313 ± 139 a | 2016 ± 26 a | 0.31 ± 0.00 a |
| AFHM | 2828 ± 58 c | 0.90 ± 0.00 a | 0.69 ± 0.14 a | 1985 ± 101 b | 1789 ± 82 b | 0.31 ± 0.00 a |
| ASHM | 2317 ± 37 d | 0.91 ± 0.01 a | 0.65 ± 0.01 a | 1516 ± 45 c | 1380 ± 46 c | 0.30 ± 0.00 a |
| Sample | T0 | Td | ΔH | |||
| CM | 95.18 ± 2.34 a | 114.46 ± 3.78 a | 329.7 ± 10.87 b | |||
| AIM | 94.66 ± 2.12 a | 114.53 ± 5.30 a | 360.9 ± 8.43 a | |||
| AFHM | 70.53 ± 1.56 c | 122.34 ± 6.04 b | 270.63 ± 1.59 d | |||
| ASHM | 75.10 ± 0.96 b | 105.49 ± 4.02 c | 283.3 ± 4.08 c | |||
| Sample | Antioxidant Capacity * | Induction Period (h:min) | |
|---|---|---|---|
| DPPH | ABTS | ||
| AI | 11.7 ± 0.44 c | 222.58 ± 15.96 b | - |
| AFHs | 18.97 ± 0.58 b | 257.01 ± 14.90 ab | - |
| ASHs | 26.41 ± 1.36 a | 294.24 ± 11.92 a | - |
| CM | 51.01 ± 1.49 D | 262.53 ± 4.7 C | 15:08 ± 0.19 d |
| AIM | 84.35 ± 2.91 C | 481.87 ± 10.9 B | 16:32 ± 0.14 c |
| AFHM | 105.46 ± 1.33 B | 489.74 ± 12.87 B | 17:28 ± 0.02 b |
| ASHM | 115.46 ± 2.22 A | 530.56 ± 5.56 A | 18:47 ± 0.12 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bekiroglu, H. Aquafaba Hydrolysates as Functional Ingredients in Muffin Cakes: Effects on Physicochemical Properties, Quality Attributes, and Antioxidant Activity. Foods 2025, 14, 3709. https://doi.org/10.3390/foods14213709
Bekiroglu H. Aquafaba Hydrolysates as Functional Ingredients in Muffin Cakes: Effects on Physicochemical Properties, Quality Attributes, and Antioxidant Activity. Foods. 2025; 14(21):3709. https://doi.org/10.3390/foods14213709
Chicago/Turabian StyleBekiroglu, Hatice. 2025. "Aquafaba Hydrolysates as Functional Ingredients in Muffin Cakes: Effects on Physicochemical Properties, Quality Attributes, and Antioxidant Activity" Foods 14, no. 21: 3709. https://doi.org/10.3390/foods14213709
APA StyleBekiroglu, H. (2025). Aquafaba Hydrolysates as Functional Ingredients in Muffin Cakes: Effects on Physicochemical Properties, Quality Attributes, and Antioxidant Activity. Foods, 14(21), 3709. https://doi.org/10.3390/foods14213709






