Influence of Purple Onion Pulp Addition Level on Oxidative, Microbial, and Sensory Characteristics of Refrigerated Beef Patties
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of the Purple Onion Pulp
2.3. Preparation of Beef Patties
2.4. pH Value Determination
2.5. Color Determination
2.6. Cooking Loss Determination
2.7. Texture Characteristics Determination
2.8. Metmyoglobin (MetMb) Content Determination
2.9. Thiobarbitururic Acid Reactive Substances (TBARSs) Value
2.10. Protein Carbonyl Content
2.11. Total Volatile Basic Nitrogen (TVB-N) Value
2.12. Total Aerobic Mesophilic Bacteria (TAMB) Determination
2.13. Sensory Evaluation Analysis
2.14. Statistical Analysis
3. Results and Discussion
3.1. pH Value
3.2. Color
3.3. Cooking Loss
3.4. Texture Characteristics
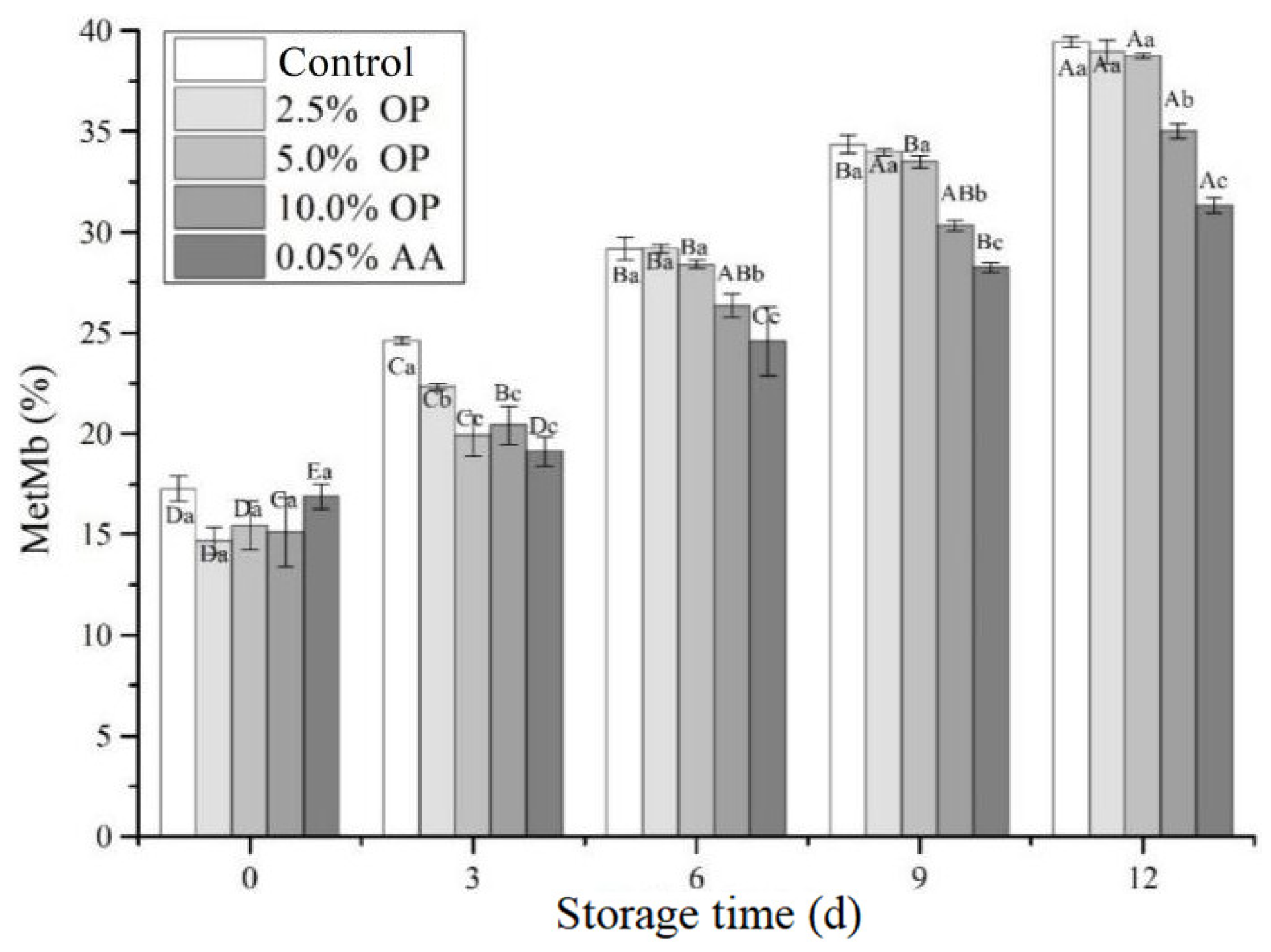
3.5. Metmyoglobin (MetMb) Content
3.6. TBARSs Value
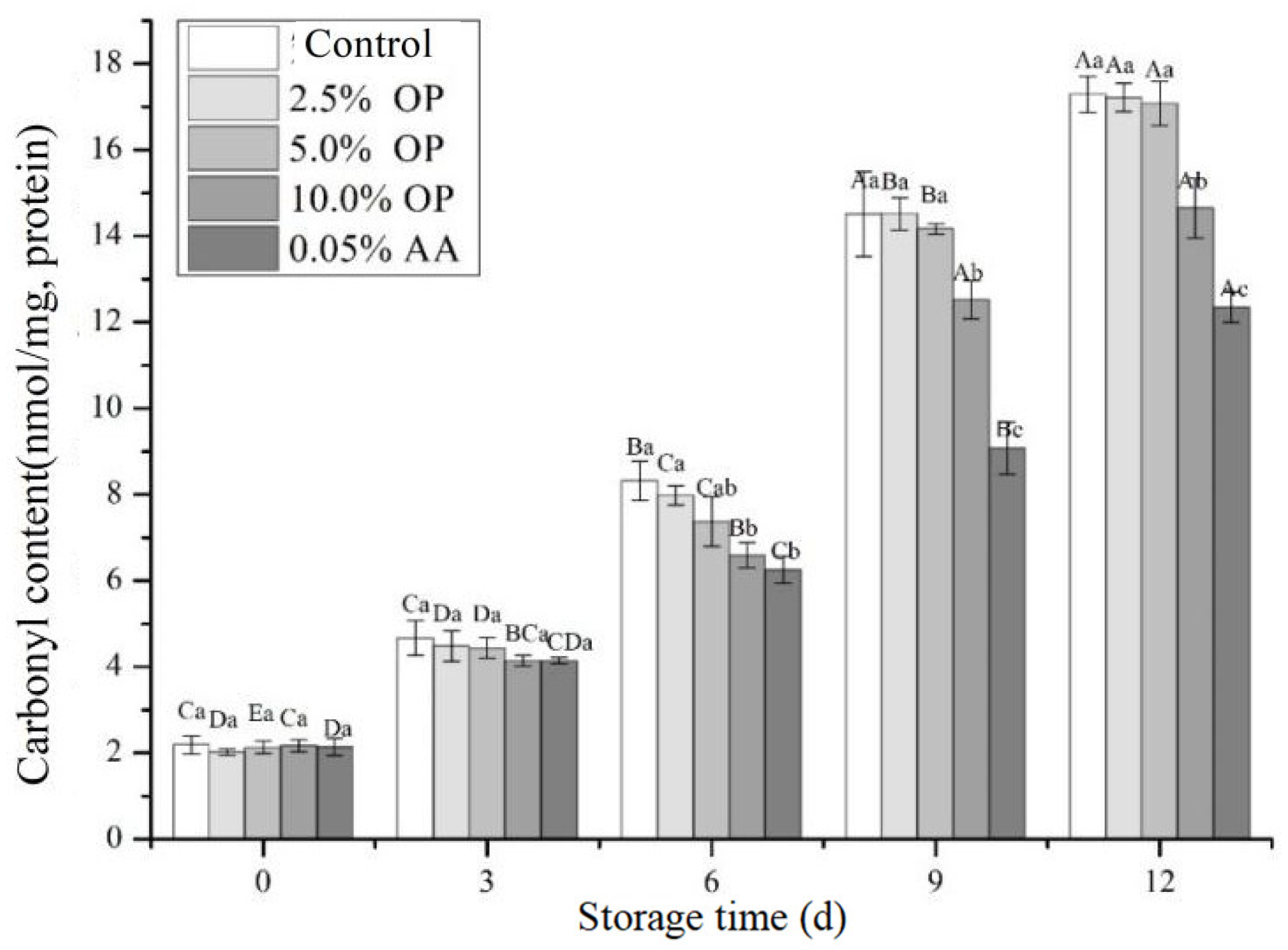
3.7. Carbonyl Content
3.8. TVB-N Value
3.9. Total Aerobic Mesophilic Bacteria (TAMB)
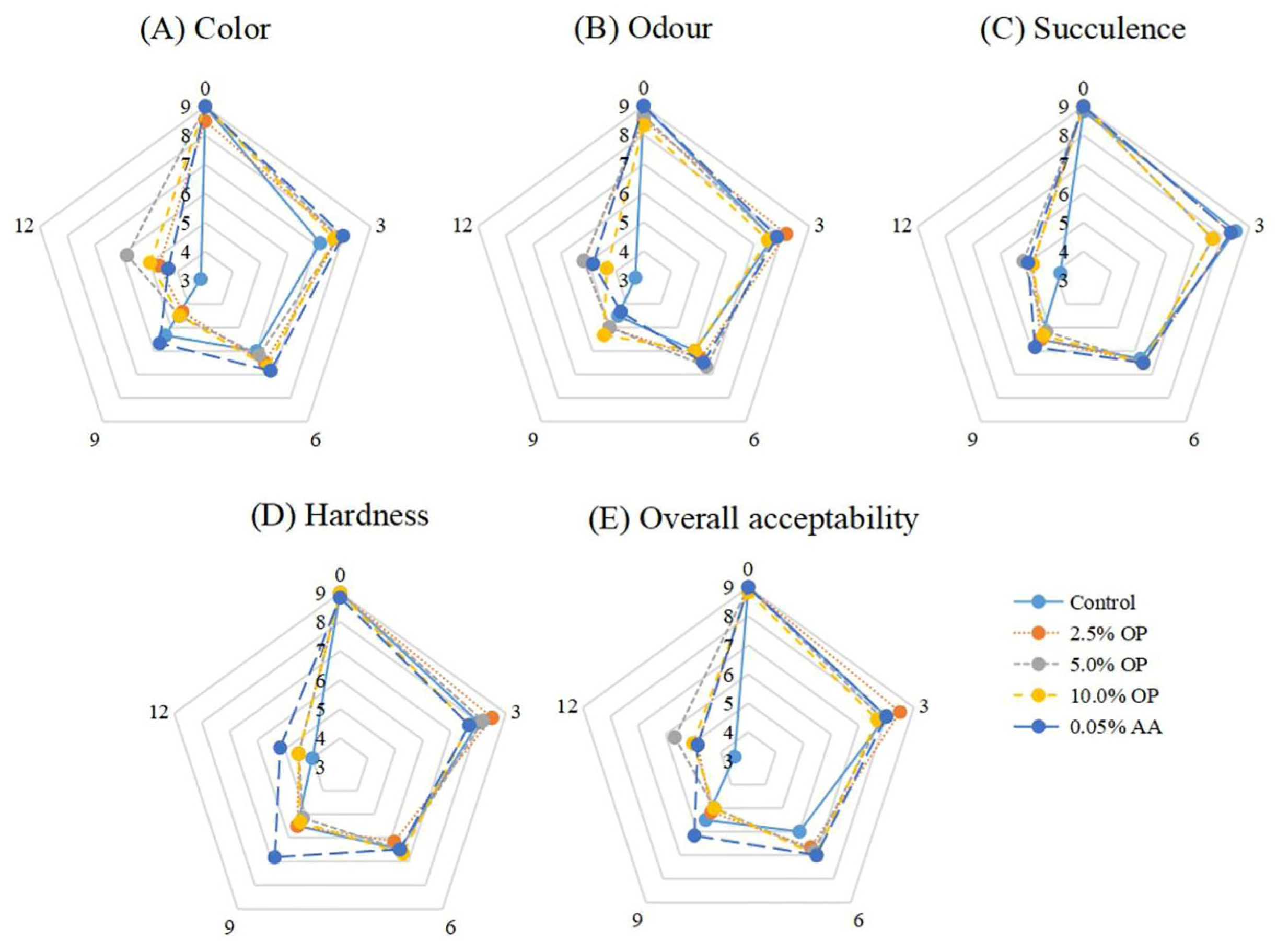
3.10. Sensory Evaluation
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Asghar, Z.; Arshad, M.S.; Khalid, W.; Saeed, F.; Imran, A.; Suleria, H.A.R. Impact of gamma irradiation and poppy seed extract on quality and storage stability of BP. Int. J. Food Prop. 2023, 26, 1645–1662. [Google Scholar] [CrossRef]
- Ngongoni, N.K.; Pfukwa, M.T.; Mapiye, C. Keeping quality of raw ground beef patties fortified with polyphenols extracted from Acacia mearnsii bark and leaves. Meat Sci. 2025, 219, 109665. [Google Scholar] [CrossRef]
- Lin, L.; Mao, X.F.; Sun, Y.H.; Rajivgandhi, G.; Cui, H. Antibacterial properties of nanofibers containing chrysanthemum essential oil and their application as beef packaging. Int. J. Food Microbiol. 2019, 292, 21–30. [Google Scholar] [CrossRef] [PubMed]
- Villasante, J.; Ouerfelli, M.; Bobet, A.; Metón, I.; Almajano, M.P. The Effects of pecan shell, roselle flower and red pepper on the quality of beef patties during chilled storage. Foods 2020, 9, 1692. [Google Scholar] [CrossRef]
- Prommachart, R.; Belem, T.S.; Uriyapongson, S.; Rayas-Duarte, P.; Uriyapongson, J.; Ramanathan, R. The effect of black rice water extract on surface color, lipid oxidation, microbial growth, and antioxidant activity of beef patties during chilled storage. Meat Sci. 2020, 164, 108091. [Google Scholar] [CrossRef]
- Cheng, J.; Xu, L.; Xiang, R.; Liu, X.; Zhu, M. Effects of mulberry polyphenols on oxidation stability of sarcoplasmic and myofibrillar proteins in dried minced pork slices during processing and storage. Meat Sci. 2020, 160, 107973. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.T.; Wang, Y.Q.; Song, Y.; Ren, M.N.; Gao, Z.M.; Ren, J. Effect of onion skin powder on color, lipid, and protein oxidative stability of premade beef patty during cold storage. Sci. Rep. 2024, 14, 1. [Google Scholar] [CrossRef] [PubMed]
- Velázquez, L.; Quiñones, J.; Martínez, A.; Pérez, I.; Velasquez, C.; Sepúlveda-Truan, G.; Díaz, R.; Campagnol, P.C.B.; Sepúlveda, N. Evaluation of Antioxidant and Antimicrobial Properties of Murtilla Leaves (Ugni molinae Turcz.) in beef patties: Effects on Quality Parameters and Shelf Life. Foods 2024, 13, 4174. [Google Scholar] [CrossRef]
- Beya, M.M.; Netzel, M.E.; Sultanbawa, Y.; Smyth, H.; Hoffman, L.C. Kakadu plum (Terminalia ferdinandiana) bioactivity against spoilage microorganisms and oxidative reactions in refrigerated raw beef patties under modified atmosphere packaging. Meat Sci. 2023, 204, 109268. [Google Scholar] [CrossRef]
- Karakaya, M.; Bayrak, E.; Ulusoy, K. Use of Natural Antioxidants in Meat and Meat Products. J. Food Sci. Eng. 2011, 1, 1–10. [Google Scholar]
- Magdalena, S.E.; Agnieszka, N.; Agata, C. Plant extracts rich in polyphenols: Antibacterial agents and natural preservatives for meat and meat products. Crit. Rev. Food Sci. Nutr. 2021, 61, 149–178. [Google Scholar]
- Zhou, X.R.; Zhang, Y.; Jiang, B. Extraction and Electrochemical Analysis of Polyphenols in Plant Samples. Int. J. Electrochem. Sci. 2019, 14, 7410–7422. [Google Scholar] [CrossRef]
- Hu, Y.Y.; Yan, B.W.; Chen, Z.S.; Wang, L.; Tang, W.; Huang, C.X. Recent Technologies for the Extraction and Separation of Polyphenols in Different Plants: A Review. J. Renew. Mater. 2022, 10, 1471–1490. [Google Scholar] [CrossRef]
- Tapp, W.N.; Yancey, J.W.; Apple, J.K.; Dikeman, M.E.; Godbee, R.G. Noni puree (Morinda citrifolia) mixed in BP enhanced color stability. Meat Sci. 2012, 91, 131–136. [Google Scholar] [CrossRef] [PubMed]
- Patinho, I.; Selani, M.M.; Saldaña, E.; Bortoluzzi, A.C.T.; Rios-Mera, J.D.; da Silva, C.M.; Kushida, M.M.; Contreras-Castillo, C.J. Agaricus bisporus mushroom as partial fat replacer improves the sensory quality maintaining the instrumental characteristics of beef burger. Meat Sci. 2020, 172, 108307. [Google Scholar] [CrossRef]
- Romero, M.C.; Fogar, R.A.; Fernández, C.L.; Doval, M.M.; Romero, A.M.; Judis, M.A. Effects of freeze-dried pulp of Eugenia uniflora L. and Opuntia ficus-indica fruits on quality attributes of beef patties enriched with n-3 fatty acids. J. Food Sci. Technol. 2020, 58, 1918–1926. [Google Scholar] [CrossRef]
- Lima Campos, B.C.D.S.; Bellucci, E.R.B.; Junior, C.A.A.; de Souza, D.P.M.; Bertuci, M.L.; Lorenzo, J.M.; Barretto, A.C.D.S. Açaí residue extract as a natural antioxidant to enhance the shelf-life of beef patties. J. Sci. Food Agric. 2025, in press. [Google Scholar] [CrossRef]
- Sharma, K.; Ko, E.Y.; Assefa, A.D.; Ha, S.; Nile, S.H.; Lee, E.T.; Park, S.W. Temperature-dependent studies on the total phenolics, favonoids, antioxidant activities, and sugar content in six onion varieties. J. Food Drug. Anal. 2015, 23, 243–252. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.A.; Kim, K.T.; Nah, S.Y.; Chung, M.S.; Cho, S.; Paik, H.D. Antimicrobial and antioxidative effects of onion peel extracted by the subcritical water. Food Sci. Biotechnol. 2011, 20, 543–548. [Google Scholar] [CrossRef]
- Berno, N.D.; Tezotto-Uliana, J.V.; dos Santos Dias, C.T.; Kluge, R.A. Storage temperature and type of cut affect the biochemical and physiological characteristics of fresh-cut purple onions. Postharvest Biol. Technol. 2014, 93, 91–96. [Google Scholar] [CrossRef]
- Mamoro, W.U.; Neuza, J. Oxidative stability of soybean oil added of purple onion (Allium cepa L.) peel extract during accelerated storage conditions. Food Control 2021, 127, 108130. [Google Scholar] [CrossRef]
- Lee, S.Y.; Kim, H.W.; Hwang, K.E.; Song, D.H.; Choi, M.S.; Ham, Y.K.; Choi, Y.S.; Lee, J.W.; Lee, S.K.; Kim, C.J. Combined Effect of Kimchi Powder and Onion Peel Extract on Quality Characteristics of Emulsion Sausages Prepared with Irradiated Pork. Korean J. Food Sci. Anim. Resour. 2015, 35, 277–285. [Google Scholar] [CrossRef]
- Bedrníček, J.; Kadlec, J.; Laknerová, I.; Mráz, J.; Samková, E.; Petrášková, E.; Hasoňová, L.; Vácha, F.; Kron, V.; Smetana, P. Onion Peel Powder as an Antioxidant-Rich Material for Sausages Prepared from Mechanically Separated Fish Meat. Antioxidants 2020, 9, 974. [Google Scholar] [CrossRef]
- Abdelhakam, O.S.; Elsebaie, E.M.; Ghazi, A.K.; Gouda, M.S. Quality characteristics of beef hamburger enriched with red grape pomace powder during freezing storage. Slov. Vet. Res. 2019, 56, 333–373. [Google Scholar] [CrossRef]
- Biswas, A.K.; Chatli, M.K.; Sahoo, J. Antioxidant potential of curry (Murraya koenigii L.) and mint (Mentha spicata) leaf extracts and their effect on colour and oxidative stability of raw ground pork meat during refrigeration storage. Food Chem. 2012, 133, 467–472. [Google Scholar] [CrossRef]
- Bellucci, E.R.B.; Munekata, P.E.; Pateiro, M.; Lorenzo, J.M.; da Silva Barretto, A.C. Red pitaya extract as natural antioxidant in pork patties with total replacement of animal fat. Meat Sci. 2021, 171, 108284. [Google Scholar] [CrossRef] [PubMed]
- Bahmanyar, F.; Hosseini, S.M.; Mirmoghtadaie, L.; Shojaee-Aliabadi, S. Effects of replacing soy protein and bread crumb with quinoa and buckwheat flour in functional beef burger formulation. Meat Sci. 2021, 172, 108305. [Google Scholar] [CrossRef]
- Cui, H.; Dong, Y.; Lu, T.; Zou, X.; Wang, M.; Yang, X.; Zhou, H. Effect of ethanolic extract from Morus alba L. leaves on the quality and sensory aspects of chilled pork under retail conditions. Meat Sci. 2021, 172, 108368. [Google Scholar] [CrossRef] [PubMed]
- Witte, V.C.; Krause, G.F.; Bailey, M.F. A new extraction method for determining 2-thiobarbituric acid values of pork and beef during storage. J. Food Sci. 1970, 35, 582–585. [Google Scholar] [CrossRef]
- Vuorela, S.; Salminen, H.; Mäkelä, M.; Kivikari, R.; Karonen, M.; Heinonen, M. Effect of plant phenolics on protein and lipid oxidation in cooked pork meat patties. J. Agric. Food Chem. 2005, 53, 8492–8497. [Google Scholar] [CrossRef]
- Fan, X.J.; Liu, S.Z.; Li, H.H.; He, J.; Feng, J.T.; Zhang, X.; Yan, H.E. Effects of Portulaca oleracea L. extract on lipid oxidation and color of pork meat during refrigerated storage. Meat Sci. 2019, 147, 82–90. [Google Scholar] [CrossRef]
- Santas, J.; Almajano, M.P.; Carbó, R. Antimicrobial and antioxidant activity of crude onion (Allium cepa L.) extracts. Int. J. Food Sci. Technol. 2010, 45, 403–409. [Google Scholar] [CrossRef]
- Demir, H.; Çelik, S.; Sezer, Y.Ç. Effect of ultrasonication and vacuum impregnation pretreatments on the quality of beef marinated in onion juice a natural meat tenderizer. Food Sci. Technol. Int. 2021, 28, 340–352. [Google Scholar] [CrossRef]
- Tang, X.; Cronin, D.A. The effects of brined onion extracts on lipid oxidation and sensory quality in refrigerated cooked turkey breast rolls during storage. Food Chem. 2007, 99, 712–718. [Google Scholar] [CrossRef]
- Jiao, Y.; Quek, S.Y.; Gu, M.; Guo, Y.; Liu, Y. Polyphenols from thinned young kiwifruit as natural antioxidant: Protective effects on beef oxidation, physicochemical and sensory properties during storage. Food Control 2020, 108, 106870. [Google Scholar] [CrossRef]
- Zahid, M.A.; Choi, J.Y.; Seo, J.K.; Parvin, R.; Ko, J.; Yang, H.S. Effects of clove extract on oxidative stability and sensory attributes in cooked beef patties at refrigerated storage. Meat Sci. 2020, 161, 107972. [Google Scholar] [CrossRef]
- Huang, X.; Sun, L.; Liu, L.; Wang, G.Z.; Luo, P.; Tang, D.B.; Huang, Q. Study on the mechanism of mulberry polyphenols inhibiting oxidation of beef myofibrillar protein. Food Chem. 2022, 372, 131241. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.T.; An, X.Z.; Gao, Z.M.; Li, Z.Y.; Tian, S.X.; Lu, Y.Y. Effects of ethanolic extract from onion skin on the quality characteristics of beef patties during refrigerated storage. Food Sci. Technol. 2022, 42, e118121. [Google Scholar] [CrossRef]
- Irkin, R.; Arslan, M. Effect of onion (Allium cepa L.) extract on microbiological quality of refrigerated beef meat. J. Muscle Foods 2010, 21, 308–316. [Google Scholar] [CrossRef]
- Ren, M.; Xie, T.; Chen, L.; Zhao, T.; Zhou, C. Pickering emulsion stabilized by hollow Zein/SSPS nanoparticles loaded with Thymol: Formation, characterization, and application in fruit preservation. Food Res. Int. 2024, 201, 115561. [Google Scholar] [CrossRef] [PubMed]
- Fadillah, A.; van den Borne, B.H.P.; Poetri, O.N.; Hogeveen, H.; Slijper, T.; Pisestyani, H.; Schukken, Y.H. Evaluation of factors associated with bulk milk somatic cell count and total plate count in Indonesian smallholder dairy farms. Front. Vet. Sci. 2023, 10, 1280264. [Google Scholar] [CrossRef]
- Suo, T.; Guo, X.N.; Zhu, K.X. Effects of tempering with plasma-activated water on total plate count and quality properties of wheat flour. J. Cereal Sci. 2022, 105, 103468. [Google Scholar] [CrossRef]
- Zhu, Y.L.; Wang, W.; Li, M.; Zhang, J.M.; Ji, L.L.; Zhao, Z.P.; Zhang, R.; Cai, D.M.; Chen, L. Microbial diversity of meat products under spoilage and its controlling approaches. Front. Nutr. 2022, 9, 1078201. [Google Scholar] [CrossRef]
- Yang, H.S.; Lee, E.J.; Moon, S.H.; Paik, H.D.; Nam, K.; Ahn, D.U. Effect of garlic, onion, and their combination on the quality and sensory characteristics of irradiated raw ground beef. Meat Sci. 2011, 89, 202–208. [Google Scholar] [CrossRef]
- Rico, C.W.; Kim, G.R.; Jo, C.R.; Nam, K.C.; Kang, H.J.; Ahn, D.U.; Kwon, J.H. Microbiological and physicochemical quality of irradiated ground beef as affected by added garlic or onion. Korean J. Food Sci. Anim. Resour. 2009, 29, 680–684. [Google Scholar] [CrossRef]
- Taylor, J.; Ahmed, I.A.M.; Al-Juhaimi, F.Y.; Bekhit, A.E.A. Consumers’ Perceptions and Sensory Properties of Beef Patty Analogues. Foods 2020, 9, 63. [Google Scholar] [CrossRef]
- Reihani, S.F.S.; Tan, T.C.; Huda, N.; Easa, A.M. Frozen storage stability of beef patties incorporated with extracts from ulam raja leaves (Cosmos caudatus). Food Chem. 2014, 155, 17–23. [Google Scholar] [CrossRef] [PubMed]
- Colle, M.C.; Richard, R.P.; Smith, D.M.; Colle, M.J.; Loucks, W.I.; Gray, S.J.; Reynolds, Z.D.; Sutton, H.A.; Nasados, J.A.; Doumit, M.E. Dry potato extracts improve water holding capacity, shelf life, and sensory characteristics of fresh and precooked beef patties. Meat Sci. 2019, 149, 156–162. [Google Scholar] [CrossRef] [PubMed]
- Richards, J.; Lammert, A.; Madden, J.; Cahn, A.; Kang, I.; Amin, S. Addition of carrot pomace to enhance the physical, sensory, and functional properties of beef patties. Foods 2024, 13, 3910. [Google Scholar] [CrossRef] [PubMed]
- Tarasevičienė, Ž; Čechovičienė, I.; Paulauskienė, A.; Gumbytė, M.; Blinstrubienė, A.; Burbulis, N. The Effect of Berry Pomace on Quality Changes of beef patties during Refrigerated Storage. Foods 2022, 11, 2180. [Google Scholar] [CrossRef]




| Content | Moisture (%) | Protein (%) | Fat (%) |
|---|---|---|---|
| Control | 74.42 ± 1.22 | 19.25 ± 0.12 | 2.15 ± 0.13 |
| 2.5% OP | 75.44 ± 0.73 | 18.26 ± 0.21 | 2.01 ± 0.14 |
| 5.0% OP | 75.87 ± 0.58 | 17.86 ± 0.31 | 1.95 ± 0.17 |
| 10.0% OP | 77.68 ± 0.37 | 17.15 ± 0.23 | 1.82 ± 0.11 |
| 0.05% AA | 74.58 ± 0.58 | 19.34 ± 0.25 | 2.19 ± 0.16 |
| Storage Time (D) | Control | 2.5% OP | 5.0% OP | 10.0% OP | 0.05% AA |
|---|---|---|---|---|---|
| 0 | 5.56 Da ± 0.01 | 5.43 Ca ± 0.01 | 5.44 Ca ± 0.02 | 5.45 Ba ± 0.03 | 5.35 Cb ± 0.04 |
| 3 | 5.50 Ca ± 0.01 | 5.44 Cb ± 0.01 | 5.44 Cb ± 0.01 | 5.45 Bb ± 0.01 | 5.42 Bc ± 0.01 |
| 6 | 5.53 Ca ± 0.01 | 5.47 Cb ± 0.03 | 5.48 Bb ± 0.01 | 5.45 Bc ± 0.02 | 5.41 Bc ± 0.01 |
| 9 | 5.62 Ba ± 0.04 | 5.55 Bab ± 0.03 | 5.50 Bb ± 0.01 | 5.46 Bb ± 0.01 | 5.33 Cb ± 0.03 |
| 12 | 5.73 Aa ± 0.02 | 5.72 Aa ± 0.02 | 5.65 Ab ± 0.01 | 5.54 Ac ± 0.01 | 5.48 Ad ± 0.01 |
| Color Parameters | Storage Time (D) | Control | 2.5% OP | 5.0% OP | 10.0% OP | 0.05% AA |
|---|---|---|---|---|---|---|
| L* | 0 | 31.4 Aa ± 0.21 | 31.1 Aa ± 1.53 | 31.4 Aa ± 0.67 | 30.0 Aa ± 0.10 | 31.1 Aa ± 0.17 |
| 3 | 31.6 Aa ± 0.39 | 31.2 Ab ± 0.06 | 30.8 Cb ± 0.29 | 31.3 Ab ± 0.44 | 31.2 Aa ± 0.16 | |
| 6 | 31.7 Aa ± 1.90 | 31.9 Aa ± 1.5 | 31.1 Ba ± 0.85 | 31.6 Aa ± 1.42 | 31.7 Aa ± 0.47 | |
| 9 | 32.0 Aa ± 1.28 | 31.8 Aa ± 2.43 | 30.9 Ca ± 0.64 | 31.9 Aa ± 0.65 | 31.3 Aa ± 0.23 | |
| 12 | 32.7 Aa ± 0.38 | 31.8 Aa ± 0.49 | 32.5 Aa ± 1.11 | 31.4 Aa ± 1.48 | 32.0 Aa ± 1.22 | |
| a* | 0 | 12.4 Aa ± 1.49 | 10.6 Bab ± 0.70 | 9.3 Cb ± 0.19 | 9.0 Cb ± 0.21 | 11.7 Aa ± 0.14 |
| 3 | 12.4 Aa ± 0.39 | 10.3 Ab ± 0.45 | 10.7 ABb ± 0.39 | 10.4 Ab ± 0.06 | 11.2 Aab ± 1.00 | |
| 6 | 12.1 Aa ± 0.53 | 11.1 Aa ± 2.35 | 11.3 Aa ± 1.28 | 10.0 Aa ± 0.59 | 11.5 Aa ± 2.25 | |
| 9 | 11.9 Aa ± 0.94 | 9.1 Ac ± 1.55 | 10.0 ABbc ± 0.11 | 9.2 BCc ± 0.56 | 11.2 Aab ± 0.76 | |
| 12 | 10.1 Aa ± 0.74 | 9.4 Aa ± 0.45 | 10.7 ABa ± 0.67 | 9.9 ABa ± 0.16 | 9.4 Aa ± 1.42 | |
| b* | 0 | 7.6 Aa ± 0.81 | 7.1 Aa ± 0.98 | 6.9 Ba ± 0.21 | 6.6 Aa ± 0.18 | 6.6 Aa ± 0.73 |
| 3 | 8.0 Aa ± 0.41 | 7.7 Aa ± 0.23 | 7.0 Ba ± 0.14 | 7.4 Aa ± 0.59 | 7.8 Aa ± 0.13 | |
| 6 | 8.2 Aa ± 0.57 | 8.0 Aa ± 0.23 | 7.9 Aa ± 0.77 | 7.4 Aa ± 0.50 | 7.7 Aa ± 0.38 | |
| 9 | 8.8 Aa ± 0.43 | 7.6 Ab ± 0.67 | 7.0 Bb ± 0.19 | 7.3 Ab ± 0.34 | 7.7 Ab ± 0.47 | |
| 12 | 9.2 Aa ± 0.64 | 7.9 Ab ± 0.30 | 8.1 Aab ± 0.47 | 7.2 Ab ± 0.79 | 7.9 Ab ± 0.66 | |
| ΔE | 0–3 | 2.63 Ba ± 0.10 | 1.99 Ba ± 0.64 | 2.72 ABa ± 0.24 | 2.63 Aa ± 0.25 | 2.45 ABa ± 0.12 |
| 0–6 | 3.35 ABa ± 0.72 | 4.11 Aa ± 1.01 | 3.39 ABa ± 0.35 | 2.60 Aa ± 0.22 | 2.52 ABa ± 0.50 | |
| 0–9 | 2.76 ABa ± 0.53 | 3.19 ABa ± 0.42 | 2.44 Ba ± 0.82 | 2.40 Aa ± 0.73 | 1.81 Ba ± 0.06 | |
| 0–12 | 4.94 Aa ± 0.92 | 2.86 ABb ± 0.75 | 4.24 Aab ± 0.61 | 2.38 Ab ± 0.57 | 3.94 Aab ± 0.94 |
| Properties | Storage Time (D) | Control | 2.5% OP | 5.0% OP | 10.0% OP | 0.05% AA |
|---|---|---|---|---|---|---|
| Hardness (N) | 0 | 24.37 Ba ± 0.80 | 26.52 Aa ± 1.77 | 26.03 Aa ± 0.63 | 25.76 Aa ± 1.23 | 26.98 Ba ± 2.47 |
| 3 | 33.72 Aa ± 3.50 | 29.09 Aab ± 1.95 | 25.17 Abc ± 1.01 | 23.43 Ac ± 1.06 | 33.44 Aa ± 4.67 | |
| 6 | 37.10 Aa ± 6.80 | 31.96 Aab ± 3.31 | 27.29 Ab ± 4.12 | 25.15 Ab ± 0.02 | 36.97 Aa ± 1.01 | |
| 9 | 36.88 Aa ± 2.39 | 31.57 Aab ± 1.17 | 28.71 Aab ± 3.06 | 25.29 Ab ± 2.23 | 34.55 Aab ± 0.35 | |
| 12 | 35.90 Aa ± 0.58 | 30.87 Aab ± 5.43 | 27.06 Aab ± 5.13 | 24.63 Ab ± 2.99 | 34.94 Aa ± 0.52 | |
| Cohesiveness | 0 | 0.24 Aa ± 0.01 | 0.26 ABa ± 0.01 | 0.26 Aa ± 0.01 | 0.25 Aa ± 0.02 | 0.26 Aa ± 0.01 |
| 3 | 0.26 Aa ± 0.01 | 0.26 ABa ± 0.00 | 0.27 Aa ± 0.01 | 0.26 Aa ± 0.02 | 0.27 Aa ± 0.01 | |
| 6 | 0.25 Ab ± 0.01 | 0.28 Aa ± 0.02 | 0.25 Ab ± 0.01 | 0.26 Ab ± 0.01 | 0.27 Aab ± 0.01 | |
| 9 | 0.25 Aa ± 0.02 | 0.25 Ba ± 0.01 | 0.26 Aa ± 0.02 | 0.25 Aa ± 0.01 | 0.26 Aa ± 0.02 | |
| 12 | 0.25 Aa ± 0.01 | 0.25 Ba ± 0.01 | 0.26 Aa ± 0.02 | 0.25 Aa ± 0.01 | 0.27 Aa ± 0.03 | |
| Springiness (mm) | 0 | 0.97 Aa ± 0.14 | 1.00 Ca ± 0.06 | 0.94 Ba ± 0.04 | 1.13 ABa ± 0.01 | 1.03 Ba ± 0.23 |
| 3 | 1.17 Aa ± 0.07 | 1.08 BCab ± 0.08 | 1.02 Bbc ± 0.06 | 0.94 Bc ± 0.04 | 1.16 Ba ± 0.05 | |
| 6 | 1.30 Aa ± 0.07 | 1.09 BCc ± 0.03 | 1.02 Bc ± 0.05 | 1.16 ABbc ± 0.015 | 1.48 Aa ± 0.16 | |
| 9 | 1.28 Aa ± 0.03 | 1.41 Aa ± 0.11 | 1.29 Aa ± 0.22 | 1.30 Aa ± 0.08 | 1.33 ABa ± 0.16 | |
| 12 | 1.21 Aa ± 0.10 | 1.23 ABa ± 0.19 | 1.32 Aa ± 0.15 | 1.12 ABa ± 0.27 | 1.11 Ba ± 0.13 | |
| Gumminess (g) | 0 | 7.82 Aa ± 0.11 | 7.84 Aa ± 0.26 | 6.79 Ab ± 0.16 | 6.58 ABb ± 0.71 | 6.86 Cb ± 0.79 |
| 3 | 8.70 Aa ± 0.99 | 7.59 Aab ± 0.60 | 6.80 Ab ± 0.14 | 6.14 Bb ± 0.63 | 8.94 ABa ± 1.28 | |
| 6 | 9.37 Aab ± 1.43 | 8.91 Aab ± 1.05 | 6.91 Ac ± 1.14 | 7.56 Abc ± 0.42 | 9.85 Aa ± 0.51 | |
| 9 | 9.85 Aa ± 0.85 | 9.46 Aa ± 2.06 | 7.32 Aa ± 0.61 | 6.22 Ba ± 0.50 | 8.25 ABa ± 1.25 | |
| 12 | 7.66 Aa ± 0.23 | 7.58 Aa ± 1.30 | 6.79 Aa ± 2.08 | 6.21 Ba ± 0.85 | 7.28 BCa ± 0.41 | |
| Chewiness (g·m) | 0 | 7.65 Ba ± 0.11 | 7.83 Ba ± 0.26 | 6.39 Ba ± 0.16 | 7.45 ABb ± 0.71 | 7.10 Ca ± 0.79 |
| 3 | 10.25 ABa ± 0.99 | 8.21 Bab ± 0.60 | 6.97 ABbc ± 0.14 | 5.77 Bc ± 0.63 | 10.22 BCa ± 0.28 | |
| 6 | 12.19 Aab ± 0.43 | 9.72 ABbc ± 1.05 | 7.09 ABc ± 1.14 | 8.80 ABc ± 0.42 | 14.58 Aa ± 0.51 | |
| 9 | 12.56 Aa ± 0.85 | 13.48 Aa ± 0.60 | 9.45 ABb ± 0.61 | 8.05 Ab ± 0.50 | 11.03 ABab ± 0.25 | |
| 12 | 9.28 ABa ± 0.23 | 9.49 ABa ± 0.30 | 9.89 Aa ± 0.18 | 6.88 ABb ± 0.85 | 8.08 Cab ± 0.41 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wei, J.; Zhang, F.; Yang, L.; Wang, C. Influence of Purple Onion Pulp Addition Level on Oxidative, Microbial, and Sensory Characteristics of Refrigerated Beef Patties. Foods 2025, 14, 3659. https://doi.org/10.3390/foods14213659
Wei J, Zhang F, Yang L, Wang C. Influence of Purple Onion Pulp Addition Level on Oxidative, Microbial, and Sensory Characteristics of Refrigerated Beef Patties. Foods. 2025; 14(21):3659. https://doi.org/10.3390/foods14213659
Chicago/Turabian StyleWei, Jiaxin, Fujuan Zhang, Li Yang, and Cuntang Wang. 2025. "Influence of Purple Onion Pulp Addition Level on Oxidative, Microbial, and Sensory Characteristics of Refrigerated Beef Patties" Foods 14, no. 21: 3659. https://doi.org/10.3390/foods14213659
APA StyleWei, J., Zhang, F., Yang, L., & Wang, C. (2025). Influence of Purple Onion Pulp Addition Level on Oxidative, Microbial, and Sensory Characteristics of Refrigerated Beef Patties. Foods, 14(21), 3659. https://doi.org/10.3390/foods14213659





