Abstract
Natamycin is widely used in other countries for the postharvest treatment of agricultural commodities to prevent fungal growth. However, since no MRL has been set in Korea, natamycin residues are regulated under the Positive List System (PLS) with a uniform limit of 0.01 mg/kg, requiring the development of highly sensitive and reliable analytical methods. In this study, a QuEChERS-based analytical method was developed and validated for the quantification of natamycin in five agricultural commodities—soybean, mandarin, hulled rice, green pepper, and potato—using liquid chromatography–tandem mass spectrometry (LC-MS/MS). Extraction using methanol with 3 g of MgSO4 resulted in high recoveries without crystallization, and clean-up with MgSO4 and C18 effectively reduced matrix interferences blow <50%. Natamycin was detected in all five matrices at 6.8 min without any interfering peaks. The MLOQ was determined at 0.01 mg/kg for all five matrices. The mean recoveries (82.2–115.4%) and %CV values (1.1–4.6%) values were within the acceptance criteria defined by the CODEX guidelines. Matrix effects were classified as “soft” for mandarin (|ME| < 20%) and “medium” for soybean, hulled rice, green pepper, and potato (20% ≤ |ME| < 50%). The analytical method for natamycin was validated as suitable for regulatory safety monitoring under the Korean PLS.
1. Introduction
Natamycin is a polyene macrolide fungicide produced by Streptomyces natalensis, which inhibits fungal spore germination by binding to ergosterol in the microbial cell membrane [1,2,3,4]. Natamycin is widely used in processed foods such as cheese, yogurt, juice, and wine to inhibit spoilage microorganisms [5,6]. In addition to its application in processed foods, it is also used postharvest as a pesticide on agricultural commodities such as citrus fruits, pineapples, and mushrooms, typically through spraying or surface coating, to control fungal contamination and maintain product quality during storage [7]. Reflecting this use, the antifungal efficacy of natamycin as a postharvest fungicide has been extensively studied in processed foods and certain fresh produce such as blueberries, grapes, and cherry tomatoes [8,9]. However, most previous studies have focused on beverages, dairy products, processed foods, or wine, whereas research on natamycin residue levels in raw agricultural produce continues to be limited. Consequently, it is difficult to determine whether and to what extent natamycin residues may be present in agricultural commodities. Therefore, from the perspective of agricultural commodity consumption, the presence of pesticide residues may pose potential risks to consumer safety [10,11,12,13,14]. Consequently, many national food regulatory agencies continue to revise guidelines for the registration of natamycin as a pesticide based on toxicological and exposure assessments. For example, the U.S. Environmental Protection Agency (EPA) has assessed the tolerances of natamycin for citrus fruits, avocados, and mangoes [15]. However, natamycin has not yet been registered as a pesticide in Korea, and consequently, no MRL has been established. As a result, under the Positive List System (PLS) enforced by the Ministry of Food and Drug Safety (MFDS), a default residue limit of 0.01 mg/kg is applied to unregistered pesticides, including natamycin [16,17]. For the safety control of natamycin in imported agricultural commodities, highly sensitive and accurate analytical methods are required to detect residues at concentrations below 0.01 mg/kg. Natamycin is an amphiphilic compound containing a lactone ring with non-polar conjugated double bonds and several polar functional groups, such as hydroxyl groups [18,19]. These structural characteristics result in poor solubility in water but high solubility in polar organic solvents, including methanol and acetonitrile [20,21,22]. In addition, the polyene structure is highly sensitive to ultraviolet (UV) light, leading to degradation with increased exposure time, and exhibits rapid degradation under both low and high pH conditions [13,23,24]. Considering these characteristics, an accurate and precise analytical method for detecting natamycin in agricultural commodities is required.
In recent years, the QuEChERS (Quick, Easy, Cheap, Effective, Rugged, and Safe) method, coupled with liquid chromatography-tandem mass spectrometry (LC-MS/MS), has been widely applied for the determination of pesticide residues in various agricultural commodities [25,26]. The QuEChERS method, developed by Anastassiades in 2003, provides a rapid and simplified alternative to conventional liquid–liquid extraction (LLE) and solid-phase extraction (SPE) [27]. However, attributable to the complex nature of agricultural matrices, non-target compounds may interfere with analytical accuracy, requiring the application of highly selective and sensitive detection techniques such as LC-MS/MS [28]. This study developed and validated a QuEChERS-based analytical method employing LC-MS/MS for the determination of natamycin in imported agricultural commodities. Thereafter, the validated method was applied to monitoring imported agricultural products, and the detected levels were evaluated to ensure compliance with regulatory limits for consumer safety.
2. Materials and Methods
2.1. Chemicals and Reagents
A natamycin standard (purity 91.13%) was purchased from HPC Standards GmbH (Cunnersdorf, Germany). The physicochemical properties of natamycin are summarized in Table A1. HPLC grade organic solvent-including methanol, and water-were purchased from J.T.Baker® (Avantor Performance Materials Korea Ltd., Suwon-Si, Republic of Korea). Extraction reagents for the QuEChERS method were obtained from Agilent Technologies Korea Ltd. (Seoul, Republic of Korea). The AOAC method kit (No. 7982-7755) contained 6 g anhydrous magnesium sulfate (MgSO4) and 1.5 g sodium acetate (NaOAc); the EN method kit (No. 5982-7650) contained 4 g MgSO4, 1 g sodium chloride (NaCl), 1 g trisodium citrate dihydrate (Na3Cit·2H2O), and 0.5 g disodium hydrogencitrate sesquihydrate (Na2HCit·1.5H2O); and the Original method kit (No. 5982-7550) contained 4 g MgSO4 and 1 g NaCl. Dispersive solid-phase extraction (d-SPE) sorbents, including MgSO4, octadecylsilane (C18), and graphitized carbon black (GCB), were also purchased from Agilent Technologies Korea Ltd. (Seoul, Republic of Korea). Formic acid (HPLC grade, purity 99%), used for acidifying the mobile phase, was supplied by FUJIFILM Wako Pure Chemical Corporation (Osaka, Japan).
2.2. LC-MS/MS Instrumental Conditions
Natamycin analysis was performed using a liquid chromatography–tandem mass spectrometry (LC-MS/MS) system comprising an AB SCIEX QTRAP 5500 mass spectrometer (AB SCIEX, Concord, ON, Canada) coupled with an AB SCIEX ExionLC™ Series UHPLC (AB SCIEX, Concord, ON, Canada). Chromatographic separation was carried out on a reversed-phase Unison UK-C18 column (100 mm × 2.0 mm, 3 µm, Imtakt, Kyoto, Japan), maintained at 35 °C, with an injection volume of 5 µL. The mobile phases consisted of 0.1% formic acid in water (A) and 0.1% formic acid in methanol (B). Gradient elution was performed at a flow rate of 0.2 mL/min under the following conditions: the initial composition of 50:50 (A:B, v/v) was held from 0.0 to 4.0 min, followed by a linear increase in B to 100% from 4.0 to 5.0 min. This condition was maintained until 7.0 min to ensure complete elution of natamycin. The mobile phase was then returned to 50:50 (A:B, v/v) from 8.0 to 12.0 min for column re-equilibration. The total run time was 12 min, and natamycin was detected at a retention time (RT) of 6.8 min.
Mass spectrometric detection was performed in positive electrospray ionization (ESI+) mode using multiple reaction monitoring (MRM) to enhance selectivity and sensitivity.
The ion spray voltage was set to +4500 V, and the source temperature was maintained at 550 °C. Nitrogen was used as the collision gas. MRM parameters were optimized by direct infusion of a 20 µg/L natamycin standard solution. The precursor ion was observed at m/z 666.2, corresponding to the protonated molecular ion [M + H]+. Two product ions with the highest signal intensities were selected as the quantifier and qualifier ions. The final MRM conditions, including precursor/product ion transitions, cone voltages, collision energies, and retention time, are summarized in Table A2.
2.3. Selection and Preparation of Samples
For the optimization and validation of the analytical method, two commodities—soybean (legume vegetable) and mandarin (fruit)—and five commodities—hulled rice (cereal grain), potato (root and tuber vegetable), soybean (legume vegetable), mandarin (fruit), and green pepper (fruiting vegetable)—were selected, respectively, based on the Codex food classification system and the national priority list of commonly consumed foods. All samples were purchased as pesticide-free organic agricultural products from a certified organic food market.
A total of 102 imported agricultural products were collected from both online and offline markets for natamycin monitoring. The samples consisted of 21 legumes (3 soybeans, 6 kidney beans, 2 lentils, 2 chickpeas, 2 adzuki beans, 2 peas, 2 cowpeas, 1 lupin bean, and 1 fava bean), 32 fruits (4 oranges, 3 cherries, 3 lemons, 2 mangos, 5 bananas, 5 grapes, and 1 each of lime, raspberry, blueberry, melon, kiwi, grapefruit, mangosteen, papaya, avocado, and pineapple), 19 cereals (4 quinoas, 4 kamuts, 4 barleys, 2 farros, 2 oat rices, and 1 each of millet, corn, and calrose rice), 28 vegetables (4 green peppers, 3 broccoli, 2 green onions, 2 garlic scapes, 2 shallots, 2 asparagus, 2 Chinese cabbages, and 1 each of spinach, okra, onion, carrot, radish, pumpkin, jicama, cabbage, burdock, ginger, and red bell pepper), and 2 root crops (2 potatoes). These agricultural products originated from 17 countries: Australia, Belgium, Canada, Chile, China, Israel, Italy, the Netherlands, New Zealand, Peru, the Philippines, the Republic of Costa Rica, the Republic of Madagascar, Spain, Thailand, the United States, and Vietnam. All samples were immediately frozen at −70 °C in a deep freezer for at least 24 h, and then homogenized using a stainless-steel homogenizer to ensure complete sample uniformity. The homogenized cereal and legume samples were further passed through a 420 μm stainless-steel mesh sieve to obtain a uniform particle size. All homogenized samples were transferred into high-density polyethylene (HDPE) amber bottles and stored at −20 °C prior to analysis.
2.4. Optimization and Validation of Extraction and Purification Conditions
For method optimization, soybean and mandarin were selected as priority matrices based on their distinct physicochemical characteristics. Soybean is characterized by a high lipid content, while mandarin exhibits high acidity and peels rich in pectin and complex aromatic compounds. These matrix characteristics are known to increase interferences and matrix effects, thereby complicating the analytical process [29,30]. In particular, soybean samples were pre-hydrated to enhance solvent accessibility, as their dried state reduces extraction efficiency when using water-miscible organic solvents [31]. The efficiency of extraction and purification was determined by recovery. For method optimization in this study, the efficiencies of extraction and purification were evaluated using the QuEChERS method. Methanol was selected as an extraction solvent based on the amphiphilic nature of natamycin—containing polar (–OH, –COOH) and nonpolar (–C=C–, –CH3) functional groups [11,12,13,14,15]. To optimize the extraction method, the QuEChERS method was compared with the Original method, EN 15662, and AOAC 2007.01 extraction. The clean-up step using dispersive solid-phase extraction (d-SPE) to minimize matrix effects was optimized with various combinations of sorbents, including C18 and graphitized carbon black (GCB).
The method validation parameters were evaluated in accordance with both the CODEX (CAC/GL 40-1993) [32] and the MFDS Guideline of Standard Procedures of Test Methods for Foods and Other Substances (2025) [33]. Validation parameters included specificity, linearity, limits of detection (LOD) and quantification (LOQ), accuracy, precision, and matrix effect. Specificity was assessed by comparing the chromatograms of blank and spiked samples to determine whether any interfering substances had the same retention time and m/z values as natamycin. The ion ratio was evaluated to confirm identification based on the consistent relative abundance of the quantifier and qualifier ions, and was calculated using data obtained from matrix-matched standard solutions (0.002, 0.005, 0.01, 0.02, 0.05, and 0.06 μg/mL) and recovery samples (0.01 mg/kg, n = 5). Linearity was performed by constructing a calibration curve using the same six concentration levels (0.002, 0.005, 0.01, 0.02, 0.05, and 0.06 μg/mL) of matrix-matched standard solutions, and the coefficient of determination (R2) was confirmed to meet the acceptance criterion (≥0.98) [34].
The instrumental limit of detection (ILOD) and instrumental limit of quantitation (ILOQ) were determined using natamycin standard solutions at six concentrations (0.002–0.06 μg/mL), each analyzed in seven replicates. The standard deviation of the y-intercept (σ) and the slope (S) from the calibration curve were used to calculate the limits according to the following equations: ILOD = 3.3 × (σ/S) and ILOQ = 10 × (σ/S). The signal-to-noise (S/N) ratios for the ILOD and ILOQ, measured by LC-MS/MS, were confirmed to meet the acceptance criteria of ≥3 and ≥10, respectively. The method limit of quantitation (MLOQ) was calculated as 0.01 mg/kg using the ILOQ and relevant analytical parameters, including the sample weight and sample solution volumes [35].
Accuracy and precision were evaluated at three fortification levels: MLOQ (0.01 mg/kg), 10× MLOQ (0.1 mg/kg), and 50× MLOQ (0.5 mg/kg). Natamycin standard solutions were spiked into blank matrices, and five replicate analyses were performed at each level. Mean recovery (%) and the coefficient of variation (CV, %) were calculated to assess accuracy and repeatability in accordance with the acceptance criteria specified by the MFDS guidelines [33].
Matrix effects on signal intensity were calculated from the ratio of the slopes of calibration curves (0.002, 0.005, 0.01, 0.02, 0.05, and 0.06 μg/mL; five replicates) constructed using standard solutions and matrix-matched solutions. The matrix-matched standard solutions were prepared by mixing the natamycin standard solution with blank sample extract to obtain a solution containing 90% blank extract. Positive values indicated ion enhancement, whereas negative values indicated ion suppression. The absolute matrix effect was classified into three levels: <20% (low/soft), 20% to <50% (moderate/medium), and ≥50% (high/strong) [36].
All data acquisition and processing were performed using Analyst software (Sciex, Version 1.6.3, Framingham, MA, USA) on an LC-MS/MS system equipped with an AB SCIEX QTRAP 5500 mass spectrometer and an ExionLC™ UHPLC. Statistical analyses were conducted using Microsoft Excel 2016 (Microsoft, Redmond, WA, USA).
2.5. Applicability of the Established Analytical Method
Monitoring was conducted to determine natamycin residues in imported agricultural products currently distributed in the Korean market. A total of 102 samples were collected from both online and offline sources and classified into five categories: legumes, fruits, cereals, vegetables, and root crops. Natamycin levels were quantified using matrix-matched calibration curves based on the validated analytical method. Finally, the detected levels in imported agricultural products were evaluated for compliance with the Korean Positive List System (PLS) to ensure consumer safety.
3. Results and Discussion
3.1. Optimization of Extraction and Purification Conditions
Soybean and mandarin were selected as representative matrices due to their complex physicochemical characteristics. Their high lipid content and acidity, respectively, enhanced matrix interferences and complicated residue analysis.
3.1.1. Optimization of Extraction Conditions
Natamycin was extracted with methanol (solubility approximately 3000 mg/L), and recoveries were evaluated using three QuEChERS methods—Original, EN 15662, and AOAC 2007.01 (Table 1)—in accordance with CODEX (CAC/GL 40-1993) and MFDS guidelines [37,38,39]. The recoveries obtained with the EN 15662 method for soybean at 0.01 mg/kg and for mandarin at 0.1 mg/kg were not within the acceptable range of 70–120%. Notably, the mean recovery for soybean at 0.01 mg/kg was 126.8%, which is likely attributable to strong salting-out effects induced by the QuEChERS procedure, resulting in disproportionately higher recoveries compared with other fortification levels [40]. In contrast, the recoveries and CV values obtained with the Original and AOAC 2007.01 methods were within the acceptance criteria for both soybean and mandarin. The Original method generally showed higher and more consistent recoveries and was selected as the optimized extraction method.

Table 1.
Recoveries of natamycin in soybean and mandarin using the Original, EN 15662, and AOAC 2007.01 extraction methods.
The QuEChERS original method includes a salting-out extraction with NaCl; therefore, it is important to remove salts that can precipitate. After extraction using the original method, salt precipitation was observed in the analytical sample solution, which may compromise analytical reproducibility [41]. In the extraction method, 3 g of MgSO4 was employed and NaCl was not used to prevent salt precipitation after extraction. The recovery and %CV of natamycin in both soybean and mandarin ranged from 79.0 to 109.2% and 0.3 to 1.3%, respectively, which were within the acceptable criteria specified by the guidelines.
3.1.2. Optimization of Purification Conditions
To reduce nonpolar and pigment-related interferences, purification efficiency was evaluated by adjusting the amounts of C18, MgSO4, and GCB. C18 is effective in removing lipids and other nonpolar substances, while GCB enhances the removal of pigments such as carotenoids, chlorophylls, and sterols [42]. For the three purification conditions tested—MgSO4 150 mg + C18 25 mg, MgSO4 900 mg + C18 150 mg, and MgSO4 900 mg + C18 150 mg + GCB 20 mg—natamycin recoveries met the acceptance criteria (60–120% with CV ≤ 32% at 0.01 mg/kg, 70–120% with CV ≤ 22% at 0.1 mg/kg, and 70–110% with CV ≤ 18% at 0.5 mg/kg). However, the addition of GCB at levels above 50 mg resulted in recoveries that fell outside the acceptable range (Table 2). Previous studies have indicated that GCB may lead to analyte loss depending on the molecular structure during the purification step [42].

Table 2.
Recoveries of natamycin in soybean and mandarin under different purification steps using various combinations of MgSO4, C18, and GCB.
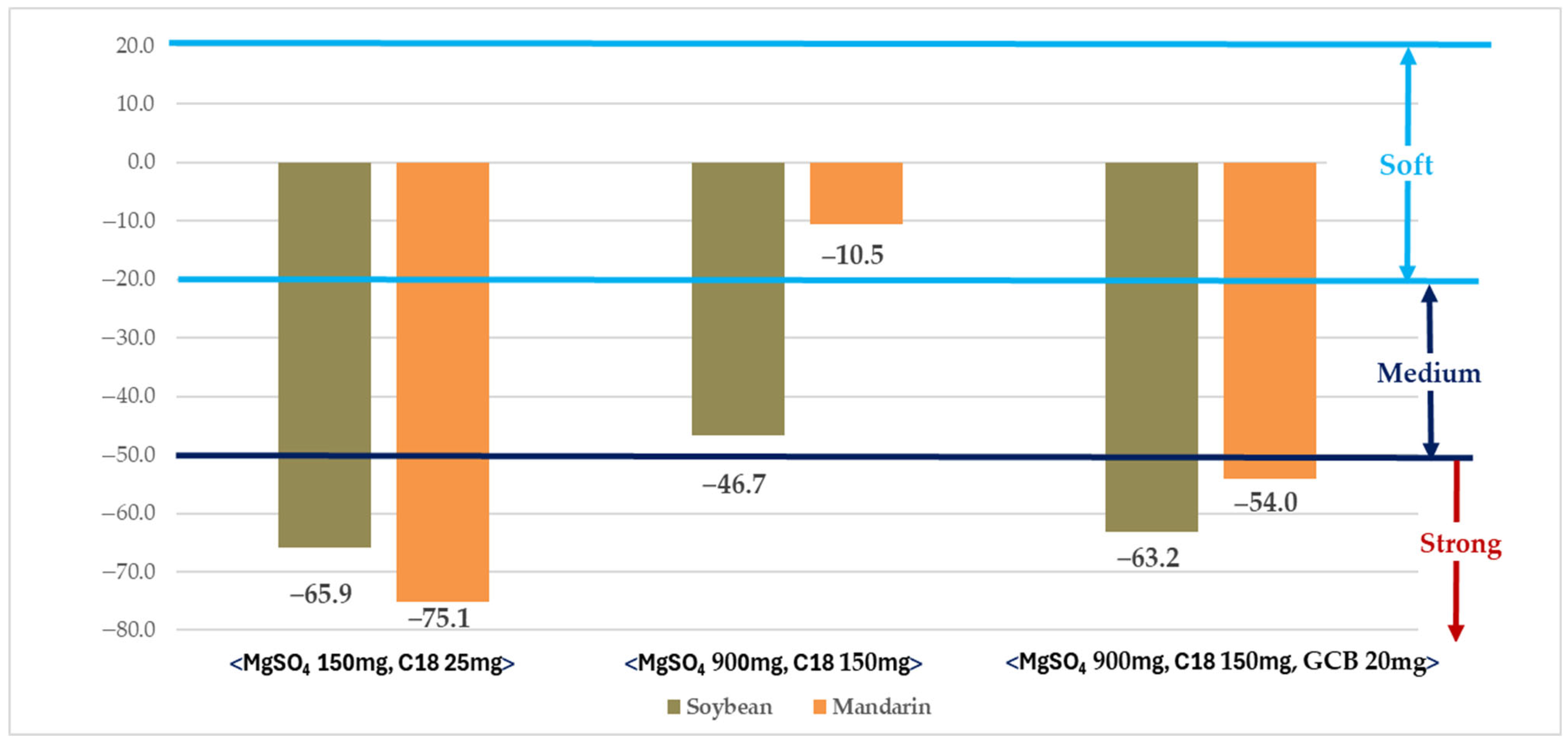
The matrix effect was evaluated using purification methods (MgSO4 150 mg + C18 25 mg, MgSO4 900 mg + C18 150 mg, and MgSO4 900 mg + C18 150 mg + GCB 20 mg) in which recoveries were within the acceptable range (Figure 1). Within these purification conditions, the method employing MgSO4 900 mg + C18 150 mg resulted in matrix effects classified within the soft or medium range, whereas the other methods exceeded the strong range [43].
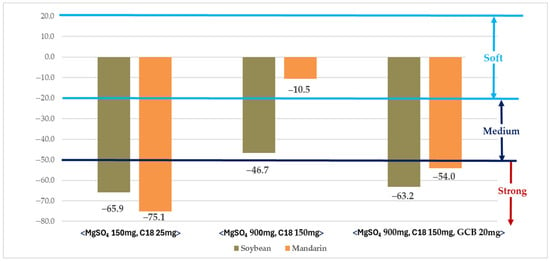
Figure 1.
Evaluation of matrix effects under different purification conditions.
Finally, the analytical method for natamycin was optimized as follows: Ten grams of sample (5 g for cereals and legumes, pre-hydrated with 5 mL distilled water for 30 min) were weighed into a 50 mL conical tube. Methanol (20 mL, or 10 mL for cereals and legumes) was added, and the mixture was vigorously shaken for 1 min. Magnesium sulfate (3 g) was then added, followed by another 1 min of shaking, and the mixture was centrifuged at 4000 rpm for 5 min. A 5 mL aliquot of the supernatant was transferred to a tube containing 900 mg MgSO4 and 150 mg C18, shaken for 1 min, and centrifuged again at 4000 rpm for 5 min for purification. The purified extract was filtered through a syringe filter and analyzed by LC–MS/MS.
3.2. Method Validation
The optimized method described above was validated for hulled rice (cereal grain), potato (root and tuber vegetable), soybean (legume vegetable), mandarin (fruit), and green pepper (fruiting vegetable) to verify its robustness and reliability.
3.2.1. Specificity and Linearity
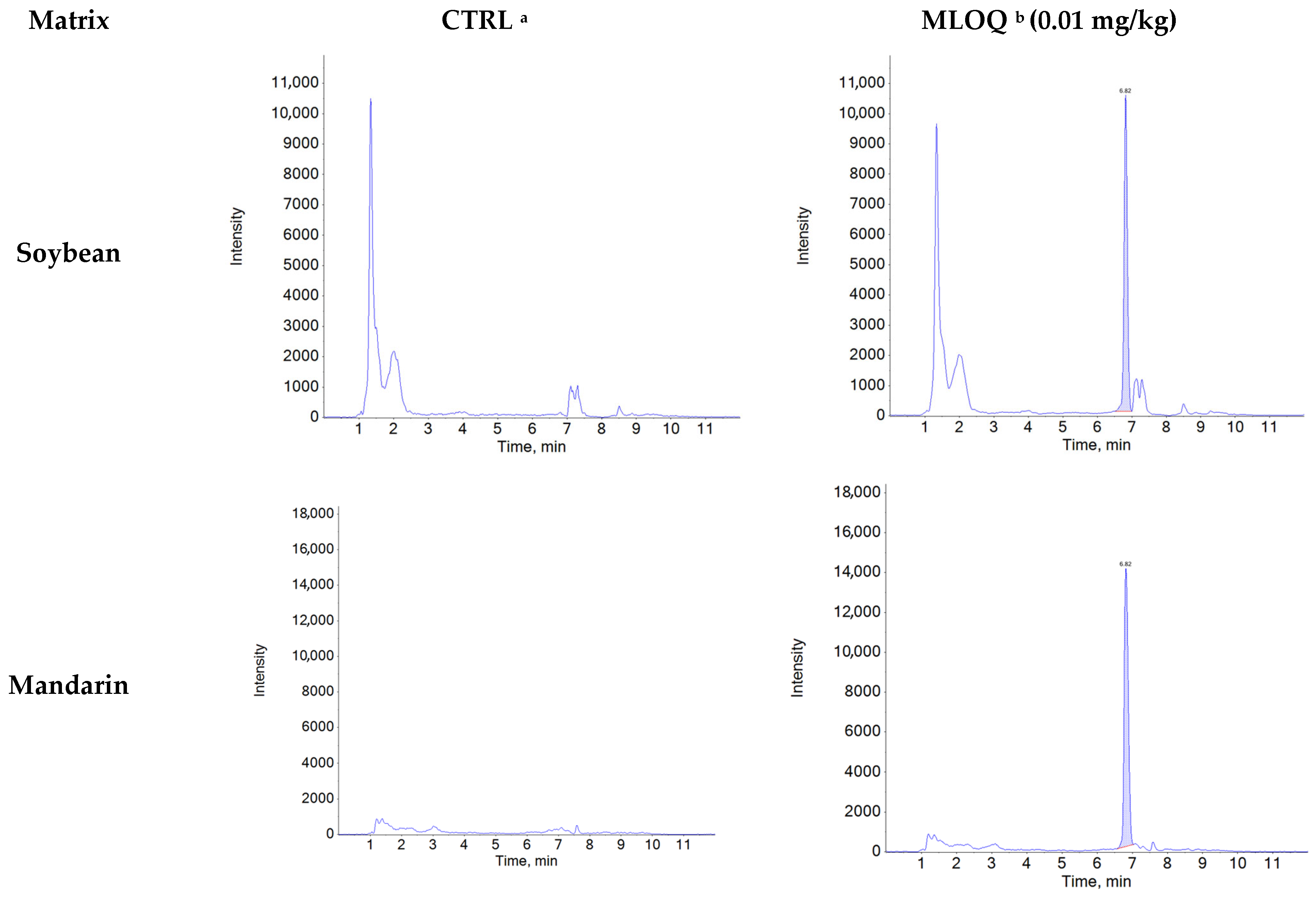
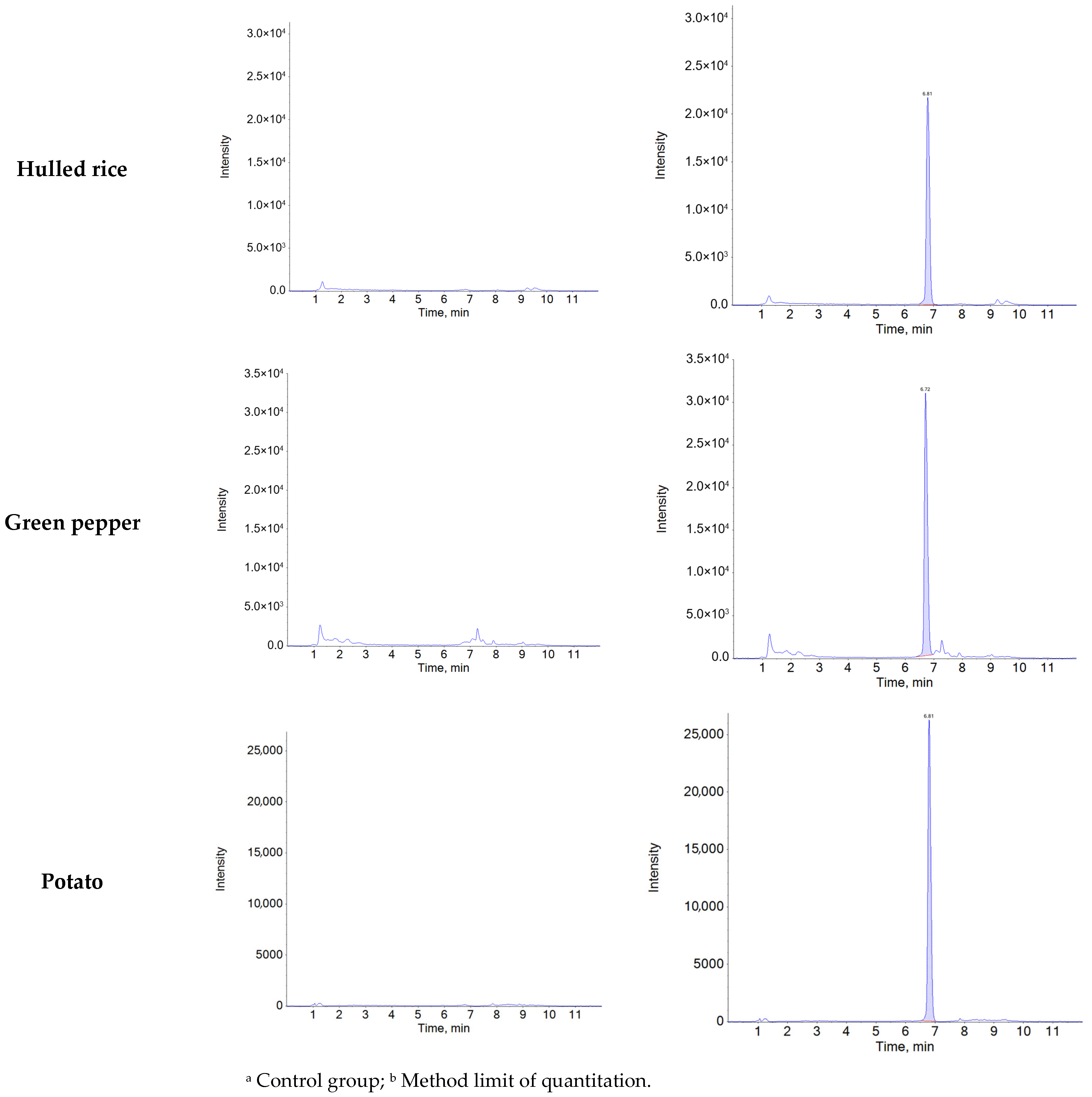
Specificity was confirmed by comparing chromatograms of blank samples with those of blank samples spiked with natamycin at the MLOQ (0.01 mg/kg). Natamycin was consistently detected at a retention time of 6.8 min in all five matrices (soybean, mandarin, hulled rice, green pepper, and potato), with no interfering peaks observed at the same retention time and mass-to-charge ratio (m/z) (Figure A1). The average ion ratios of the matrix-matched standard solutions (0.002–0.06 μg/mL) were below 10% across all matrices: soybean (0.51%), mandarin (0.51%), hulled rice (0.47%), green pepper (0.51%), and potato (0.48%). The relative tolerances were calculated as −3.18% for soybean, −0.81% for mandarin, 2.15% for hulled rice, 3.34% for green pepper, and 0.53% for potato, all of which were within the acceptable tolerance limit (±50%) according to the 2002/657/EC guideline [44]. Linearity was confirmed using matrix-matched standard solutions at six concentration levels (0.002–0.06 μg/mL), with coefficients of determination (R2 > 0.99) obtained for matrices (Table A3).
3.2.2. Method Limit of Quantitation (MLOQ)
The ILOD and ILOQ, calculated from the standard deviation of the y-intercept (σ) and the slope (S) of the calibration curve (0.002–0.06 μg/mL, 7 replicates), were 0.002 μg/mL and 0.005 μg/mL, respectively. The signal-to-noise (S/N) ratios of the ILOD and ILOQ measured by LC–MS/MS met the acceptance criteria of ≥3 and ≥10, respectively. The MLOQ was determined for all five matrices by multiplying the calculated ILOQ (0.005 μg/mL) by the sample solution volume (20 mL, or 10 mL for cereals and legumes) and dividing by the sample weight (10 g, or 5 g for cereals and legumes), resulting in a value of 0.01 mg/kg, which complied with the requirements of the Korean PLS.
3.2.3. Accuracy and Precision
Accuracy and precision were assessed by spiking blank matrices with natamycin at three concentration levels—MLOQ (0.01 mg/kg), 10× MLOQ (0.1 mg/kg), and 50× MLOQ (0.5 mg/kg)—followed by recovery testing in five replicates at each level. The mean recoveries (82.2–115.4%) and %CV values (1.1–4.6%) values were within the acceptance criteria defined by the CODEX guidelines, which specify recovery ranges of 60–120% with CV ≤ 32% at the LOQ, 70–120% with CV ≤ 22% at 10× LOQ, and 70–110% with CV ≤ 18% at 50× LOQ [32] (Table 3). These results demonstrate the high accuracy and analytical reliability of the method.

Table 3.
Recoveries and %CV values for natamycin in five representative agricultural commodities.
3.2.4. Matrix Effect
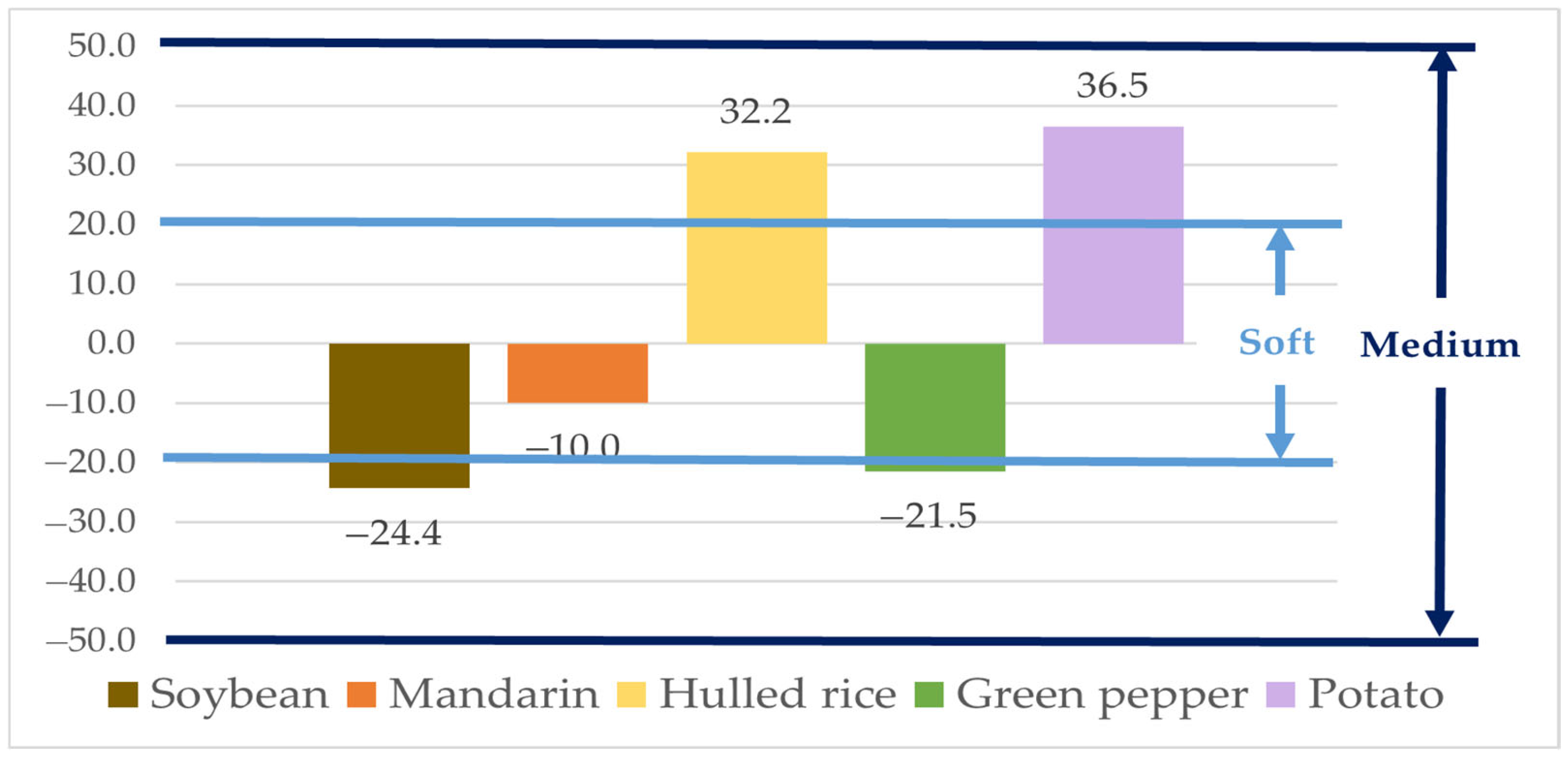
The matrix effects (MEs), based on the slopes of the calibration curves, were −24.4% for soybean, −10.0% for mandarin, 32.2% for hulled rice, −21.5% for green pepper, and 36.5% for potato (Figure 2). The ME for mandarin fell within the “soft” matrix effect range (|ME| < 20%), indicating negligible interference. The MEs for soybean, hulled rice, green pepper, and potato were classified as “medium” matrix effects (20% ≤ |ME| < 50%). Therefore, natamycin was quantified using the matrix-matched calibration curve for accurate determination [45].
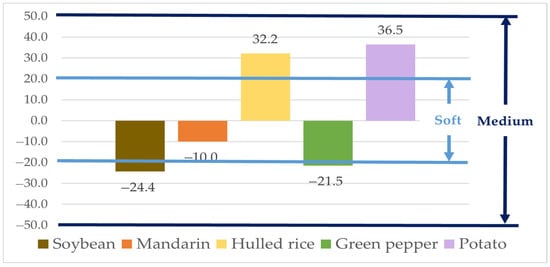
Figure 2.
Matrix effect of Natamycin in agricultural matrices.
3.3. Monitoring of Imported Agricultural Products
Monitoring of natamycin was conducted on 102 imported agricultural products, including 21 legumes, 32 fruits, 19 cereals, 28 vegetables, and 2 root crops, originating from 17 countries including Australia, China, Spain, the United States, and Vietnam. Natamycin was not detected in any of the 102 imported agricultural products. This result is attributable to the photo-degradation characteristics of natamycin, which is known to degrade upon exposure to light sources such as ultraviolet (UV), LED, and fluorescent lighting during distribution and retail processes associated with importation [46]. In a previous study, natamycin was not detected in wine due to degradation during the production process [47]. The absence of detectable residues indicates compliance with Korea’s PLS (≤0.01 mg/kg) and provides reassurance regarding consumer safety. Although this study was limited to 102 samples, the findings provide meaningful evidence on the residue status of natamycin in agricultural commodities. The 2017 JMPR evaluation noted that the lack of sufficient residue data, particularly for commodities such as citrus fruits, pineapples, and mushrooms, makes it difficult to establish reliable MRLs [48]. The present study provides a scientifically validated method and initial monitoring data that may serve as a basis for addressing this gap. Future studies incorporating a wider range of samples and expanded stability evaluations, building on the monitoring results of this study, will further strengthen the evidence base and support more comprehensive consumer protection.
4. Conclusions
Natamycin, although widely used in agricultural practices in other countries, is not registered for use in Korea. Therefore, an analytical method is required to manage imported agricultural products at the enforcement level. In this study, a QuEChERS-based analytical method using LC–MS/MS was developed and validated for the determination of natamycin residues in agricultural commodities. Method validation was performed for five representative crops—soybean, mandarin, hulled rice, green pepper, and potato—in compliance with CODEX guidelines (CAC/GL 40-1993) and the standard testing protocol issued by the Ministry of Food and Drug Safety (MFDS) [34] confirming the reliability of the method. The application of the validated method to 102 imported agricultural products from 17 countries revealed no detectable residues of natamycin. These results were within the default tolerance level (≤0.01 mg/kg) established under Korea’s PLS for regulating agricultural products. Although this study was limited to 102 samples, it provides meaningful evidence regarding the status of natamycin residues in agricultural commodities. In particular, given the limited international monitoring data for commodities such as citrus fruits, pineapples, and mushrooms, which makes establishing reliable maximum residue limits (MRLs) challenging, these findings offer important evidence on the occurrence of natamycin residues in agricultural commodities. Building on these findings, future studies incorporating a broader range of samples and extended stability evaluations will further reinforce the evidence base and support more comprehensive consumer protection. Thus, the analytical method developed and validated in this study is reliable and reproducible for monitoring natamycin residues under Korea’s PLS and contributes to ensuring consumer safety.
Author Contributions
Conceptualization, G.-E.-H.A. and H.-R.C.; methodology, G.-E.-H.A.; software, G.-E.-H.A.; validation, G.-E.-H.A., J.-K.O. and J.-H.K.; formal analysis, G.-E.-H.A.; investigation, G.-E.-H.A., J.-K.O. and J.-H.K.; resources, H.-R.C.; data curation, G.-E.-H.A.; writing—original draft preparation, G.-E.-H.A.; writing—review and editing, G.-E.-H.A. and H.-R.C.; visualization, G.-E.-H.A. and H.-R.C.; supervision, H.-R.C.; project administration, H.-R.C.; funding acquisition, H.-R.C. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Ministry of Food and Drug Safety, Republic of Korea, grant number: 23192MFDS282.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The original contributions presented in this study are included in the article. Further inquiries can be directed to the corresponding author.
Acknowledgments
The authors would like to thank Wonkwang University, Iksan, Republic of Korea, for their assistance with the monitoring work in this study.
Conflicts of Interest
The authors declare no conflicts of interest.
Appendix A

Table A1.
Chemical structure and physicochemical properties of Natamycin.
Table A1.
Chemical structure and physicochemical properties of Natamycin.
| Structure | 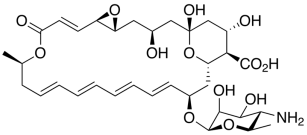 |
| IUPAC name | (8E,14E,16E,18E,20E)-(1R,3S,5R,7R,12R,22R,24S,25R,26S)-22-(3-amino-3,6-dideoxy-β-D-mannopyranosyloxy)-1,3,26-trihydroxy-12-methyl-10-oxo-6,11,28-trioxatricyclo[22.3.1.05,7]octacosa-8,14,16,18,20-pentaene-25-carboxylic acid |
| Molecular formula | C33H47NO13 |
| Molecular weight (g/mol) | 665.7 |
| Log Kow | −3.67 |
| Log P | −1.1 |
| pKa | 3.58 (strongest acidic) 9.11 (strongest basic) |
| Water solubility (g/L, 20–25 °C) | 0.04 |

Table A2.
MRM conditions for Natamycin.
Table A2.
MRM conditions for Natamycin.
| Exact Mass (g/mol) | Precursor Ion (m/z) | Product Ion (m/z) | CV (V) a | CE b | RT c (min) |
|---|---|---|---|---|---|
| 665.3 | 666.2 | 503.0 d | 24 | 17 | 6.8 |
| 485.0 e | 15 | 20 |
a CV: Cone voltage; b CE: Collision energy; c RT: Retention time; d Quantitation ion; e Confirmation ion.

Table A3.
Linear equations and coefficients of determination (R2) for matrix-matched calibration of natamycin in agricultural matrices.
Table A3.
Linear equations and coefficients of determination (R2) for matrix-matched calibration of natamycin in agricultural matrices.
| Agricultural Matrix | Linear Equation | R2 |
|---|---|---|
| Soybean | y = 2,720,216.3832x + 7403.6597 | 0.9985 |
| Mandarin | y = 4,564,924.8555x + 17,922.3719 | 0.9954 |
| Hulled Rice | y = 7,831,874.1206x − 4558.4131 | 0.9979 |
| Green Pepper | y = 5,781,466.9529x + 12,118.6316 | 0.9936 |
| Potato | y = 7,565,558.2164x − 2894.3815 | 0.9926 |
Appendix B
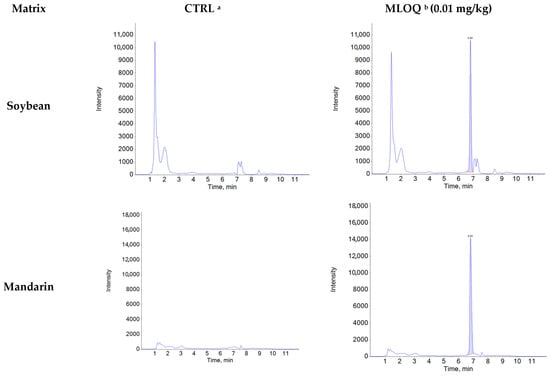
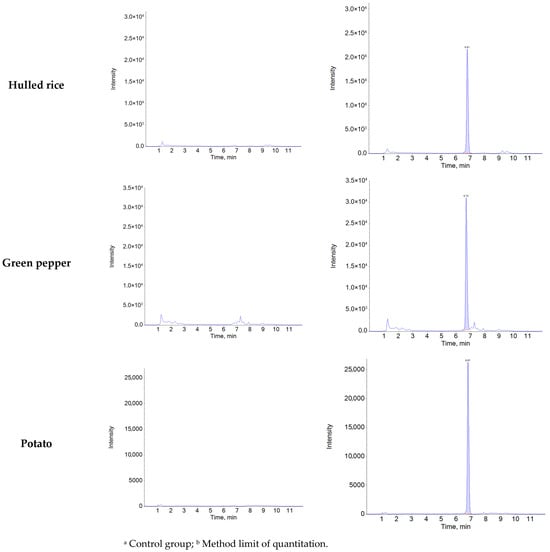
Figure A1.
Representative chromatograms of control and recovery samples (0.01 mg/kg) for natamycin in agricultural matrices.
Figure A1.
Representative chromatograms of control and recovery samples (0.01 mg/kg) for natamycin in agricultural matrices.
References
- Haack, S.E.; Ivors, K.L.; Holmes, G.J.; Förster, H.; Adaskaveg, J.E. Natamycin, a New Biofungicide for Managing Crown Rot of Strawberry Caused by QoI-Resistant Colletotrichum acutatum. Plant Dis. 2018, 102, 1687–1695. [Google Scholar] [CrossRef] [PubMed]
- Meena, M.; Prajapati, P.; Ravichandran, C.; Sehrawat, R. Natamycin: A natural preservative for food applications-a review. Food Sci. Biotechnol. 2021, 30, 1481–1496. [Google Scholar] [CrossRef] [PubMed]
- Welscher, Y.M.t.; Jones, L.; van Leeuwen, M.R.; Dijksterhuis, J.; de Kruijff, B.; Eitzen, G.; Breukink, E. Natamycin Inhibits Vacuole Fusion at the Priming Phase via a Specific Interaction with Ergosterol. Antimicrob. Agents Chemother. 2010, 54, 2618–2625. [Google Scholar] [CrossRef] [PubMed]
- Welscher, Y.M.t.; Napel, H.H.t.; Balagué, M.M.; Souza, C.M.; Riezman, H.; de Kruijff, B.; Breukink, E. Natamycin Blocks Fungal Growth by Binding Specifically to Ergosterol without Permeabilizing the Membrane. J. Biol. Chem. 2008, 283, 6393–6401. [Google Scholar] [CrossRef]
- Eissa, S.; Abdou, A.M.; Mohamed, H. The role of natamycin fortification to extend shelf life of plain yoghurt. Benha Vet. Med. J. 2014, 27, 140–149. [Google Scholar]
- Dalhoff, A.A.H.; Levy, S.B. Does use of the polyene natamycin as a food preservative jeopardise the clinical efficacy of amphotericin B? A word of concern. Int. J. Antimicrob. Agents 2015, 45, 564–567. [Google Scholar] [CrossRef][Green Version]
- Saito, S.; Wang, F.; Xiao, C.L. Natamycin as a postharvest treatment to control gray mold on stored blueberry fruit caused by multi-fungicide resistant Botrytis cinerea. Postharvest Biol. Technol. 2022, 187, 111862. [Google Scholar] [CrossRef]
- Wang, F.; Saito, S.; Xiao, C.-L. Postharvest application of natamycin to control gray mold in table grapes. Postharvest Biol. Technol. 2024, 210, 112777. [Google Scholar] [CrossRef]
- Yang, Y.; Huan, C.; Liang, X.; Fang, S.; Wang, J.; Chen, J. Development of Starch-Based Antifungal Coatings by Incorporation of Natamycin/Methyl-β-Cyclodextrin Inclusion Complex for Postharvest Treatments on Cherry Tomato against Botrytis cinerea. Molecules 2019, 24, 3962. [Google Scholar] [CrossRef]
- Nougadère, A.; Sirot, V.; Kadar, A.; Fastier, A.; Truchot, E.; Vergnet, C.; Hommet, F.; Baylé, J.; Gros, P.; Leblanc, J.-C. Total diet study on pesticide residues in France: Levels in food as consumed and chronic dietary risk to consumers. Environ. Int. 2012, 45, 135–150. [Google Scholar] [CrossRef]
- Tisza, B.B.; Járomi, L.; Háhn, J.; Bérczi, B.; Horváth-Sarródi, A.; Gubicskóné Kisbenedek, A.; Gerencsér, G. Possible Genotoxic Effects of Post-Harvest Fungicides Applied on Citrus Peels: Imazalil, Pyrimethanil, Thiabendazole and Their Mixtures. Foods 2025, 14, 1264. [Google Scholar] [CrossRef] [PubMed]
- Detry, P.; Willame, P.; Van Hoeck, E.; Van Loco, J.; Goscinny, S. Development, validation and application of multi-class methods for the analysis of food additives by liquid chromatography coupled to tandem mass spectrometry. Food Addit. Contam. Part A 2022, 39, 1349–1364. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Li, X.; Wang, P.; Ma, T.; Huang, W.; Han, S.; Zhan, J. Detection method optimization, content analysis and stability exploration of natamycin in wine. Food Chem. 2016, 194, 928–937. [Google Scholar] [CrossRef] [PubMed]
- Şanlı, S.; Kılıçarslan, S.; Şanlı, N. Evaluation of natamycin in commercial dairy products by a green capillary zone electrophoresis method and confirmation with a Liquid Chromatography-Mass Spectrometry. Food Biosci. 2022, 50, 102114. [Google Scholar] [CrossRef]
- Environmental Protection Agency (EPA). Natamycin; Exemption From the Requirement of a Tolerance. Fed. Regist. 2016, 81, 58407–58410. [Google Scholar]
- Kim, H.-H.; Han, Y.; Lee, H.-H.; Yang, Y.; Cho, S.; Park, J.; Kim, D.; Kim, A.; Park, D.; Mun, S.; et al. Monitoring of Positive List System on Residual Pesticides Analysis of Agricultural Products in Southwest of Korea. Agric. Res. Technol. 2022, 27, 556368. [Google Scholar] [CrossRef]
- Kang, H.-R.; Park, Y.-B.; Do, Y.-S.; Jeong, J.-A.; Lee, S.-B.; Cho, S.-H.; Lee, H.-K.; Son, J.-H.; Lee, M.-K.; Lee, B.-H.; et al. A Safety Survey on Pesticide Residues in Tropical Fruits Depending on Implementation of Positive List System. J. Food Hyg. Saf. 2018, 33, 310–315. [Google Scholar] [CrossRef]
- Zotchev, S.B. Polyene Macrolide Antibiotics and their Applications in Human Therapy. Curr. Med. Chem. 2003, 10, 211–223. [Google Scholar] [CrossRef]
- Kidwai, M.; Venkataramanan, R.; Rastogi, S.; Sapra, P. Discovery and Development of Antifungal Compounds. Curr. Med. Chem. Anti Infect. Agents 2003, 2, 27–71. [Google Scholar] [CrossRef]
- Ban, Z.J.; Chen, F.Y.; Liu, L.L.; Zhang, S.; Wang, L.J.; Wang, H.P.; Wang, L.Y.; Zhu, Y. Gliadin nanoparticles stabilized by sodium carboxymethyl cellulose as carriers for improved dispersibility, stability and bacteriostatic activity of Natamycin. Food Biosci. 2023, 53, 102575. [Google Scholar] [CrossRef]
- Capitán-Vallvey, L.F.; Checa-Moreno, R.; Navas, N. Rapid Ultraviolet Spectrophotometric and Liquid Chromatographic Methods for the Determination of Natamycin in Lactoserum Matrix. J. AOAC Int. 2000, 83, 802–808. [Google Scholar] [CrossRef]
- Zeng, X.; Danquah, M.K.; Jing, K.; Woo, M.W.; Chen, X.D.; Xie, Y.; Lu, Y. Solubility properties and diffusional extraction behavior of natamycin from streptomyces gilvosporeus biomass. Biotechnol. Prog. 2012, 29, 109–115. [Google Scholar] [CrossRef]
- Brik, H. New high-molecular decomposition products of natamycin (pimaricin) with intact lactone-ring. J. Antibiot. 1976, 29, 632–637. [Google Scholar] [CrossRef]
- Koontz, J.L.; Marcy, J.E.; Barbeau, W.E.; Duncan, S.E. Stability of natamycin and its cyclodextrin inclusion complexes in aqueous solution. J. Agric. Food Chem. 2003, 51, 7111–7114. [Google Scholar] [CrossRef] [PubMed]
- Patel, N.G.; Dhale, D.A.; Rathod, M.C. Analysis of Pesticide Residue in Fruit Using the QuEChERS Method Coupled With LC-MS/MS Detection. Curr. Agric. Res. J. 2024, 12, 865–872. [Google Scholar] [CrossRef]
- Rejczak, T.; Tuzimski, T. A review of recent developments and trends in the QuEChERS sample preparation approach. Open Chem. 2015, 13, 980–1010. [Google Scholar] [CrossRef]
- Santana-Mayor, Á.; Socas-Rodríguez, B.; Herrera-Herrera, A.V.; Rodríguez-Delgado, M.Á. Current trends in QuEChERS method. A versatile procedure for food, environmental and biological analysis. TrAC Trends Anal. Chem. 2019, 116, 214–235. [Google Scholar] [CrossRef]
- Chawla, S.; Patel, H.K.; Gor, H.N.; Vaghela, K.M.; Solanki, P.P.; Shah, P.G. Evaluation of Matrix Effects in Multiresidue Analysis of Pesticide Residues in Vegetables and Spices by LC-MS/MS. J. Aoac Int. 2017, 100, 616–623. [Google Scholar] [CrossRef]
- Guan, W.B.; Li, Z.N.; Zhang, H.Y.; Hong, H.J.; Rebeyev, N.; Ye, Y.; Ma, Y.Q. Amine modified graphene as reversed-dispersive solid phase extraction materials combined with liquid chromatography-tandem mass spectrometry for pesticide multi-residue analysis in oil crops. J. Chromatogr. A 2013, 1286, 1–8. [Google Scholar] [CrossRef]
- Lehotay, S.J.; Son, K.A.; Kwon, H.; Koesukwiwat, U.; Fu, W.S.; Mastovska, K.; Hoh, E.; Leepipatpiboon, N. Comparison of QuEChERS sample preparation methods for the analysis of pesticide residues in fruits and vegetables. J. Chromatogr. A 2010, 1217, 2548–2560. [Google Scholar] [CrossRef]
- Cho, S.M.; Do, J.-A.; Lee, H.S.; Park, J.-S.; Shin, H.-S.; Jang, D.E.; Cho, M.-S.; Jung, Y.-h.; Lee, K. Development and Validation of the Analytical Method for Oxytetracycline in Agricultural Products using QuEChERS and LC-MS/MS. J. Food Hyg. Saf. 2019, 34, 227–234. [Google Scholar] [CrossRef]
- Codex Alimentarius Commission (CAC). Guidelines on Good Laboratory Practice in Pesticide Residue Analysis; CAC/GL 40-1993; Rev.1-2003; CAC: Rome, Italy, 2003. [Google Scholar]
- Ministry of Food and Drug Safety (MFDS). Guideline of Standard Procedures of Test Methods for Foods and Other Substances; Ministry of Food and Drug Safety (MFDS): Cheongju, Republic of Korea, 2025. [Google Scholar]
- Oh, J.K.; Kim, J.H.; An, G.E.H.; Chang, H.R. Simultaneous Determination of Six Acidic Pesticides, Including 2,4-DB and 2,4,5-T with No Established MRL in Korea Using LC-MS/MS and QuEChERS for the Safety of Imported Agricultural Products. Foods 2025, 14, 904. [Google Scholar] [CrossRef]
- Lee, S.-H.; Kwak, S.-Y.; Sarker, A.; Moon, J.-K.; Kim, J.-E. Optimization of a Multi-Residue Analytical Method during Determination of Pesticides in Meat Products by GC-MS/MS. Foods 2022, 11, 2930. [Google Scholar] [CrossRef] [PubMed]
- Łozowicka, B.; Rutkowska, E.; Jankowska, M. Influence of QuEChERS modifications on recovery and matrix effect during the multi-residue pesticide analysis in soil by GC/MS/MS and GC/ECD/NPD. Environ. Sci. Pollut. Res. 2017, 24, 7124–7138. [Google Scholar] [CrossRef] [PubMed]
- Stark, J. PRESERVATIVES | Permitted Preservatives—Natamycin. In Encyclopedia of Food Microbiology; Elsevier: Amsterdam, The Netherlands, 1999; pp. 1776–1781. [Google Scholar]
- U.S. Environmental Protection Agency (EPA); Office of Pesticide Programs, Biopesticides and Pollution Prevention Division. Natamycin (PC Code 051102): Risk Assessment & Tolerance Exemption; U.S. Environmental Protection Agency (EPA): Washington, DC, USA, 2011. [Google Scholar]
- González-Curbelo, M.Á.; Varela-Martínez, D.A.; Riaño-Herrera, D.A. Pesticide-Residue Analysis in Soils by the QuEChERS Method: A Review. Molecules 2022, 27, 4323. [Google Scholar] [CrossRef] [PubMed]
- Tongchai, P.; Sawarng, N.; Wongta, A.; Jaitham, U.; Sutan, K.; Kawichai, S.; Jung, C.; Chuttong, B.; Hongsibsong, S. Optimization and Validation of a QuEChERS-Based Method Combined with Gas Chromatography–Tandem Mass Spectrometry for Analyzing Pesticide Residues in Edible Insect Samples. Molecules 2025, 30, 2293. [Google Scholar] [CrossRef]
- Williams, M.L.; Olomukoro, A.A.; Emmons, R.V.; Goodage, N.H.; Gionfriddo, E. Matrix effects demystified: Strategies for resolving challenges in analytical separations of complex samples. J. Sep. Sci. 2023, 46, 2300571. [Google Scholar] [CrossRef]
- Casado, N.; Morante-Zarcero, S.; Sierra, I. Application of the QuEChERS Strategy as a Useful Sample Preparation Tool for the Multiresidue Determination of Pyrrolizidine Alkaloids in Food and Feed Samples: A Critical Overview. Appl. Sci. 2022, 12, 4325. [Google Scholar] [CrossRef]
- Kmellár, B.; Fodor, P.; Pareja, L.; Ferrer, C.; Martínez-Uroz, M.A.; Valverde, A.; Fernandez-Alba, A.R. Validation and uncertainty study of a comprehensive list of 160 pesticide residues in multi-class vegetables by liquid chromatography–tandem mass spectrometry. J. Chromatogr. A 2008, 1215, 37–50. [Google Scholar] [CrossRef]
- European Commission. Commission Decision of 12 August 2002 implementing Council Directive 96/23/EC concerning the performance of analytical methods and the interpretation of results. 2002/657/EC. Off. J. Eur. Union 2002, L 221, 228–236. [Google Scholar]
- Rad, R.; Hosu, A.; Cimpoiu, C. Matrix-matching calibration as a new approach for quantitation of monosodium glutamate in food seasoning powders using high performance thin layer chromatography. J. Food Compos. Anal. 2025, 139, 107153. [Google Scholar] [CrossRef]
- Teixeira, G.H.A.; O’Keefe, S.F. Short communication: Mycosporine-like amino acids protect natamycin against photodegradation in milk exposed to fluorescent or light-emitting diode light. J. Dairy Sci. 2019, 102, 4972–4977. [Google Scholar] [CrossRef]
- Bernardi, G.; Rizzetti, T.; Adaime, M.; Zanella, R.; Prestes, O. Fast Sample Preparation Method Using Ultra-High Performance Liquid Chromatography Coupled to Tandem Mass Spectrometry for Natamycin Determination in Wine Samples. J. Braz. Chem. Soc. 2016, 28, 831–837. [Google Scholar] [CrossRef]
- FAO/WHO Joint Meeting on Pesticide Residues (JMPR). Evaluation of Natamycin; FAO Plant Production and Protection Paper 230; Food and Agriculture Organization of the United Nations (FAO): Rome, Italy, 2017; pp. 1745–1764. Available online: https://www.fao.org/fileadmin/templates/agphome/documents/Pests_Pesticides/JMPR/Evaluation2017/NATAMYCIN__300_.pdf (accessed on 29 September 2025).
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).