Impact of High Hydrostatic Pressure, Ultrasound, and Pulsed Electric Field in Beverages Fermentation: A Review of Nutritional, Functional, and Sensory Aspects and the Future
Abstract
1. Introduction
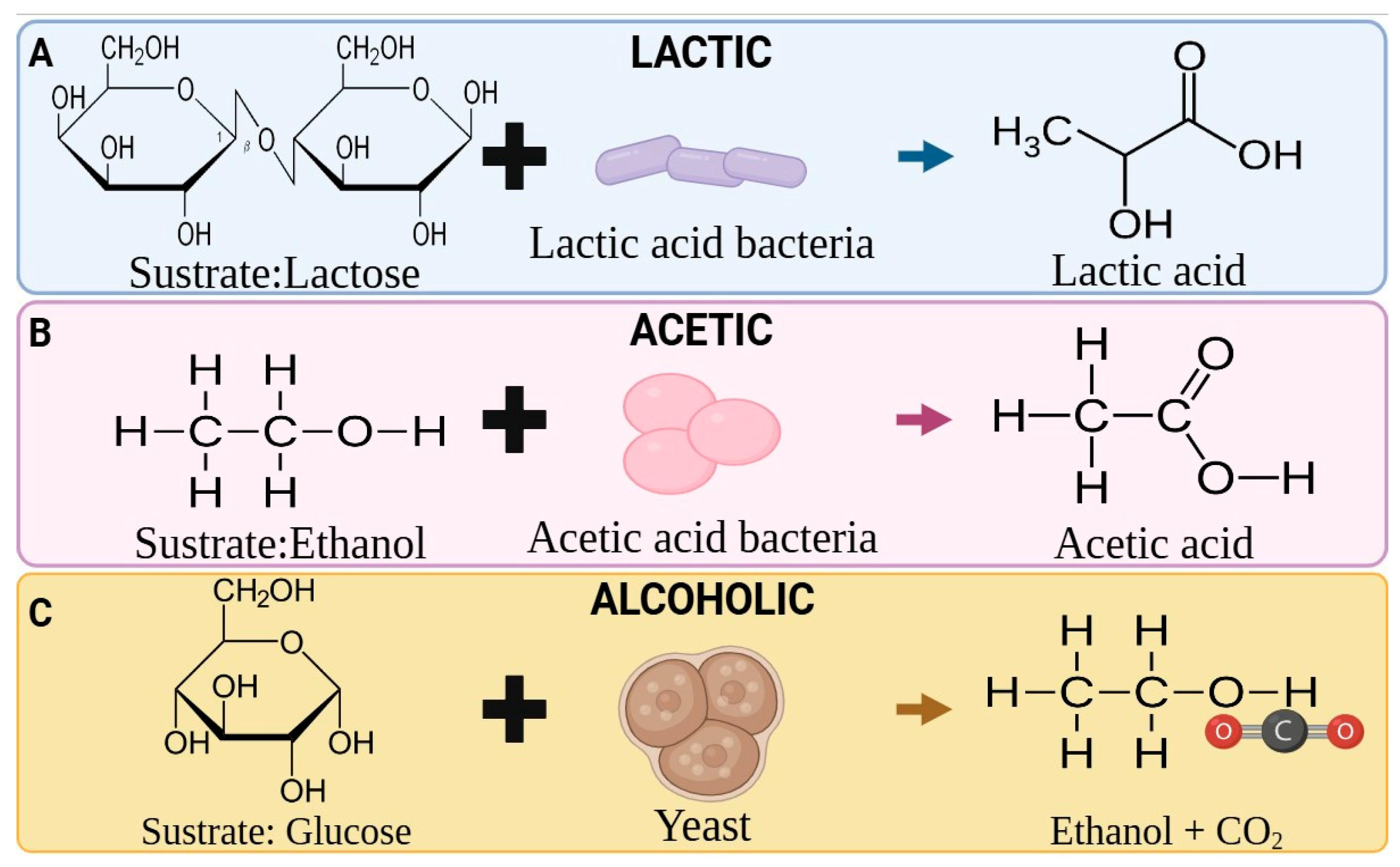
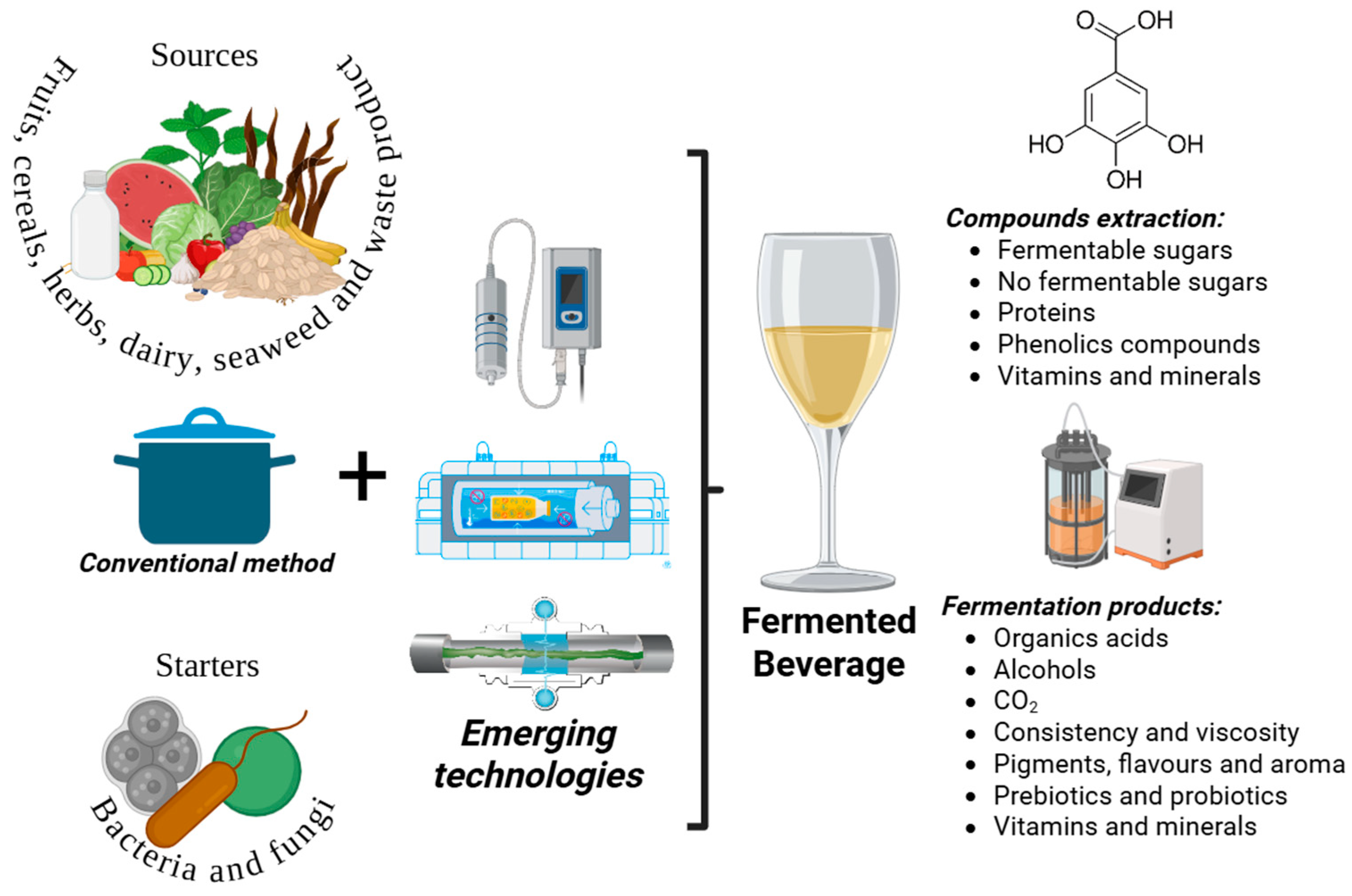
2. Types of Fermented Beverages
2.1. Fruits and Vegetables
2.2. Cereals
2.3. Herbs
2.4. Dairy Products
2.5. Seaweed
2.6. Waste Products
3. Fermented Beverage Processing
3.1. Conventional Method
| Fermented Beverage | Source | Fermentation Conditions | Microorganisms | References |
|---|---|---|---|---|
| Beers | barley, malted barley, wheat, rice, rye, corn, oats, sorghum, bread | Lager 7–21 days; 5–15 °C; pH 4.0–5.5 Ale: 2–6 weeks;18–27 °C; pH 4.0–5.0 Lambic: 3–9 months; 15–25 °C; pH 3.8–4.4 | Saccharomyces spp., Brettanomyces spp., Pediococcus spp., Hanseniaspora spp., Saccharomyces spp., Brettanomyces spp., Pediococcus spp., Hanseniaspora spp., Lactobacillus spp., Acetobacter spp. and Gluconobacter spp. | [34,72,73] |
| Fruit beers | blueberries, cherries, peaches, raspberries, strawberries, mangoes, apples, pears, pineapple, banana | |||
| Free gluten beers | rice, corn, quinoa, buckwheat, amaranth, oats, and sorghum | |||
| Wine | grapes | Alcoholic: 10–15 days; 14–30 °C; pH 4.5–6.5 Malolactic: 2–12 weeks; 20–30 °C; pH 3.5–6.5 | Saccharomyces spp., Lactobacillus spp., Leuconostoc spp., Oenococcus spp., and Pediococcus spp. | [33,36] |
| Fruit wine | blackberry, pineapple, passion fruit, banana, and watermelon | [27,35] | ||
| Rice wine | rice | First fermentation: 5–14 days; 15–30 °C; pH 4.5–5.5 Second fermentation: 1–2 months; 10–15 °C; pH 4.0–5.0 | Saccharomyces spp., Mucor spp., Bacillus spp., Aspergillus spp., Rhizopus spp. and Lactobacillus spp. | [74] |
| Vinegar | blueberry, persimmon, sugar cane, beer, citron, plum, dates, pomegranate, grains, soursop, cherry, kombucha, malt, mango, apple, molasses, honey, rice must, orange, pears, pineapple and its by-products, banana and papaya, whey, grape, wine | Alcoholic: 7–14 days; 18–20 °C; pH ~5.0 Acetic: 1–2 weeks; 25–40 °C; pH 5.5–7.0 | Saccharomyces spp., Acetobacter spp. | [34,75] |
| Cider | apple | First fermentation: 1–4 weeks; 15–30 °C; pH 3.5–6.5 Second fermentation: 1–3 weeks; 10–17 °C; pH 3.5–5.0 | Saccharomyces spp., Hanseniaspora spp., Torulaspora spp. | [22,34] |
| Kefir | milk, water, sugar, nuts, herbs | 1–4 days; 18–30 °C; pH 2.5–7.0 | Lactobacillus spp. | [10] |
| Kombucha | sugar, black tea and green tea | 1–3 weeks; 20–30 °C; pH 3.5–4.5 | Komagataeibacter spp., Gluconobacter spp., Acetobacter spp., Brettanomyces spp. | [15,44,45,76,77] |
| Kvass | Rye, malt, sugar, water | 1–2 weeks; 18–25 °C; pH 3.5–4.5 | Lactobacillus spp., Leuconostoc spp., Saccharomyces spp., Brettanomyces spp. | [48] |
| Yogurt | milk | 4–8 h; 40–45 °C; pH 4.3–4.6 | Streptococcus spp., Lactobacillus spp., Bifidobacterium spp. | [4,50,78] |
| Seaweed fermented | brown, red and green algae | 50 °C for 24 h | Lactobacillus spp., Saccharomyces spp., Lactiplantibacillus spp. | [57,58,59] |
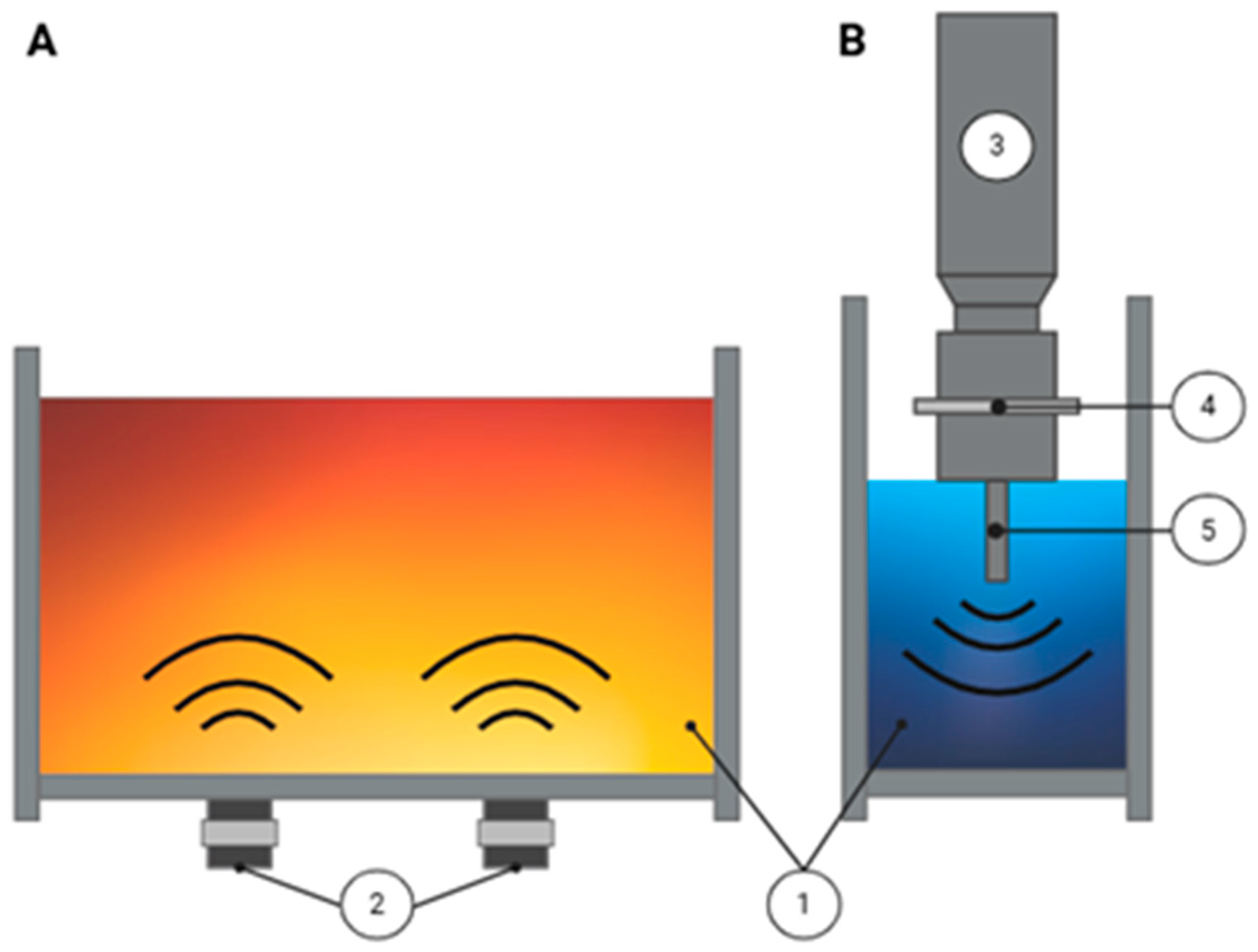
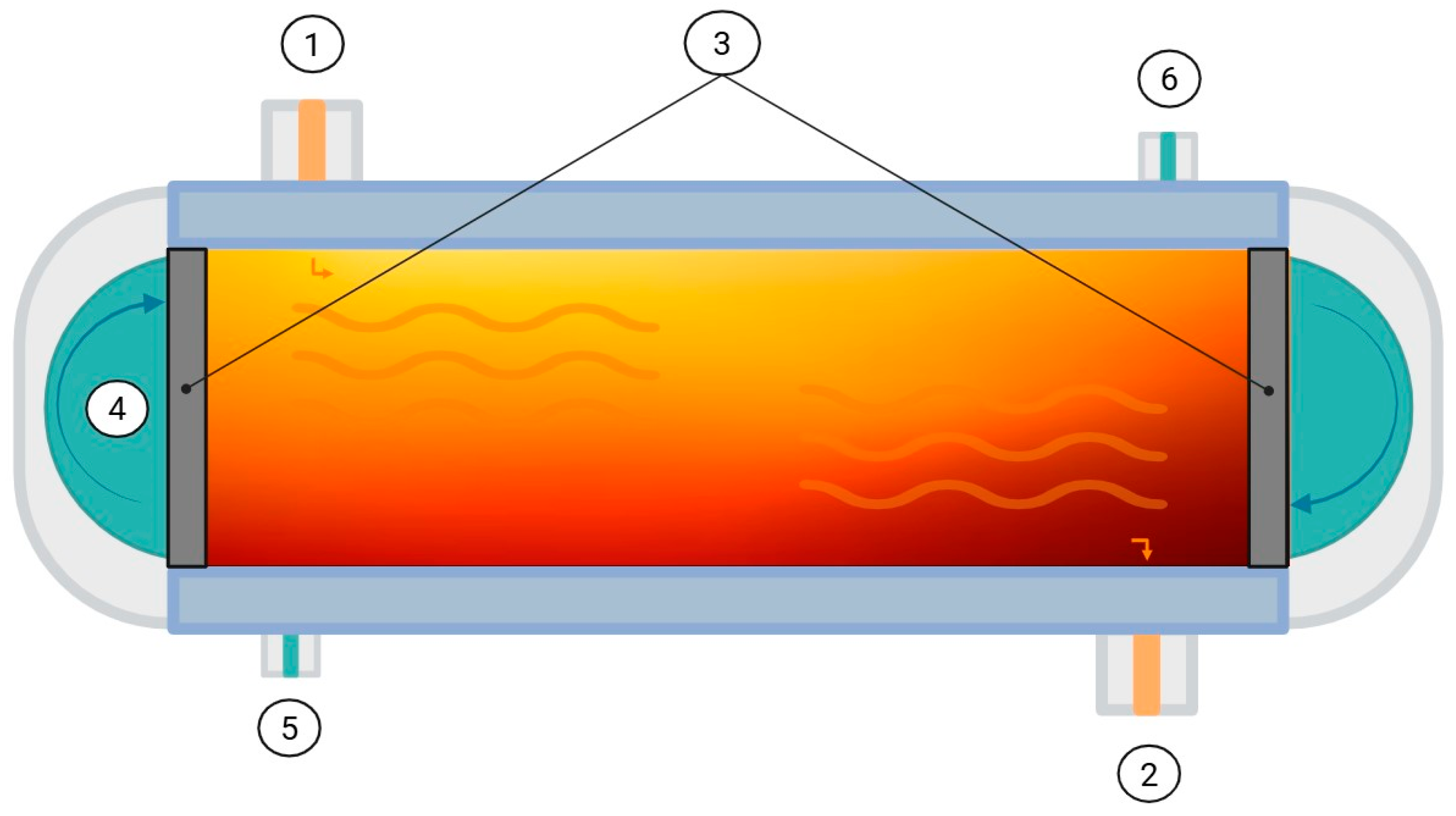
3.2. Emerging Technologies
3.2.1. Ultrasound (US)
3.2.2. High Hydrostatic Pressure (HHP)
3.2.3. Pulsed Electric Fields (PEF)
4. HHP, US, and PEF in Fermented Beverage Production
4.1. Effects on Microorganisms
4.2. Nutritional and Functional Properties
| Assisted Technology | FB | Fermented Beverage Properties | Reference |
|---|---|---|---|
| US | Mixed kefir | Increase antioxidant activity by 28% (22 kHz, 90 W/L, 3 min) Improved sensory properties (22 kHz, 90 W/L, 3 min) | [83] |
| Yogurt fermenter | Increase vitamin C content by 28% (22 kHz, 120 W/L, 3 min) Increase antioxidant activity by 67% (22 kHz, 90 W/L, 3 min) | ||
| Industrial kefir | Increase vitamin C content by 30% (22 kHz, 120 W/L, 3 min) Increase antioxidant activity by 67% (22 kHz, 90 W/L, 3 min) | ||
| Active peptide yogurt | Increases peptide content (28 kHz, 100 W/L, 35 min) | [98] | |
| Tepache | Maintains titratable acidity and pH (25 kHz, 20–100%, 5–10 min) Promotes changes in the microstructure and composition (25 kHz, 20–100%, 5–10 min) | [84] | |
| Chinese rice wine | Decreases sugar content (28 kHz, 35 W/L, 1 h, seventh day) Increases total acid content and content of ester (28 kHz, 35 W/L, 1 h, seventh day) Maintains pH value (28 kHz, 35 W/L, 1 h, seventh day) | [5] | |
| White millet beverage | Increases total phenol content and antioxidant activity (20 kHz, 3 W/L, 40.11%, 11.09 min) Increases flavonoid content (20 kHz, 3 W/L, 41.42%, 2.63 min) Decreases particle size (20 kHz, 3 W/L, 41.42%, 2.63 min) | [85] | |
| HPP | Yogurt | Increases pH level (700 MPa, 10 min) Decreases total solids content (500–600–700 MPa, 10 min) Enhanced firmness (700 MPa, 10 min) Decreases wheying off level (500–600–700 MPa, 10 min) Improves sensory properties like color, flavors, taste, and firmness (700 MPa, 10 min) | [108] |
| Yacon-Litchi-Longan juice | Decreases 13.75% of free amino acids content (500 MPa, 25 °C, 15 min) Loss of 3.67% of total volatile flavors compounds (500 MPa, 25 °C, 15 min) | [100] | |
| Shalgam | Maintains pH and total soluble solids (100–500 MPa, 20–40 °C, 5–15 min) | [93] | |
| Apple Juice | Increases caffeic, ferric, and chlorogenic acid levels after 24 h (200 MPa, 10 min) Decreases pH level (200 MPa, 10 min) Decreases color an 80% (300 MPa, 10 min) Increases total phenol content (200 MPa, 10 min) Maintains antioxidant activity (200–300–400 MPa, 10 min) | [96] | |
| PEF | Yogurt | Slightly decreases in pH (1 kV/cm, 4 Hz, 50 pulses) Fastest decreases in oxidation reduction potential (3.67 kV/cm, 0.5 Hz, 50 pulses) | [101] |
| Yogurt | Decreases pH (1 kV/cm, 150 Hz, 8μs, 400μs, 3.8 J) | [50] | |
| Wines | Up to 41.5 kV/cm, 49.4 kJ/kg. improved the extraction of anthocyanins and phenols such as catechin, as well as reducing 2-hexenal. Increases in total phenol content (8 kV/cm, 344 Hz, 300 s) | [109,115] |
4.3. Organoleptic and Physicochemical Characteristics
5. Future Perspectives, Challenges and Opportunities
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| FB | Fermented beverages |
| US | Ultrasounds |
| HHP | High hydrostatic pressures |
| PEF | Pulsed electric field |
References
- Blandino, A.; Al-Aseeri, M.E.; Pandiella, S.S.; Cantero, D.; Webb, C. Cereal-Based Fermented Foods and Beverages. Food Res. Int. 2003, 36, 527–543. [Google Scholar] [CrossRef]
- Patrignani, F.; D’Alessandro, M.; Vannini, L.; Lanciotti, R. Use of Functional Microbial Starters and Probiotics to Improve Functional Compound Availability in Fermented Dairy Products and Beverages; Elsevier Inc.: Amsterdam, The Netherlands, 2020; ISBN 9780128182932. [Google Scholar]
- Terefe, N.S. Emerging Trends and Opportunities in Food Fermentation; Elsevier: Amsterdam, The Netherlands, 2016; ISBN 9780081005965. [Google Scholar]
- Ayed, L.; M’hir, S.; Asses, N. Sustainable Whey Processing Techniques: Innovations in Derivative and Beverage Production. Food Biosci. 2023, 53, 102642. [Google Scholar] [CrossRef]
- Guo, Z.H.; Wang, Q.; Zhao, J.H.; Xu, Y.P.; Mu, G.Q.; Zhu, X.M. Lactic Acid Bacteria with Probiotic Characteristics in Fermented Dairy Products Reduce Cow Milk Allergy. Food Biosci. 2023, 55, 103055. [Google Scholar] [CrossRef]
- Gille, D.; Schmid, A.; Walther, B.; Vergères, G. Fermented Food and Non-Communicable Chronic Diseases: A Review. Nutrients 2018, 10, 448. [Google Scholar] [CrossRef] [PubMed]
- Shokri, S.; Terefe, N.S.; Shekarforoush, S.S.; Hosseinzadeh, S. Ultrasound-Assisted Fermentation for Enhancing Metabolic and Probiotic Activities of LactoBacillus brevis. Chem. Eng. Process.-Process Intensif. 2021, 166, 108470. [Google Scholar] [CrossRef]
- Morales-de la Peña, M.; Miranda-Mejía, G.A.; Martín-Belloso, O. Recent Trends in Fermented Beverages Processing: The Use of Emerging Technologies. Beverages 2023, 9, 51. [Google Scholar] [CrossRef]
- Canonico, L.; Agarbati, A.; Galli, E.; Comitini, F.; Ciani, M. Metschnikowia pulcherrima as Biocontrol Agent and Wine Aroma Enhancer in Combination with a Native Saccharomyces cerevisiae. LWT 2023, 181, 114758. [Google Scholar] [CrossRef]
- De Roos, J.; De Vuyst, L. Acetic Acid Bacteria in Fermented Foods and Beverages. Curr. Opin. Biotechnol. 2018, 49, 115–119. [Google Scholar] [CrossRef]
- Sun-Waterhouse, D. The Development of Fruit-Based Functional Foods Targeting the Health and Wellness Market: A Review. Int. J. Food Sci. Technol. 2011, 46, 899–920. [Google Scholar] [CrossRef]
- Ayed, L.; M’Hir, S.; Hamdi, M. Microbiological, Biochemical, and Functional Aspects of Fermented Vegetable and Fruit Beverages. J. Chem. 2020, 2020, 5790432. [Google Scholar] [CrossRef]
- Patra, M.; Bashir, O.; Amin, T.; Wani, A.W.; Shams, R.; Chaudhary, K.S.; Mirza, A.A.; Manzoor, S. A Comprehensive Review on Functional Beverages from Cereal Grains-Characterization of Nutraceutical Potential, Processing Technologies and Product Types. Heliyon 2023, 9, e16804. [Google Scholar] [CrossRef]
- Reboleira, J.; Silva, S.; Chatzifragkou, A.; Niranjan, K.; Lemos, M.F.L. Seaweed Fermentation within the Fields of Food and Natural Products. Trends Food Sci. Technol. 2021, 116, 1056–1073. [Google Scholar] [CrossRef]
- de Miranda, J.F.; Ruiz, L.F.; Silva, C.B.; Uekane, T.M.; Silva, K.A.; Gonzalez, A.G.M.; Fernandes, F.F.; Lima, A.R. Kombucha: A Review of Substrates, Regulations, Composition, and Biological Properties. J. Food Sci. 2022, 87, 503–527. [Google Scholar] [CrossRef] [PubMed]
- Aung, T.; Eun, J.B. Impact of Time and Temperature on the Physicochemical, Microbiological, and Nutraceutical Properties of Laver Kombucha (Porphyra dentata) during Fermentation. LWT 2022, 154, 112643. [Google Scholar] [CrossRef]
- Siddiqui, S.A.; Erol, Z.; Rugji, J.; Taşçı, F.; Kahraman, H.A.; Toppi, V.; Musa, L.; Di Giacinto, G.; Bahmid, N.A.; Mehdizadeh, M.; et al. An Overview of Fermentation in the Food Industry—Looking Back from a New Perspective. Bioresour. Bioprocess. 2023, 10, 85. [Google Scholar] [CrossRef]
- Mota, M.J.; Lopes, R.P.; Koubaa, M.; Roohinejad, S.; Barba, F.J.; Delgadillo, I.; Saraiva, J.A. Fermentation at Non-Conventional Conditions in Food- and Bio-Sciences by the Application of Advanced Processing Technologies. Crit. Rev. Biotechnol. 2018, 38, 122–140. [Google Scholar] [CrossRef]
- Sahu, L.; Panda, S.K. Innovations in Technologies for Fermented Food and Beverage Industries; Springer: Cham, Switzerland, 2018. [Google Scholar] [CrossRef]
- Gupta, A.; Sanwal, N.; Bareen, M.A.; Barua, S.; Sharma, N.; Joshua Olatunji, O.; Prakash Nirmal, N.; Sahu, J.K. Trends in Functional Beverages: Functional Ingredients, Processing Technologies, Stability, Health Benefits, and Consumer Perspective. Food Res. Int. 2023, 170, 113046. [Google Scholar] [CrossRef]
- Hidalgo-Fuentes, B.; De Jes, E.; Cabrera-hidalgo, A.D.J.; Sandoval-castilla, O.; Liceaga, A.M.; Aguilar-toal, J.E. Plant-Based Fermented Beverages: Nutritional Composition, Sensory Properties, and Health Benefits. Foods 2024, 13, 844. [Google Scholar] [CrossRef]
- Al Daccache, M.; Koubaa, M.; Salameh, D.; Vorobiev, E.; Maroun, R.G.; Louka, N. Control of the Sugar/Ethanol Conversion Rate during Moderate Pulsed Electric Field-Assisted Fermentation of a Hanseniaspora Sp. Strain to Produce Low-Alcohol Cider. Innov. Food Sci. Emerg. Technol. 2020, 59, 102258. [Google Scholar] [CrossRef]
- dos Santos Rocha, C.; Magnani, M.; Jensen Klososki, S.; Aparecida Marcolino, V.; dos Santos Lima, M.; Queiroz de Freitas, M.; Carla Feihrmann, A.; Eduardo Barão, C.; Colombo Pimentel, T. High-Intensity Ultrasound Influences the Probiotic Fermentation of Baru Almond Beverages and Impacts the Bioaccessibility of Phenolics and Fatty Acids, Sensory Properties, and in Vitro Biological Activity. Food Res. Int. 2023, 173, 113372. [Google Scholar] [CrossRef]
- Loghavi, L.; Sastry, S.K.; Yousef, A.E. Effect of Moderate Electric Field on the Metabolic Activity and Growth Kinetics of Lactobacillus acidophilus. Biotechnol. Bioeng. 2007, 98, 872–881. [Google Scholar] [CrossRef]
- Alexandre, E.M.C.; Aguiar, N.F.B.; Voss, G.B.; Pintado, M.E. Properties of Fermented Beverages from Food Wastes/By-Products. Beverages 2023, 9, 45. [Google Scholar] [CrossRef]
- Allahgholi, L.; Jönsson, M.; Christensen, M.D.; Jasilionis, A.; Nouri, M.; Lavasani, S.; Linares-Pastén, J.A.; Hreggviðsson, G.Ó.; Karlsson, E.N. Fermentation of the Brown Seaweed Alaria esculenta by a Lactic Acid Bacteria Consortium Able to Utilize Mannitol and Laminari-Oligosaccharides. Fermentation 2023, 9, 499. [Google Scholar] [CrossRef]
- Lugo, L.; del Socorro Cruz-Cansino, N.; Cervantes-Elizarrarás, A.; Delgado-Olivares, L.; Ariza-Ortega, J.A.; Zafra-Rojas, Q.Y. Bacterias Ácido Lácticas de Bebidas Vegetales Fermentadas: Producción de Metabolitos y Propiedades Antimicrobianas. Educ. Salud Bol. Cient. Inst. Cienc. Salud Univ. Autón. Estado Hidalgo 2021, 9, 175–186. [Google Scholar] [CrossRef]
- Ruíz Rodríguez, L.G.; Mendoza, L.; Van, C.; Ar, N.C.O.; Pescuma, M.; Mozzi, F. Fermentación de Jugos y Bebidas a Base de Frutas. Aliment. Ferment. Microbiol. Nutr. Salud Cult. 2020, 1, 273–306. [Google Scholar]
- Devaki, C.S.; Premavalli, K.S. Fermented Vegetable Beverages; Woodhead Publishing: Sawston, UK, 2019; ISBN 9780128152713. [Google Scholar]
- Di Cagno, R.; Coda, R.; De Angelis, M.; Gobbetti, M. Exploitation of Vegetables and Fruits through Lactic Acid Fermentation. Food Microbiol. 2013, 33, 1–10. [Google Scholar] [CrossRef]
- Garcia, C.; Guerin, M.; Souidi, K.; Remize, F. Lactic Fermented Fruit or Vegetable Juices: Past, Present and Future. Beverages 2020, 6, 8. [Google Scholar] [CrossRef]
- Laureys, D.; De Vuyst, L. Microbial Species Diversity, Community Dynamics, and Metabolite Kinetics of Water Kefir Fermentation. Appl. Environ. Microbiol. 2014, 80, 2564–2572. [Google Scholar] [CrossRef] [PubMed]
- James, A.; Yao, T.; Ke, H.; Wang, Y. Microbiota for Production of Wine with Enhanced Functional Components. Food Sci. Hum. Wellness 2023, 12, 1481–1492. [Google Scholar] [CrossRef]
- Keșa, A.-L.; Pop, C.R.; Mudura, E.; Salanță, L.C.; Pasqualone, A.; Dărab, C.; Burja-Udrea, C.; Zhao, H.; Coldea, T.E. Strategies to Improve the Potential Functionality of Fruit-Based Fermented Beverages. Plants 2021, 10, 2263. [Google Scholar] [CrossRef] [PubMed]
- Matei, F.; Kosseva, M.R. Microbiology of Fruit Wine Production; Academic Press: Cambridge, MA, USA, 2017; ISBN 9780128010341. [Google Scholar]
- Wang, Y.; Li, Y.; Sun, T.; Milne, E.; Yang, Y.; Liu, K.; Li, J.; Yan, P.; Zhao, C.; Li, S.; et al. Environmental Impact of Organic and Conventional Wine Grape Production, a Case Study from Wuwei Wine Region, Gansu Province, China. Ecol. Indic. 2023, 154, 110730. [Google Scholar] [CrossRef]
- Andreou, V.; Dimopoulos, G.; Dermesonlouoglou, E.; Taoukis, P. Application of Pulsed Electric Fields to Improve Product Yield and Waste Valorization in Industrial Tomato Processing. J. Food Eng. 2020, 270, 109778. [Google Scholar] [CrossRef]
- Paredes, J.L.; Escudero-Gilete, M.L.; Vicario, I.M. A New Functional Kefir Fermented Beverage Obtained from Fruit and Vegetable Juice: Development and Characterization. LWT 2022, 154, 112728. [Google Scholar] [CrossRef]
- Pérez-Armendáriz, B.; Cardoso-Ugarte, G.A. Traditional Fermented Beverages in Mexico: Biotechnological, Nutritional, and Functional Approaches. Food Res. Int. 2020, 136, 109307. [Google Scholar] [CrossRef]
- Basinskiene, L.; Cizeikiene, D. Cereal-Based Nonalcoholic Beverages; Academic Press: Cambridge, MA, USA, 2020; ISBN 9780128169384. [Google Scholar]
- Alemayehu, G.F.; Forsido, S.F.; Tola, Y.B.; Amare, E. Optimization of Nutritional and Sensory Properties of Fermented Oat-Based Composite Beverage. Heliyon 2022, 8, e10771. [Google Scholar] [CrossRef]
- González-Salitre, L.; Basilio-Cortés, U.A.; Rodríguez-Serrano, G.M.; Contreras-López, E.; Cardelle-Cobas, A.; González-Olivares, L.G. Physicochemical and Microbiological Parameters during the Manufacturing of a Beer-Type Fermented Beverage Using Selenized Saccharomyces boulardii. Heliyon 2023, 9, e21190. [Google Scholar] [CrossRef]
- Khayatan, D.; Nouri, K.; Momtaz, S.; Roufogalis, B.D.; Alidadi, M.; Jamialahmadi, T.; Abdolghaffari, A.H.; Sahebkar, A. Plant-Derived Fermented Products: An Interesting Concept for Human Health. Curr. Dev. Nutr. 2024, 8, 102162. [Google Scholar] [CrossRef] [PubMed]
- Leonarski, E.; Guimarães, A.C.; Cesca, K.; Poletto, P. Production Process and Characteristics of Kombucha Fermented from Alternative Raw Materials. Food Biosci. 2022, 49, 101841. [Google Scholar] [CrossRef]
- Freitas, A.; Sousa, P.; Wurlitzer, N. Alternative Raw Materials in Kombucha Production. Int. J. Gastron. Food Sci. 2022, 30, 100594. [Google Scholar] [CrossRef]
- Kaashyap, M.; Cohen, M.; Mantri, N. Microbial Diversity and Characteristics of Kombucha as Revealed by Metagenomic and Physicochemical Analysis. Nutrients 2021, 13, 4446. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, I.; de Sousa Melo, D.; Menezes, A.G.T.; Fonseca, H.C.; de Assis, B.B.T.; Ramos, C.L.; Magnani, M.; Dias, D.R.; Schwan, R.F. Evaluation of Potentially Probiotic Yeasts and Lactiplantibacillus plantarum in Co-Culture for the Elaboration of a Functional Plant-Based Fermented Beverage. Food Res. Int. 2022, 160, 111697. [Google Scholar] [CrossRef]
- Sudarsini, B.; Venkateswarulu, T.V.T.; Krupanidhi, S.; Sumalatha, B.; Indira, M. Advancing Fermented Food Products: Exploring Bioprocess Technologies and Overcoming Challenges. Food Bioprocess Technol. 2024, 17, 3461–3482. [Google Scholar] [CrossRef]
- Don, M.M.; Rambli, M.; Nore, B.F. Developing Herbal-Based Beverage Fermentation Using Saccharomyces cerevisiae: The Physico-Chemical Properties. ASEAN J. Sci. Technol. Dev. 2023, 40, 39–44. [Google Scholar] [CrossRef]
- Miranda-Mejía, G.A.; Martín del Campo-Barba, S.T.; Arredondo-Ochoa, T.; Tejada-Ortigoza, V.; la Peña, M.M.-d. Low-Intensity Pulsed Electric Fields Pre-Treatment on Yogurt Starter Culture: Effects on Fermentation Time and Quality Attributes. Innov. Food Sci. Emerg. Technol. 2024, 95, 103708. [Google Scholar] [CrossRef]
- Santos, H.C.; Leonel, G.V.F.; da Silva Ramos, L.C.; Hudson, E.A.; Pinto, M.S.; de Paula Rezende, J.; Vidigal, M.C.T.R.; Pires, A.C.d.S. Enhancing Dairy Sustainability: Rheological, Sensory, and Physical-Chemical Properties of Low-Fat Fermented Beverages Incorporating Buttermilk. J. Clean. Prod. 2024, 443, 141159. [Google Scholar] [CrossRef]
- Mazorra-Manzano, M.A.; Robles-Porchas, G.R.; González-Velázquez, D.A.; Torres-Llanez, M.J.; Martínez-Porchas, M.; García-Sifuentes, C.O.; González-Córdova, A.F.; Vallejo-Córdoba, B. Cheese Whey Fermentation by Its Native Microbiota: Proteolysis and Bioactive Peptides Release with ACE-Inhibitory Activity. Fermentation 2020, 6, 19. [Google Scholar] [CrossRef]
- Sun, H.; Chen, X.; Xiang, Y.; Hu, Q.; Zhao, L. Fermentation Characteristics and Flavor Properties of Hericium Erinaceus and Tremella Fuciformis Fermented Beverage. Food Biosci. 2022, 50, 102017. [Google Scholar] [CrossRef]
- Wang, Y.; Qu, S.; Chen, M.; Cui, Y.; Shi, C.; Pu, X.; Gao, W.; Li, Q.; Han, J.; Zhang, A. Effects of Buckwheat Milk Co-Fermented with Two Probiotics and Two Commercial Yoghurt Strains on Gut Microbiota and Production of Short-Chain Fatty Acids. Food Biosci. 2023, 53, 102537. [Google Scholar] [CrossRef]
- Monteiro, P.; Lomartire, S.; Cotas, J.; Pacheco, D.; Marques, J.C.; Pereira, L.; Gonçalves, A.M.M. Seaweeds as a Fermentation Substrate: A Challenge for the Food Processing Industry. Processes 2021, 9, 1953. [Google Scholar] [CrossRef]
- Rupert, R.; Rodrigues, K.F.; Thien, V.Y.; Yong, W.T.L. Carrageenan from Kappaphycus alvarezii (Rhodophyta, Solieriaceae): Metabolism, Structure, Production, and Application. Front. Plant Sci. 2022, 13, 859635. [Google Scholar] [CrossRef]
- Trung, V.T.; Van Huynh, T.; Thinh, P.D.; San, P.T.; Bang, T.H.; Hang, N.T. Probiotic Fermented Beverage from Macroalgae. Nat. Prod. Commun. 2021, 16, 1934578X211066145. [Google Scholar] [CrossRef]
- Admassu, H.; Gasmalla, M.A.A.; Yang, R.; Zhao, W. Identification of Bioactive Peptides with α-Amylase Inhibitory Potential from Enzymatic Protein Hydrolysates of Red Seaweed (Porphyra spp.). J. Agric. Food Chem. 2018, 66, 4872–4882. [Google Scholar] [CrossRef]
- Yue, Q.; Wang, Z.; Tang, X.; Zhao, C.; Li, K.; Su, L.; Zhang, S.; Sun, X.; Liu, X.; Zhao, L. Hypolipidemic Effects of Fermented Seaweed Extracts by Saccharomyces cerevisiae and Lactiplantibacillus plantarum. Front. Microbiol. 2021, 12, 772585. [Google Scholar] [CrossRef] [PubMed]
- Gaur, V.K.; Sharma, P.; Sirohi, R.; Varjani, S.; Taherzadeh, M.J.; Chang, J.S.; Yong Ng, H.; Wong, J.W.C.; Kim, S.H. Production of Biosurfactants from Agro-Industrial Waste and Waste Cooking Oil in a Circular Bioeconomy: An Overview. Bioresour. Technol. 2022, 343, 126059. [Google Scholar] [CrossRef]
- Cheok, C.Y.; Mohd Adzahan, N.; Abdul Rahman, R.; Zainal Abedin, N.H.; Hussain, N.; Sulaiman, R.; Chong, G.H. Current Trends of Tropical Fruit Waste Utilization. Crit. Rev. Food Sci. Nutr. 2018, 58, 335–361. [Google Scholar] [CrossRef]
- Mohd Ali, M.; Hashim, N.; Abd Aziz, S.; Lasekan, O. Pineapple (Ananas comosus): A Comprehensive Review of Nutritional Values, Volatile Compounds, Health Benefits, and Potential Food Products. Food Res. Int. 2020, 137, 109675. [Google Scholar] [CrossRef] [PubMed]
- Selvanathan, Y.; Masngut, N. Optimization of Process Factor and Characterization of Vinegar-like Beverage Production via Spontaneous Fermentation from Pineapple Peel Waste. LWT 2023, 182, 114818. [Google Scholar] [CrossRef]
- Kumar, P.; Tanwar, R.; Gupta, V.; Upadhyay, A.; Kumar, A.; Gaikwad, K.K. Pineapple Peel Extract Incorporated Poly(Vinyl Alcohol)-Corn Starch Film for Active Food Packaging: Preparation, Characterization and Antioxidant Activity. Int. J. Biol. Macromol. 2021, 187, 223–231. [Google Scholar] [CrossRef] [PubMed]
- Bamforth, C.W. Beer; Oxford University Press: New York, NY, USA, 2023; ISBN 0199996741. [Google Scholar]
- Mohd Ariff, R.; Chai, X.Y.; Chang, L.S.; Fazry, S.; Othman, B.A.; Babji, A.S.; Lim, S.J. Recent Trends in Kombucha: Conventional and Alternative Fermentation in Development of Novel Beverage. Food Biosci. 2023, 53, 102714. [Google Scholar] [CrossRef]
- Aleixandre-Tudo, J.L.; du Toit, W. Cold Maceration Application in Red Wine Production and Its Effects on Phenolic Compounds: A Review. LWT 2018, 95, 200–208. [Google Scholar] [CrossRef]
- Prezioso, I.; Fioschi, G.; Rustioni, L.; Mascellani, M.; Natrella, G.; Venerito, P.; Gambacorta, G.; Paradiso, V.M. Influence of Prolonged Maceration on Phenolic Compounds, Volatile Profile and Sensory Properties of Wines from Minutolo and Verdeca, Two Apulian White Grape Varieties. LWT 2024, 192, 115698. [Google Scholar] [CrossRef]
- Liang, S.; Granato, D.; Zou, C.; Gao, Y.; Zhu, Y.; Zhang, L.; Yin, J.F.; Zhou, W.; Xu, Y.Q. Processing Technologies for Manufacturing Tea Beverages: From Traditional to Advanced Hybrid Processes. Trends Food Sci. Technol. 2021, 118, 431–446. [Google Scholar] [CrossRef]
- Liang, S.; Gao, Y.; Fu, Y.; Chen, J.; Yin, J.; Xu, Y. Innovative Technologies in Tea-Beverage Processing for Quality Improvement. Curr. Opin. Food Sci. 2022, 47, 100870. [Google Scholar] [CrossRef]
- Roda, A.; De Faveri, D.M.; Giacosa, S.; Dordoni, R.; Lambri, M. Effect of Pre-Treatments on the Saccharification of Pineapple Waste as a Potential Source for Vinegar Production. J. Clean. Prod. 2016, 112, 4477–4484. [Google Scholar] [CrossRef]
- Romero-Rodríguez, R.; Durán-Guerrero, E.; Castro, R.; Díaz, A.B.; Lasanta, C. Evaluation of the Influence of the Microorganisms Involved in the Production of Beers on Their Sensory Characteristics. Food Bioprod. Process. 2022, 135, 33–47. [Google Scholar] [CrossRef]
- Villacreces, S.; Blanco, C.A.; Caballero, I. Developments and Characteristics of Craft Beer Production Processes. Food Biosci. 2022, 45, 101495. [Google Scholar] [CrossRef]
- Tian, S.; Zeng, W.; Fang, F.; Zhou, J.; Du, G. The Microbiome of Chinese Rice Wine (Huangjiu). Curr. Res. Food Sci. 2022, 5, 325–335. [Google Scholar] [CrossRef] [PubMed]
- Ho, C.W.; Lazim, A.M.; Fazry, S.; Zaki, U.K.H.H.; Lim, S.J. Varieties, Production, Composition and Health Benefits of Vinegars: A Review. Food Chem. 2017, 221, 1621–1630. [Google Scholar] [CrossRef]
- Abaci, N.; Senol Deniz, F.S.; Orhan, I.E. Kombucha—An Ancient Fermented Beverage with Desired Bioactivities: A Narrowed Review. Food Chem. X 2022, 14, 100302. [Google Scholar] [CrossRef]
- Cardoso, R.R.; Neto, R.O.; dos Santos D’Almeida, C.T.; do Nascimento, T.P.; Pressete, C.G.; Azevedo, L.; Martino, H.S.D.; Cameron, L.C.; Ferreira, M.S.L.; de Barros, F.A.R. Kombuchas from Green and Black Teas Have Different Phenolic Profile, Which Impacts Their Antioxidant Capacities, Antibacterial and Antiproliferative Activities. Food Res. Int. 2020, 128, 108782. [Google Scholar] [CrossRef]
- Hasegawa, Y.; Bolling, B.W. Yogurt Consumption for Improving Immune Health. Curr. Opin. Food Sci. 2023, 51, 101017. [Google Scholar] [CrossRef]
- Adebo, O.A.; Njobeh, P.B.; Adeboye, A.S.; Adebiyi, J.A.; Sobowale, S.S.; Ogundele, O.M.; Kayitesi, E. Advances in Fermentation Technology for Novel Food Products. In Innovations in Technologies for Fermented Food and Beverage Industries; Springer: Cham, Switzerland, 2018; pp. 71–87. [Google Scholar] [CrossRef]
- Vinicius De Melo Pereira, G.; De Carvalho Neto, D.P.; Junqueira, A.C.D.O.; Karp, S.G.; Letti, L.A.J.; Magalhães Júnior, A.I.; Soccol, C.R. A Review of Selection Criteria for Starter Culture Development in the Food Fermentation Industry. Food Rev. Int. 2020, 36, 135–167. [Google Scholar] [CrossRef]
- Mannaa, M.; Han, G.; Seo, Y.S.; Park, I. Evolution of Food Fermentation Processes and the Use of Multi-Omics in Deciphering the Roles of the Microbiota. Foods 2021, 10, 2861. [Google Scholar] [CrossRef]
- Roobab, U.; Abida, A.; Chacha, J.S.; Athar, A.; Madni, G.M.; Ranjha, M.M.A.N.; Rusu, A.V.; Zeng, X.A.; Aadil, R.M.; Trif, M. Applications of Innovative Non-Thermal Pulsed Electric Field Technology in Developing Safer and Healthier Fruit Juices. Molecules 2022, 27, 4031. [Google Scholar] [CrossRef] [PubMed]
- Potoroko, I.; Kalinina, I.; Botvinnikova, V.; Krasulya, O.; Fatkullin, R.; Bagale, U.; Sonawane, S.H. Ultrasound Effects Based on Simulation of Milk Processing Properties. Ultrason. Sonochem. 2018, 48, 463–472. [Google Scholar] [CrossRef]
- Aguilar, K. Evaluating Ultrasound Pre-Treatment as a Tool for Improving the Process of a Fermented Beverage Made from Pineapple by-Products. Braz. J. Food Technol. 2022, 25, e2021116. [Google Scholar] [CrossRef]
- Meena, L.; Buvaneswaran, M.; Byresh, T.S.; Sunil, C.K.; Rawson, A.; Venkatachalapathy, N. Effect of Ultrasound Treatment on White Finger Millet-Based Probiotic Beverage. Meas. Food 2023, 10, 100090. [Google Scholar] [CrossRef]
- Sirohi, R.; Negi, T.; Rawat, N.; Sagar, N.A.; Sindhu, R.; Tarafdar, A. Emerging Technologies for the Extraction of Bioactives from Mushroom Waste. J. Food Sci. Technol. 2024, 61, 1069–1082. [Google Scholar] [CrossRef] [PubMed]
- Hu, H.; Yonezawa, Y.; Matsuda, A.; Ishida, N.; Noguchi, A. Influence of Low Intensity Ultrasound and Ohmic Heating on Growth of Sake Yeast. Food Sci. Technol. Res. 2012, 18, 611–616. [Google Scholar] [CrossRef]
- Meneguzzo, F.; Zabini, F. Industrialization of Hydrodynamic Cavitation in Plant Resource Extraction. Curr. Opin. Chem. Eng. 2025, 48, 101140. [Google Scholar] [CrossRef]
- Ahmed, Z.; Uddin, N.; Latif, A.; Tufail, T.; Qayum, A.; Manzoor, M.F.; Khan, K.A.; Ashraf, J.; Khalid, N.; Xu, B. Improving the Quality and Digestibility of Wheat Flour Starch and Protein for Noodles through Ultrasound, High Hydrostatic Pressure, and Plasma Technologies: A Review. Int. J. Biol. Macromol. 2024, 282, 137383. [Google Scholar] [CrossRef] [PubMed]
- Farid, M.S.; Anjum, R.; Yang, Y.; Tu, M.; Zhang, T.; Pan, D.; Sun, Y.; Wu, Z. Recent Trends in Fermented Plant-Based Analogues and Products, Bioactive Peptides, and Novel Technologies-Assisted Fermentation. Trends Food Sci. Technol. 2024, 149, 104529. [Google Scholar] [CrossRef]
- Ntourtoglou, G.; Tsapou, E.A.; Drosou, F.; Bozinou, E.; Lalas, S.; Tataridis, P.; Dourtoglou, V. Pulsed Electric Field Extraction of α and β-Acids from Pellets of Humulus lupulus (Hop). Front. Bioeng. Biotechnol. 2020, 8, 297. [Google Scholar] [CrossRef] [PubMed]
- Martín-García, B.; Tylewicz, U.; Verardo, V.; Pasini, F.; Gómez-Caravaca, A.M.; Caboni, M.F.; Dalla Rosa, M. Pulsed Electric Field (PEF) as Pre-Treatment to Improve the Phenolic Compounds Recovery from Brewers’ Spent Grains. Innov. Food Sci. Emerg. Technol. 2020, 64, 102402. [Google Scholar] [CrossRef]
- Ozturk, E.; Alpas, H.; Arici, M. Effect of the High Hydrostatic Pressure Process on the Microbial and Physicochemical Quality of Shalgam. ACS Omega 2024, 9, 10400–10414. [Google Scholar] [CrossRef]
- Milani, E.A.; Silva, F.V.M. Pasteurization of Beer by Non-Thermal Technologies. Front. Food Sci. Technol. 2021, 1, 798676. [Google Scholar] [CrossRef]
- Speranza, B.; Campaniello, D.; Altieri, C.; Sinigaglia, M.; Bevilacqua, A.; Corbo, M.R. Increase of Acidification of Synthetic Brines by Ultrasound-Treated Lactiplantibacillus plantarum Strains Isolated from Olives. Ultrason. Sonochem. 2021, 74, 105583. [Google Scholar] [CrossRef]
- Ma, J.; Wang, Y.; Zhao, M.; Tong, P.; Lv, L.; Gao, Z.; Liu, J.; Long, F. High Hydrostatic Pressure Treatments Improved Properties of Fermentation of Apple Juice Accompanied by Higher Reserved Lactobacillus plantarum. Foods 2023, 12, 441. [Google Scholar] [CrossRef] [PubMed]
- Barukčić, I.; Lisak Jakopović, K.; Herceg, Z.; Karlović, S.; Božanić, R. Influence of High Intensity Ultrasound on Microbial Reduction, Physico-Chemical Characteristics and Fermentation of Sweet Whey. Innov. Food Sci. Emerg. Technol. 2015, 27, 94–101. [Google Scholar] [CrossRef]
- Huang, G.; Chen, S.; Tang, Y.; Dai, C.; Sun, L.; Ma, H.; He, R. Stimulation of Low Intensity Ultrasound on Fermentation of Skim Milk Medium for Yield of Yoghurt Peptides by Lactobacillus paracasei. Ultrason. Sonochem. 2019, 51, 315–324. [Google Scholar] [CrossRef]
- Shokri, S.; Shekarforoush, S.S.; Hosseinzadeh, S. Stimulatory Effects of Low Intensity Ultrasound on the Growth Kinetics and Metabolic Activity of Lactococcus lactis subsp. Lactis. Process Biochem. 2020, 89, 1–8. [Google Scholar] [CrossRef]
- Chen, H.; Xiao, G.; Xu, Y.; Yu, Y.; Wu, J.; Zou, B. High Hydrostatic Pressure and Co-Fermentation by Lactobacillus rhamnosus and Gluconacetobacter xylinus Improve Flavor of Yacon-Litchi-Longan Juice. Foods 2019, 8, 308. [Google Scholar] [CrossRef] [PubMed]
- Chanos, P.; Warncke, M.C.; Ehrmann, M.A.; Hertel, C. Application of Mild Pulsed Electric Fields on Starter Culture Accelerates Yogurt Fermentation. Eur. Food Res. Technol. 2020, 246, 621–630. [Google Scholar] [CrossRef]
- Durazzo, A.; Carocho, M.; Heleno, S.A.; Pedrosa, M.C.; Ueda, J.M.; Barros, L.; Souto, E.B.; Santini, A.; Lucarini, M. Fermented Food/Beverage and Health: Current Perspectives. Rend. Lincei 2022, 33, 729–738. [Google Scholar] [CrossRef]
- Guan, Q.; Xiong, T.; Xie, M. Influence of Probiotic Fermented Fruit and Vegetables on Human Health and the Related Industrial Development Trend. Engineering 2021, 7, 212–218. [Google Scholar] [CrossRef]
- Briguglio, G.; Costa, C.; Pollicino, M.; Giambò, F.; Catania, S.; Fenga, C. Polyphenols in Cancer Prevention: New Insights (Review). Int. J. Funct. Nutr. 2020, 1, 9. [Google Scholar] [CrossRef]
- Filosa, S.; Di Meo, F.; Crispi, S. Polyphenols-Gut Microbiota Interplay and Brain Neuromodulation. Neural Regen. Res. 2018, 13, 2055–2059. [Google Scholar] [CrossRef]
- Brüssow, H. Probiotics and Prebiotics in Clinical Tests: An Update. F1000Research 2019, 8, 1157. [Google Scholar] [CrossRef]
- Herrera-Ponce, A.L.; Salmeron-Ochoa, I.; Rodriguez-Figueroa, J.C.; Santellano-Estrada, E.; Garcia-Galicia, I.A.; Alarcon-Rojo, A.D. High-Intensity Ultrasound as Pre-Treatment in the Development of Fermented Whey and Oat Beverages: Effect on the Fermentation, Antioxidant Activity and Consumer Acceptance. J. Food Sci. Technol. 2022, 59, 796–804. [Google Scholar] [CrossRef]
- Swelam, S. Impact of High Hydrostatic Pressure on Composition and Quality of Yoghurt. J. Food Dairy Sci. 2018, 9, 31–35. [Google Scholar] [CrossRef]
- Arcena, M.R.; Leong, S.Y.; Then, S.; Hochberg, M.; Sack, M.; Mueller, G.; Sigler, J.; Kebede, B.; Silcock, P.; Oey, I. The Effect of Pulsed Electric Fields Pre-Treatment on the Volatile and Phenolic Profiles of Merlot Grape Musts at Different Winemaking Stages and the Sensory Characteristics of the Finished Wines. Innov. Food Sci. Emerg. Technol. 2021, 70, 102698. [Google Scholar] [CrossRef]
- El Darra, N.; Turk, M.F.; Ducasse, M.A.; Grimi, N.; Maroun, R.G.; Louka, N.; Vorobiev, E. Changes in Polyphenol Profiles and Color Composition of Freshly Fermented Model Wine Due to Pulsed Electric Field, Enzymes and Thermovinification Pretreatments. Food Chem. 2016, 194, 944–950. [Google Scholar] [CrossRef]
- Souza, F.E.B.; Rodrigues, S.; Fonteles, T.V. Non-Thermal Technologies in Food Fermentation: Mechanisms, Benefits, and Industrial Perspectives for Sustainable Development. Processes 2025, 13, 2988. [Google Scholar] [CrossRef]
- Yousfi, N.; Merbahi, N.; Bouajila, J.; Taillandier, P.; Debouba, M. Microbial Fermentation Assisted by Pulsed Electric Fields, Magnetic Fields and Cold Atmospheric Plasma: State of the Art. Fermentation 2025, 11, 417. [Google Scholar] [CrossRef]
- Galván-D’Alessandro, L.; Carciochi, R.A. Fermentation Assisted by Pulsed Electric Field and Ultrasound: A Review. Fermentation 2018, 4, 1. [Google Scholar] [CrossRef]
- Yu, H.; Cheng, X.; Li, H.; Du, Q.; Zeng, X.; Wu, Z.; Guo, Y.; Pan, D. Effects and Improvement Mechanisms of Ultrasonic Pretreatment on the Quality of Fermented Skim Milk. Ultrason. Sonochem. 2024, 108, 106958. [Google Scholar] [CrossRef]
- Feng, Y.; Yang, T.; Zhang, Y.; Zhang, A.; Gai, L.; Niu, D. Potential Applications of Pulsed Electric Field in the Fermented Wine Industry. Front. Nutr. 2022, 9, 1048632. [Google Scholar] [CrossRef]
- De Oliveira, P.V.; da Silva Júnior, A.H.; de Oliveira, C.R.S.; Assumpção, C.F.; Ogeda, C.H. Kombucha Benefits, Risks and Regulatory Frameworks: A Review. Food Chem. Adv. 2023, 2, 100288. [Google Scholar] [CrossRef]
- Li, M.; He, Z.; He, L.; Li, C.; Tao, H.; Ye, C.; Liu, L.; Zeng, X.; Ran, G. Effect of Fermentation Parameters on the Anthocyanin Content, Sensory Properties, and Physicochemical Parameters of Potato Blueberry Yogurt. Fermentation 2022, 8, 489. [Google Scholar] [CrossRef]
- Bebek Markovinović, A.; Stulić, V.; Putnik, P.; Janči, T.; Pavlić, B.; Milošević, S.; Herceg, Z.; Khaneghah, A.M.; Bursać Kovačević, D. Optimizing Pulsed Electric Field and High-Power Ultrasound Treatments to Preserve Anthocyanin Stability and Physicochemical Quality in Stored Strawberry Juice. Qual. Assur. Saf. Crop. Foods 2025, 17, 129–142. [Google Scholar] [CrossRef]
- Labrador Fernández, L.; Pérez-Porras, P.; Díaz-Maroto, M.C.; Gómez-Plaza, E.; Pérez-Coello, M.S.; Bautista-Ortín, A.B. The Technology of High-power Ultrasound and Its Effect on the Color and Aroma of Rosé Wines. J. Sci. Food Agric. 2023, 103, 6616–6624. [Google Scholar] [CrossRef]
- Costa, M.G.M.; Fonteles, T.V.; de Jesus, A.L.T.; Rodrigues, S. Sonicated Pineapple Juice as Substrate for L. casei Cultivation for Probiotic Beverage Development: Process Optimisation and Product Stability. Food Chem. 2013, 139, 261–266. [Google Scholar] [CrossRef] [PubMed]
- Coskun, F. A Traditional Turkish Fermented Non-Alcoholic Beverage, “Shalgam”. Beverages 2017, 3, 49. [Google Scholar] [CrossRef]
- Rios-Corripio, G.; Welti-Chanes, J.; Rodríguez-Martínez, V.; Guerrero-Beltrán, J.Á. High Hydrostatic Pressure Processing of Fresh Juice and a Fermented Beverage of Black Cherry (Prunus serotina). J. Agric. Food Res. 2024, 15, 100937. [Google Scholar] [CrossRef]
- Choi, Y.-J.; Kim, H.E.; Lee, M.J.; Park, S.J.; Park, S.H.; Lee, M.-A. Impact of High Hydrostatic Pressure and Thermal Processing on the Quality of Kimchi Juice. Int. J. Food Prop. 2025, 28, 1–15. [Google Scholar] [CrossRef]
- Türkol, M.; Yıkmış, S.; Ganimet, Ş.; Gezer, G.E.; Abdi, G.; Hussain, S.; Aadil, R.M. Optimization of Sensory Properties of Ultrasound-Treated Strawberry Vinegar. Ultrason. Sonochem. 2024, 105, 106874. [Google Scholar] [CrossRef]
- Ramaswamy, R.; Krishnan, S.B.; Leong, S.S.J. Pulsed Electric Field Technology for Recovery of Proteins from Waste Plant Resources and Deformed Mushrooms: A Review. Processes 2024, 12, 342. [Google Scholar] [CrossRef]
- Landi, G.; Benedetti, M.; Sforzini, M.; Eslami, E.; Pataro, G. Comparative Analysis of Cost, Energy Efficiency, and Environmental Impact of Pulsed Electric Fields and Conventional Thermal Treatment with Integrated Heat Recovery for Fruit Juice Pasteurization. Foods 2025, 14, 2239. [Google Scholar] [CrossRef]
- Nurjati, S.K.; Purnawan, M.A.; Stevviani, R. High-Pressure Processing (HPP) Energy Efficiency and Scalability Challenges in Ultra-Processed Meat: A Review. J. Clean Technol. 2025, 2, 45–56. [Google Scholar] [CrossRef]
- Silva, F.V.M.; Borgo, R.; Guanziroli, A.; Ricardo-da-Silva, J.M.; Aguiar-Macedo, M.; Redondo, L.M. Pilot Scale Continuous Pulsed Electric Fields Treatments for Vinification and Stabilization of Arinto and Moscatel Graúdo (Vitis vinifera L.) White Grape Varieties: Effects on Sensory and Physico-Chemical Quality of Wines. Beverages 2024, 10, 6. [Google Scholar] [CrossRef]
- Peng, K.; Koubaa, M.; Bals, O.; Vorobiev, E. Effect of Pulsed Electric Fields on the Growth and Acidification Kinetics of Lactobacillus delbrueckii Subsp. bulgaricus. Foods 2020, 9, 1146. [Google Scholar] [CrossRef]
- Perić, K.; Tomašević, M.; Ćurko, N.; Brnčić, M.; Kovačević Ganić, K. Non-Thermal Technology Approaches to Improve Extraction, Fermentation, Microbial Stability, and Aging in the Winemaking Process. Appl. Sci. 2024, 14, 6612. [Google Scholar] [CrossRef]
- Prempeh, N.Y.A.; Nunekpeku, X.; Murugesan, A.; Li, H. Ultrasound in the Food Industry: Mechanisms and Applications for Non-Invasive Texture and Quality Analysis. Foods 2025, 14, 2057. [Google Scholar] [CrossRef] [PubMed]
- Arruda, T.R.; Vieira, P.; Silva, B.M.; Freitas, T.D.; do Amaral, A.J.B.; Vieira, E.N.R.; Leite Júnior, B.R.d.C. What Are the Prospects for Ultrasound Technology in Food Processing? An Update on the Main Effects on Different Food Matrices, Drawbacks, and Applications. J. Food Process Eng. 2021, 44, e13872. [Google Scholar] [CrossRef]
- Bariya, A.R.; Rathod, N.B.; Patel, A.S.; Nayak, J.K.B.; Ranveer, R.C.; Hashem, A.; Abd_Allah, E.F.; Ozogul, F.; Jambrak, A.R.; Rocha, J.M. Recent Developments in Ultrasound Approach for Preservation of Animal Origin Foods. Ultrason. Sonochem. 2023, 101, 106676. [Google Scholar] [CrossRef]
- Sebastià, A.; Calleja-Gómez, M.; Pallarés, N.; Barba, F.J.; Berrada, H.; Ferrer, E. Impact of Combined Processes Involving Ultrasound and Pulsed Electric Fields on ENNs, and OTA Mitigation of an Orange Juice-Milk Based Beverage. Foods 2023, 12, 1582. [Google Scholar] [CrossRef]
- Kumar, A.; Hailu, G.G.; Xv, M.; Qiu, H.; Choic, S.I.; Lee, O.H.; Wang, Z.; Han, Z.; Liu, S.; Wei, S. Synergistic Integration of High-Pressure Processing, Pulsed Electric Fields, Cold Plasma, and UV Light with Bioactive Compounds for Enhanced Food Safety, Quality, and Shelf-Life: New Advances and Mechanisms. Food Humanit. 2025, 5, 100757. [Google Scholar] [CrossRef]
- Al-Sharify, Z.T.; Al-Najjar, S.Z.; Anumudu, C.K.; Hart, A.; Miri, T.; Onyeaka, H. Non-Thermal Technologies in Food Processing: Implications for Food Quality and Rheology. Appl. Sci. 2025, 15, 3049. [Google Scholar] [CrossRef]
- Singla, M.; Sit, N. Application of Ultrasound in Combination with Other Technologies in Food Processing: A Review. Ultrason. Sonochem. 2021, 73, 105506. [Google Scholar] [CrossRef]

| Assisted Technology | Substrate | Microorganism | Effect of Treatment Conditions on Microbial Behavior | Reference |
|---|---|---|---|---|
| US | Sweet whey | Strep. thermophilus, L. delbrueckii bulgaricus and L. acidophilus | Minimum content of viable cells (20 kHz, 480 W, 8 min, 55 °C) | [97] |
| Slightly increases pH, electrical conductivity, and viscosity (20 kHz, 480–600 W, 6.5–10 min, 45–55 °C) | ||||
| Maintains titratable acidity (20 kHz, 480–600 W, 6.5–10 min, 45–55 °C) | ||||
| Decreases fermentation time by 0.5 h (20 kHz, 84 W, 160 s, 55 °C) | ||||
| Milk | Lactobacillus | Increases metabolic activity and biomass (120 and 90 W/L, 3 min) | [83] | |
| Buffer solution | L. lactis | Increases viable cell count and cell permeability (24 kHz, 400 W, 30%, 5 min) | [99] | |
| Increases β-galactosidase activity (24 kHz, 400 W, 30%, 5 min) | ||||
| Increases lactic acid yield (24 kHz, 400 W, 20%, 5 min) | ||||
| Decreases protein concentration (24 kHz, 400 W, 30%, 5 min) | ||||
| Buffer solution | L. brevis | Increases viable cell count (23 kHz, 150 W, 10 μm, 5 min, 30 °C) | [7] | |
| Increases cell permeability and proteolysis (24 kHz, 150 W, 15 μm, 5 min, 30 °C) | ||||
| Increases acidity of the medium (23 kHz, 150 W, 10 μm, 5 min, 30 °C) | ||||
| Increase γ-aminobutyric acid production (23 kHz, 150 W, 10 μm, 5 min, 30 °C) | ||||
| Decreases pH of the medium (23 kHz, 150 W, 10 μm, 5 min, 30 °C) | ||||
| Pineapple beverage by-product | S. cerevisiae | Favors the releasing of ethanol (25 kHz, 20–100%, 5–10 min) | [84] | |
| Dairy | S. cerevisiae | Increases ethanol yield (28 kHz, 35 W/L, 1 h, first day) | [5] | |
| Decreases fermentation time (28 kHz, 35 W/L, 1 h, first day) | ||||
| White millet drink | L. rhamnosus | Increases viable cells count (20 kHz, 3 W/L, 40.11%, 11.09 min) | [85] | |
| Decreases fermentation time (20 kHz, 0.83 W/L, 41.42%, 2.63 min) | ||||
| HHP | Yacon, lychee and longan juice | L. rhamnosus and G. xylinus | Glucose, fructose, and sucrose content was partially reduced (300–500 MPa, 25 °C, 15 min) | [100] |
| Apple juice | L. plantarum | Survival of L. plantarum in simulated gastric fluid reached 97.37% after fermentation. | [96] | |
| After 24 h, caffeic, ferric, and chlorogenic acid levels increase (200–400 MPa, 10 min) | ||||
| PEF | Buffer solution | L. acidophilus | Higher bacteriocin formation (1 V/cm, 60 Hz, first 5 h, 30 °C) | [24] |
| Higher biomass production (1 V/cm, 60 Hz, 2 min on/off, 37 °C) | ||||
| Apple juice | Hanseniaspora spp. | Increases the biomass concentration by around 25% (285 V/cm, 10 pulses each 100 μs, Δt = 1 ms, Δtt = 1 s, during and after fermentation) | [22] | |
| Decreases ethanol content by 1.6% (285 V/cm, 10 pulses each 100 μs, Δt = 1 ms, Δtt = 1 s, during log phase) | ||||
| Yogurt | Strep. thermophilus and L. bulgaricus | 15.4% of the initial inoculum of Strep. thermophilus and 24.3% of that of L. bulgaricus survived (1–3.67 kV/cm, 0.5–4 Hz, 5–50 pulses) | [101] | |
| Yogurt | Strep. thermophilus and L. delbrueckii bulgaricus | Shortest fermentation time (1 kV/cm, 150 Hz, 8 μs, 400 μs, 3.8 J) | [50] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pizarro-Oteíza, S.; Salazar, F.; Cea, R.; Cavieres, O.; Meenu, M. Impact of High Hydrostatic Pressure, Ultrasound, and Pulsed Electric Field in Beverages Fermentation: A Review of Nutritional, Functional, and Sensory Aspects and the Future. Foods 2025, 14, 3576. https://doi.org/10.3390/foods14203576
Pizarro-Oteíza S, Salazar F, Cea R, Cavieres O, Meenu M. Impact of High Hydrostatic Pressure, Ultrasound, and Pulsed Electric Field in Beverages Fermentation: A Review of Nutritional, Functional, and Sensory Aspects and the Future. Foods. 2025; 14(20):3576. https://doi.org/10.3390/foods14203576
Chicago/Turabian StylePizarro-Oteíza, Sebastián, Fernando Salazar, Romina Cea, Oscar Cavieres, and Maninder Meenu. 2025. "Impact of High Hydrostatic Pressure, Ultrasound, and Pulsed Electric Field in Beverages Fermentation: A Review of Nutritional, Functional, and Sensory Aspects and the Future" Foods 14, no. 20: 3576. https://doi.org/10.3390/foods14203576
APA StylePizarro-Oteíza, S., Salazar, F., Cea, R., Cavieres, O., & Meenu, M. (2025). Impact of High Hydrostatic Pressure, Ultrasound, and Pulsed Electric Field in Beverages Fermentation: A Review of Nutritional, Functional, and Sensory Aspects and the Future. Foods, 14(20), 3576. https://doi.org/10.3390/foods14203576





