Assessment of the Impact of Metals in Wild Edible Mushrooms from Dambovita County, Romania, on Human Health
Abstract
1. Introduction
2. Materials and Methods
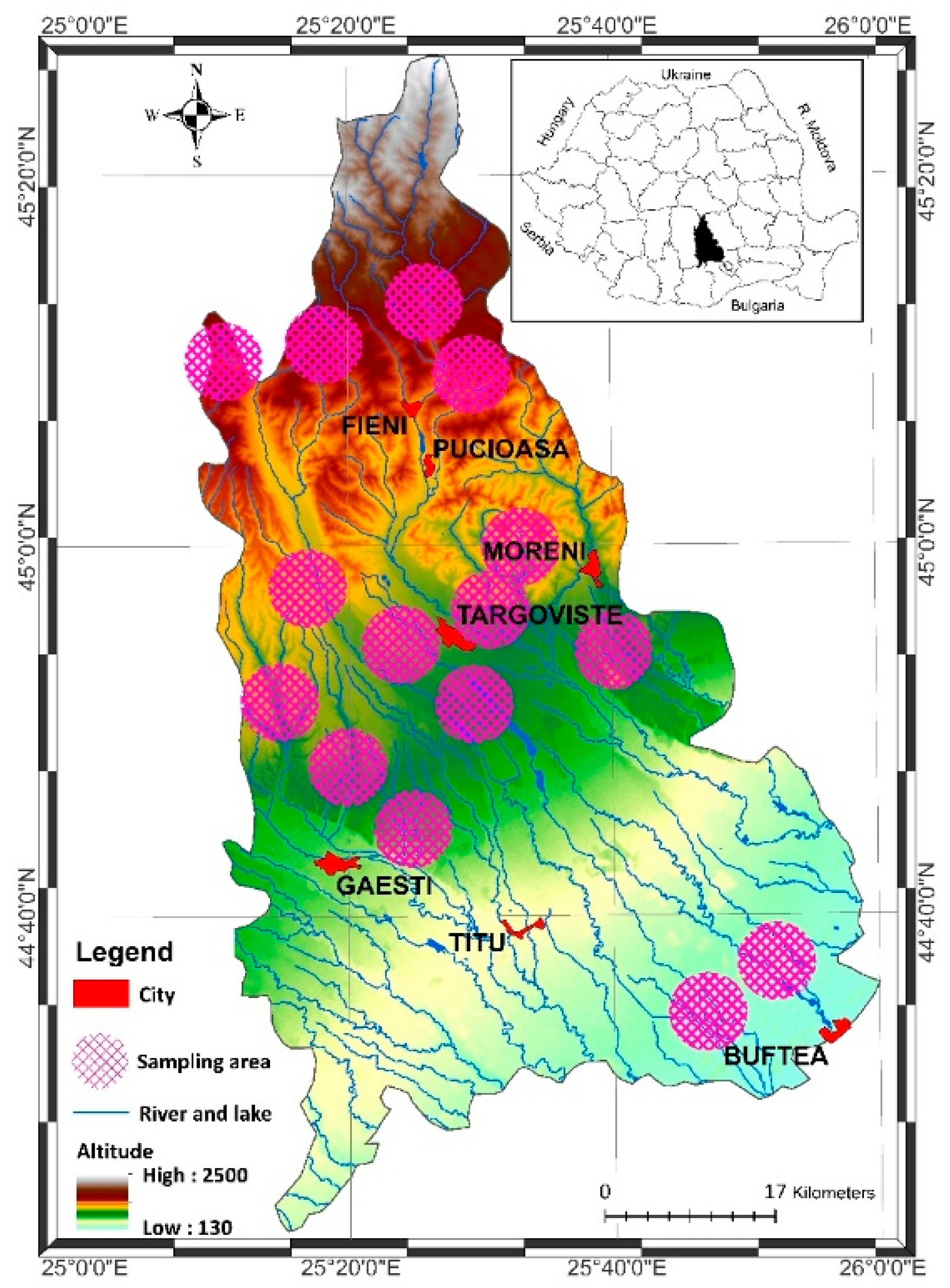
2.1. Study Area
2.2. Sampling and Samples Preparation
2.3. Energy-Dispersive X-Ray Fluorescence Quantitative Analysis with Fundamental Parameters Method (EDXRF-FP)
2.4. Human Health Risk Assessment
3. Results and Discussion
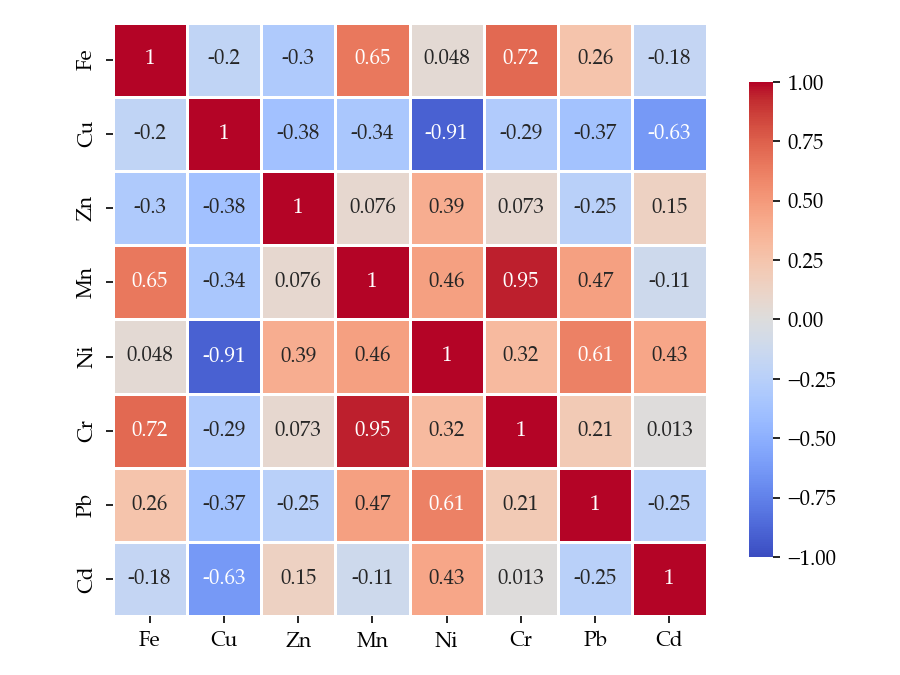
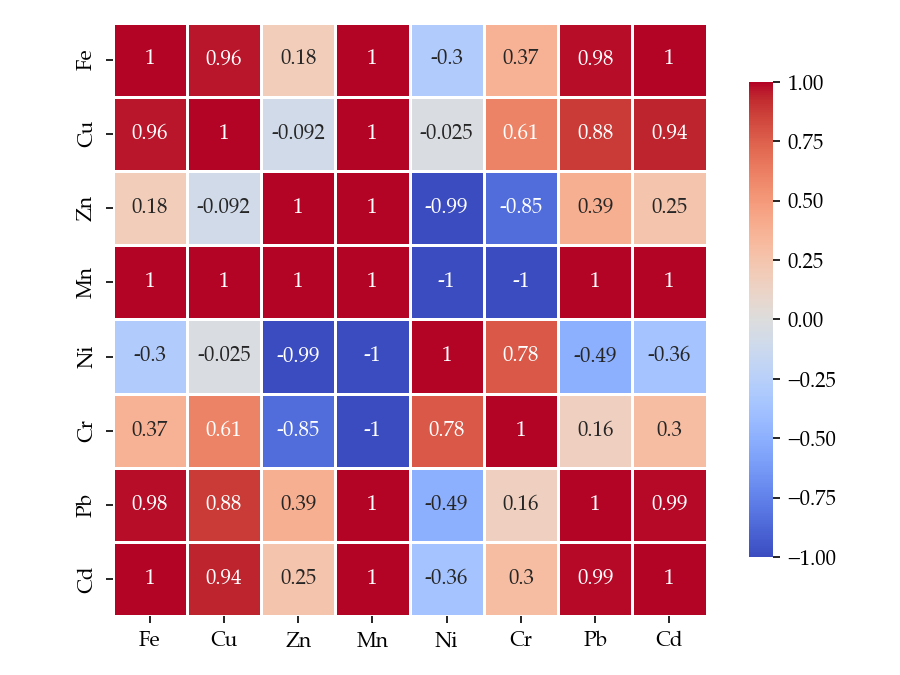
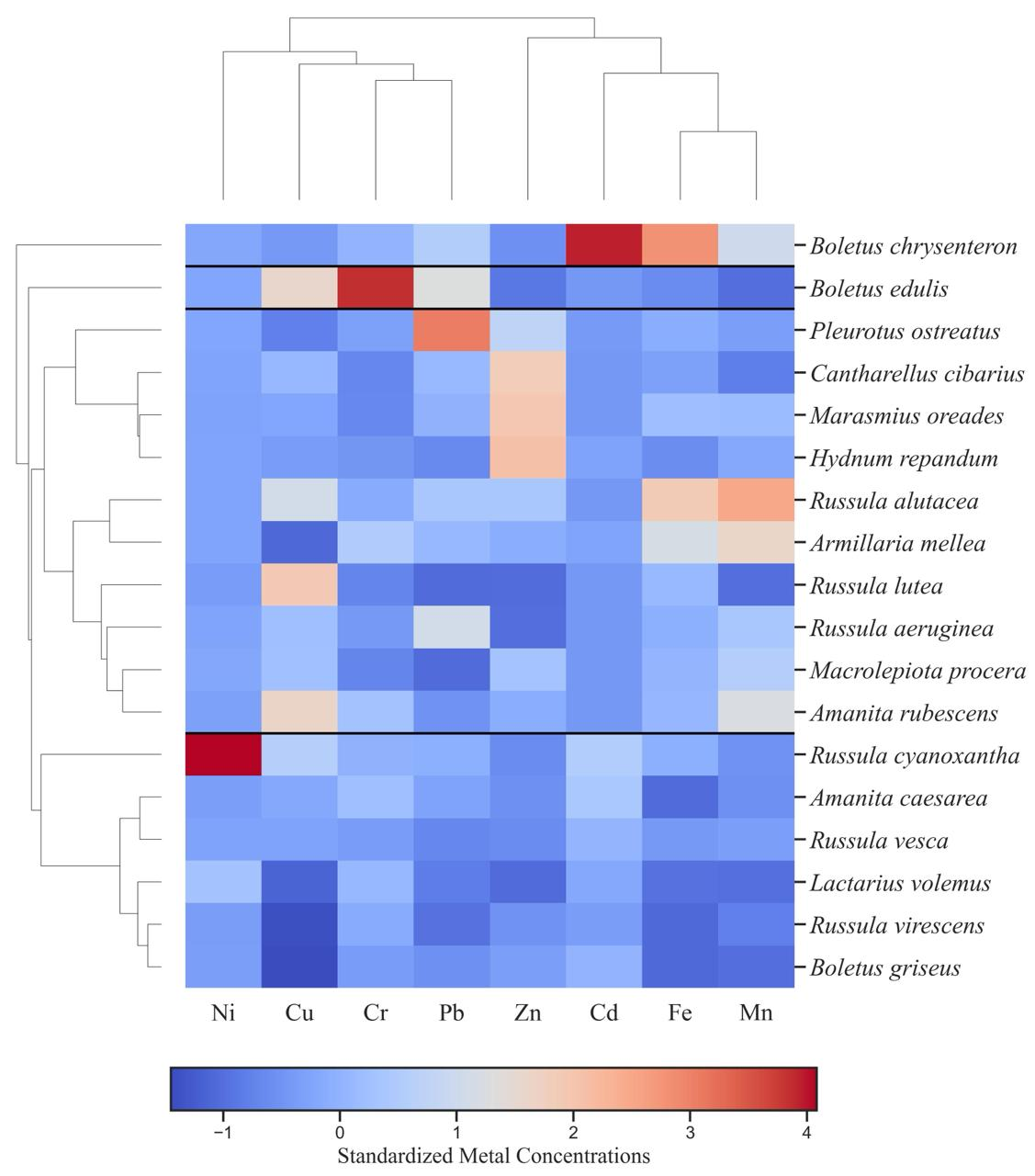
3.1. Metal Content of Studied Wild Mushroom Samples
3.2. The Daily Metal Intake Estimated
3.3. Non-Carcinogenic Risk Assessment
3.4. Carcinogenic Risk Assessment
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| EDXRF-FP | Energy Dispersive X-Ray Fluorescence Quantitative Analysis with Fundamental Parameters |
| NIST | National Institute of Standards and Technology |
| USEPA | United States Environmental Protection Agency |
| EPA | Environmental Protection Agency |
| HQ | Hazard quotients |
| HI | Hazard indices |
| EDI | Estimated daily intake |
| RfD | Reference dose |
| ILCR | Incremental lifetime cancer risk |
| ADI | Average daily intake |
| CSF | Cancer slope factor |
| DRI | Dietary reference intake |
| DMI | Daily metal intake |
References
- Losoya-Sifuentes, C.; Loredo-Treviño, A.; Belmares, R.; Cruz, M.; Refugio Rocha-Pizaña, M. Edible Mushrooms: A Nutrient-Rich Ingredient for Healthier Food Products—A Review. Curr. Nutr. Rep. 2025, 14, 9. [Google Scholar] [CrossRef] [PubMed]
- Krishnamoorthi, R.; Srinivash, M.; Mahalingam, P.U.; Malaikozhundan, B. Dietary nutrients in edible mushroom, Agaricus Bisporus and their radical scavenging, antibacterial, and antifungal efects. Process Biochem. 2022, 121, 10–17. [Google Scholar] [CrossRef]
- Gernand, A.D.; Schulze, K.J.; Stewart, C.P.; West, K.P.; Christian, P. Micronutrient deficiencies in pregnancy worldwide: Health efects and prevention. Nat. Rev. Endocrinol. 2016, 12, 274–289. [Google Scholar] [CrossRef]
- Mattila, P.; Könkö, K.; Eurola, M.; Pihlava, J.M.; Astola, J.; Vahteristo, L.; Hietaniemi, V.; Kumpulainen, J.; Valtonen, M.; Piironen, V. Contents of vitamins, mineral elements, and some phenolic compounds in cultivated mushrooms. J. Agric. Food Chem. 2001, 49, 2343–2348. [Google Scholar] [CrossRef]
- Yang, Y.; Zhu, D.; Qi, R.; Chen, Y.; Sheng, B.; Zhang, X. Association between Intake of Edible Mushrooms and Algae and the Risk of Cognitive Impairment in Chinese Older Adults. Nutrients 2024, 16, 637. [Google Scholar] [CrossRef] [PubMed]
- Jo Feeney, M.; Miller, A.M.; Roupas, P. Mushrooms-Biologically Distinct and Nutritionally Unique: Exploring a “Third Food Kingdom”. Nutr. Today 2014, 49, 301–307. [Google Scholar] [CrossRef]
- Fulgoni, V.L.; Agarwal, S. Nutritional impact of adding a serving of mushrooms on usual intakes and nutrient adequacy using National Health and Nutrition Examination Survey 2011–2016 data. Food Sci. Nutr. 2021, 9, 1504–1511. [Google Scholar] [CrossRef]
- Cha, S.; Bell, L.; Williams, C.M. The Relationship between Mushroom Intake and Cognitive Performance: An Epidemiological Study in the European Investigation of Cancer-Norfolk Cohort (EPIC-Norfolk). Nutrients 2024, 16, 353. [Google Scholar] [CrossRef]
- Zhang, X.; Huang, F.; Zhang, J.; Wei, Y.; Bai, J.; Wang, H.; Jia, X. Association between Micronutrient-Related Dietary Pattern and Cognitive Function among Persons 55 Years and Older in China: A Longitudinal Study. Nutrients 2023, 15, 481. [Google Scholar] [CrossRef]
- Gou, R.; Qin, J.; Pang, W.; Cai, J.; Luo, T.; He, K.; Xiao, S.; Tang, X.; Zhang, Z.; Li, Y. Correlation between dietary patterns and cognitive function in older Chinese adults: A representative cross-sectional study. Front. Nutr. 2023, 10, 1093456. [Google Scholar] [CrossRef] [PubMed]
- Solfrizzi, V.; Custodero, C.; Lozupone, M.; Imbimbo, B.P.; Valiani, V.; Agosti, P.; Schilardi, A.; D’Introno, A.; La Montagna, M.; Calvani, M.; et al. Relationships of Dietary Patterns, Foods, and Micro- and Macronutrients with Alzheimer’s Disease and Late-Life Cognitive Disorders: A Systematic Review. J. Alzheimers Dis. 2017, 59, 815–849. [Google Scholar] [CrossRef]
- Yao, Y.; Chen, H.; Chen, L.; Ju, S.-Y.; Yang, H.; Zeng, Y.; Gu, D.; Ng, T.P. Type of tea consumption and depressive symptoms in Chinese older adults. BMC Geriatr. 2021, 21, 331. [Google Scholar] [CrossRef]
- Aoki, S.; Yamagishi, K.; Maruyama, K. Mushroom intake and risk of incident disabling dementia: The Circulatory Risk in Communities Study (CIRCS). Br. J. Nutr. 2024, 131, 1641–1647. [Google Scholar] [CrossRef]
- Silvestri, L.; Pettinato, M.; Furiosi, V.; Bavuso Volpe, L.; Nai, A.; Pagani, A. Managing the dual nature of iron to preserve health. Int. J. Mol. Sci. 2023, 24, 3995. [Google Scholar] [CrossRef]
- Rolić, T.; Yazdani, M.; Mandić, S.; Distante, S. Iron Metabolism, Calcium, Magnesium and Trace Elements: A Review. Biol. Trace Elem. Res. 2025, 203, 2216–2225. [Google Scholar] [CrossRef]
- Weiland, J.L.; Sherrow, L.K.; Jayant, D.A.; Katz, K.D. Chemical Hand Warmer Packet Ingestion: A Case of Elemental Iron Exposure. Wilderness Environ. Med. 2017, 28, 246–248. [Google Scholar] [CrossRef] [PubMed]
- Sousa, C.; Vinha, A.F.; Moutinho, C.; Matos, C. Trace minerals in human health: Iron, zinc, copper, manganese and fluorine. IJSRM Human. 2019, 13, 57–80. [Google Scholar]
- Oros, V. Contribuții la Protecția și Ingineria Mediului cu Aplicații în Zonele Miniere Afectate de Scurgeri Acide. Teza de abilitare, Universitatea Tehnica din Cluj-Napoca, Cluj-Napoca, Romania. 2015. Available online: https://www.utcluj.ro/media/documents/2015/Teza_abilitare_Vasile_Oros.pdf (accessed on 1 August 2025).
- Nezami, H.; Kooshki, A.; Esmaily, H.; Sanjari, M.; Ahmadi, Z.; Sadeghi, M.; Mirzaei, S.M.M. Cerebrovascular accident and essential and toxic metals: Cluster analysis and principal component analysis. BMC Pharmacol. Toxicol. 2025, 26. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Li, W.; Wang, Y.; Wang, T.; Ma, M.; Tian, C. Association between the change of serum copper and ischemic stroke: A systematic review and meta-analysis. J. Mol. Neurosci. 2020, 70, 475–480. [Google Scholar] [CrossRef] [PubMed]
- Briffa, J.; Sinagra, E.; Blundell, R. Heavy metal pollution in the environment and their toxicological effects on humans. Heliyon 2020, 6, e04691. [Google Scholar] [CrossRef]
- Masindi, V.; Muedi, K.L. Environmental contamination by heavy metals. In Heavy Metals; InTech: London, UK, 2018. [Google Scholar]
- Walker, C.H.; Sibly, R.M.; Hopkin, S.P.; Peakal, D.B. Principles of Ecotoxicology, 4th ed.; Taylor&Francis Group, Ed.; CRC Press: Boca Raton, FL, USA, 2012. [Google Scholar]
- Gautam, P.K.; Gautam, R.K.; Chattopadhyaya, M.C.; Banerjee, S.; Pandey, J.D. Heavy metals in the environment: Fate, transport, toxicity and remediation technologies. Nova Sci. Publ. 2016, 60, 101–130. [Google Scholar]
- Tchounwou, P.B.; Yedjou, C.G.; Patlolla, A.K.; Sutton, D.J. Heavy metal toxicity and the environment. EXS 2012, 101, 133–164. [Google Scholar] [PubMed]
- Duffus, J.H. Heavy metals a meaningless term? Pure Appl. Chem. 2002, 74, 793–807. [Google Scholar] [CrossRef]
- Wang, L.K. Heavy Metals in the Environment; CRC Press: Boca Raton, FL, USA, 2009. [Google Scholar]
- Jat Baloch, M.Y.; Su, C.; Talpur, S.A.; Iqbal, J.; Bajwa, K. Arsenic removal from groundwater using Iron pyrite: Influence factors and removal mechanism. J. Earth Sci. 2023, 34, 857–867. [Google Scholar] [CrossRef]
- Talpur, S.A.; Baloch, M.Y.J.; Su, C.; Iqbal, J.; Ahmed, A.; Talpur, H.A. Application of synthetic Iron Oxyhydroxide with influencing factors for removal of As(V) and As(III) from groundwater. J. Earth Sci. 2024, 35, 998–1009. [Google Scholar] [CrossRef]
- Talpur, S.A.; Cinosi, A.; Stoppa, F.; Talpur, H.A.; Novembre, D.; Rosatelli, G. Heavy metals pollution of Pescara River (southern Italy): Risk assessment based on total reflection X-ray fluorescence analyses. Mar. Pollut. Bull. 2025, 211, 117397. [Google Scholar] [CrossRef] [PubMed]
- Lopez, A.R.; Elena Ortega-Caneda, E.; Espada-Bellido, E.; Spanu, D.; Zava, M.; Monticelli, D. Decoding trace element speciation in mushrooms: Analytical techniques, comprehensive data review, and health implications. Food Chem. 2025, 463, 141460. [Google Scholar] [CrossRef]
- Zavastin, D.E.; Biliuta, G.; Dodi, G.; Macsim, A.M.; Lisa, G.; Gherman, S.P.; Breaban, I.G.; Miron, A.; Coseri, S. Metal content and crude polysaccharide characterization of selected mushrooms growing in Romania. J. Food Compos. Anal. 2018, 67, 149–158. [Google Scholar] [CrossRef]
- Sun, L.; Chang, W.; Bao, C.; Zhuang, Y. Metal Contents, Bioaccumulation, and Health Risk Assessment in Wild Edible Boletaceae Mushrooms. J. Food Sci. 2017, 82, 1500–1508. [Google Scholar] [CrossRef]
- Liu, S.; Fu, Y.; Shi, M.; Wan, H.; Guo, J. Pollution level and risk assessment of lead, cadmium, mercury, and arsenic in edible mushrooms from Jilin Province, China. J. Food Sci. 2021, 86, 3374–3383. [Google Scholar] [CrossRef]
- Kalač, P. Trace element contents in European species of wild growing edible mushrooms: A review for the period 2000–2009. Food Chem. 2010, 122, 2–15. [Google Scholar] [CrossRef]
- Senila, M.; Senila, L.; Resz, M.A. Chemical composition and nutritional characteristics of popular wild edible mushroom species collected from North-Western Romania. J. Food Compos. Anal. 2024, 134, 106504. [Google Scholar] [CrossRef]
- Vamanu, E. Bioactive capacity of some Romanian wild edible mushrooms consumed mainly by local communities. Nat. Prod. Res. 2017, 32, 440–443. [Google Scholar] [CrossRef] [PubMed]
- Vasile, D.; Dinca, L.; Enescu, C.M. Impact of collecting mushrooms from the spontaneous flora on forest ecosystems in Romania. AgroLife Sci. J. 2017, 6, 268–275. Available online: https://agrolifejournal.usamv.ro/index.php/agrolife/article/view/180 (accessed on 13 August 2025).
- Enescu, C.M. What is the potential for collecting and marketing of non-timber forest products in Horezu (Valcea County)? Sci. Pap. Ser. Manag. Econ. Eng. Agruculture Rural. Dev. 2022, 22, 217–222. Available online: https://managementjournal.usamv.ro/index.php/scientific-papers/2767-what-is-the-potential-for-collecting-and-marketing-of-non-timber-forest-products-in-horezu-valcea-county (accessed on 13 August 2025).
- Radulescu, C.; Stihi, C.; Busuioc, G.; Gheboianu, A.I.; Popescu, I.V. Studies concerning heavy metals bioaccumulation of wild edible mushrooms from industrial area by using spectrometric techniques. Bull. Environ. Contam. Toxicol. 2010, 84, 641–646. [Google Scholar] [CrossRef]
- Buruleanu, L.; Radulescu, C.; Georgescu, A.A.; Nicolescu, M.C.; Olteanu, R.L.; Dulama, I.D.; Stanescu, G.S. Chemometric Assessment of the Interactions between the Metal Contents, Antioxidant Activity, Total Phenolics, and Flavonoids in Mushrooms. Anal. Lett. 2019, 52, 1195–1214. [Google Scholar] [CrossRef]
- Stihi, C.; Radulescu, C.; Busuioc, G.; Popescu, I.V.; Gheboianu, A.; Ene, A. Studies on Accumulation of Heavy Metals from Substrate to Edible Wild Mushrooms. Rom. J. Phys. 2011, 56, 257–264. [Google Scholar]
- Busuioc, G.; Elkes, C.C.; Stihi, C.; Iordache, S.; Ciulei, S.C. The bioaccumulation and translocation of Fe, Zn, and Cu in species of mushrooms from Russula genus. Environ. Pollut. Res. 2011, 18, 890–896. [Google Scholar] [CrossRef]
- Senila, M. Metal and metalloid monitoring in water by passive sampling—A review. Rev. Anal. Chem. 2023, 42, 20230065. [Google Scholar] [CrossRef]
- Bucurica, I.A.; Dulama, I.D.; Radulescu, C.; Banica, A.L.; Stanescu, S.G. Heavy Metals and Associated Risks of Wild Edible Mushrooms Consumption: Transfer Factor, Carcinogenic Risk, and Health Risk Index. J. Fungi 2024, 10, 844. [Google Scholar] [CrossRef]
- Fogarasi, M.; Diaconeasa, Z.M.; Pop, C.R.; Fogarasi, S.; Semeniuc, C.A.; Fărcaş, A.C.; Țibulcă, D.; Sălăgean, C.-D.; Tofană, M.; Socaci, S.A. Elemental Composition, Antioxidant and Antibacterial Properties of Some Wild Edible Mushrooms from Romania. Agronomy 2020, 10, 1972. [Google Scholar] [CrossRef]
- Bielli, E. Ciuperci: Cunoaşterea, Recunoaşterea şi Căutarea Celor Mai Cunoscute Specii de Ciuperci; All Educational: Bucuresti, Romania, 1999. [Google Scholar]
- Jenkins, R. X-Ray Fluorescence Spectrometry; Wiley: Hoboken, NJ, USA, 1998. [Google Scholar]
- USEPA. Risk Assessment Guidance for Superfund Volume I Human Health Evaluation Manual (Part A); USEPA: Washington, DC, USA, 1989. Available online: https://www.epa.gov/sites/default/files/2015-09/documents/rags_a.pdf (accessed on 1 August 2025).
- Ravanipour, M.; Nabipour, I.; Yunesian, M.; Rastkari, N.; Mahvi, A.H. Exposure sources of polychlorinated biphenyls (PCBs) and health risk assessment: A systematic review in Iran. Environ. Sci. Pollut. Res. 2022, 29, 55437–55456. [Google Scholar] [CrossRef] [PubMed]
- Wu, B.; Zhao, D.Y.; Jia, H.Y.; Zhang, Y.; Zhang, X.X.; Cheng, S.P. Preliminary risk assessment of trace metal pollution in surface water from Yangtze River in Nanjing Section, China. Bull. Environ. Contam. Toxicol. 2009, 82, 405–409. [Google Scholar] [CrossRef] [PubMed]
- Hubal, E.A.C.; de Wet, T.; Du Toit, L.; Firestone, M.P.; Ruchirawat, M.; van Engelen, J.; Vickers, C. Identifying important life stages for monitoring and assessing risks from exposures to environmental contaminants: Results of a World Health Organization review. Regul. Toxicol. Pharmacol. 2014, 69, 113–124. [Google Scholar] [CrossRef]
- Okumuş, E.; Canbolat, F.; Acar, I. Evaluation of antioxidant activity, anti-lipid peroxidation effect and elemental impurity risk of some wild Agaricus species mushrooms. BMC Plant Biol. 2025, 25, 476. [Google Scholar] [CrossRef]
- Sarikurkcu, C.; Tepe, B.; Kocak, M.S.; Uren, M.C. Metal concentration and antioxidant activity of edible mushrooms from Turkey. Food Chem. 2015, 175, 549–555. [Google Scholar] [CrossRef]
- Sarikurkcu, C.; Popovic-Djordjevi, J.; Solak, M.H. Wild edible mushrooms from Mediterranean region: Metal concentrations and health risk assessment. Ecotoxicol. Environ. Saf. 2020, 190, 110058. [Google Scholar] [CrossRef]
- Fu, Z.; Liu, G.; Wang, L. Assessment of potential human health risk of trace element in wild edible mushroom species collected from Yunnan Province, China. Environ. Sci. Pollut. Res. 2020, 27, 29218–29227. [Google Scholar] [CrossRef]
- Canbolat, F. Analysis of non-carcinogenic health risk assessment of elemental impurities in vitamin C supplements. Iran. J. Basic Med. Sci. 2023, 26, 216–227. [Google Scholar] [CrossRef]
- Hashempour-Baltork, F.; Jannat, B.; Tajdar-Oranj, B.; Aminzare, M.; Sahebi, H. A comprehensive systematic review and health risk assessment of potentially toxic element intakes via fish consumption in Iran. Ecotoxicol. Environ. Saf. 2023, 249, 114349. [Google Scholar] [CrossRef]
- Haj Heidary, R.; Golzan, S.A.; Mirza Alizadeh, A.; Hamedi, H.; Ataee, M. Probabilistic health risk assessment of potentially toxic elements in the traditional and industrial Olive products. Environ. Sci. Pollut. Res. 2023, 30, 10213–10225. [Google Scholar] [CrossRef]
- Ebrahimzadeh, G.; Alimohammadi, M.; Rezaei Kahkha, M.R.; Mahvi, A.H. Contamination level and human non-carcinogenic risk assessment of diazinon pesticide residue in drinking water resources—A case study, IRAN. Int. J. Environ. Anal. Chem. 2020, 102, 4726–4737. [Google Scholar] [CrossRef]
- USEPA. Chapter 7: Characterizing Risk and Hazard; U.S. EPA Region 6: Dallas, TX, USA, 2005. Available online: https://archive.epa.gov/epawaste/hazard/tsd/td/web/pdf/05hhrap7.pdf (accessed on 1 August 2025).
- Dumitrescu, C.; Stihi, C.; Costinel, D.; Geana, E.I.; Ciucure, C.T.; Popescu, D.I.; Tanislav, D.; Bretcan, P. Assessment of Pesticide Contamination of Groundwater from Titu-Sarata Plain, Romania. Appl. Sci. 2025, 15, 5880. [Google Scholar] [CrossRef]
- Bamuwamye, M.; Ogwok, P.; Tumuhairwe, V. Cancer and non-cancer risks associated with heavy metal exposures from street foods: Evaluation of roasted meats in an urban setting. J. Environ. Pollut. Hum. Health 2015, 3, 24–30. [Google Scholar]
- Huang, M.; Zhou, S.; Sun, B.; Zhao, Q. Heavy metals in wheat grain: Assessment of potential health risk for inhabitants in Kunshan. China. Sci. Total Environ. 2008, 405, 54–61. [Google Scholar] [CrossRef] [PubMed]
- Meftaul, I.M.; Venkateswarlu, K.; Annamalai, P.; Parven, A.; Megharaj, M. Degradation of four pesticides in five urban landscape soils: Human and environmental health risk assessment. Environ. Geochem. Health 2022, 45, 1–16. [Google Scholar]
- Guerra, F.; Trevizam, A.R.; Muraoka, T.; Marcante, N.C.; Canniatti-Brazaca, S.G. Heavy metals in vegetables and potential risk for human health. Sci. Agric. 2012, 69, 54–60. [Google Scholar] [CrossRef]
- Kalantary, R.R.; Barzegar, G.; Jorfi, S. Monitoring of pesticides in surface water, pesticides removal efficiency in drinking water treatment plant and potential health risk to consumers using Monte Carlo simulation in Behbahan City. Iran. Chemosphere 2022, 286, 131667. [Google Scholar] [CrossRef]
- Sultana, M.S.; Rana, S.; Yamazaki, S.; Aono, T.; Yoshida, S. Health risk assessment for carcinogenic and non-carcinogenic heavy metal exposures from vegetables and fruits of Bangladesh. Cogent Environ. Sci. 2017, 3, 1291107. [Google Scholar] [CrossRef]
- Official Journal of the European Union. Commission Regulation (EU) No. 915/2023; Official Journal of the European Union: Luxembourg, 2023; Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:32023R0915 (accessed on 1 August 2025).
- Murtagh, F.; Legendre, P. Ward’s hierarchical agglomerative clustering method: Which algorithms implement Ward’s criterion? J. Classif. 2014, 31, 274–295. [Google Scholar] [CrossRef]
- National Institutes of Health. Office of Dietary Supplements. Nutrient Recommendations and Databases. Available online: https://ods.od.nih.gov/HealthInformation/nutrientrecommendations.aspx (accessed on 1 August 2025).
- Dietary Reference Intakes (DRIs): Recommended Dietary Allowances and Adequate Intakes, Elements. Food and Nutrition Board, National Academies. Available online: https://www.ncbi.nlm.nih.gov/books/NBK545442/table/appJ_tab3/?report=objectonly (accessed on 1 August 2025).
- Falandysz, J.; Saba, M.; Rutkowska, M.; Konieczka, P. Total mercury and methylmercury (MeHg) in braised and crude boletus edulis carpophores during various developmental stages. Environ. Sci. Pollut. Res. 2022, 29, 3107–3115. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Chen, S.; Li, Q.; Liu, L. Changes in arsenic speciation in wild edible Fungi after different cooking processes and gastrointestinal digestion. Molecules 2023, 28, 603. [Google Scholar] [CrossRef] [PubMed]
- Atique Ullah, A.K.M.; Maksud, M.A.; Khan, S.R.; Lutfa, L.N.; Quraishi, S.B. Dietary intake of heavy metals from eight highly consumed species of cultured fish and possible human health risk implications in Bangladesh. Toxicol. Rep. 2017, 4, 574–579. [Google Scholar] [CrossRef] [PubMed]
- Likuku, A.S.; Obuseng, G. Health Risk Assessment of Heavy Metals via Dietary Intake of Vegetables Irrigated with Treated Wastewater around Gaborone, Botswana. In Proceedings of the International Conference on Plant, Marine and Environmental Sciences (PMES-2015), Kuala Lumpur, Malaysia, 1–2 January 2015. [Google Scholar]
- US Environmental Protection Agency. Integrated Risk Information System (IRIS), Manganese; CASRN 7439-96-5|DTXSID2024169. Available online: https://iris.epa.gov/ChemicalLanding/&substance_nmbr=373 (accessed on 1 August 2025).
- US Environmental Protection Agency. Integrated Risk Information System (IRIS), Nickel, Soluble Salts; CASRN Various. Available online: https://cfpub.epa.gov/ncea/iris/iris_documents/documents/subst/0271_summary.pdf (accessed on 1 August 2025).
- US Environmental Protection Agency. Integrated Risk Information System (IRIS), Chromium (VI); CASRN 18540-29-9. Available online: https://cfpub.epa.gov/ncea/iris/iris_documents/documents/subst/0144_summary.pdf (accessed on 1 August 2025).
- US Environmental Protection Agency. Integrated Risk Information System (IRIS), Chromium (III), Insoluble Salts; CASRN 16065-83-1. Available online: https://cfpub.epa.gov/ncea/iris/iris_documents/documents/subst/0028_summary.pdf (accessed on 1 August 2025).
- US Environmental Protection Agency. Integrated Risk Information System (IRIS), Lead and Compounds (Inorganic); CASRN 7439-92-1. Available online: https://cfpub.epa.gov/ncea/iris/iris_documents/documents/subst/0277_summary.pdf (accessed on 1 August 2025).
- Nowakowski, P.; Markiewicz-Żukowska, R.; Soroczyńska, J.; Puścion-Jakubik, A.; Mielcarek, K. Evaluation of toxic element content and health risk assessment of edible wild mushrooms. J. Food Compos. Anal. 2021, 96, 103698. [Google Scholar] [CrossRef]

| Sample ID | Taxonomic Family | Species | Associated Habitat/Topography | Vernacular Name (Romanian/ English) | Growth Period |
|---|---|---|---|---|---|
| Highly Edible | |||||
| WM1 | Russulaceae | Russula virescens | Beech and mixed forests/hills, mountains | Hulubite/ Dove Mushroom | Summer–Autumn |
| WM2 | Russulaceae | Russula cyanoxantha | Mixed deciduous and coniferous forests, clearings/hills, mountains, meadows, and gardens in plains | Vinetica porumbeilor/ Pigeon Mushroom | Summer–Autumn |
| WM3 | Russulaceae | Russula alutacea | Deciduous forests, calcareous soils/hills, mountains | Painisoara/ Fairy Ring Mushroom | Summer–Autumn |
| WM4 | Pleurotaceae | Pleurotus ostreatus | On deciduous trunks/plains, hills, mountains | Pastrav de fag/Beech Salmon Mushroom | Autumn–Winter |
| WM5 | Amanitaceae | Amanita caesarea | Deciduous forests, sandy or gravelly soils/plains, mountains | Buretele domnesc/Royal Bolete | Summer–Autumn |
| WM6 | Boletaceae | Boletus edulis | Deciduous forests from sunny areas/hills, mountains | Manatarca pietroasa/Boletes/Stone Mushroom | Summer–Autumn |
| WM7 | Lepiotaceae | Macrolepiota procera | Deciduous forest clearings; sandy or gravelly soils/plains, hills, mountains | Palaria sarpelui/Snake’s Cap | Summer–Early Autumn |
| WM8 | Cantharellaceae | Cantharellus cibarius | Coniferous and deciduous forests; among moss or leaf litter/mostly in mountains | Bureti galbeni/Chanterelle | Late Spring– Autumn |
| WM9 | Marasmiaceae | Marasmius oreades | Meadows, clearings; sandy soils/along roads | Ghebe de lunca/Meadow Mushroom | Spring– Autumn |
| Generally Edible | |||||
| WM10 | Russulaceae | Russula vesca | Deciduous and coniferous forests/hills and mountains | Painea pamantului/Earth Bread | Summer |
| WM11 | Russulaceae | Russula lutea | Deciduous and coniferous forests/hills and mountains | Burete galben/Yellow Mushroom | Summer–Early Autumn |
| WM12 | Russulaceae | Russula aeruginea | Coniferous and deciduous forests, especially under birch/plains, hills, mountains | Vinetica porcului/Piglet Mushroom | Summer–Autumn |
| WM13 | Amanitaceae | Amanita rubescens | Deciduous and coniferous forests/plains, hills, mountains | Buretele rosu brobonat/Red-staining Mushroom | Spring–Autumn |
| WM14 | Hydnaceae | Hydnum repandum | Deciduous and coniferous forests; hills, mountains | Flocoșel/Hedgehog Mushroom | Late Summer–Autumn |
| WM15 | Physalacriaceae | Armillaria mellea | Decaying deciduous and coniferous trunks/hills, mountains | Ghebe/Honey Fungus | Autumn–Late Autumn |
| Moderately Edible | |||||
| WM16 | Russulaceae | Lactarius volemus | Deciduous and coniferous forests/mountains | Laptuca dulce/Sweet Milkcap | Summer |
| WM17 | Boletaceae | Boletus chrysenteron | Deciduous forests/mossy areas | Hribul de muschi/Moss Bolete | Summer–Autumn |
| WM18 | Boletaceae | Boletus griseus | Deciduous forests/plains, hills, mountains | Buretele cenusiu/Gray Bolete | Summer–Early Autumn |
| Metals | SRM * Concentration (mg/kg dw **) | Measured Concentration (mg/kg dw **) | Recovery (%) |
|---|---|---|---|
| Fe | 46.00 ± 2.00 | 40.15 ± 1.80 | 87.3 |
| Cu | 2.80 ± 0.20 | 2.89 ± 0.35 | 103.5 |
| Zn | 38.00 ± 2.00 | 34.99 ± 2.50 | 92.1 |
| Mn | 488.00 ± 12.00 | 466.04 ± 17.40 | 95.5 |
| Ni | 1.47 ± 0.10 | 1.46 ± 0.25 | 99.7 |
| Cr | *** | 6.20 ± 0.72 | Nd **** |
| Pb | 0.167 ± 0.015 | 0.179 ± 0.021 | 105.3 |
| Cd | 0.233 ± 0.004 | 0.252 ± 0.035 | 108.2 |
| Sample ID | Fe | Cu | Zn | Mn | Ni | Cr | Pb | Cd |
|---|---|---|---|---|---|---|---|---|
| WM1 | 6.503 ± 0.055 | 0.718 ± 0.052 | 8.728 ± 0.070 | 2.219 ± 0.020 | 0.061 ± 0.008 | 0.094 ± 0.007 | 0.017 ± 0.001 | 0.017 ± 0.001 |
| WM2 | 28.289 ± 1.815 | 2.385 ± 0.151 | 8.004 ± 0.350 | 4.507 ± 0.335 | 4.507 ± 0.200 | 0.115 ± 0.006 | 0.214 ± 0.020 | 0.187 ± 0.007 |
| WM3 | 68.996 ± 4.450 | 2.805 ± 0.120 | 14.675 ± 0.520 | 32.025 ± 1.800 | 0.163 ± 0.007 | 0.095 ± 0.006 | 0.310 ± 0.010 | nd ** |
| WM4 | 27.160 ± 2.318 | 1.252 ± 0.052 | 17.128 ± 0.761 | 6.137 ± 0.290 | 0.187 ± 0.007 | 0.066 ± 0.004 | 0.886 ± 0.040 | 0.012 ± 0.001 |
| WM5 | 6.946 ± 0.447 | 1.731 ± 0.077 | 8.446 ± 0.382 | 4.146 ± 0.200 | 0.069 ± 0.002 | 0.155 ± 0.009 | 0.167 ± 0.007 | 0.167 ± 0.005 |
| WM6 | 16.245 ± 1.722 | 3.205 ± 0.240 | 6.311 ± 0.300 | nd ** | 0.182 ± 0.008 | 0.760 ± 0.041 | 0.507 ± 0.020 | nd ** |
| WM7 | 29.975 ± 2.500 | 2.088 ± 0.104 | 14.183 ± 0.950 | 14.549 ± 0.620 | 0.196 ± 0.009 | nd ** | nd ** | nd ** |
| WM8 | 22.497 ± 2.100 | 1.968 ± 0.085 | 24.281 ± 1.140 | 2.018 ± 0.100 | 0.151 ± 0.007 | 0.003 ± 0.001 | 0.249 ± 0.010 | nd ** |
| WM9 | 33.661 ± 1.968 | 1.691 ± 0.082 | 25.114 ± 1.051 | 10.737 ± 0.650 | 0.141 ± 0.006 | 0.006 ± 0.002 | 0.216 ± 0.009 | nd ** |
| WM10 | 20.340 ± 1.732 | 1.656 ± 0.071 | 8.086 ± 0.370 | 6.207 ± 0.220 | 0.139 ± 0.006 | 0.053 ± 0.002 | 0.077 ± 0.005 | 0.090 ± 0.006 |
| WM11 | 31.624 ± 1.500 | 3.480 ± 0.150 | 5.296 ± 0.200 | nd ** | 0.033 ± 0.002 | nd ** | nd ** | nd ** |
| WM12 | 27.536 ± 1.700 | 2.075 ± 0.120 | 5.423 ± 0.300 | 12.587 ± 0.550 | 0.158 ± 0.007 | 0.045 ± 0.002 | 0.461 ± 0.015 | nd ** |
| WM13 | 30.891 ± 1.940 | 3.245 ± 0.150 | 11.529 ± 0.460 | 20.595 ± 1.120 | 0.092 ± 0.006 | 0.166 ± 0.008 | 0.113 ± 0.008 | nd ** |
| WM14 | 16.742 ± 1.270 | 1.567 ± 0.085 | 25.942 ± 1.127 | 7.609 ± 0.340 | 0.157 ± 0.007 | 0.040 ± 0.001 | 0.089 ± 0.005 | 0.033 ± 0.001 |
| WM15 | 54.573 ± 2.290 | 0.979 ± 0.050 | 11.442 ± 0.620 | 23.954 ± 2.100 | 0.165 ± 0.007 | 0.202 ± 0.015 | 0.247 ± 0.018 | 0.038 ± 0.001 |
| WM16 | 8.871 ± 0.350 | 0.942 ± 0.032 | 5.115 ± 0.245 | 0.236 ± 0.010 | 0.700 ± 0.030 | 0.134 ± 0.008 | 0.050 ± 0.002 | 0.048 ± 0.002 |
| WM17 | 88.745 ± 4.337 | 1.539 ± 0.080 | 8.405 ± 0.400 | 18.511 ± 0.870 | 0.200 ± 0.009 | 0.119 ± 0.005 | 0.344 ± 0.012 | 0.850 ± 0.020 |
| WM18 | 6.309 ± 0.525 | 0.679 ± 0.040 | 9.914 ± 0.520 | nd ** | 0.071 ± 0.005 | 0.053 ± 0.004 | 0.108 ± 0.003 | 0.088 ± 0.002 |
| Elements | Children ≥ 4 Years | Male | Female | Pregnant Women |
|---|---|---|---|---|
| Fe (mg/day) | 10–11 | 8 | 18 | 27 |
| Cu (mg/day) | 0.440–0.890 | 0.900 | 0.900 | 1 |
| Zn (mg/day) | 5–11 | 11 | 8 | 11 |
| Mn (mg/day) | 1.5–2.2 | 2.3 | 1.8 | 2 |
| Cr (mg/day) | 0.015–0.035 | 0.035 | 0.025 | 0.030 |
| Sample ID | Fe (mg/day) | Cu (mg/day) | Zn (mg/day) | Mn (mg/day) | Ni (mg/day) | Cr (mg/day) | Pb (mg/day) | Cd (mg/day) |
|---|---|---|---|---|---|---|---|---|
| WM1 | 1.950 | 0.215 | 2.618 | 0.665 | 0.018 | 0.028 | 0.005 | 0.005 |
| WM2 | 8.486 | 0.715 | 2.401 | 1.352 | 1.352 | 0.034 | 0.064 | 0.056 |
| WM3 | 20.698 | 0.841 | 4.402 | 9.607 | 0.048 | 0.028 | 0.093 | na * |
| WM4 | 8.148 | 0.375 | 5.138 | 1.841 | 0.056 | 0.019 | 0.265 | 0.002 |
| WM5 | 2.083 | 0.519 | 2.533 | 1.243 | 0.020 | 0.046 | 0.050 | 0.050 |
| WM6 | 4.873 | 0.961 | 1.893 | na * | 0.054 | 0.228 | 0.152 | na * |
| WM7 | 8.992 | 0.626 | 4.254 | 4.364 | 0.058 | na * | na * | na * |
| WM8 | 6.749 | 0.590 | 7.284 | 0.605 | 0.045 | 0.000 | 0.074 | na * |
| WM9 | 10.098 | 0.507 | 7.534 | 3.221 | 0.042 | 0.001 | 0.064 | na * |
| WM10 | 6.102 | 0.496 | 2.425 | 1.862 | 0.041 | 0.015 | 0.023 | 0.027 |
| WM11 | 9.487 | 1.044 | 1.588 | na * | 0.009 | na * | na * | na * |
| WM12 | 8.260 | 0.622 | 1.626 | 3.776 | 0.047 | 0.013 | 0.138 | na * |
| WM13 | 9.267 | 0.973 | 3.458 | 6.178 | 0.027 | 0.049 | 0.033 | na * |
| WM14 | 5.022 | 0.470 | 7.782 | 2.282 | 0.047 | 0.012 | 0.026 | 0.009 |
| WM15 | 16.371 | 0.293 | 3.432 | 7.186 | 0.049 | 0.060 | 0.074 | 0.011 |
| WM16 | 2.661 | 0.282 | 1.534 | 0.070 | 0.210 | 0.040 | 0.015 | 0.014 |
| WM17 | 26.623 | 0.461 | 2.521 | 5.553 | 0.060 | 0.035 | 0.103 | 0.255 |
| WM18 | 1.892 | 0.203 | 2.974 | na * | 0.021 | 0.015 | 0.032 | 0.026 |
| Sample | EDI (mg kg−1day−1) | |||||||
|---|---|---|---|---|---|---|---|---|
| Fe | Cu | Zn | Mn | Ni | Cr | Pb | Cd | |
| Russula virescens | 0.002 | <10−3 | 0.003 | <10−3 | <10−3 | <10−3 | <10−3 | <10−3 |
| Russula cyanoxantha | 0.011 | <10−3 | 0.003 | 0.001 | 0.001 | <10−3 | <10−3 | <10−3 |
| Russula alutacea | 0.026 | 0.001 | 0.005 | 0.012 | <10−3 | <10−3 | <10−3 | na * |
| Pleurotus ostreatus | 0.010 | <10−3 | 0.006 | 0.002 | <10−3 | <10−3 | <10−3 | <10−3 |
| Amanita caesarea | 0.002 | <10−3 | 0.003 | 0.001 | <10−3 | <10−3 | <10−3 | <10−3 |
| Boletus edulis | 0.006 | 0.001 | 0.002 | na * | <10−3 | <10−3 | <10−3 | na * |
| Macrolepiota procera | 0.011 | <10−3 | 0.005 | 0.005 | <10−3 | na * | na * | na * |
| Cantharellus cibarius | 0.008 | <10−3 | 0.009 | <10−3 | <10−3 | <10−3 | <10−3 | na * |
| Marasmius oreades | 0.013 | <10−3 | 0.009 | 0.004 | <10−3 | <10−3 | <10−3 | na * |
| Russula vesca | 0.007 | <10−3 | 0.003 | 0.002 | <10−3 | <10−3 | <10−3 | <10−3 |
| Russula lutea | 0.012 | 0.001 | 0.002 | na * | <10−3 | na * | na * | na * |
| Russula aeruginea | 0.010 | <10−3 | 0.002 | 0.004 | <10−3 | <10−3 | <10−3 | na * |
| Amanita rubescens | 0.012 | 0.001 | 0.004 | 0.008 | <10−3 | <10−3 | <10−3 | na * |
| Hydnum repandum | 0.006 | <10−3 | 0.010 | 0.002 | <10−3 | <10−3 | <10−3 | <10−3 |
| Armillaria mellea | 0.021 | <10−3 | 0.004 | 0.009 | <10−3 | <10−3 | <10−3 | <10−3 |
| Lactarius volemus | 0.003 | <10−3 | 0.001 | <10−3 | <10−3 | <10−3 | <10−3 | <10−3 |
| Boletus chrysenteron | 0.034 | <10−3 | 0.003 | 0.007 | <10−3 | <10−3 | <10−3 | <10−3 |
| Boletus griseus | 0.002 | <10−3 | 0.003 | na * | <10−3 | <10−3 | <10−3 | <10−3 |
| Sample | EDI (mg kg−1day−1) | |||||||
|---|---|---|---|---|---|---|---|---|
| Fe | Cu | Zn | Mn | Ni | Cr | Pb | Cd | |
| Russula virescens | 0.024 | 0.002 | 0.032 | 0.008 | <10−3 | <10−3 | <10−3 | <10−3 |
| Russula cyanoxantha | 0.104 | 0.008 | 0.029 | 0.016 | <10−3 | <10−3 | <10−3 | <10−3 |
| Russula alutacea | 0.255 | 0.010 | 0.054 | 0.118 | <10−3 | <10−3 | 0.001 | 0 |
| Pleurotus ostreatus | 0.100 | 0.004 | 0.063 | 0.022 | <10−3 | <10−3 | 0.003 | 0 |
| Amanita caesarea | 0.025 | 0.006 | 0.031 | 0.015 | <10−3 | <10−3 | <10−3 | <10−3 |
| Boletus edulis | 0.060 | 0.011 | 0.023 | na * | <10−3 | 0.002 | 0.001 | na * |
| Macrolepiota procera | 0.110 | 0.007 | 0.052 | 0.053 | <10−3 | na * | na * | na * |
| Cantharellus cibarius | 0.083 | 0.007 | 0.089 | 0.007 | <10−3 | na * | <10−3 | na * |
| Marasmius oreades | 0.124 | 0.006 | 0.092 | 0.039 | <10−3 | na * | <10−3 | na * |
| Russula vesca | 0.075 | 0.006 | 0.029 | 0.022 | <10−3 | <10−3 | <10−3 | <10−3 |
| Russula lutea | 0.116 | 0.012 | 0.019 | na * | <10−3 | na * | na * | na * |
| Russula aeruginea | 0.101 | 0.007 | 0.020 | 0.046 | <10−3 | <10−3 | 0.001 | na * |
| Amanita rubescens | 0.114 | 0.012 | 0.042 | 0.076 | <10−3 | <10−3 | <10−3 | na * |
| Hydnum repandum | 0.061 | 0.005 | 0.095 | 0.028 | <10−3 | <10−3 | <10−3 | <10−3 |
| Armillaria mellea | 0.201 | 0.003 | 0.042 | 0.088 | <10−3 | <10−3 | <10−3 | <10−3 |
| Lactarius volemus | 0.032 | 0.003 | 0.018 | <10−3 | 0.002 | <10−3 | <10−3 | <10−3 |
| Boletus chrysenteron | 0.328 | 0.005 | 0.031 | 0.068 | <10−3 | <10−3 | 0.001 | 0.003 |
| Boletus griseus | 0.023 | 0.002 | 0.036 | na * | <10−3 | <10−3 | <10−3 | <10−3 |
| Fe | Cu | Zn | Mn | Ni | Cr | Pb | Cd | |
|---|---|---|---|---|---|---|---|---|
| RfD (mg kg−1day−1) | 0.7 | 0.04 | 0.3 | 0.14 | 0.02 | 0.003 | 0.0035 | 0.001 |
| CSF (mg kg−1day−1) | - | 1.7 | - | - | not established * | 0.5 | 0.0085 | 6.3 |
| Sample ID | HQ | HI | ||||||
|---|---|---|---|---|---|---|---|---|
| Fe | Cu | Mn | Ni | Cr | Pb | Cd | ||
| WM1 | 0.00 | 0.01 | 0.01 | 0.00 | 0.01 | 0.00 | 0.01 | 0.04 |
| WM2 | 0.02 | 0.02 | 0.01 | 0.09 | 0.01 | 0.02 | 0.07 | 0.24 |
| WM3 | 0.04 | 0.03 | 0.09 | 0.00 | 0.01 | 0.03 | na * | 0.20 |
| WM4 | 0.02 | 0.01 | 0.02 | 0.00 | 0.01 | 0.10 | 0.00 | 0.16 |
| WM5 | 0.00 | 0.02 | 0.01 | 0.00 | 0.02 | 0.02 | 0.07 | 0.14 |
| WM6 | 0.01 | 0.03 | na * | 0.00 | 0.10 | 0.06 | na * | 0.20 |
| WM7 | 0.02 | 0.02 | 0.04 | 0.00 | na * | na * | na * | 0.08 |
| WM8 | 0.01 | 0.02 | 0.01 | 0.00 | 0.00 | 0.03 | na * | 0.07 |
| WM9 | 0.02 | 0.02 | 0.03 | 0.00 | 0.00 | 0.02 | na * | 0.09 |
| WM10 | 0.01 | 0.02 | 0.02 | 0.00 | 0.01 | 0.01 | 0.04 | 0.11 |
| WM11 | 0.02 | 0.03 | na * | 0.00 | na * | na * | na * | 0.05 |
| WM12 | 0.02 | 0.02 | 0.04 | 0.00 | 0.01 | 0.05 | na * | 0.14 |
| WM13 | 0.02 | 0.03 | 0.06 | 0.00 | 0.02 | 0.01 | na * | 0.14 |
| WM14 | 0.01 | 0.02 | 0.02 | 0.00 | 0.01 | 0.01 | 0.01 | 0.08 |
| WM15 | 0.03 | 0.01 | 0.07 | 0.00 | 0.03 | 0.03 | 0.01 | 0.18 |
| WM16 | 0.00 | 0.01 | 0.00 | 0.01 | 0.02 | 0.01 | 0.02 | 0.07 |
| WM17 | 0.05 | 0.02 | 0.05 | <10−2 | 0.02 | 0.04 | 0.33 | 0.51 |
| WM18 | <10−2 | 0.01 | na * | <10−2 | 0.01 | 0.01 | 0.03 | 0.06 |
| Sample ID | HQ | HI | ||||||
|---|---|---|---|---|---|---|---|---|
| Fe | Cu | Mn | Ni | Cr | Pb | Cd | ||
| WM1 | 0.03 | 0.07 | 0.06 | 0.01 | 0.12 | 0.02 | 0.06 | 0.37 |
| WM2 | 0.15 | 0.22 | 0.12 | 0.83 | 0.14 | 0.23 | 0.69 | 2.38 |
| WM3 | 0.36 | 0.26 | 0.85 | 0.03 | 0.12 | 0.33 | na * | 1.95 |
| WM4 | 0.14 | 0.12 | 0.16 | 0.03 | 0.08 | 0.94 | 0.03 | 1.5 |
| WM5 | 0.04 | 0.16 | 0.11 | 0.01 | 0.19 | 0.18 | 0.62 | 1.31 |
| WM6 | 0.09 | 0.30 | na * | 0.03 | 0.94 | 0.54 | na * | 1.9 |
| WM7 | 0.16 | 0.19 | 0.38 | 0.04 | na * | na * | na * | 0.77 |
| WM8 | 0.12 | 0.18 | 0.05 | 0.03 | 0.00 | 0.26 | na * | 0.64 |
| WM9 | 0.18 | 0.16 | 0.28 | 0.03 | 0.01 | 0.23 | na * | 0.89 |
| WM10 | 0.11 | 0.15 | 0.16 | 0.03 | 0.07 | 0.08 | 0.33 | 0.93 |
| WM11 | 0.17 | 0.32 | na * | 0.01 | na * | na * | na * | 0.5 |
| WM12 | 0.15 | 0.19 | 0.33 | 0.03 | 0.06 | 0.49 | na * | 1.25 |
| WM13 | 0.16 | 0.30 | 0.54 | 0.02 | 0.20 | 0.12 | na * | 1.34 |
| WM14 | 0.09 | 0.14 | 0.20 | 0.03 | 0.05 | 0.09 | 0.12 | 0.72 |
| WM15 | 0.29 | 0.09 | 0.63 | 0.03 | 0.25 | 0.26 | 0.14 | 1.69 |
| WM16 | 0.05 | 0.09 | 0.01 | 0.13 | 0.17 | 0.05 | 0.18 | 0.68 |
| WM17 | 0.47 | 0.14 | 0.49 | 0.04 | 0.15 | 0.36 | 3.14 | 4.79 |
| WM18 | 0.03 | 0.06 | na * | 0.01 | 0.07 | 0.11 | 0.33 | 0.61 |
| Sample ID | ILCR Adults | Total ILCR Adults | ILCR Children | Total ILCR Children | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Cu | Cr | Pb | Cd | Cu | Cr | Pb | Cd | |||
| WM1 | 5.22 × 10−3 | 2.01 × 10−4 | 6.2 × 10−7 | 4.58 × 10−4 | 5.88 × 10−3 | 3.32 × 10−2 | 12.82 × 10−4 | 4 × 10−6 | 29.21 × 10−4 | 3.74 × 10−2 |
| WM2 | 1.73 × 10−2 | 2.46 × 10−4 | 7.79 × 10−6 | 5.04 × 10−3 | 2.26 × 10−2 | 11.05 × 10−2 | 15.68 × 10−4 | 5.0 × 10−5 | 3.21 × 10−2 | 14.43 × 10−2 |
| WM3 | 2.04 × 10−2 | 2.03 × 10−4 | 1.12 × 10−5 | na * | 2.06 × 10−2 | 13.00 × 10−2 | 12.95 × 10−4 | 7.2 × 10−5 | na * | 13.14 × 10−2 |
| WM4 | 9.10 × 10−3 | 1.41 × 10−4 | 3.22 × 10−5 | 1.88 × 10−4 | 9.47 × 10−3 | 5.80 × 10−2 | 9.00 × 10−4 | 2.05 × 10−4 | 12.03 × 10−4 | 6.03 × 10−2 |
| WM5 | 1.25 × 10−2 | 3.31 × 10−4 | 6.08 × 10−6 | 4.50 × 10−3 | 1.74 × 10−2 | 8.02 × 10−2 | 21.13 × 10−4 | 3.9 × 10−5 | 2.86 × 10−2 | 11.10 × 10−2 |
| WM6 | 2.33 × 10−2 | 1.62 × 10−3 | 1.84 × 10−5 | na * | 2.49 × 10−2 | 14.85 × 10−2 | 1.03 × 10−2 | 1.18 × 10−4 | na * | 15.90 × 10−2 |
| WM7 | 1.51 × 10−2 | na * | na * | na * | 1.51 × 10−2 | 9.67 × 10−2 | na * | na * | na * | 9.67 × 10−2 |
| WM8 | 1.43 × 10−2 | 6.42 × 10−6 | 9.06 × 10−6 | na * | 1.43 × 10−2 | 9.12 × 10−2 | 4.1 × 10−5 | 5.8 × 10−5 | na * | 9.13 × 10−2 |
| WM9 | 1.23 × 10−2 | 1.28 × 10−5 | 7.86 × 10−6 | na * | 1.23 × 10−2 | 7.83 × 10−2 | 8.2 × 10−5 | 5.0 × 10−5 | na * | 7.85 × 10−2 |
| WM10 | 1.20 × 10−2 | 1.13 × 10−4 | 2.80 × 10−6 | 2.42 × 10−3 | 1.45 × 10−2 | 7.67 × 10−2 | 7.23 × 10−4 | 1.8 × 10−5 | 1.54 × 10−2 | 9.29 × 10−2 |
| WM11 | 2.53 × 10−2 | na * | na * | na * | 2.53 × 10−2 | 16.13 × 10−2 | na * | na * | na * | 16.13 × 10−2 |
| WM12 | 1.50 × 10−2 | 9.63 × 10−5 | 1.67 × 10−5 | na * | 1.52 × 10−2 | 9.61 × 10−2 | 6.14 × 10−4 | 1.07 × 10−4 | na * | 9.69 × 10−2 |
| WM13 | 2.36 × 10−2 | 3.55 × 10−4 | 4.11 × 10−6 | na * | 2.39 × 10−2 | 15.04 × 10−2 | 22.63 × 10−4 | 2.6 × 10−5 | na * | 15.27 × 10−2 |
| WM14 | 1.14 × 10−2 | 8.56 × 10−5 | 3.24 × 10−6 | 8.89 × 10−4 | 1.23 × 10−2 | 7.26 × 10−2 | 5.45 × 10−4 | 2.1 × 10−5 | 56.69 × 10−4 | 7.88 × 10−2 |
| WM15 | 7.12 × 10−3 | 4.32 × 10−4 | 8.99 × 10−6 | 1.02 × 10−3 | 8.58 × 10−3 | 4.53 × 10−2 | 27.54 × 10−4 | 5.7 × 10−5 | 65.28 × 10−4 | 5.47 × 10−2 |
| WM16 | 6.85 × 10−3 | 2.86 × 10−4 | 1.82 × 10−6 | 1.29 × 10−3 | 8.43 × 10−3 | 4.36 × 10−2 | 18.27 × 10−4 | 1.2 × 10−5 | 82.46 × 10−4 | 5.37 × 10−2 |
| WM17 | 1.11 × 10−2 | 2.54 × 10−4 | 1.25 × 10−5 | 2.29 × 10−2 | 3.43 × 10−2 | 7.13 × 10−2 | 16.23 × 10−4 | 8.0 × 10−5 | 14.60 × 10−2 | 21.90 × 10−2 |
| WM18 | 4.94 × 10−3 | 1.13 × 10−4 | 3.93 × 10−6 | 2.37 × 10−3 | 7.43 × 10−3 | 3.14 × 10−2 | 7.23 × 10−4 | 2.5 × 10−5 | 1.51 × 10−2 | 4.73 × 10−2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stihi, C.; Dumitrescu, C. Assessment of the Impact of Metals in Wild Edible Mushrooms from Dambovita County, Romania, on Human Health. Foods 2025, 14, 3540. https://doi.org/10.3390/foods14203540
Stihi C, Dumitrescu C. Assessment of the Impact of Metals in Wild Edible Mushrooms from Dambovita County, Romania, on Human Health. Foods. 2025; 14(20):3540. https://doi.org/10.3390/foods14203540
Chicago/Turabian StyleStihi, Claudia, and Crinela Dumitrescu. 2025. "Assessment of the Impact of Metals in Wild Edible Mushrooms from Dambovita County, Romania, on Human Health" Foods 14, no. 20: 3540. https://doi.org/10.3390/foods14203540
APA StyleStihi, C., & Dumitrescu, C. (2025). Assessment of the Impact of Metals in Wild Edible Mushrooms from Dambovita County, Romania, on Human Health. Foods, 14(20), 3540. https://doi.org/10.3390/foods14203540







