Polycyclic Aromatic Hydrocarbons (PAHs) and Phthalate Esters (PAEs) in the Farmed Fishes from Khanh Hoa, Viet Nam: Level and Health Risk Assessment
Abstract
1. Introduction
2. Materials and Methods
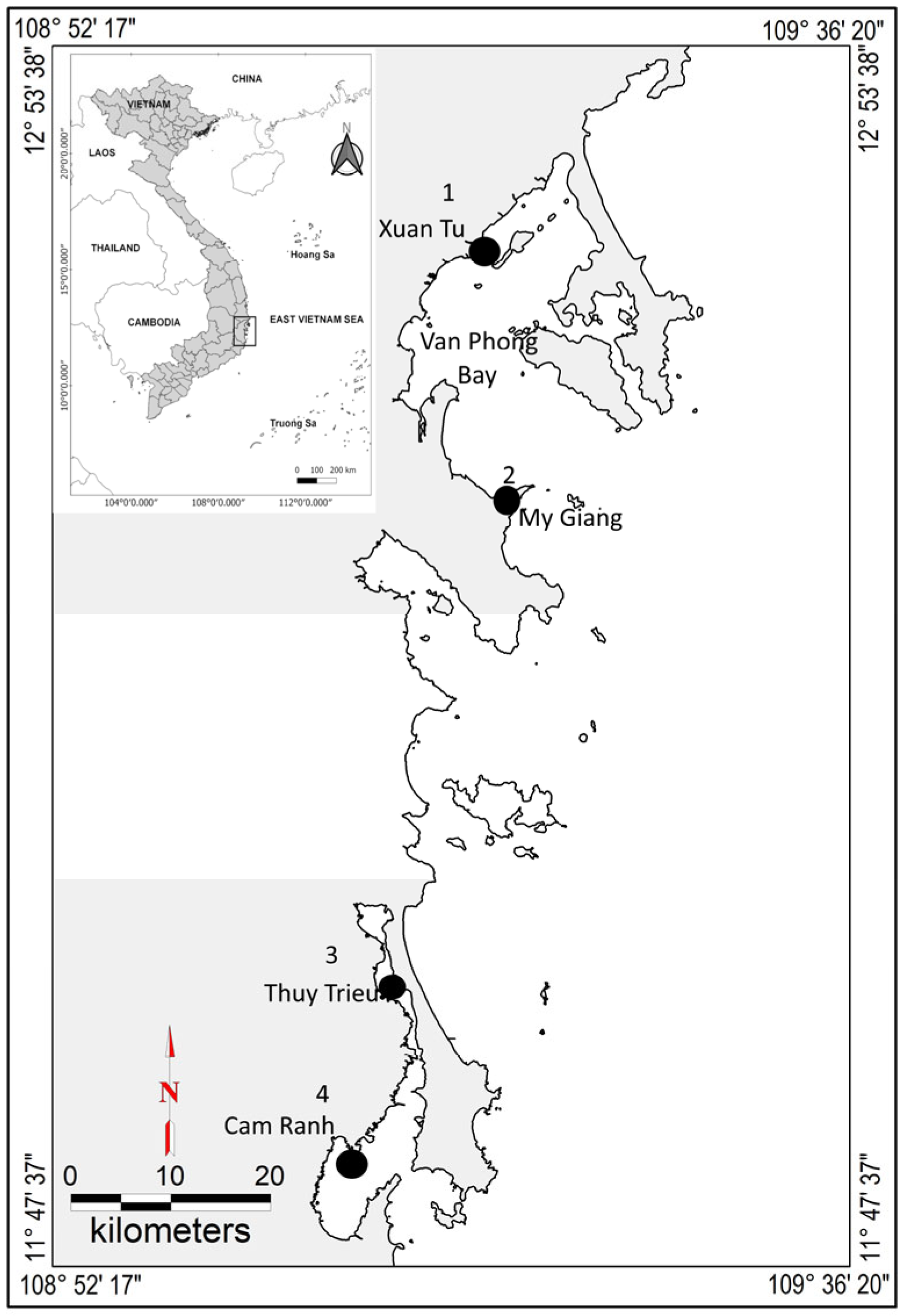
2.1. Study Sites
2.2. Sampling and Extraction
2.3. Sample Analysis
2.4. Statistical Analysis
2.5. Health Risk Assessment
3. Results
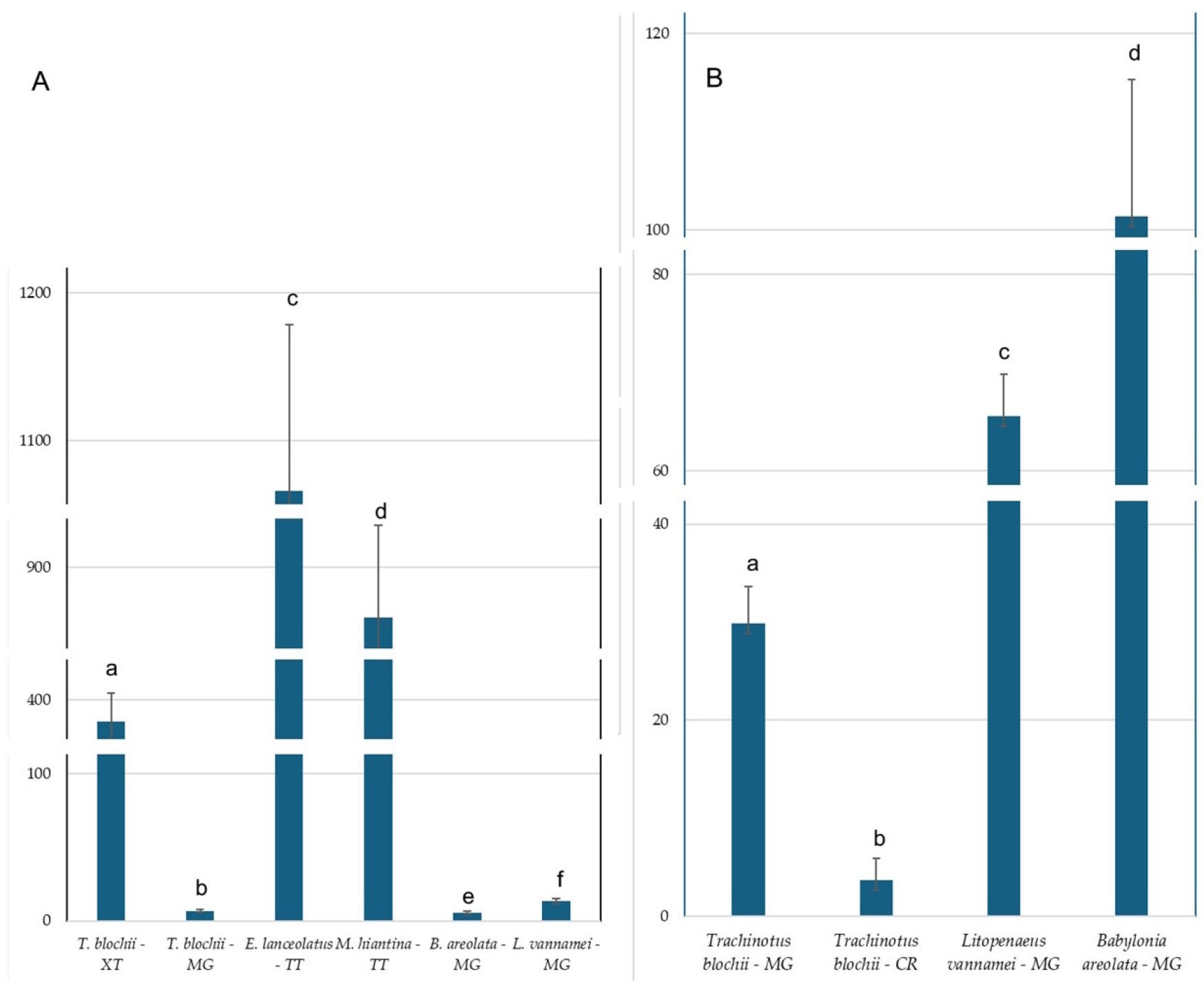
3.1. Occurrence of the PAEs in Farmed Species
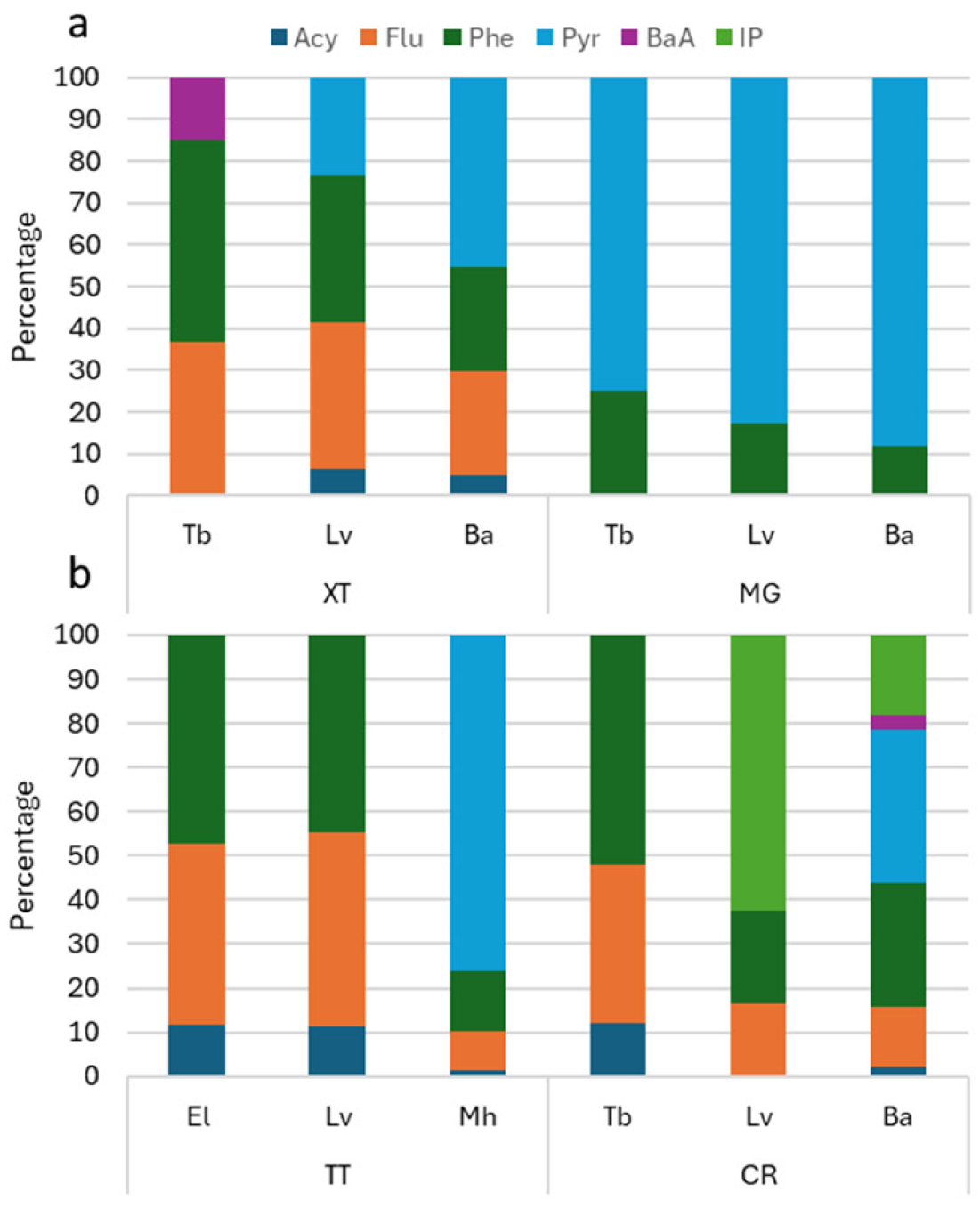
3.2. Occurrence of the PAHs in Farmed Species
3.3. Health Risk Assessment
4. Discussion
| Species/Name | Location | ∑PAHs | Sample Processing | Sources |
|---|---|---|---|---|
| Fishes | ||||
| Trachinotus blochii | Viet Nam | 17.6–25.7 | Sample: muscle tissues Extraction: water bath ultrasonication using an acetone/n-hexane mixture (1:1, v/v) Detection and Quantification: GC–MS/MS 1 | This study |
| Trachinotus blochii | Hong Kong | 48.7–67.2 | Sample: muscle tissues Extraction: Soxhlet extraction using an acetone and dichloromethane mixture (1:1, v/v) Detection and Quantification: GC–MS 2 | [45] Cheung |
| Epinephelus lanceolatus | Viet Nam | 16.43 | 1 | This study |
| Epinephelus coioides | Hong Kong | 30.2–50.2 | 2 | [45] |
| Epinephelus bleekeri | Hong Kong | 37.6–45.8 | 2 | [45] |
| Lateolabrax japonicus | Taiwan | 35.16 | Sample: muscle tissues Extraction: water bath ultrasonication with the mixture of acetone/n-hexane (1:1, v/v) Detection and Quantification: GC–MS | [29] |
| Freshwater fishes | Viet Nam | 22–228 | Sample: muscle tissues Extraction: ultrasound with the n-hexane and dichloromethane mix solvent. Detection and Quantification: GC × GC-TOF/MS | [43] |
| Mollusks | ||||
| Babylonia areolata | Viet Nam | 13.32–46.33 | 1 | This study |
| Marcia hiantina | Viet Nam | 47.34 | 1 | This study |
| Crassostrea belcheri | Malaysia | 309–2225 | Sample: soft tissues Extraction: cyclohexane Detection and Quantification: GC-MS | [46] |
| Crassostrea gigas | Japan | 289–450 | Sample: soft tissues Extraction: acetone Detection and Quantification: GC-MS | [47] |
| Perna viridis | Viet Nam | 3.65–15.79 | Sample: soft tissues Extraction: HCl/Chloroform Detection and Quantification: HPLC-FLD | [39] |
| Perna viridis | Viet Nam | 34–110 | Sample: soft tissues Extraction: mixture of acetone, dichloromethane, n-hexane (1:1:1, v/v/v) in a Soxhlet apparatus Detection and Quantification: GC-MS | [48] |
| Crustaceans | ||||
| Litopenaeus vannamei | Viet Nam | 9.14–27.94 | 1 | This study |
| Metapenaeus affinis | Iran | 1644–3792 | Extraction: Soxhlet system using dichloromethane Detection and Quantification: GC-MS | [49] |
| Portunus trituberculatus | China | 119.11 | Sample: edible tissues Extraction: Soxhlet system using n-hexane/acetone (1:1 v/v) Detection and Quantification: GC-MS/MS | [50] |
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| Acy | Acenaphthylene |
| ADI | Average Daily Intake |
| Ant | Anthracene |
| Ba | Babylonia areolata |
| BaA | benzo[a]anthracene |
| BBP | butyl benzyl phthalate |
| BbF | benzo[b]fluoranthene |
| BkF | benzo[k]fluoranthene |
| BP | benzo[g,h,i] perylene |
| Chr | chrysene |
| CR | Cam Ranh |
| DA | dibenzo[a,h]anthracene |
| DBP | dibutyl phthalate |
| DEHA | di(2-ethylhexyl) adipate |
| DEHP | bis(2-ethylhexyl) phthalate |
| DEP | diethyl phthalate |
| DA | dibenz[a,h]anthracene |
| DMP | dimethyl phthalate |
| DnOP | di-n-octyl phthalate |
| El | Epinephelus lanceolatus |
| Flu | Fluorene |
| GC-MS/MS | Gas Chromatograph–Mass Spectrometer |
| HI | Hazard Index |
| HQ | Hazard Quotient |
| IARC | International Agency for Research on Cancer |
| ILCR | Incremental Lifetime Cancer Risk |
| IP | Indeno[1,2,3-cd] pyrene |
| Lv | Litopenaeus vannamei |
| MDL | Method Detection Limit |
| Mh | Marcia hiantina |
| MG | My Giang |
| PAE | Phthalate Esters |
| PAH | Polycyclic Aromatic Hydrocarbons |
| Phe | Phenanthrene |
| Pyr | Pyrene |
| Tb | Trachinotus blochii |
| TT | Thuy Trieu |
| XT | Xuan Tu |
Appendix A
| PAHs | LOD (µg kg−1) | LOQ (µg kg−1) | Recovery (%) | RSD (%) | Calibration Equation |
|---|---|---|---|---|---|
| Acy | 0.12 | 0.37 | 109.43 | 0.6–4.9 | y = 1.0069x + 0.1188 |
| Flu | 0.08 | 0.28 | 86.32 | 1.5–5.2 | y = 1.1624x − 0.3277 |
| Phe | 0.12 | 0.40 | 92.44 | 0.8–5.5 | y = 1.0952x − 0.2113 |
| Ant | 0.09 | 0.31 | 98.94 | 1.4–6.4 | y = 0.9673x + 0.0846 |
| Pyr | 0.17 | 0.54 | 90.99 | 0.8–6.2 | y = 0.9683x + 0.135 |
| Chr | 0.21 | 0.65 | 94.72 | 2.3–6.5 | y = 0.9743x + 0.0594 |
| BaA | 0.20 | 0.61 | 93.98 | 1.8–5.6 | y = 1.0555x − 0.101 |
| BbF | 0.15 | 0.42 | 95.95 | 2.0–6.4 | y = 1.084x − 0.1535 |
| BkF | 0.14 | 0.51 | 91.17 | 1.5–5.7 | y = 1.0531x − 0.1488 |
| IP | 0.14 | 0.45 | 95.36 | 0.4–5.5 | y = 1.0336x − 0.0323 |
| DA | 0.17 | 0.57 | 96.75 | 1.9–6.3 | y = 0.9507x + 0.0325 |
| BP | 0.31 | 0.89 | 91.28 | 0.7–5.7 | y = 0.9391x + 0.3194 |
| PEAs | |||||
| DMP | 0.75 | 2.48 | 109.62 | 3.5–9.6 | y = 1.1541x − 0.2843 |
| DBP | 0.62 | 2.07 | 98.45 | 2.5–10.8 | y = 0.9607x + 0.2362 |
| DEP | 0.71 | 2.33 | 103.76 | 3.0–11.7 | y = 0.9641x + 0.1654 |
| BBP | 0.98 | 3.23 | 95.31 | 2.8–11.5 | y = 1.0178x − 0.1243 |
| DEHP | 0.12 | 0.41 | 111.84 | 5.7–14.8 | y = 1.1781x − 0.4312 |
| CPEA | ADI | HQ | |||||
|---|---|---|---|---|---|---|---|
| Overall | RfD (mg kg−1 day−1) | 95th | Mean | 95th | Mean | 95th | Mean |
| DBP | 752 | 9.6 × 10−3 | 1.4 × 10−3 | 1.6 × 10−3 | 2.3 × 10−3 | 2.1 × 10−6 | 3.0 × 10−7 |
| DEP | 3160 | 9.1 × 10−2 | 3.7 × 10−3 | 1.5 × 10−2 | 6 × 10−3 | 4.7 × 10−6 | 1.9 × 10−7 |
| DEHP | 2000 | 1.0 | 0.2 | 0.17 | 0.03 | 8.4 × 10−5 | 1.6 × 10−5 |
| HI | 9.1 × 10−5 | 1.7 × 10−5 | |||||
| Fishes | |||||||
| DBP | 752 | 1.0 × 10−2 | 4.6 × 10−3 | 1.7 × 10−3 | 7.5 × 10−4 | 2.3 × 10−6 | 1.0 × 10−6 |
| DEP | 3160 | 2.9 × 10−2 | 1.7 × 10−2 | 4.7 × 10−3 | 2.8 × 10−3 | 1.5 × 10−6 | 8.7 × 10−7 |
| DEHP | 2000 | 1.0 | 0.39 | 0.16 | 6.4 × 10−2 | 8.2 × 10−5 | 3.2 × 10−5 |
| HI | 8.6 × 10−5 | 3.4 × 10−5 | |||||
| Crustacean | |||||||
| DBP | 752 | 1.3 × 10−3 | 1.3 × 10−3 | 2.1 × 10−4 | 2.1 × 10−4 | 2.8 × 10−7 | 2.7 × 10−7 |
| DEP | 3160 | 6.2 × 10−2 | 3.3 × 10−2 | 1.0 × 10−2 | 5.5 × 10−3 | 3.3 × 10−6 | 1.7 × 10−6 |
| DEHP | 2000 | 1.3 × 10−2 | 1.3 × 10−2 | 2.2 × 10−3 | 2.2 × 10−3 | 1.0 × 10−6 | 1.1 × 10−6 |
| HI | 4.6 × 10−6 | 3.1 × 10−6 | |||||
| Mollusk | |||||||
| DBP | 752 | 1.3 × 10−3 | 1.3 × 10−3 | 2.2 × 10−4 | 2.2 × 10−4 | 2.9 × 10−7 | 2.9 × 10−7 |
| DEP | 3160 | 9.1 × 10−2 | 1.4 × 10−3 | 1.5 × 10−2 | 2.3 × 10−4 | 4.8 × 10−6 | 7.1 × 10−8 |
| DEHP | 2000 | 0.82 | 0.44 | 0.14 | 7.2 × 10−2 | 6.8 × 10−5 | 3.6 × 10−5 |
| HI | 7.3 × 10−5 | 3.6 × 10−5 | |||||
| CPEA | ADI | HQ | |||||
|---|---|---|---|---|---|---|---|
| Xuan Tu | RfD (mg kg−1 day−1) | 95th | Mean | 95th | Mean | 95th | Mean |
| DBP | 752 | 4.4 × 10−3 | 4.6 × 10−3 | 7.5 × 10−4 | 7.5 × 10−4 | 1.0 × 10−6 | 1.0 × 10−6 |
| DEP | 3160 | 1.3 × 10−3 | 1.3 × 10−3 | 2.2 × 10−4 | 2.2 × 10−4 | 7.0 × 10−8 | 7.0 × 10−8 |
| DEHP | 2000 | 3.9 × 10−1 | 3.9 × 10−1 | 6.4 × 10−3 | 6.4 × 10−3 | 3.2 × 10−5 | 3.2 × 10−5 |
| HI | 3.3 × 10−5 | 3.3 × 10−5 | |||||
| My Giang | |||||||
| DBP | 752 | 1.4 × 10−3 | 1.3 × 10−3 | 2.3 × 10−4 | 2.2 × 10−4 | 3.1 × 10−7 | 2.9 × 10−7 |
| DEP | 3160 | 9.8 × 10−2 | 6.6 × 10−2 | 1.6 × 10−2 | 1.1 × 10−2 | 5.1 × 10−6 | 3.4 × 10−6 |
| DEHP | 2000 | 1.3 × 10−2 | 6.9 × 10−3 | 2.1 × 10−3 | 1.1 × 10−3 | 1.0 × 10−6 | 5.7 × 10−7 |
| HI | 6.5 × 10−6 | 4.3 × 10−6 | |||||
| Thuy Trieu | |||||||
| DBP | 752 | 1.3 × 10−3 | 1.3 × 10−3 | 2.1 × 10−4 | 2.1 × 10−4 | 2.8 × 10−7 | 2.9 × 10−7 |
| DEP | 3160 | 1.4 × 10−2 | 1.3 × 10−3 | 2.2 × 10−4 | 2.2 × 10−3 | 3.3 × 10−6 | 6.8 × 10−8 |
| DEHP | 2000 | 1.1 | 9.7 × 10−1 | 1.7 × 10−1 | 1.6 × 10−1 | 1.0 × 10−6 | 8.0 × 10−5 |
| HI | 4.6 × 10−6 | 8.0 × 10−5 | |||||
| Cam Ranh | |||||||
| DBP | 752 | 1.1 × 10−2 | 1.1 × 10−2 | 1.5 × 10−3 | 1.9 × 10−3 | 2.5 × 10−6 | 2.5 × 10−6 |
| DEP | 3160 | 3.7 × 10−3 | 3.7 × 10−3 | 4.9 × 10−4 | 6.0 × 10−4 | 1.9 × 10−7 | 1.9 × 10−7 |
| DEHP | 2000 | 0.0 | 0 | 0 | 0 | 0 | 0 |
| HI | 2.7 × 10−6 | 2.7 × 10−6 | |||||
References
- Barathi, S.; J, G.; Rathinasamy, G.; Sabapathi, N.; Aruljothi, K.N.; Lee, J.; Kandasamy, S. Recent trends in polycyclic aromatic hydrocarbons pollution distribution and counteracting bio-remediation strategies. Chemosphere 2023, 337, 139396. [Google Scholar] [CrossRef] [PubMed]
- Duran, R.; Cravo-Laureau, C. Role of environmental factors and microorganisms in determining the fate of polycyclic aromatic hydrocarbons in the marine environment. FEMS Microbiol. Rev. 2016, 40, 814–830. [Google Scholar] [CrossRef] [PubMed]
- Ji, S.H.; Yin, F.; Zhang, W.W.; Song, Z.B.; Qin, B.Y.; Su, P.H.; Zhang, J.P.; Kitazawa, D. Occurrences, sources, and human health risk assessments of polycyclic aromatic hydrocarbons in marine organisms from temperate coastal area. Front. Ecol. Evol. 2022, 10, 850247. [Google Scholar] [CrossRef]
- Pourebrahimi, S.; Pirooz, M. Microplastic pollution in the marine environment: A review. J. Hazard. Mater. Adv. 2023, 10, 100327. [Google Scholar] [CrossRef]
- Alfaro-Núñez, A.; Astorga, D.; Cáceres-Farías, L.; Bastidas, L.; Soto Villegas, C.; Macay, K.; Christensen, J.H. Microplastic pollution in seawater and marine organisms across the Tropical Eastern Pacific and Galápagos. Sci. Rep. 2021, 11, 6424. [Google Scholar] [CrossRef]
- Barboza, L.G.A.; Frias, J.P.G.L.; Booth, A.M.; Vieira, L.R.; Masura, J.; Baker, J.; Foster, G.; Guilhermino, L. Microplastics Pollution in the Marine Environment. In World Seas: An Environmental Evaluation, 2nd ed.; Sheppard, C., Ed.; Elsevier Ltd.: London, UK, 2019; pp. 329–351. [Google Scholar]
- Sharma, S.; Bhardwaj, A.; Thakur, M.; Saini, A. Understanding microplastic pollution of marine ecosystem: A review. Environ. Sci. Pollut. Res. 2023, 31, 41402–41445. [Google Scholar] [CrossRef]
- Amelia, T.S.M.; Khalik, W.M.A.W.M.; Ong, M.C.; Shao, Y.T.; Pan, H.-J.; Bhubalan, K. Marine microplastics as vectors of major ocean pollutants and its hazards to the marine ecosystem and humans. Prog. Earth Planet Sci. 2021, 8, 12. [Google Scholar]
- Xia, Y.; Niu, S.; Yu, J. Microplastics as vectors of organic pollutants in aquatic environment: A review on mechanisms, numerical models, and influencing factors. Sci. Total Environ 2023, 887, 164008. [Google Scholar]
- Tang, G.; Liu, M.; Zhou, Q.; He, H.; Chen, K.; Zhang, H.; Hu, J.H.; Huang, Q.H.; Luo, Y.M.; Ke, H.W.; et al. Microplastics and polycyclic aromatic hydrocarbons (PAHs) in Xiamen coastal areas: Implications for anthropogenic impacts. Sci. Total Environ. 2018, 634, 811–820. [Google Scholar]
- Zhao, X.; Jin, H.; Ji, Z.; Li, D.; Kaw, H.Y.; Chen, J.; Xie, Z.; Zhang, T. PAEs and PAHs in the surface sediments of the East China Sea: Occurrence, distribution and influence factors. Sci. Total Environ. 2020, 703, 134763. [Google Scholar]
- Mehraie, A.; Shariatifar, N.; Arabameri, M.; Moazzen, M.; Mortazavian, A.M.; Sheikh, F.; Sohrabvandi, S. Determination of phthalate acid esters (PAEs) in bottled water distributed in Tehran: A health risk assessment study. Int. J. Environ. Anal. Chem. 2022, 104, 2417–2431. [Google Scholar] [CrossRef]
- Yang, Y.; Zhao, Z.; Chang, Y.; Wang, H.; Wang, H.; Dong, W.; Yan, G. PAHs and PAEs in the surface sediments from Nenjiang Rriver and the second Songhua River, China: Distribution, composition and risk assessment. Process Saf. Environ. Prot. 2023, 178, 765–775. [Google Scholar]
- Grmasha, R.A.; Abdulameer, M.H.; Stenger-Kovács, C.; Al-Sareji, O.J.; Al-Gazali, Z.; Al-Juboori, R.A.; Meiczinger, M.; Hashim, K.S. Polycyclic aromatic hydrocarbons in the surface water and sediment along Euphrates River system: Occurrence, sources, ecological and health Risk assessment. Mar. Pollut. Bull. 2023, 187, 114568. [Google Scholar] [CrossRef] [PubMed]
- Paluselli, A.; Kim, S.K. Horizontal and vertical distribution of phthalates acid ester (PAEs) in seawater and sediment of East China Sea and Korean South Sea: Traces of plastic debris? Mar. Pollut. Bull. 2020, 151, 110831. [Google Scholar] [CrossRef] [PubMed]
- Honda, M.; Suzuki, N. Toxicities of polycyclic aromatic hydrocarbons for aquatic animals. Int. J. Environ. Res. Public Health 2020, 17, 1363. [Google Scholar] [PubMed]
- Tian, J.; Lu, Z.; Sanganyado, E.; Wang, Z.; Du, J.; Gao, X.; Gan, Z.W.; Wu, J. Trophic transfer of polycyclic aromatic hydrocarbons in marine mammals based on isotopic determination. Sci. Total Environ. 2023, 875, 162531. [Google Scholar]
- Oliva, A.L.; La Colla, N.S.; Arias, A.H.; Blasina, G.E.; Lopez Cazorla, A.; Marcovecchio, J.E. Distribution and human health risk assessment of pahs in four fish species from a SW Atlantic estuary. Environ. Sci. Pollut. Res. 2017, 24, 18979–18990. [Google Scholar] [CrossRef]
- Ferrante, M.; Zanghì, G.; Cristaldi, A.; Copat, C.; Grasso, A.; Fiore, M.; Signorelli, S.S.; Zuccarello, P.; Oliveri Conti, G. PAHs in seafood from the Mediterranean Sea: An exposure risk assessment. Food Chem. Toxicol. 2018, 115, 385–390. [Google Scholar] [CrossRef]
- Sun, J.; Pan, L.; Cao, Y.; Li, Z. Biomonitoring of polycyclic aromatic hydrocarbons (PAHs) from Manila clam Ruditapes philippinarum in Laizhou, Rushan and Jiaozhou, Bays of China, and investigation of its relationship with human carcinogenic risk. Mar. Pollut. Bull. 2020, 160, 111556. [Google Scholar] [CrossRef]
- Eça, G.F.; Albergaria-Barbosa, A.C.R.; de Souza, M.M.; Costa, P.G.; Leite, A.S.; Fillmann, G.; Hatje, V. Polycyclic aromatic hydrocarbons in sediments and shellfish from Todos os Santos Bay, Brazil. Mar. Pollut. Bull. 2021, 173, 112944. [Google Scholar] [CrossRef]
- Liu, B.; Liang, L.V.; Ding, L.; Gao, L.; Li, J.; Ma, X.; Yu, Y. Comparison of phthalate esters (PAEs) in freshwater and marine food webs: Occurrence, bioaccumulation, and trophodynamics. J. Hazard. Mater. 2024, 466, 133534. [Google Scholar] [CrossRef] [PubMed]
- Thuy, H.T.T.; Loan, T.T.C.; Phuong, T.H. The potential accumulation of polycyclic aromatic hydrocarbons in phytoplankton and bivalves in Can Gio coastal wetland, Vietnam. Environ. Sci. Pollut. Res. 2018, 25, 17240–17249. [Google Scholar] [CrossRef] [PubMed]
- Tran-Lam, T.-T.; Quan, T.C.; Bui, M.Q.; Dao, Y.H.; Le, G.T. Endocrine-disrupting chemicals in Vietnamese marine fish: Occurrence, distribution, and risk assessment. Sci. Total Environ. 2024, 908, 168305. [Google Scholar] [PubMed]
- WHO/FAO. Evaluation of Certain Food Additives and Contaminants (Technical Report No. 751); Cambridge University Press: Cambridge, UK, 1987. [Google Scholar]
- Abhishek; Chakkaravarthi, S.; Agarwal, T. Fish consumption patterns and health risk assessment of polycyclic aromatic hydrocarbons and polychlorinated biphenyls in fried and grilled fish products and mitigation strategies. Toxicol. Rep. 2025, 14, 101953. [Google Scholar] [CrossRef]
- USEPA. Exposure Factors Handbook; United States Environmental Protection Agency: Philadelphia, PA, USA, 1997. [Google Scholar]
- EPA. Guidance for Assessing Chemical Contaminant Data for Use in Fish Advisories; U.S. Environmental Protection Agency: Washington, DC, USA, 2000. [Google Scholar]
- Ju, Y.-R.; Chen, C.-F.; Wang, M.-H.; Chen, C.-W.; Dong, C.-D. Assessment of polycyclic aromatic hydrocarbons in seafood collected from coastal aquaculture ponds in Taiwan and human health risk assessment. J. Hazard. Mater. 2022, 421, 126708. [Google Scholar] [CrossRef]
- Hu, H.; Mao, L.; Fang, S.; Xie, J.; Zhao, M.; Jin, H. Occurrence of phthalic acid esters in marine organisms from Hangzhou Bay, China: Implications for human exposure. Sci. Total Environ. 2020, 721, 137605. [Google Scholar]
- Su, Z.H.; Wang, C.; Zhou, X.; He, M.J. Organophosphate esters and phthalate esters in marine fishes from a coastal area of China: Occurrence, tissue distribution, trophic transfer, and human exposure. Mar. Environ. Res. 2025, 208, 107135. [Google Scholar] [CrossRef]
- Savoca, D.; Barreca, S.; Lo Coco, R.; Punginelli, D.; Orecchio, S.; Maccotta, A. Environmental aspect concerning phthalates contamination: Analytical approaches and assessment of biomonitoring in the aquatic environment. Environments 2023, 10, 99. [Google Scholar] [CrossRef]
- Marmara, D.; Brundo, M.V.; Pecoraro, R.; Scalisi, E.M.; Contino, M.; Sica, C.; Ferruggia, G.; Indelicato, S.; Velardita, R.; Tiralongo, F.; et al. plastic additives in commercial fish of Aegean and Ionian Seas and potential hazard to human health. Front. Mar. Sci. 2024, 11, 1334237. [Google Scholar] [CrossRef]
- Cho, Y.; Shim, W.J.; Jang, M.; Han, G.M.; Hong, S.H. Abundance and characteristics of microplastics in market bivalves from South Korea. Environ. Pollut. 2019, 245, 1107–1116. [Google Scholar] [CrossRef]
- Li, J.; Lusher, A.L.; Rotchell, J.M.; Deudero, S.; Turra, A.; Bråte, I.L.N.; Sun, C.G.; Hossain, M.S.; Li, Q.P.; Kolandhasamy, P.; et al. Using mussel as a global bioindicator of coastal microplastic pollution. Environ. Pollut. 2019, 244, 522–533. [Google Scholar] [CrossRef]
- Cheng, Z.; Nie, X.-P.; Wang, H.-S.; Wong, M.-H. Risk assessments of human exposure to bioaccessible phthalate esters through market fish consumption. Environ. Int. 2013, 57–58, 75–80. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Wang, Y.; Tan, Y.; Jiang, C.; Li, T.; Yang, Y.; Zhang, Z. Phthalate esters in the largest river of Asia: An exploration as indicators of microplastics. Sci. Total Environ. 2023, 902, 166058. [Google Scholar] [PubMed]
- Cao, Y.; Lin, H.; Zhang, K.; Xu, S.; Yan, M.; Leung, K.M.Y.; Lam, P.K.S. Microplastics: A major source of phthalate esters in aquatic environments. J. Hazard. Mater. 2022, 32, 128731. [Google Scholar]
- Hoang, T.T.T.; Luu, P.T.; Loan, T.T.C.; Dong, N.V.; Bao, L.D.; Yen, T.T.H.; Huy, D.X. Bioaccumulation of polycyclic aromatic hydrocarbons (PAHs) in green mussels (Perna viridis) from Cangio Area, Hochiminh City. VNU J. Sci. Earth Environ. Sci. 2020, 36, 38–45. [Google Scholar] [CrossRef]
- Pham, L.T.; Hoang, T.T.T.; Tu, L.C.T.; Tran, Y.H.T.; Le, B.D.; Nguyen, D.V.; Do, X.H.; Thai, N.V. Bioaccumulation and health risk assessment of polycyclic aromatic hydrocarbons in oyster (Crassostrea sp.) and gastropod (Cymatium sp.) species from the Can Gio coastal wetland in Vietnam. J. Mar. Freshw. Res. 2020, 71, 617–626. [Google Scholar]
- Silva, D.C.C.; Marques, J.C.; Gonçalves, A.M.M. Polycyclic aromatic hydrocarbons in commercial marine bivalves: Abundance, main impacts of single and combined exposure and potential impacts for human health. Mar. Pollut. Bull. 2024, 209, 117295. [Google Scholar] [CrossRef]
- Bouzidi, I.; Fkiri, A.; Sellami, B.; Harrath, A.H.; Boufahja, F.; Mezni, A.; Vidal, L.; Vaulot, C.; Josien, L.; Beyrem, H.; et al. Does the photocatalytic activity of nanoparticles protect the marine mussel Mytilus galloprovincialis from polycyclic aromatic hydrocarbon toxicity? Environ. Sci. Pollut. Res. 2021, 28, 44301–44314. [Google Scholar] [CrossRef]
- Phan, D.Q.; Vi, T.P.; Tran, T.M.; Nguyen, T.N.; Truong, T.K.; Dang, L.H.B.; Pham, H.V.; Le, H.T. Monitoring of polycyclic aromatic hydrocarbons (PAHs) in several fish species collected from some lakes in Hanoi area. Vietnam J. Sci. Technol. 2017, 22, 19–23. [Google Scholar]
- Baumard, P.; Budzinski, H.; Garrigues, P. Polycyclic aromatic hydrocarbons in sediments and mussels of the western Mediterranean sea. Environ. Toxicol. Chem. 1998, 17, 765–776. [Google Scholar] [CrossRef]
- Cheung, K.C.; Leung, H.M.; Kong, K.Y.; Wong, M.H. Residual levels of DDTs and PAHs in freshwater and marine fish from Hong Kong markets and their health risk assessment. Chemosphere 2007, 66, 460–468. [Google Scholar] [CrossRef] [PubMed]
- Vaezzadeh, V.; Zakaria, M.P.; Bong, C.W.; Masood, N.; Mohsen Magam, S.; Alkhadher, S. Mangrove oyster (Crassostrea belcheri) as a biomonitor species for bioavailability of polycyclic aromatic hydrocarbons (PAHs) from sediment of the West Coast of Peninsular Malaysia. Polycycl. Aromat. Compd. 2019, 39, 470–485. [Google Scholar]
- Onozato, M.; Nishigaki, A.; Okoshi, K. Polycyclic aromatic hydrocarbons in sediments and bivalves on the Pacific Coast of Japan: Influence of tsunami and fire. PLoS ONE 2016, 11, e0156447. [Google Scholar]
- Isobe, T.; Takada, H.; Kanai, M.; Tsutsumi, S.; Isobe, K.O.; Boonyatumanond, R.; Zakaria, M.P. Distribution of polycyclic aromatic hydrocarbons (PAHs) and phenolic endocrine disrupting chemicals in South and Southeast Asian mussels. Environ. Monit. Assess. 2007, 135, 423–440. [Google Scholar] [CrossRef]
- Monjezi, S.D.; Bakhtiyari, A.R.; Alavi-Yeganeh, M.S. Sourcing aliphatic and polycyclic aromatic hydrocarbons (PAHs) in Jinga shrimp (Metapenaeus affinis) muscle tissues and surface sediments (study case: Northwest Persian Gulf). Environ. Sci. Pollut. Res. 2024, 31, 28644–28657. [Google Scholar] [CrossRef]
- Zhang, C.; Li, Y.; Wang, C.; Feng, Z.; Hao, Z.; Yu, W.; Wang, T.; Zou, X. Polycyclic aromatic hydrocarbons (PAHs) in marine organisms from two fishing grounds, South Yellow Sea, China: Bioaccumulation and human health risk assessment. Mar. Pollut. Bull. 2020, 153, 110995. [Google Scholar] [CrossRef]
- Li, Y.; Guo, N.; Zou, X.; Li, P.; Zou, S.; Luo, J.; Yang, Y. Pollution level and health risk assessment of polycyclic aromatic hydrocarbons in marine fish from two coastal regions, the South China Sea. Mar. Pollut. Bull. 2021, 168, 112376. [Google Scholar] [CrossRef]
- Hassaan, M.A.; Ragab, S.; Sikaily, A.E.; Nemr, A.E. Sources of hydrocarbons and their risk assessment in seawater and sediment samples collected from the Nile Delta coast of the Mediterranean Sea. Sci. Rep. 2024, 14, 5082. [Google Scholar] [CrossRef]
- Shi, W.; Xu, M.; Liu, Q.; Xie, S. Polycyclic aromatic hydrocarbons in seawater, surface sediment, and marine organisms of Haizhou Bay in Yellow Sea, China: Distribution, source apportionment, and health risk assessment. Mar. Pollut. Bull. 2022, 174, 113280. [Google Scholar] [CrossRef]
- IARC. Some non-heterocyclic polycyclic aromatic hydrocarbons and some related exposures; Working group on the evaluation of carcinogenic risks to humans. IARC Monogr. Eval. Carcinog. Risks Hum. 2010, 92, 1–183. [Google Scholar]
- EFSA. Polycyclic aromatic hydrocarbons in food—Scientific opinion of the panel on contaminants in the food. EFSA J. 2008, 724, 1–114. [Google Scholar]
- Wang, Q.; Lu, D.; Xiong, Y.R.; Peng, F.; Li, J.Y.; Wu, F.; Chu, Y.P.; Sun, R.H.; Tian, S.Q. Occurrence of polycyclic aromatic hydrocarbons (PAHs) in the seafood from an important fishing area in the East China Sea and a comparison between seafood from different origins. Environ. Monit. Assess. 2022, 194, 528. [Google Scholar] [CrossRef] [PubMed]
- de Melo, A.P.Z.; Hoff, R.B.; Molognoni, L.; Kleemann, C.R.; de Oliveira, T.; de Oliveira, L.V.A.; Daguer, H.; Barreto, P.L.M. Determination of polycyclic aromatic hydrocarbons in seafood by PLE-LC-APCI-MS/MS and preliminary risk assessment of the Northeast Brazil oil spill. Food Anal. Methods 2022, 15, 1826–1842. [Google Scholar] [CrossRef]
- European Union. Commission Regulation (EC) No. 1881/2006 of 19 December 2006 Setting Maximum Levels for Certain Contaminants in Foodstuffs. Off. J. Eur. Union 2006, 364, 5–24. [Google Scholar]
- Said, T.O.; Idris, A.M.; Sahlabji, T. Combining relationship indices, human risk indices, multivariate statistical analysis and international guidelines for assessing the residue levels of USEPA-PAHs in seafood. Polycycl. Aromat. Compd. 2020, 40, 758–773. [Google Scholar] [CrossRef]
- Shi, J.C.; Zheng, G.J.S.; Wong, M.H.; Liang, H.; Li, Y.L.; Wu, Y.L.; Li, P.; Liu, W.H. Health risks of polycyclic aromatic hydrocarbons via fish consumption in Haimen Bay (China), downstream of an e-waste recycling site (Guiyu). Environ. Res. 2016, 147, 233–240. [Google Scholar] [CrossRef]
- USEPA. Proposed Guidelines for Carcinogenic Risk Assessment; Office of Science and Technology: Washington, DC, USA, 1996. [Google Scholar]
- Kpoclou, Y.E.; Adinsi, L.; Anihouvi, V.B.; Douny, C.; Brose, F.; Igout, A.; Scippo, M.L.; Hounhouigan, D.J. Steam precooking, an effective pretreatment to reduce contamination with polycyclic aromatic hydrocarbons in traditionally smoked shrimp. J. Food Sci. Technol. 2021, 58, 4646–4653. [Google Scholar] [CrossRef]
- Sharifiarab, G.; Ahmadi, M.; Shariatifar, N.; Ariaii, P. Investigating the effect of type of fish and different cooking methods on the residual amount of polycyclic aromatic hydrocarbons (PAHs) in some Iranian fish: A health risk assessment. Food Chem. 2023, 19, 100789. [Google Scholar] [CrossRef]

| PAHs | Abb. | Fish | |||
|---|---|---|---|---|---|
| Tb-XT | Tb-MG | Tb-CR | El-TT | ||
| Acenaphthylene | Acy | ND | ND | 3.06 ± 0.13 b | 1.95 ± 0.10 a |
| Fluorene | Flu | 7.74 ± 1.58 a | ND | 8.95 ± 0.25 ab | 6.70 ± 0.22 ab |
| Phenanthrene | Phe | 10.17 ± 1.86 a | 4.21 ± 0.64 b | 13.16 ± 050 d | 7.78 ± 0.40 c |
| Pyrene | Pyr | ND | 13.34 ± 0.61 | ND | ND |
| Benz[a]anthracene | BaA | ND | ND | ||
| Indeno[1,2,3-cd] pyrene | IP | 3.10 ± 1.06 | ND | ND | ND |
| PAHs | Abb. | Shrimp | |||
| Lv-XT | Lv-MG | Lv-TT | Lv-CR | ||
| Acenaphthylene | Acy | 0.62 ± 0.29 a | ND | 1.03 ± 0.13 a | ND |
| Fluorene | Flu | 3.36 ± 0.65 a | ND | 4.02 ± 0.42 b | 4.60 ± 0.55 a |
| Phenanthrene | Phe | 3.33 ± 0.92 a | 2.91 ± 0.19 a | 4.09 ± 0.20 ab | 5.83 ± 0.37 abc |
| Pyrene | Pyr | 2.25 ± 0.36 a | 14.09 ± 0.35 b | ND | ND |
| Benz[a]anthracene | BaA | ND | ND | ND | ND |
| Indeno[1,2,3-cd] pyrene | IP | ND | ND | ND | 17.51 ± 2.05 |
| PAHs | Abb. | Mollusk | |||
| Ba-XT | Ba-MG | Ba-CR | Mh-TT | ||
| Acenaphthylene | Acy | 0.68 ± 0.10 a | ND | 0,71* | 0.69 ± 0.06 a |
| Fluorene | Flu | 3.48 ± 0.72 a | ND | 4.55 ± 0.67 a | 4.19 ± 0.29 a |
| Phenanthrene | Phe | 3.42 ±0.97 a | 5.47 ± 0.58 ab | 9.22 ± 1.74 abc | 6.37 ± 0.56 ab |
| Pyrene | Pyr | 6.34 ±2.15 a | 40.86 ± 2.90 b | 11.56 ± 1.47 c | 36.09 ± 1.24 d |
| Benz[a]anthracene | BaA | ND | 1.10 * | ND | |
| Indeno[1,2,3-cd] pyrene | IP | ND | 5.95 ± 2.45 | ND | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nguyen, X.-V.; Hoang, T.-D.; Nguyen-Nhat, N.-T.; Nguyen, Q.-H.; Nguyen, X.-T.; Nguyen, T.-H.; Truong, S.H.T.; Nguyen, M.-N.T.; Dao, V.-H. Polycyclic Aromatic Hydrocarbons (PAHs) and Phthalate Esters (PAEs) in the Farmed Fishes from Khanh Hoa, Viet Nam: Level and Health Risk Assessment. Foods 2025, 14, 3518. https://doi.org/10.3390/foods14203518
Nguyen X-V, Hoang T-D, Nguyen-Nhat N-T, Nguyen Q-H, Nguyen X-T, Nguyen T-H, Truong SHT, Nguyen M-NT, Dao V-H. Polycyclic Aromatic Hydrocarbons (PAHs) and Phthalate Esters (PAEs) in the Farmed Fishes from Khanh Hoa, Viet Nam: Level and Health Risk Assessment. Foods. 2025; 14(20):3518. https://doi.org/10.3390/foods14203518
Chicago/Turabian StyleNguyen, Xuan-Vy, Trung-Du Hoang, Nhu-Thuy Nguyen-Nhat, Quoc-Hoi Nguyen, Xuan-Thuy Nguyen, Trung-Hieu Nguyen, Si Hai Trinh Truong, My-Ngan T. Nguyen, and Viet-Ha Dao. 2025. "Polycyclic Aromatic Hydrocarbons (PAHs) and Phthalate Esters (PAEs) in the Farmed Fishes from Khanh Hoa, Viet Nam: Level and Health Risk Assessment" Foods 14, no. 20: 3518. https://doi.org/10.3390/foods14203518
APA StyleNguyen, X.-V., Hoang, T.-D., Nguyen-Nhat, N.-T., Nguyen, Q.-H., Nguyen, X.-T., Nguyen, T.-H., Truong, S. H. T., Nguyen, M.-N. T., & Dao, V.-H. (2025). Polycyclic Aromatic Hydrocarbons (PAHs) and Phthalate Esters (PAEs) in the Farmed Fishes from Khanh Hoa, Viet Nam: Level and Health Risk Assessment. Foods, 14(20), 3518. https://doi.org/10.3390/foods14203518






